Recent Advances in Tissue Adhesives for Clinical Medicine
Abstract
1. Introduction
2. The Mechanisms of Adhesion
2.1. Van der Waals Force
2.2. Capillary Forces
2.3. Hydrogen Bonding
2.4. Static-Electric Force
2.5. Chemical Bonds
3. Adhesion Measurements
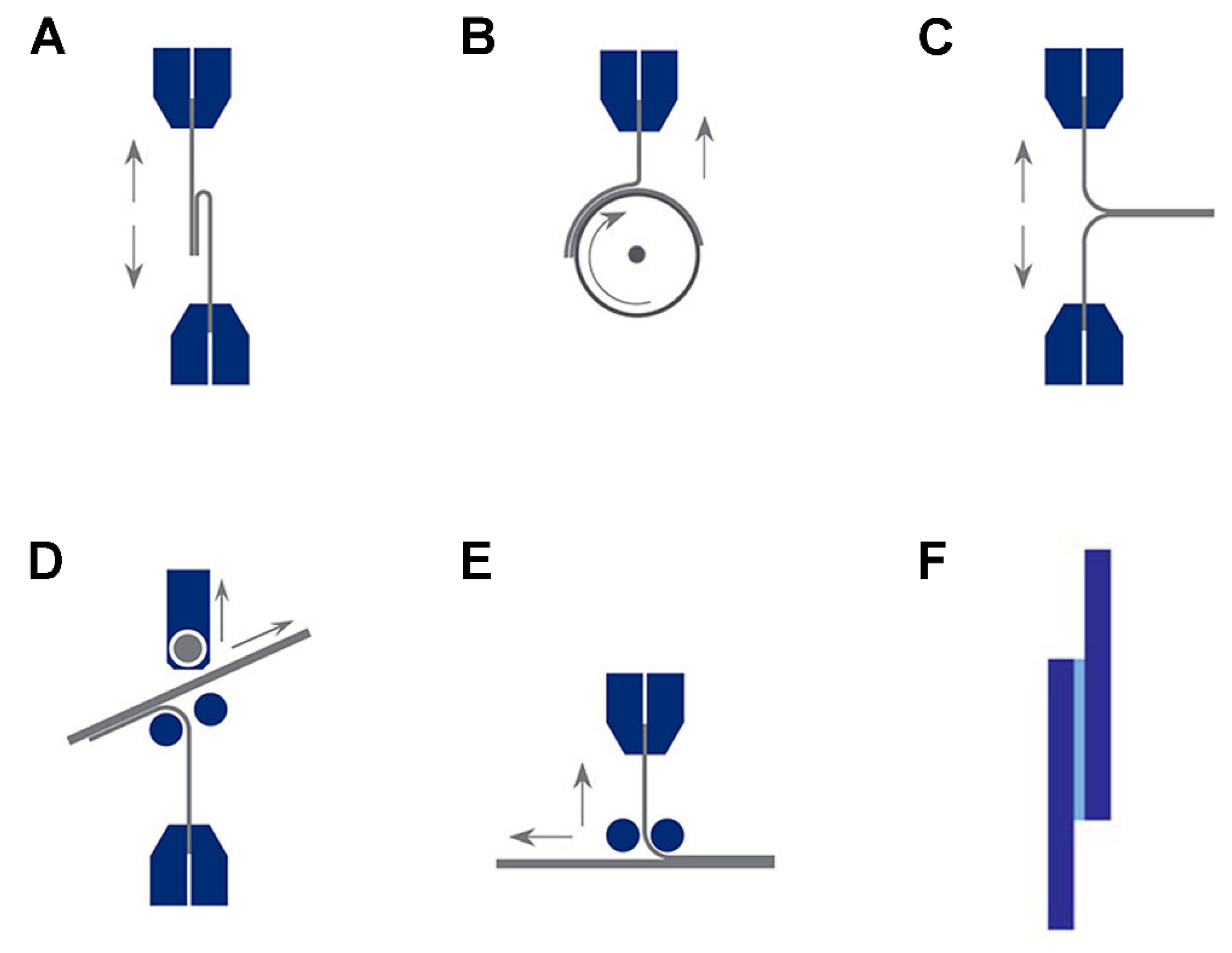
3.1. Tension Test
3.2. Peel Tests
3.3. Lap Shear Tests
3.4. Pull out/Pull-off Test
4. Adhesives in the Clinic
4.1. Natural or Biological Adhesives
4.1.1. Fibrin-Based Adhesives
4.1.2. Collagen-Based Adhesives
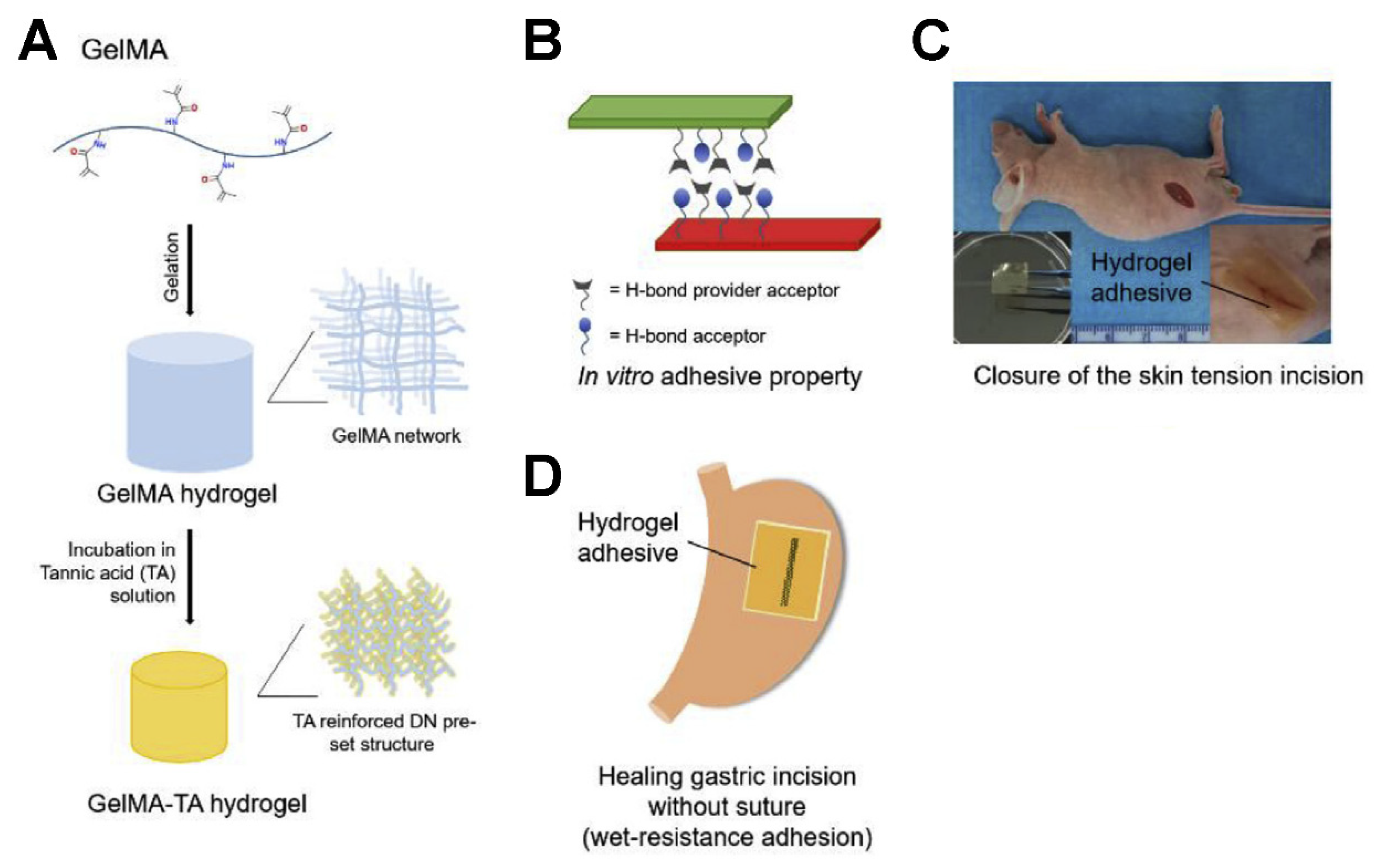
4.1.3. Gelatin-Based Adhesives
4.1.4. Albumin-Based Adhesives
4.1.5. Chitosan-Based Adhesives
4.1.6. Dextran-Based Adhesives
4.1.7. Chondroitin Sulfate-Based Adhesives
4.2. Synthetic and Semisynthetic Adhesives
4.2.1. Polycyanoacrylates
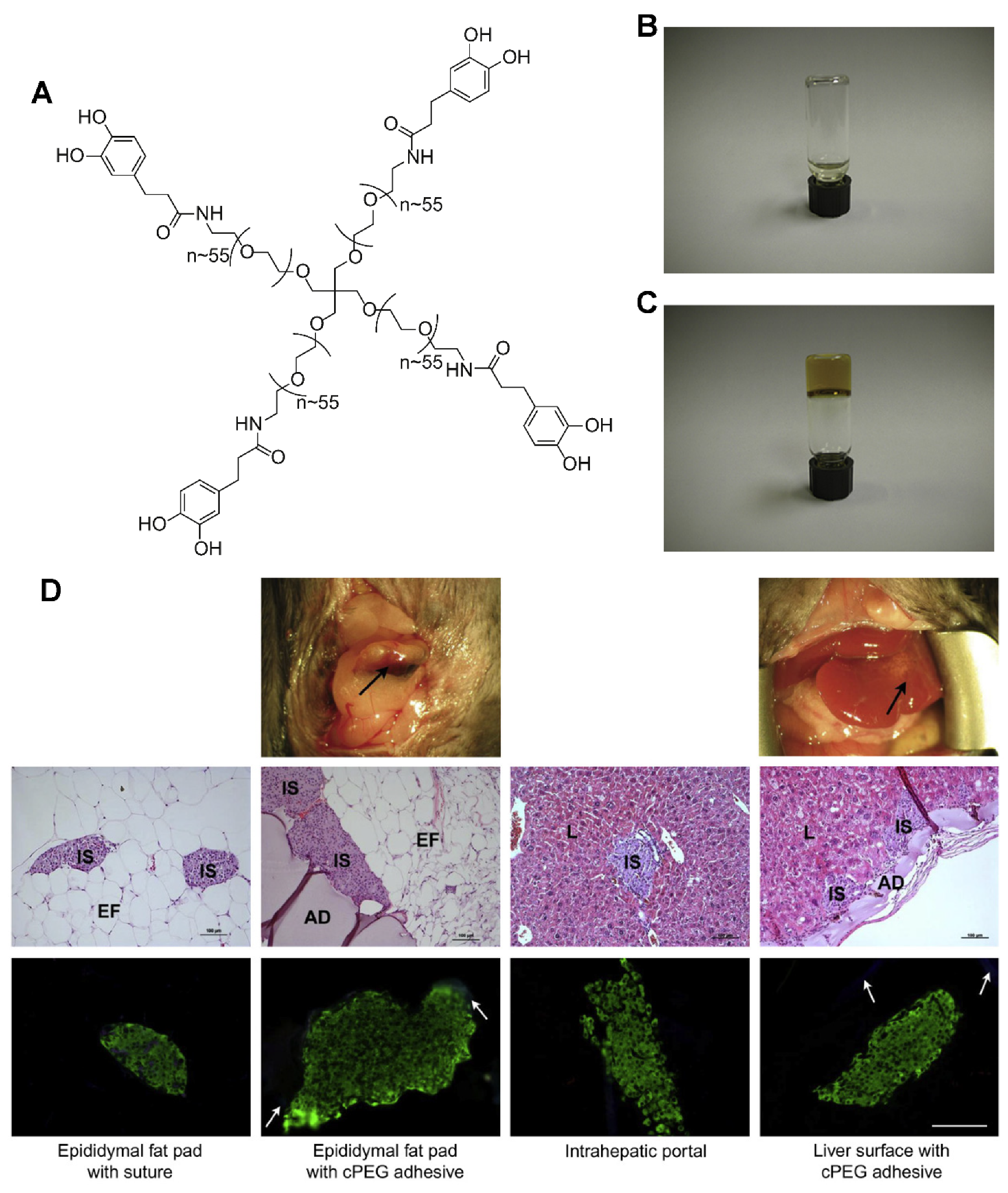
4.2.2. Poly (ethylene glycol)
4.2.3. Polyurethanes
4.2.4. Dendrimers and Hyperbranched Polymers
4.3. Biomimetic Adhesives
4.3.1. Mussel-Inspired Adhesives
4.3.2. Gecko-Inspired Adhesives
5. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Bouten, P.J.M.; Zonjee, M.; Bender, J.; Yauw, S.T.K.; van Goor, H.; van Hest, J.C.M.; Hoogenboom, R. The chemistry of tissue adhesive materials. Prog. Polymer Sci. 2014, 39, 1375–1405. [Google Scholar] [CrossRef]
- Duarte, A.P.; Coelho, J.F.; Bordado, J.C.; Cidade, M.T.; Gil, M.H. Surgical adhesives: Systematic review of the main types and development forecast. Prog. Polymer Sci. 2012, 37, 1031–1050. [Google Scholar] [CrossRef]
- Spotnitz, W.D.; Burks, S. Hemostats, sealants, and adhesives: Components of the surgical toolbox. Transfusion 2008, 48, 1502–1516. [Google Scholar] [CrossRef] [PubMed]
- Spotnitz, W.D.; Burks, S. State-of-the-art review: Hemostats, sealants, and adhesives II: Update as well as how and when to use the components of the surgical toolbox. Clin. Appl. Thromb Hemost. 2010, 16, 497–514. [Google Scholar] [CrossRef] [PubMed]
- Spotnitz, W.D.; Burks, S. Hemostats, sealants, and adhesives III: A new update as well as cost and regulatory considerations for components of the surgical toolbox. Transfusion 2012, 52, 2243–2255. [Google Scholar] [CrossRef] [PubMed]
- Jarrett, P.; Coury, A. Tissue adhesives and sealants for surgical applications. In Joining and Assembly of Medical Materials and Devices; Elsevier: Amsterdam, The Netherlands, 2013; pp. 449–490. [Google Scholar]
- Bold, E.L.; Wanamaker, J.R.; Zins, J.E.; Lavertu, P. The use of fibrin glue in the healing of skin flaps. Am. J. Otolaryngol. 1996, 17, 27–30. [Google Scholar] [CrossRef]
- Lund, C. Medical adhesives in the NICU. Newborn Infant Nurs. Rev. 2014, 14, 160–165. [Google Scholar] [CrossRef]
- Mati-Baouche, N.; Elchinger, P.-H.; de Baynast, H.; Pierre, G.; Delattre, C.; Michaud, P. Chitosan as an adhesive. Eur. Polymer J. 2014, 60, 198–212. [Google Scholar] [CrossRef]
- Sanders, R.P.; Goodman, N.C.; Amiss, J.L.R.; Pierce, R.A.; Moore, M.M.; Marx, G.; Morgan, R.F.; Spotnitz, W.D. Effect of fibrinogen and thrombin concentrations on mastectomy seroma prevention. J. Surg. Res. 1996, 61, 65–70. [Google Scholar] [CrossRef]
- Moore, M.; Burak, W.E., Jr.; Nelson, E.; Kearney, T.; Simmons, R.; Mayers, L.; Spotnitz, W.D. Fibrin sealant reduces the duration and amount of fluid1 drainage after axillary dissection: A randomized prospective clinical trial. Am. Coll. Surg. 2001, 192, 591–599. [Google Scholar] [CrossRef]
- Garcia-Rinaldi, R.; Simmons, P.; Salcedo, V.; Howland, C. A technique for spot application of fibrin glue during open heart operations. Ann. Thorac. Surg. 1989, 47, 59–61. [Google Scholar] [CrossRef]
- Spotnitz, W.D.; Dalton, M.S.; Baker, J.W.; Nolan, S.P. Reduction of perioperative hemorrhage by anterior mediastinal spray application of fibrin glue during cardiac operations. Ann. Thorac. Surg. 1987, 44, 529–531. [Google Scholar] [CrossRef]
- Matthew, T.L.; Spotnitz, W.D.; Kron, I.L.; Daniel, T.M.; Tribble, C.G.; Nolan, S.P. Four years’ experience with fibrin sealant in thoracic and cardiovascular surgery. Ann. Thorac. Surg. 1990, 50, 40–43. [Google Scholar] [CrossRef]
