Pyrolysis of Low Density Polyethylene: Kinetic Study Using TGA Data and ANN Prediction
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Thermal Decomposition of LDPE
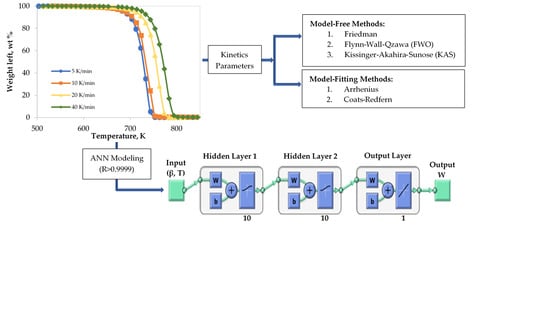
2.3. Kinetic Theory
2.4. Topology of ANNs
3. Results and Discussion
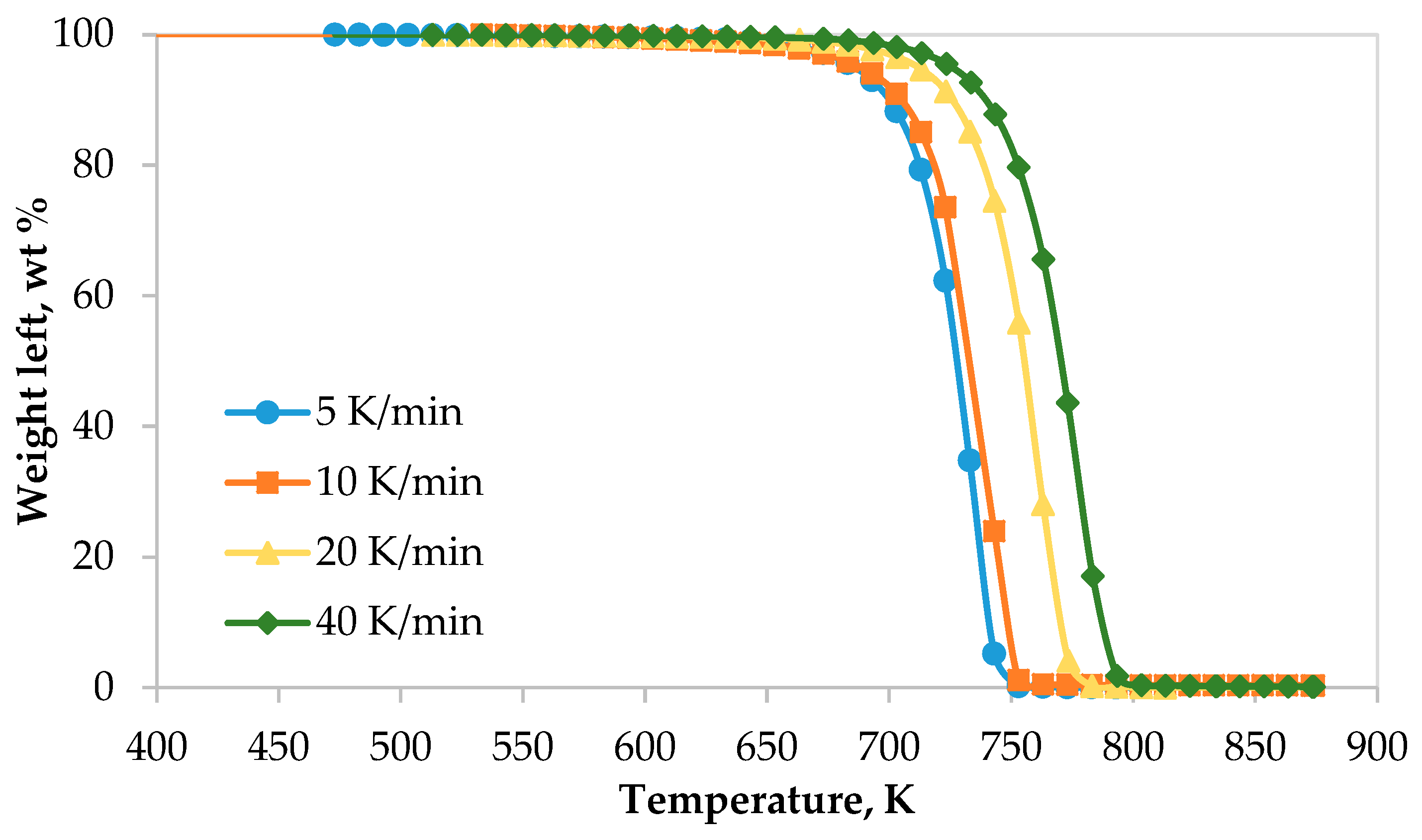
3.1. TG-DTG Analysis of LDPE
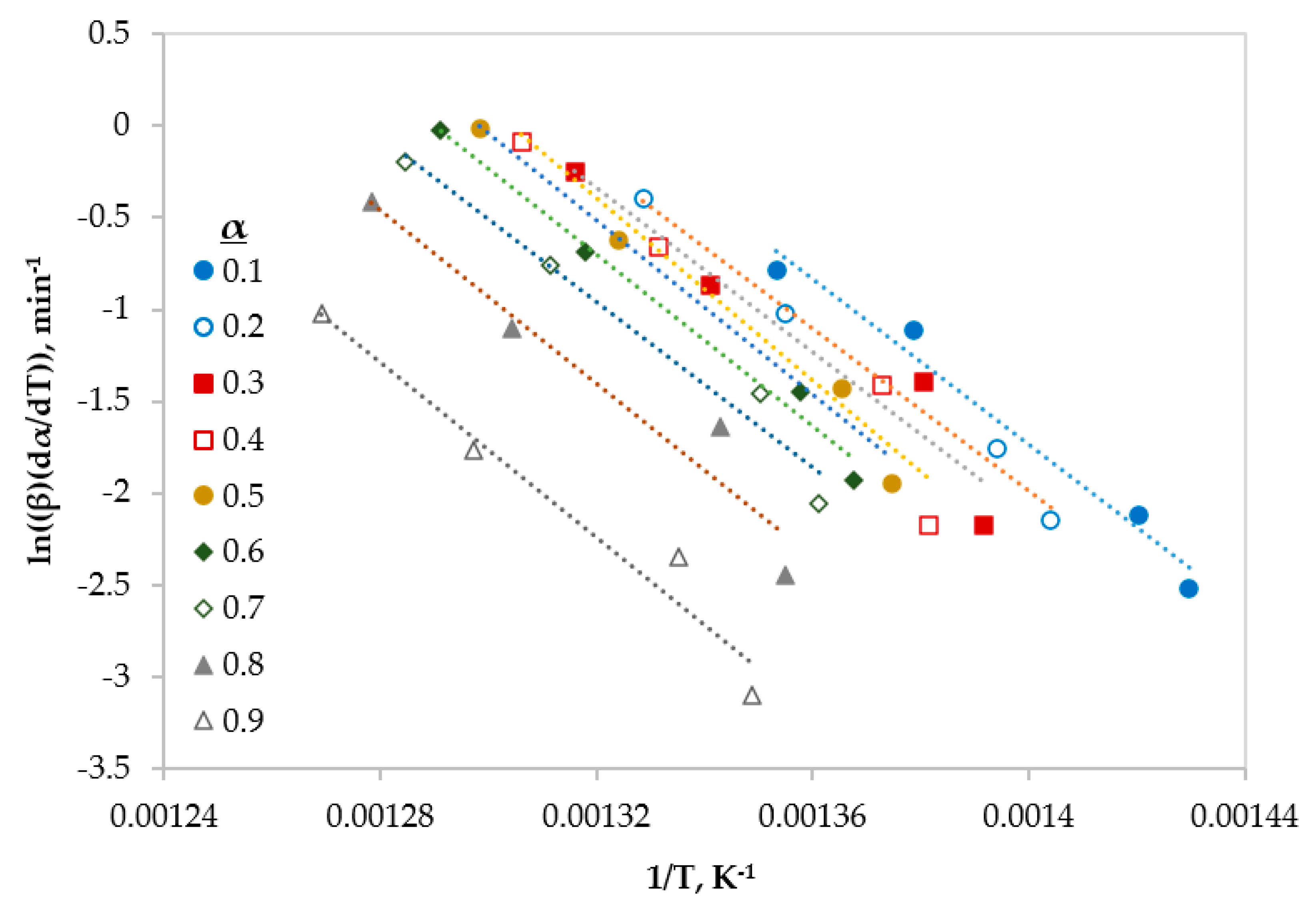
3.2. Model-Free Kinetics Calculation
3.3. Model-Fitting Kinetics Calculation
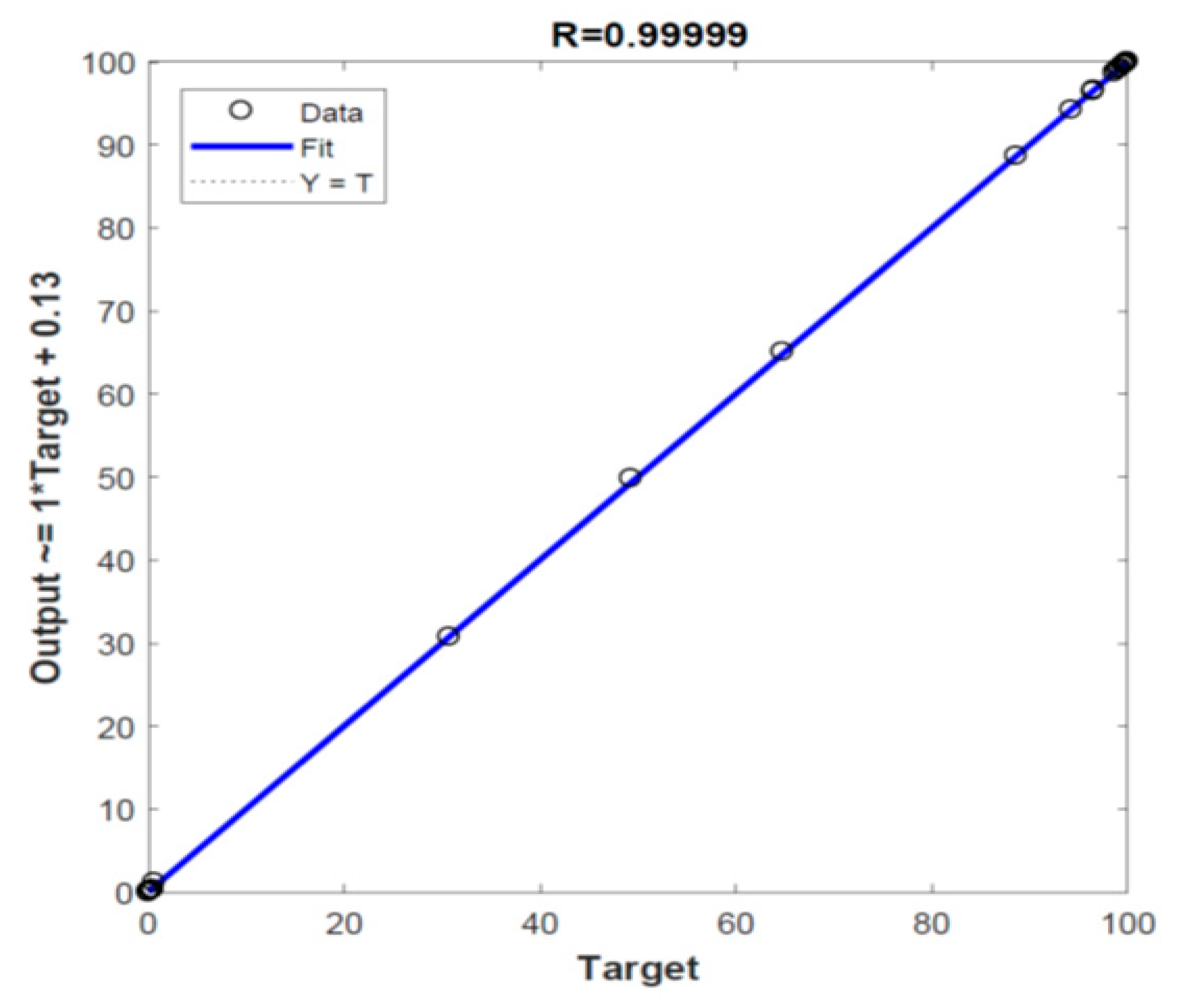
3.4. Pyrolysis Prediction by ANN Model
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Miskolczi, N.; Bartha, L.; Deák, G. Thermal degradation of polyethylene and polystyrene from the packaging industry over different catalysts into fuel-like feed stocks. Polym. Degrad. Stabil. 2006, 91, 517–526. [Google Scholar] [CrossRef]
- Diaz Silvarrey, L.S.; Phan, A.N. Kinetic study of municipal plastic waste. Int. J. Hydrogen Energy 2016, 41, 16352–16364. [Google Scholar] [CrossRef]
- Cardona, S.C.; Corma, A. Tertiary recycling of polypropylene by catalytic cracking in a semi batch stirred reactor. Appl. Catalysis B 2000, 25, 151–162. [Google Scholar] [CrossRef]
- Arandes, J.; Abajo, I.; Lopez-Valerio, D.; Fernandez, I.; Azkoiti, M.J.; Olazar, M.; Bilbao, J. Transformation of several plastic wastes into fuels by catalytic cracking. Ind. Eng. Chem. Res. 1997, 36, 4523–4529. [Google Scholar] [CrossRef]
- Kaminsky, W.; Schlesselmann, B.; Simon, C.M. Thermal degradation of mixed plastic waste to aromatic and gas. Poly. Degrad. Stab. 1996, 53, 189–197. [Google Scholar] [CrossRef]