- Vakalopoulos, K.A.; Daams, F.; Wu, Z.; Timmermans, L.; Jeekel, J.J.; Kleinrensink, G.-J.; van der Ham, A.; Lange, J.F. Tissue adhesives in gastrointestinal anastomosis: A systematic review. J. Surg. Res. 2013, 180, 290–300. [Google Scholar] [CrossRef] [PubMed]
- Lustgarten, L.; Abadi, J.R.; Sancevic, R.; Meneses, P.; Morrel, A.P.; Lugo, J. Use of a protein-based tissue adhesive as an aid for the surgical reconstruction of advanced and recurrent skin cancer tumors to the head and neck region: A technical. Surg. Neurol. 2007, 68, 53–59. [Google Scholar] [CrossRef] [PubMed]
- Yoo, J. PD23. Autologous platelet adhesives: Applications in soft-tissue surgery of the head and neck. Oral Oncol. Suppl. 2009, 3, 15–16. [Google Scholar] [CrossRef]
- Ishitani, M.B.; McGahren, E.D.; Sibley, D.A.; Spotnitz, W.D.; Rodgers, B.M. Laparoscopically applied fibrin glue in experimental liver trauma. J. Pediatric Surg. 1989, 24, 867–871. [Google Scholar] [CrossRef]
- Han, B.; Meng, B.; Cui, G.; Wu, Z.; Yu, L.; Zhu, H.; Ma, H.; Shi, J.; Lv, Y. Regeneration of splenic autotransplants attached on liver by a tissue adhesive. Transplant. Proc. 2010, 42, 1944–1948. [Google Scholar] [CrossRef]
- Kumar, A.; Maartens, N.F.; Kaye, A.H. Evaluation of the use of BioGlue® in neurosurgical procedures. J. Clin. Neurosci. 2003, 10, 661–664. [Google Scholar] [CrossRef]
- Buckley, M.J.; Beckman, E.J. Adhesive use in oral and maxillofacial surgery. Oral Maxillofac. Surg. Clin. N. Am. 2010, 22, 195–199. [Google Scholar] [CrossRef]
- Hewitt, C.W.; Marra, S.W.; Kann, B.R.; Tran, H.S.; Puc, M.M.; Chrzanowski, F.A.; Tran, J.-L.V.; Lenz, S.D.; Cilley, J.H.; Simonetti, V.A.; et al. BioGlue surgical adhesive for thoracic aortic repair during coagulopathy: Efficacy and histopathology. Ann. Thorac. Surg. 2001, 71, 1609–1612. [Google Scholar] [CrossRef]
- Wilkinson, J.; Chikhani, M. The use of Histoacryl® skin adhesive to secure thoracic epidural catheters: A volunteer study. Reg. Anesth. Pain Med. 2007, 32, 148. [Google Scholar] [CrossRef]
- Farrar, D.F. Bone adhesives for trauma surgery: A review of challenges and developments. Int. J. Adhes. Adhes. 2012, 33, 89–97. [Google Scholar] [CrossRef]
- Van Landuyt, K.L.; Snauwaert, J.; de Munck, J.; Peumans, M.; Yoshida, Y.; Poitevin, A.; Coutinho, E.; Suzuki, K.; Lambrechts, P.; van Meerbeek, B. Systematic review of the chemical composition of contemporary dental adhesives. Biomaterials 2007, 28, 3757–3785. [Google Scholar] [CrossRef]
- Spotnitz, W.D. Hemostats, sealants, and adhesives: A practical guide for the surgeon. Am. Surg. 2012, 78, 1305–1321. [Google Scholar]
- Sheikh, B.Y. Efficacy of acrylate tissue adhesive as vascular repair and hemostatic material. Ann. Vasc. Surg. 2007, 21, 56–60. [Google Scholar] [CrossRef]
- Awaja, F.; Gilbert, M.; Kelly, G.; Fox, B.; Pigram, P.J. Adhesion of polymers. Prog. Polymer Sci. 2009, 34, 948–968. [Google Scholar] [CrossRef]
- Peppas, N.A.; Buri, P.A. Surface, interfacial and molecular aspects of polymer bioadhesion on soft tissues. J. Control. Release 1985, 2, 257–275. [Google Scholar] [CrossRef]
- Chen, Q.; Pugno, N.M. Bio-mimetic mechanisms of natural hierarchical materials: A review. J. Mech. Behav. Biomed. Mater. 2013, 19, 3–33. [Google Scholar] [CrossRef]
- Delrio, F.W.; de Boer, M.P.; Knapp, J.A.; Reedy, E.D., Jr.; Clews, P.J.; Dunn, M.L. The role of van der Waals forces in adhesion of micromachined surfaces. Nat. Mater. 2005, 4, 629–634. [Google Scholar] [CrossRef]
- Ji, A.; Han, L.; Dai, Z. Adhesive contact in animal: Morphology, mechanism and bio-inspired application. Bionic Eng. 2011, 8, 345–356. [Google Scholar] [CrossRef]
- Autumn, K.; Sitti, M.; Liang, Y.A.; Peattie, A.M.; Hansen, W.R.; Sponberg, S.; Kenny, T.W.; Fearing, R.; Israelachvili, J.N.; Full, R.J. Evidence for van der Waals adhesion in gecko setae. Proc. Natl. Acad. Sci. USA 2002, 99, 12252–12256. [Google Scholar] [CrossRef] [PubMed]
- Hanna, G.B. Adhesion and detachment of the toe pads of tree frogs. WJP J. Exp. Biol. 1991, 155, 103–125. [Google Scholar]