- Aguado, J.; Serrano, D.P.; Miguel, G.S.; Castro, M.C.; Madrid, S. Feedstock recycling of polyethylene in a two-step thermo-catalytic reaction system. J. Anal. Appl. Pyrolysis 2007, 79, 415–423. [Google Scholar] [CrossRef]
- Jan, M.R.; Shah, J.; Gulab, H. Catalytic conversion of waste high-density polyethylene into useful hydrocarbons. Fuel 2013, 105, 595–602. [Google Scholar] [CrossRef]
- Lyon, R.E. An integral method of non-isothermal kinetic analysis. Thermochim. Acta 1997, 297, 117–124. [Google Scholar] [CrossRef]
- Saha, B.; Ghoshal, A.K. Model-free kinetics analysis of ZSM-5 catalyzed pyrolysis of waste LDPE. Thermochim. Acta 2007, 453, 120–127. [Google Scholar] [CrossRef]
- Aboulkas, A.; El Harfi, K.; Bouadili, A. Pyrolysis of olive residue/low density polyethylene mixture: Part I Thermogravimetric kinetics. J. Fuel Chem. Technol. 2008, 36, 672–678. [Google Scholar] [CrossRef]
- Aboulkas, A.; El Harfi, K.; Bouadili, A. Thermal degradation behaviors of polyethylene and polypropylene. Part I: Pyrolysis kinetics and mechanisms. Energy Convers. Manag. 2010, 51, 1363–1369. [Google Scholar] [CrossRef]
- Aguado, R.; Olaza, M.; Gaisan, B.; Prieto, R.; Bilbao, J. Kinetic study of polyolefin pyrolysis in a conical spouted bed reactor, Chem. Eng. J. 2003, 92, 91–99. [Google Scholar] [CrossRef]
- Sorum, L.; Gronli, M.; Hustad, J.E. Pyrolysis characteristics and kinetics of municipal solid wastes. Fuel 2001, 80, 1217–1227. [Google Scholar] [CrossRef]
- Wu, C.H.; Chang, C.Y.; Hor, J.L.; Shih, S.M.; Chen, L.W.; Chang, F.W. On the thermal treatment of plastic mixture of MSW: Pyrolysis kinetics. Waste Manag. 1993, 13, 221–235. [Google Scholar] [CrossRef]