- Drotlef, D.-M.; Stepien, L.; Kappl, M.; Barnes, W.J.P.; Butt, H.-J.; del Campo, A. Insights into the adhesive mechanisms of tree frogs using artificial mimics. Adv. Funct. Mater. 2013, 23, 1137–1146. [Google Scholar] [CrossRef]
- Lin, A.Y.M.; Brunner, R.; Chen, P.Y.; Talke, F.E.; Meyers, M.A. Underwater adhesion of abalone: The role of van der Waals and capillary forces. Acta Mater. 2009, 57, 4178–4185. [Google Scholar] [CrossRef]
- Autumn, K.; Liang, Y.A.; Hsieh, S.T.; Zesch, W.; Chan, W.P.; Kenny, T.W.; Fearing, R.; Full, R.J. Adhesive force of a single gecko foot-hair. Nature 2000, 405, 681–685. [Google Scholar] [CrossRef]
- Geim, A.K.D.; Grigorieva, S.V.; Novoselov, I.V.; Zhukov, K.S.; Shapoval, A.A.; Yu, S. Microfabricated adhesive mimicking gecko foot-hair. Nat. Mater. 2003, 2, 461–463. [Google Scholar]
- Butt, H.J.; Kappl, M. Normal capillary forces. Adv. Colloid Interface Sci. 2009, 146, 48–60. [Google Scholar] [CrossRef]
- Lee, H.; Lee, B.P.; Messersmith, P.B. A reversible wet/dry adhesive inspired by mussels and geckos. Nature 2007, 448, 338–341. [Google Scholar] [CrossRef]
- Huber, G.; Mantz, H.; Spolenak, R.; Mecke, K.; Jacobs, K.; Gorb, S.N.; Arzt, E. Evidence for capillarity contributions to gecko adhesion from single spatula nanomechanical measurements. Proc. Natl. Acad. Sci. USA 2005, 102, 16293–16296. [Google Scholar] [CrossRef]
- Eisner, T.; Aneshansley, D.J. Defense by foot adhesion in a beetle (Hemisphaerota cyanea). Proc. Natl. Acad. Sci. USA 2000, 97, 6568–6573. [Google Scholar] [CrossRef] [PubMed]
- Qian, J.; Gao, H. Scaling effects of wet adhesion in biological attachment systems. Acta Biomater. 2006, 2, 51–58. [Google Scholar] [CrossRef] [PubMed]
- Walker, G.; Yulf, A.B.; Ratcliffe, J. The adhesive organ of the blowfly, Calliphora vomitoria: A functional approach (Diptera: Calliphoridae). J. Zool. 1985, 205, 297–307. [Google Scholar] [CrossRef]
- Federle, W.; Baumgartner, W.; Holldobler, B.J. Biomechanics of ant adhesive pads: Frictional forces are rate-and temperature-dependent. Exp. Biol. 2004, 207, 67–74. [Google Scholar] [CrossRef]
- Kollman, P.A. Hydrogen bonding. Curr. Biol. 1999, 9, R501. [Google Scholar] [CrossRef][Green Version]
- Sijbesma, R.P.; Beijer, F.H.; Brunsveld, L.; Folmer, B.J.; Hirschberg, J.H.; Lange, R.F.; Lowe, J.K.; Meijer, E.W. Reversible polymers formed from self-complementary monomers using quadruple hydrogen bonding. Science 1997, 278, 1601–1604. [Google Scholar] [CrossRef]
- Faghihnejad, A.; Feldman, K.E.; Yu, J.; Tirrell, M.V.; Israelachvili, J.N.; Hawker, C.J.; Kramer, E.J.; Zeng, H. Adhesion and surface interactions of a self-healing polymer with multiple hydrogen-bonding groups. Adv. Funct. Mater. 2014, 24, 2322–2333. [Google Scholar] [CrossRef]
- Liu, B.; Wang, Y.; Miao, Y.; Zhang, X.; Fan, Z.; Singh, G.; Zhang, X.; Xu, K.; Li, B.; Hu, Z. Hydrogen bonds autonomously powered gelatin methacrylate hydrogels with super-elasticity, self-heal and underwater self-adhesion for sutureless skin and stomach surgery and E-skin. Biomaterials 2018, 171, 83–96. [Google Scholar] [CrossRef]
- Ahn, B.K.; Lee, D.W.; Israelachvili, J.N.; Waite, J.H. Underwater contact adhesion and microarchitecture in polyelectrolyte complexes actuated by solvent exchange. Nat. Mater. 2014, 13, 867–872. [Google Scholar] [CrossRef]
- Maboudian, R. Critical review: Adhesion in surface micromechanical structures. J. Vac. Sci. Technol. B 1997, 15, 1. [Google Scholar] [CrossRef]
- Oyane, A.; Ootsuka, T.; Hayama, K.; Sogo, Y.; Ito, A. Enhanced immobilization of acidic proteins in the apatite layer via electrostatic interactions in a supersaturated calcium phosphate solution. Acta Biomater. 2011, 7, 2969–2976. [Google Scholar] [CrossRef] [PubMed]
- Lee, I.C.; Wu, Y.C. Assembly of polyelectrolyte multilayer films on supported lipid bilayers to induce neural stem/progenitor cell differentiation into functional neurons. ACS Appl. Mater. Interfaces 2014, 6, 14439–14450. [Google Scholar] [CrossRef] [PubMed]
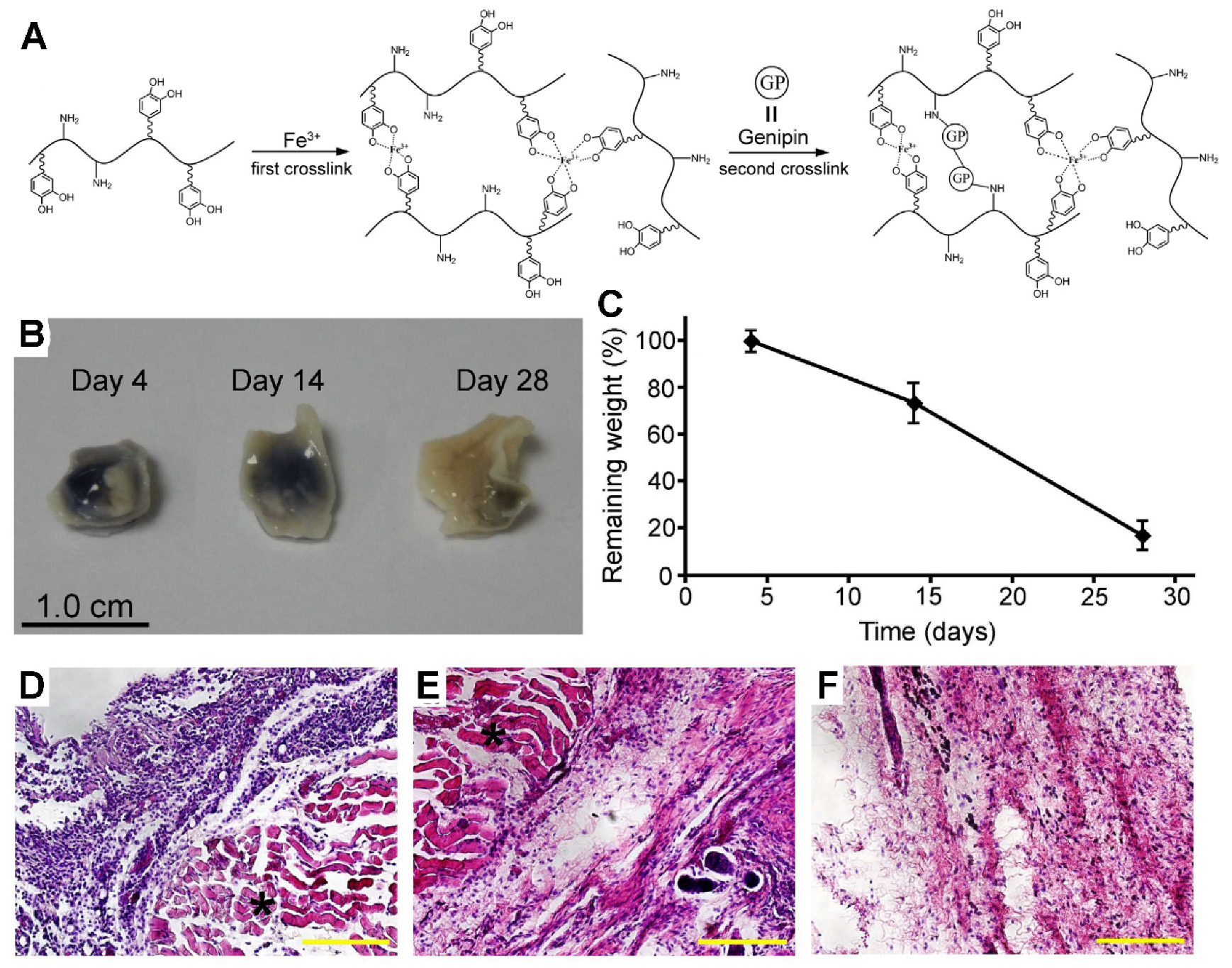