- Das, P.; Tiwari, P. Thermal degradation kinetics of plastics and model selection. Thermochim. Acta 2017, 654, 191–202. [Google Scholar] [CrossRef]
- Conesa, J.A.; Caballero, J.A.; Reyes-Labarta, A.J. Artificial neural network for modelling thermal decompositions. J. Anal. Appl. Pyrolysis 2004, 71, 343–352. [Google Scholar] [CrossRef]
- Yıldız, Z.; Uzun, H.; Ceylan, S.; Topcu, Y. Application of artificial neural networks to co-combustion of hazelnut husk–lignite coal blends. Bioresour. Technol. 2016, 200, 42–47. [Google Scholar] [CrossRef]
- Çepelioĝullar, O.; Mutlu, I.; Yaman, S.; Haykiri-Acma, H. A study to predict pyrolytic behaviors of refuse-derived fuel (RDF): Artificial neural network application. J. Anal. Appl. Pyrolysis 2016, 122, 84–94. [Google Scholar] [CrossRef]
- Charde, S.J.; Sonawane, S.S.; Sonawane, S.H.; Shimpi, N.G. Degradation Kinetics of Polycarbonate Composites: Kinetic Parameters and Artificial Neural Network. Chem. Biochem. Eng. Q. 2018, 32, 151–165. [Google Scholar] [CrossRef]
- Chen, J.; Xie, C.; Liu, J.; He, Y.; Xie, W.; Zhang, X.; Chang, K.; Kuo, J.; Sun, J.; Zheng, L.; et al. Co-combustion of sewage sludge and coffee grounds under increased O2/CO2 atmospheres: Thermodynamic characteristics, kinetics and artificial neural network modeling. Bioresour. Technol. 2018, 250, 230–238. [Google Scholar] [CrossRef]
- Naqvi, S.R.; Tariq, R.; Hameed, Z.; Ali, I.; Taqvi, S.A.; Naqvi, M.; Niazi, M.B.K.; Noor, T.; Farooq, W. Pyrolysis of high-ash sewage sludge: Thermo-kinetic study using TGA and artificial neural networks. Fuel 2018, 233, 529–538. [Google Scholar] [CrossRef]