- Lu, X.; Shi, S.; Li, H.; Gerhard, E.; Lu, Z.; Tan, X.; Li, W.; Rahn, K.M.; Xie, D.; Xu, G.; et al. Magnesium oxide-crosslinked low-swelling citrate-based mussel-inspired tissue adhesives. Biomaterials 2020, 232, 119719. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Ge, L.; Mueller, A.; Carlson, M.A.; Teusink, M.J.; Shuler, F.D.; Xie, J. Twisting electrospun nanofiber fine strips into functional sutures for sustained co-delivery of gentamicin and silver. Nanomed. NBM 2017, 13, 1435–1445. [Google Scholar] [CrossRef]
- Noble, C.; Smulders, N.; Lewis, R.; Carré, M.J.; Franklin, S.E.; MacNeil, S.; Taylor, Z.A. Controlled peel testing of a model tissue for diseased aorta. J. Biomech. 2016, 49, 3667–3675. [Google Scholar] [CrossRef][Green Version]
- Diez, M.; Kang, M.-H.; Kim, S.-M.; Kim, H.-E.; Song, J. Hydroxyapatite (HA)/poly-l-lactic acid (PLLA) dual coating on magnesium alloy under deformation for biomedical applications. J. Mater. Sci. Mater. Med. 2016, 27, 34. [Google Scholar] [CrossRef]
- Bitton, R.; Josef, E.; Shimshelashvili, I.; Shapira, K.; Seliktar, D.; Bianco-Peled, H. Phloroglucinol-based biomimetic adhesives for medical applications. Acta Biomater. 2009, 5, 1582–1587. [Google Scholar] [CrossRef]
- Reece, T.B.; Maxey, T.S.; Kron, I.L. A prospectus on tissue adhesives. Am. J. Surg. 2001, 182, 40S–44S. [Google Scholar] [CrossRef]
- Anema, J.G.; Morey, A.F.; Harris, R.; MacPhee, M.; Cornum, R.L. Potential uses of absorbable fibrin adhesive bandage for genitourinary trauma. World J. Surg. 2001, 25, 1573–1577. [Google Scholar] [CrossRef]
- Borst, H.G.; Haverich, A.; Walterbusch, G.; Maatz, W. Fibrin adhesive: An important hemostatic adjunct in cardiovascular operations. J. Thorac. Cardiovasc. Surg. 1982, 84, 548–553. [Google Scholar] [CrossRef]
- Sierra, D.H. Fibrin sealant adhesive systems: A review of their chemistry, material properties and clinical applications. J. Biomater. Appl. 1993, 7, 309–352. [Google Scholar] [CrossRef] [PubMed]
- Albala, D.M. Fibrin sealants in clinical practice. Cardiovasc. Surg. 2003, 1, 5–11. [Google Scholar] [CrossRef]
- Wax, M.K.; Ramadan, H.H.; Ortiz, O.; Wetmore, S.J. Contemporary management of cerebrospinal fluid rhinorrhea. Otolaryngol. Head Neck. Surg. 1997, 116, 442–449. [Google Scholar] [CrossRef]
- Sawamura, Y.; Asaoka, K.; Terasaka, S.; Tada, M.; Uchida, T. Evaluation of application techniques of fibrin sealant to prevent cerebrospinal fluid leakage: A new device for the application of aerosolized fibrin glue. Neurosurgery 1999, 44, 332–337. [Google Scholar] [CrossRef]
- Shaffrey, C.I.; Spotnitz, W.D.; Shaffrey, M.E.; Jane, J.A. Neurosurgical applications of fibrin glue: Augmentation of dural closure in 134 patients. Neurosurgery 1990, 26, 207–210. [Google Scholar] [CrossRef]
- Stuart, J.D.; Kenney, J.G.; Lettieri, J.; Spotnitz, W.; Baker, J. Application of single-donor fibrin glue to burns. J. Burn Care Rehabil. 1988, 9, 619–622. [Google Scholar] [CrossRef]
- Jackson, M.R. Fibrin sealants in surgical practice: An overview. Am. J. Surg. 2001, 182, 1S–7S. [Google Scholar] [CrossRef]
- De la Garza, J.L.; Rumsey, E., Jr. Fibrin glue and hemostasis in liver trauma: A case report. J. Trauma 1990, 30, 512–513. [Google Scholar] [CrossRef]
- Kram, H.B.; del Junco, T.; Clark, S.R.; Ocampo, H.P.; Shoemaker, W.C. Techniques of splenic preservation using fibrin glue. J. Trauma 1990, 30, 97–101. [Google Scholar] [CrossRef]
- Ochsner, M.G.; Maniscalco-Theberge, M.E.; Champion, H.R. Fibrin glue as a hemostatic agent in hepatic and splenic trauma. J. Trauma 1990, 30, 884–887. [Google Scholar] [CrossRef]
- Sierra, D.H.; Eberhardt, A.W.; Lemons, J.E. Failure characteristics of multiple-component fibrin-based adhesives. J. Biomed. Mater. Res. 2002, 59, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Strausberg, R.L.; Link, R.P. Protein-based medical adhesives. Trends Biotechnol. 1990, 8, 53–57. [Google Scholar] [CrossRef]
- Spotnitz, W.D.; Falstrom, J.K.; Rodeheaver, G.T. The role of sutures and fibrin sealant in wound healing. Surg. Clin. N. Am. 1997, 77, 651–669. [Google Scholar] [CrossRef]
- Schenk, W.G., III; Burks, S.G.; Gagne, P.J.; Kagan, S.A.; Lawson, J.H.; Spotnitz, W.D. Fibrin sealant improves hemostasis in peripheral vascular surgery: A randomized prospective trial. Ann. Surg. 2003, 237, 871–876. [Google Scholar] [CrossRef]