- Chan, J.H.; Balke, S.T. The thermal degradation kinetics of polypropylene: Part III. thermogravimetric analyses. Polym. Degrad. Stabil. 1997, 57, 135–149. [Google Scholar] [CrossRef]
- Khedri, S.; Elyasi, S. Kinetic analysis for thermal cracking of HDPE: A new isoconversional approach. Polym. Degrad. Stabil. 2016, 129, 306–318. [Google Scholar] [CrossRef]
- Xu, F.; Wang, B.; Yang, D.; Hao, J.; Qiao, Y.; Tiana, Y. Thermal degradation of typical plastics under high heating rate conditions by TG-FTIR: Pyrolysis behaviors and kinetic analysis. Energy Convers. Manag. 2018, 171, 1106–1115. [Google Scholar] [CrossRef]
- Gundogar, A.S.; Kok, M.V. Thermal characterization, combustion and kinetics of different crude oils. Fuel 2014, 123, 59–65. [Google Scholar] [CrossRef]
- Baloch, M.K.; Khurran, M.J.Z.; Durrani, G.F. Application of different methods for the Thermogravimetric analysis of polyethylene samples. J. Appl. Polym. Sci. 2011, 120, 3511–3518. [Google Scholar] [CrossRef]
- Quantrille, T.E.; Liu, Y.A. Artificial Intelligence in Chemical Engineering; Academic Press: San Diego, LA, USA, 1991. [Google Scholar]
- Halali, M.A.; Azari, V.; Arabloo, M.; Mohammadi, A.H.; Bahadori, A. Application of a radial basis function neural network to estimate pressure gradient in water–oil pipelines. J. Taiwan Inst. Chem. Eng. 2016, 58, 189–202. [Google Scholar] [CrossRef]
- Govindan, B.; Jakka, S.C.B.; Radhakrishnan, T.K.; Tiwari, A.K.; Sudhakar, T.M.; Shanmugavelu, P.; Kalburgi, A.K.; Sanyal, A.; Sarkar, S. Investigation on kinetic parameters of combustion and oxy-combustion of calcined pet coke employing thermogravimetric analysis coupled to artificial neural network modeling. Energy Fuels 2018, 32, 3995–4007. [Google Scholar] [CrossRef]