- Hino, M.; Ishiko, O.; Honda, K.I.; Yamane, T.; Ohta, K.; Takubo, T.; Tatsumi, N. Transmission of symptomatic parvovirus B19 infection by fibrin sealant used during surgery. Br. J. Haematol. 2000, 108, 194–195. [Google Scholar] [CrossRef]
- Horowitz, B.; Busch, M. Estimating the pathogen safety of manufactured human plasma products: Application to fibrin sealants and to thrombin. Transfusion 2008, 48, 1739–1753. [Google Scholar] [CrossRef]
- CoStasis Multi-center Collaborative Writing Committee. A novel collagen-based composite offers effective hemostasis for multiple surgical indications: Results of a randomized controlled trial. Surgery 2001, 129, 445–450. [Google Scholar] [CrossRef]
- Farndale, R.W.; Sixma, J.J.; Barnes, M.J.; de Groot, P.G. The role of collagen in thrombosis and hemostasis. J. Thromb Haemost. 2004, 2, 561–573. [Google Scholar] [CrossRef]
- Chvapil, M.; Kronenthal, L.; van Winkle, W., Jr. Medical and surgical applications of collagen. Int. Rev. Connect Tissue Res. 1973, 6, 1–61. [Google Scholar]
- Doillon, C.J.; Whyne, C.F.; Brandwein, S.; Silver, F.H. Collagen-based wound dressings: Control of the pore structure and morphology. J. Biomed. Mater. Res. 1986, 20, 1219–1228. [Google Scholar] [CrossRef]
- Fleck, C.A.; Simman, R. Modern collagen wound dressings: Function and purpose. J. Am. Col. Certif Wound Spec. 2010, 2, 50–54. [Google Scholar] [CrossRef] [PubMed]
- Grabska-Liberek, I.; Galus, R.; Owczarek, W.; Wlodarsk, K.; Zabielski, S.; Malejczyk, J.; Sladowski, D. Collagen based dressings in the treatment of wound healing. Pol. Merkur. Lekarski. 2013, 35, 51–54. [Google Scholar] [PubMed]
- Taguchi, T.; Saito, H.; Uchida, Y.; Sakane, M.; Kobayashi, H.; Kataoka, K.; Tanaka, J. Bonding of soft tissues using a novel tissue adhesive consisting of a citric acid derivative and collagen. Mater. Sci. Eng. C 2004, 24, 775–780. [Google Scholar] [CrossRef]
- Taguchi, T.; Saito, H.; Aoki, H.; Uchida, Y.; Sakane, M.; Kobayashi, H.; Tanaka, J. Biocompatible high-strength glue consisting of citric acid derivative and collagen. Mater. Sci. Eng. C 2006, 26, 9–13. [Google Scholar] [CrossRef]
- Baik, S.H.; Kim, J.H.; Cho, H.H.; Park, S.N.; Kim, Y.S.; Suh, H. Development and analysis of a collagen-based hemostatic adhesive. J. Surg. Res. 2010, 164, e221–e228. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Kopelman, D.; Wu, L.Q.; Hijji, K.; Attar, I.; Preiss-Bloom, O.; Payne, G.F. Biomimetic sealant based on gelatin and microbial transglutaminase: An initial in vivo investigation. J. Biomed. Mater. Res. B Appl. Biomater. 2009, 91, 5–16. [Google Scholar] [CrossRef]
- Bonchek, L.I.; Braunwald, N.S. Experimental evaluation of a cross-linked gelatin adhesive in gastrointestinal surgery. Ann. Surg. 1967, 165, 420–424. [Google Scholar] [CrossRef]
- Nomori, H.; Horio, H.; Morinaga, S.; Suemasu, K. Gelatin-resorcinol–formaldehyde-glutaraldehyde glue for sealing pulmonary air leaks during thoracoscopic operation. Ann. Thorac Surg. 1999, 67, 212–216. [Google Scholar] [CrossRef]
- Albes, J.M.; Krettek, C.; Hausen, B.; Rohde, R.; Haverich, A.; Borst, H.G. Biophysical properties of the gelatin-resorcinformaldehyde/glutaraldehyde adhesive. Ann. Thorac Surg. 1993, 56, 910–915. [Google Scholar] [CrossRef]
- Matsuda, M.; Ueno, M.; Endo, Y.; Inoue, M.; Sasaki, M.; Taguchi, T. Enhanced tissue penetration-induced high bonding strength of a novel tissue adhesive composed of cholesteryl group-modified gelatin and disuccinimidyl tartarate. Colloids Surf. B Biointerfaces 2012, 91, 48–56. [Google Scholar] [CrossRef]
- Haines-Butterick, L.; Rajagopal, K.; Branco, M.; Salick, D.; Rughani, R.; Pilarz, M.; Lamm, M.S.; Pochan, D.J.; Schneider, J.P. Controlling hydrogelation kinetics by peptide design for three-dimensional encapsulation and injectable delivery of cells. Proc. Natl. Acad. Sci. USA 2007, 104, 7791–7796. [Google Scholar] [CrossRef] [PubMed]
- Ulijn, R.V.; Smith, A.M. Designing peptide based nanomaterials. Chem. Soc. Rev. 2008, 37, 664–675. [Google Scholar] [CrossRef] [PubMed]
- Suderman, R.J.; Dittmer, N.T.; Kanost, M.R.; Kramer, K.J. Model reactions for insect cuticle sclerotization: Cross-linking of recombinant cuticular proteins upon their laccase-catalyzed oxidative conjugation with catechols. Insect Biochem. Mol. Biol. 2006, 36, 353–365. [Google Scholar] [CrossRef] [PubMed]
- Elvin, C.M.; Vuocolo, T.; Brownlee, A.G.; Sando, L.; Huson, M.G.; Liyou, N.E.; Stockwell, P.R.; Lyons, R.E.; Kim, M.; Edwards, G.A.; et al. A highly elastic tissue sealant based on photopolymerised gelatin. Biomaterials 2010, 31, 8323–8331. [Google Scholar] [CrossRef]
- Wedmore, I.; McManus, J.G.; Pusateri, A.E.; Holcomb, J.B. A special report on the chitosan-based hemostatic dressing: Experience in current combat operations. J. Trauma 2006, 60, 655–658. [Google Scholar] [CrossRef]
- Brown, M.A.; Daya, M.R.; Worley, J.A. Experience with chitosan dressings in a civilian EMS system. J. Emerg. Med. 2009, 37, 1–7. [Google Scholar] [CrossRef]
- Klokkevold, P.R.; Fukayama, H.; Sung, E.C.; Bertolami, C.N. The effect of chitosan (poly-N-acetyl glucosamine) on lingual hemostasis in heparinized rabbits. J. Oral Maxillofac Surg. 1999, 57, 49–52. [Google Scholar] [CrossRef]
- Bhatia, S.K.; Arthur, S.D.; Chenault, H.K.; Kodokian, G.K. Interactions of polysaccharide-based tissue adhesives with clinically relevant fibroblast and macrophage cell lines. Biotechnol. Lett. 2007, 29, 1645–1649. [Google Scholar] [CrossRef]
- Bhatia, S.K.; Arthur, S.D.; Chenault, H.K.; Figuly, G.D.; Kodokian, G.K. Sealing and healing of clear corneal incisions with an improved dextran aldehyde-PEG amine tissue adhesive. Curr. Eye Res. 2007, 32, 1045–1050. [Google Scholar] [CrossRef]
- Wang, D.A.; Varghese, S.; Sharma, B.; Strehin, I.; Fermanian, S.; Gorham, J.; Fairbrother, D.H.; Cascio, B.; Elisseeff, J.H. Multifunctional chondroitin sulphate for cartilage tissue–biomaterial integration. Nat. Mater. 2007, 6, 385–392. [Google Scholar] [CrossRef]
- Strehin, I.; Nahas, Z.; Arora, K.; Nguyen, T.; Elisseeff, J. A versatile pH sensitive chondroitin sulfate–PEG tissue adhesive and hydrogel. Biomaterials 2010, 31, 2788–2797. [Google Scholar] [CrossRef] [PubMed]
- Strehin, I.; Ambrose, W.M.; Schein, O.; Salahuddin, A.; Elisseeff, J. Synthesis and characterization of a chondroitin sulfate-polyethylene glycol corneal adhesive. J. Cataract Refract. Surg. 2009, 35, 567–576. [Google Scholar] [CrossRef] [PubMed]
- Messi, G.; Marchi, A.G. Evaluation of skin laceration repair by tissue adhesive in the pediatric emergency room. Panminerva Med. 1992, 34, 77–80. [Google Scholar] [PubMed]
- Quinn, J.; Wells, G.; Sutcliffe, T.; Jarmuske, M.; Maw, J.; Stiell, I.; Johns, P. A randomized trial comparing octylcyanoacrylate tissue adhesive and sutures in the management of lacerations. Jama 1997, 277, 1527–1530. [Google Scholar] [CrossRef] [PubMed]
- Qureshi, A.; Drew, P.J.; Duthie, G.S.; Roberts, A.C.; Monson, J.R. n-Butyl cyanoacrylate adhesive for skin closure of abdominal wounds: Preliminary results. Ann. R. Coll. Surg. Engl. 1997, 79, 414–415. [Google Scholar] [PubMed]
- Singer, A.J.; Thode, H.C., Jr. A review of the literature on octylcyanoacrylate tissue adhesive. Am. J. Surg. 2004, 187, 238–248. [Google Scholar] [CrossRef]
- Edmonson, M.B. Foreign body reactions to dermabond. Am. J. Emerg. Med. 2001, 19, 240–241. [Google Scholar] [CrossRef]
- Caliceti, P.; Veronese, F.M. Pharmacokinetic and biodistribution properties of poly (ethylene glycol)–protein conjugates. Adv. Drug Deliv. Rev. 2003, 55, 1261–1277. [Google Scholar] [CrossRef]
- Tanaka, K.; Takamoto, S.; Ohtsuka, T.; Kotsuka, Y.; Kawauchi, M. Application of AdvaSeal for acute aortic dissection: Experimental study. Ann. Thorac. Surg. 1999, 68, 1308–1312. [Google Scholar] [CrossRef]
- Ye, Q.; Zhou, F.; Liu, W. Bioinspired catecholic chemistry for surface modification. Chem. Soc. Rev. 2011, 40, 4244–4258. [Google Scholar] [CrossRef]
- Ferreira, P.; Pereira, R.; Coelho, J.F.; Silva, A.F.; Gil, M.H. Modification of the biopolymer castor oil with free isocyanate groups to be applied as bioadhesive. Int. J. Biol. Macromol. 2008, 40, 144–152. [Google Scholar] [CrossRef] [PubMed]