- Boostani, M.; Karimi, H.; Azizi, S. Heat transfer to oil-water flow in horizontal and inclined pipes: Experimental investigation and ANN modeling. Int. J. Therm. Sci. 2017, 111, 340–350. [Google Scholar] [CrossRef]
- Beale, M.H.; Hagan, M.T.; Demuth, H.B. Neural Network Toolbox TM User’s Guide; MathWorks: Natick, MA, USA, 2018. [Google Scholar]
- Quan, B.; Kun, C.; Wei, X.; Yuanyuan, L.; Mengjie, C.; Xianghai, K.; Qiulu, C.; Hanping, M. Hydrocarbon rich bio-oil production, thermal behavior analysis and kinetic study of microwave-assisted co-pyrolysis of microwave-torrefied lignin with low density polyethylene. Bioresour. Technol. 2019, 291, 121860. [Google Scholar] [CrossRef]





| Reference | Activation Energy (kJ mol−1) |
|---|---|
| Diaz Silvarrey and Phan [2] | 267.61 ± 3.23 |
| Lyon [8] | 130–200 |
| Saha and Ghoshal [9] | 190 |
| Aboulkas et al. [10] | 215 |
| Aboulkas et al. [11] | 215–221 |
| Aguado et al. [12] | 261 ± 21 |
| Sorum et al. [13] | 340 |
| Wu et al. [14] | 194–206 |
| Manufacturer | Ipoh SY Recycle Plastic, Perak, Malaysia |
|---|---|
| Polymer Type | Recycled LDPE |
| Appearance (at 25 °C) | Solid |
| Physical State | Pellets |
| Colour | Black |
| Density (Kg/m3) | 910–940 |
| Melting Temperature (°C) | 115 ± 10 |
| Method | Equation | Integral (I) or Differential (D) | Plot | |
|---|---|---|---|---|
| Friedman | (6) | D | ||
| Flynn-Wall-Qzawa (FWO) | (7) | I | ||
| Kissinger-Akahira-Sunose (KAS) | (8) | I | ||
| Method | Equation | Plot | |
|---|---|---|---|
| Arrhenius | (9) | ||
| Coats-Redfern | n ≠ 1 | (10) | |