- Gilbert, T.W.; Badylak, S.F.; Gusenoff, J.; Beckman, E.J.; Clower, D.M.; Daly, P.; Rubin, J.P. Lysine-derived urethane surgical adhesive prevents seroma formation in a canine abdominoplasty model. Plast Reconstr. Surg. 2008, 122, 95–102. [Google Scholar] [CrossRef] [PubMed]
- Phaneuf, M.D.; Dempsey, D.J.; Bide, M.J.; Quist, W.C.; LoGerfo, F.W. Coating of Dacron vascular grafts with an ionic polyurethane: A novel sealant with protein binding properties. Biomaterials 2001, 22, 463–469. [Google Scholar] [CrossRef]
- Darney, P.D.; Monroe, S.E.; Klaisle, C.M.; Alvarado, A. Clinical evaluation of the Capronor contraceptive implant: Preliminary report. Am. J. Obstet. Gynecol. 1989, 160, 1292–1295. [Google Scholar] [CrossRef]
- Ferreira, P.; Coelho, J.F.; Gil, M.H. Development of a new photocrosslinkable biodegradable bioadhesive. Int. J. Pharm. 2008, 352, 172–181. [Google Scholar] [CrossRef] [PubMed]
- Duncan, R.; Izzo, L. Dendrimer biocompatibility and toxicity. Adv. Drug Deliv. Rev. 2005, 57, 2215–2237. [Google Scholar] [CrossRef] [PubMed]
- Grinstaff, M.W. Dendritic macromers for hydrogel formation: Tailored materials for ophthalmic, orthopedic, and biotech applications. J. Polymer Sci. Part A Polymer Chem. 2008, 46, 383–400. [Google Scholar] [CrossRef]
- Carnahan, M.A.; Middleton, C.; Kim, J.; Kim, T.; Grinstaff, M.W. Hybrid dendritic− linear polyester− ethers for in situ photopolymerization. J. Am. Chem. Soc. 2002, 124, 5291–5293. [Google Scholar] [CrossRef]
- Wathier, M.; Jung, P.J.; Carnahan, M.A.; Kim, T.; Grinstaff, M.W. Dendritic macromers as in situ polymerizing biomaterials for securing cataract incisions. J. Am. Chem. Soc. 2004, 126, 12744–12745. [Google Scholar] [CrossRef]
- Cha, H.J.; Hwang, D.S.; Lim, S. Development of bioadhesives from marine mussels. Biotechnol. J. 2008, 3, 631–638. [Google Scholar] [CrossRef]
- Lee, H.; Dellatore, S.M.; Miller, W.M.; Messersmith, P.B. Mussel-inspired surface chemistry for multifunctional coatings. Science 2007, 318, 426–430. [Google Scholar] [CrossRef] [PubMed]
- Yamada, K.; Chen, T.; Kumar, G.; Vesnovsky, O.; Topoleski, L.D.; Payne, G.F. Chitosan based water-resistant adhesive. Analogy to mussel glue. Biomacromolecules 2000, 1, 252–258. [Google Scholar] [CrossRef] [PubMed]
- Ge, L.; Li, Q.; Huang, Y.; Yang, S.; Ouyang, J.; Bu, S.; Zhong, W.; Liu, Z.; Xing, M.M.Q. Polydopamine-coated paper-stack nanofibrous membranes enhancing adipose stem cells’ adhesion and osteogenic differentiation. J. Mater. Chem. B 2014, 2, 6917–6923. [Google Scholar] [CrossRef] [PubMed]
- Filpula, D.R.; Lee, S.M.; Link, R.P.; Strausberg, S.L.; Strausberg, R.L. Structural and functional repetition in a marine mussel adhesive protein. Biotechnol. Prog. 1990, 6, 171–177. [Google Scholar] [CrossRef] [PubMed]
- Salerno, A.J.; Goldberg, I. Cloning, expression, and characterization of a synthetic analog to the bioadhesive precursor protein of the sea mussel Mytilus edulis. Appl. Microbiol. Biotechnol. 1993, 39, 221–226. [Google Scholar] [CrossRef]
- Huang, K.; Lee, B.P.; Ingram, D.R.; Messersmith, P.B. Synthesis and characterization of self-assembling block copolymers containing bioadhesive end groups. Biomacromolecules 2002, 3, 397–406. [Google Scholar] [CrossRef] [PubMed]
- Lee, B.P.; Dalsin, J.L.; Messersmith, P.B. Synthesis and gelation of DOPA-modified poly (ethylene glycol) hydrogels. Biomacromolecules 2002, 3, 1038–1047. [Google Scholar] [CrossRef]
- Murphy, J.L.; Vollenweider, L.; Xu, F.; Lee, B.P. Adhesive performance of biomimetic adhesive-coated biologic scaffolds. Biomacromolecules 2010, 11, 2976–2984. [Google Scholar] [CrossRef]
- Brubaker, C.E.; Kissler, H.; Wang, L.J.; Kaufman, D.B.; Messersmith, P.B. Biological performance of mussel-inspired adhesive in extrahepatic islet transplantation. Biomaterials 2010, 31, 420–427. [Google Scholar] [CrossRef]