| n = 1 | (11) | ||
| Heating Rate (K/min) | On-Set (K) | End-Set (K) | Peak (K) |
|---|---|---|---|
| 5 | 665 | 750 | 741 |
| 10 | 668 | 755 | 744 |
| 20 | 688 | 782 | 765 |
| 40 | 700 | 794 | 785 |
| Conversion | Friedman | FWO | KAS | ||||||
|---|---|---|---|---|---|---|---|---|---|
| E (kJ/mol) | A (min−1) | R2 | E (kJ/mol) | A (min−1) | R2 | E (kJ/mol) | A (min−1) | R2 | |
| 0.1 | 197 | 2.63 × 1013 | 0.9772 | 193 | 8.14 × 1012 | 0.9532 | 191 | 5.51 × 1012 | 0.9474 |
| 0.2 | 185 | 4.85 × 1012 | 0.9265 | 198 | 2.17 × 1013 | 0.9575 | 196 | 1.49 × 1013 | 0.9523 |
| 0.3 | 186 | 6.68 × 1012 | 0.9288 | 198 | 2.46 × 1013 | 0.9629 | 196 | 1.65 × 1013 | 0.9582 |
| 0.4 | 206 | 1.97 × 1014 | 0.9387 | 195 | 1.70 × 1013 | 0.9498 | 193 | 1.09 × 1013 | 0.9435 |
| 0.5 | 198 | 5.20 × 1013 | 0.9793 | 194 | 1.55 × 1013 | 0.9527 | 191 | 9.74 × 1012 | 0.9467 |
| 0.6 | 194 | 3.06 × 1013 | 0.9844 | 194 | 1.97 × 1013 | 0.9567 | 192 | 1.23 × 1013 | 0.9511 |
| 0.7 | 188 | 1.17 × 1013 | 0.9674 | 196 | 3.07 × 1013 | 0.9612 | 194 | 1.94 × 1013 | 0.9562 |
| 0.8 | 196 | 4.31 × 1013 | 0.9345 | 197 | 4.15 × 1013 | 0.9665 | 195 | 2.62 × 1013 | 0.9621 |
| 0.9 | 198 | 4.50 × 1013 | 0.9559 | 192 | 2.16 × 1013 | 0.9720 | 190 | 1.28 × 1013 | 0.9681 |
| Average | 194 | 4.63 × 1013 | 0.9547 | 195 | 2.23 × 1013 | 0.9592 | 193 | 1.43 × 1013 | 0.9540 |
| Heating Rate (K/min) | Arrhenius Method | Coats-Redfern Method | ||||
|---|---|---|---|---|---|---|
| E (kJ/mol) | A (min−1) | R2 | E (kJ/mol) | A (min−1) | R2 | |
| 5 | 207 | 1.42 × 1014 | 0.9673 | 193 | 4.22 × 1010 | 0.9295 |
| 10 | 200 | 2.29 × 1013 | 0.985 | 193 | 6.75 × 1010 | 0.9436 |