- Bilic, G.; Brubaker, C.; Messersmith, P.B.; Mallik, A.S.; Quinn, T.M.; Haller, C.; Done, E.; Gucciardo, L.; Zeisberger, S.M.; Zimmermann, R.; et al. Injectable candidate sealants for fetal membrane repair: Bonding and toxicity in vitro. Am. J. Obstet. Gynecol. 2010, 202, 85.e1-9. [Google Scholar] [CrossRef]
- Fan, C.; Fu, J.; Zhu, W.; Wang, D.A. A mussel-inspired double-crosslinked tissue adhesive intended for internal medical use. Acta Biomater. 2016, 33, 51–63. [Google Scholar] [CrossRef] [PubMed]
- Mahdavi, A.; Ferreira, L.; Sundback, C.; Nichol, J.W.; Chan, E.P.; Carter, D.J.; Bettinger, C.J.; Patanavanich, S.; Chignozha, L.; Ben-Joseph, E.; et al. A biodegradable and biocompatible gecko-inspired tissue adhesive. Proc. Natl. Acad. Sci. USA 2008, 105, 2307–2312. [Google Scholar] [CrossRef] [PubMed]
| Categories | Commerical Product | Manufacturer | Constituents |
|---|---|---|---|
| Natural or biological adhesives | Crosseal | Omrix | Human fibrinogen, human thrombin, human fibronectin, human factor XIII, calcium chloride |
| TachoSil | Pharmaceuticals International GmbH | Equine collagen patch, human fibrinogen, human thrombin | |
| Vitagel | Stryker | Bovine collagen, bovine thrombin, patients own plasma | |
| GRF | Microval | Gelatin, resorcinol, formaldehyde, glutaraldehyde | |
| ProGel | NeoMend | Human Serum Albumin, PEG di NHS | |
| Tisseel | Baxter | Human fibrinogen, human fibronectin, human thrombin, human Factor XIII, bovine aprotinin, calcium chloride | |
| Artiss | Baxter | Human pooled plasma | |
| Evicel | Ethicon | Human fibrinogen, human thrombin, human factor XIII, calcium chloride | |
| CryoSeal | Thermogen | Human fibrinogen, human thrombin, human fibronectin, human Factor XIII, human Factor VIII, human vWF, human thrombin from individual units of plasma | |
| Hemaseel | Haemacure Corp. | Human fibrinogen, human fibronectin, human factor XIII, bovine thrombin, calcium chloride | |
| BioGlue | CryoLife | Albumin, glutaraldehyde | |
| synthetic polymer-based tissue adhesive | Histoacryl | B. Braun | n-Butyl-2-cyanoacrylate |
| Dermabond | Ethicon | 2-Octyl-2-cyanoacrylate | |
| Octylseal | Medline Industries | 2-Octyl-2-cyanoacrylate | |
| Surgiseal | Adhezion Biomedical | 2-Octyl-2-cyanoacrylate | |
| Omnex | Ethicon | n-Octyl-2-cyanoacrylate/butyl lactoyl-2-cyano acrylate | |
| Indermil | Henkel | n-Butyl-2-cyanoacrylate | |
| Liquiband | Advanced Medical Solutions | n-Butyl-2-cyanoacrylate | |
| Histoacryl Histoactryl Blue | Tissueseal | n-Butyl-2-cyanoacrylate | |
| Glubran Glubran2 | GEM Italy | n-Butyl-2-cyanoacrylate/methacryloxysulpholane | |
| IFABond | IFA medical | N-Hexyl-2-cyanoacrylate | |
| TissuGlu | Cohera medical | Lysine di/tri isocyanate-PEG prepolymers | |
| HemCon | Bandage Pro | Chitosan | |
| Actamax | Actamax Surgical Material LLC | Dextran aldehyde, 8-arm PEG amine MW 10,000 functionalized with tris(2-aminoethyl)amine | |
| FocalSeal-L | Focal Inc. | Photopolymerizable PEG-co-poly(lactic acid)/poly(trimethylene carbonate) | |
| DuraSeal | Covidien | Tetra-NHS-derivatized PEG and trilysine | |
| CoSeal | Cohesion Technologies | Tetra-NHS-derivatized PEG and tetra-thiol-derivatized PEG | |
| SprayGel | Covidien | Tetra-NHS-derivatized PEG and tetra-amine-derivatized PEG | |
| TissuePatch | TissueMed | poly-((N-vinylpyrrolidone)50-co- (acrylic acid)25-co-(acrylic acid N-hydroxysuccinimide ester)25) | |
| OcuSeal | Hyperbranch Medical Technology | poly(glycerol succinic acid) and PEG–aldehyde | |
| Adherus | Hyperbranch | Activated PEG and branched poly(ethylene imine) |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ge, L.; Chen, S. Recent Advances in Tissue Adhesives for Clinical Medicine. Polymers 2020, 12, 939. https://doi.org/10.3390/polym12040939
Ge L, Chen S. Recent Advances in Tissue Adhesives for Clinical Medicine. Polymers. 2020; 12(4):939. https://doi.org/10.3390/polym12040939
Chicago/Turabian StyleGe, Liangpeng, and Shixuan Chen. 2020. "Recent Advances in Tissue Adhesives for Clinical Medicine" Polymers 12, no. 4: 939. https://doi.org/10.3390/polym12040939
APA StyleGe, L., & Chen, S. (2020). Recent Advances in Tissue Adhesives for Clinical Medicine. Polymers, 12(4), 939. https://doi.org/10.3390/polym12040939