| 20 | 213 | 9.13 × 1013 | 0.9724 | 197 | 8.66 × 1010 | 0.9413 |
| 40 | 187 | 1.11 × 1012 | 0.9649 | 201 | 1.61 × 1011 | 0.9459 |
| Average | 202 | 6.43 × 1013 | 0.9724 | 196 | 8.92 × 1010 | 0.9401 |
| Model | Network Topology | 1st Transfer Function | 2nd Transfer Function | R |
|---|---|---|---|---|
| ANN1 | NN-2-10-1 | TANSIG | - | 0.99943 |
| ANN2 | NN-2-15-1 | TANSIG | - | 0.99981 |
| ANN3 | NN-2-5-1 | TANSIG | - | 0.99724 |
| ANN4 | NN-2-10-1 | LOGSIG | - | 0.99865 |
| ANN5 | NN-2-15-1 | LOGSIG | - | 0.98047 |
| ANN6 | NN-2-5-1 | LOGSIG | - | 0.99544 |
| ANN7 | NN-2-15-15-1 | TANSIG | TANSIG | 0.99978 |
| ANN8 | NN-2-15-15-1 | LOGSIG | TANSIG | 0.99961 |
| ANN9 | NN-2-15-15-1 | TANSIG | LOGSIG | 0.99989 |
| ANN10 | NN-2-10-15-1 | TANSIG | LOGSIG | 0.99990 |
| ANN11 | NN-2-10-10-1 | TANSIG | LOGSIG | 0.99993 |
| ANN12 | NN-2-10-10-1 | LOGSIG | LOGSIG | 1.00000 |
| ANN13 | NN-2-15-15-1 | LOGSIG | LOGSIG | 0.99998 |
| ANN14 | NN-2-10-15-1 | LOGSIG | LOGSIG | 0.99997 |
| ANN15 | NN-2-15-10-1 | LOGSIG | LOGSIG | 0.99996 |
| Set | Statistical Parameters | |||
|---|---|---|---|---|
| R | RMSE | MAE | MBE | |
| Training | 0.99999 | 0.09786 | 0.04177 | 0.00583 |
| Validation | 0.99999 | 0.04578 | 0.03291 | −0.01063 |
| Test | 0.99999 | 0.05197 | 0.03713 | 0.002655 |
| All | 0.99999 | 0.08621 | 0.03975 | 0.002897 |
| No. | Input Data | Predicted-Output Data | |
|---|---|---|---|
| Heating Rate (K min−1) | Temperature (K) | Weight Left (%) | |
| 1 | 5 | 528.036 | 99.87579 |
| 2 | 5 | 578.09 | 99.6904 |
| 3 | 5 | 628.072 | 99.328 |
| 4 | 5 | 678.062 | 96.50681 |
| 5 | 5 | 728.025 | 49.30348 |
| 6 | 5 | 778.043 | 0.048376 |
| 7 | 5 | 828.05 | −0.01156 |
| 8 | 10 | 528.014 | 100.0249 |
| 9 | 10 | 578.026 | 99.66833 |
| 10 | 10 | 628.017 | 98.99761 |
| 11 | 10 | 678 | 96.5807 |
| 12 | 10 | 728.002 | 64.78094 |
| 13 | 10 | 778 | 0.450783 |
| 14 | 10 | 828.018 | 0.344112 |
| 15 | 20 | 528.148 | 99.97255 |
| 16 | 20 | 578.205 | 99.85724 |
| 17 | 20 | 628.006 | 99.64173 |
| 18 | 20 | 678.273 | 98.72066 |
| 19 | 20 | 728.203 | 88.68082 |
| 20 | 20 | 778.291 | 0.577601 |
| 21 | 20 | 828.075 | −0.04285 |
| 22 | 40 | 528.194 | 99.98355 |
| 23 | 40 | 578.397 | 99.8972 |
| 24 | 40 | 628.452 | 99.74232 |
| 25 | 40 | 678.12 | 99.26672 |
| 26 | 40 | 728.501 | 94.30099 |
| 27 | 40 | 778.047 | 30.7278 |
| 28 | 40 | 828.38 | 0.264529 |
| Set | Statistical Parameters | |||
|---|---|---|---|---|
| R | RMSE | MAE | MBE | |
| simulated | 0.99998 | 0.17017 | 0.07941 | 0.04903 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dubdub, I.; Al-Yaari, M. Pyrolysis of Low Density Polyethylene: Kinetic Study Using TGA Data and ANN Prediction. Polymers 2020, 12, 891. https://doi.org/10.3390/polym12040891
Dubdub I, Al-Yaari M. Pyrolysis of Low Density Polyethylene: Kinetic Study Using TGA Data and ANN Prediction. Polymers. 2020; 12(4):891. https://doi.org/10.3390/polym12040891
Chicago/Turabian StyleDubdub, Ibrahim, and Mohammed Al-Yaari. 2020. "Pyrolysis of Low Density Polyethylene: Kinetic Study Using TGA Data and ANN Prediction" Polymers 12, no. 4: 891. https://doi.org/10.3390/polym12040891
APA StyleDubdub, I., & Al-Yaari, M. (2020). Pyrolysis of Low Density Polyethylene: Kinetic Study Using TGA Data and ANN Prediction. Polymers, 12(4), 891. https://doi.org/10.3390/polym12040891