Melting Kinetics of Nascent Poly(tetrafluoroethylene) Powder
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Kinetic Analysis
3. Results and Discussion
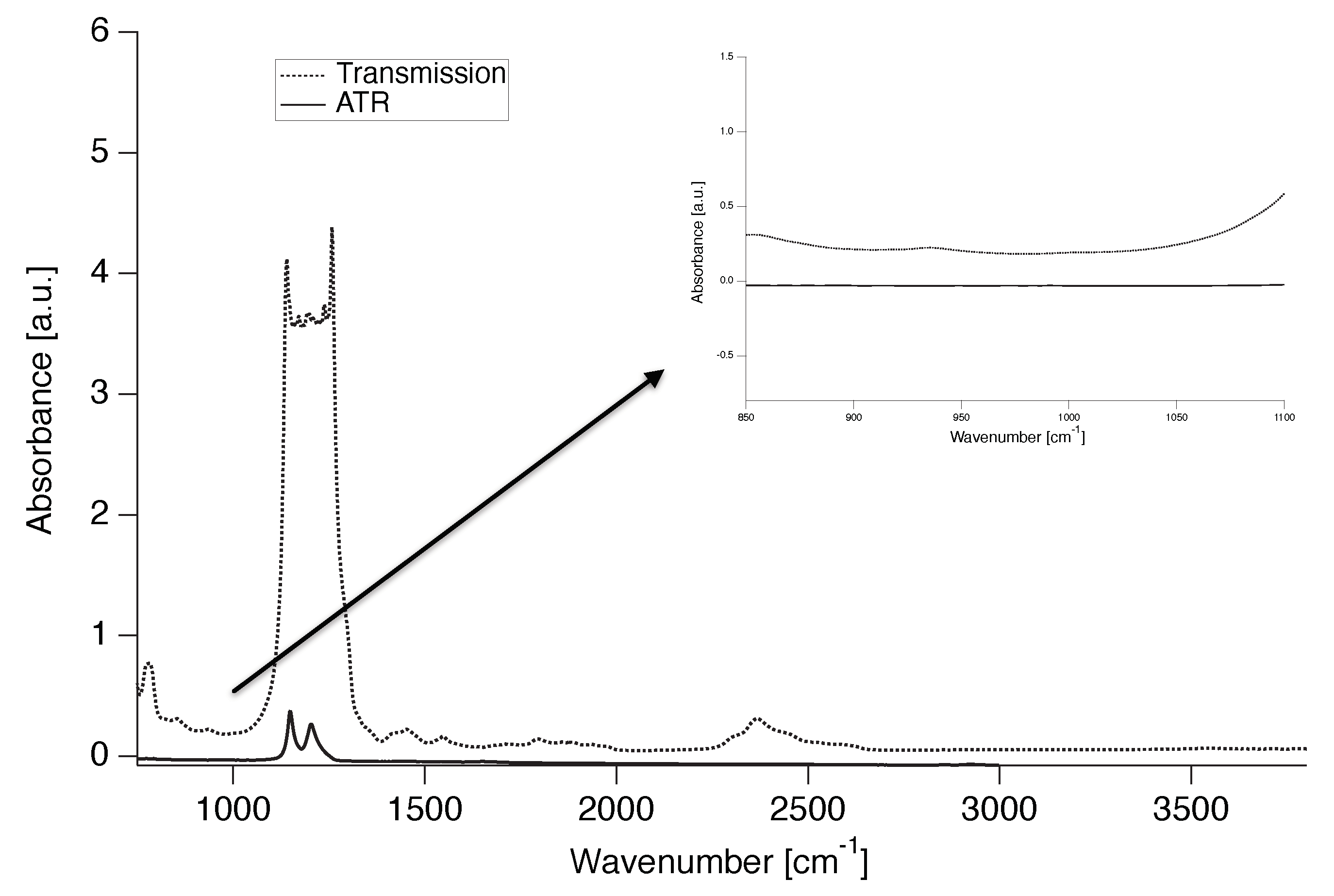
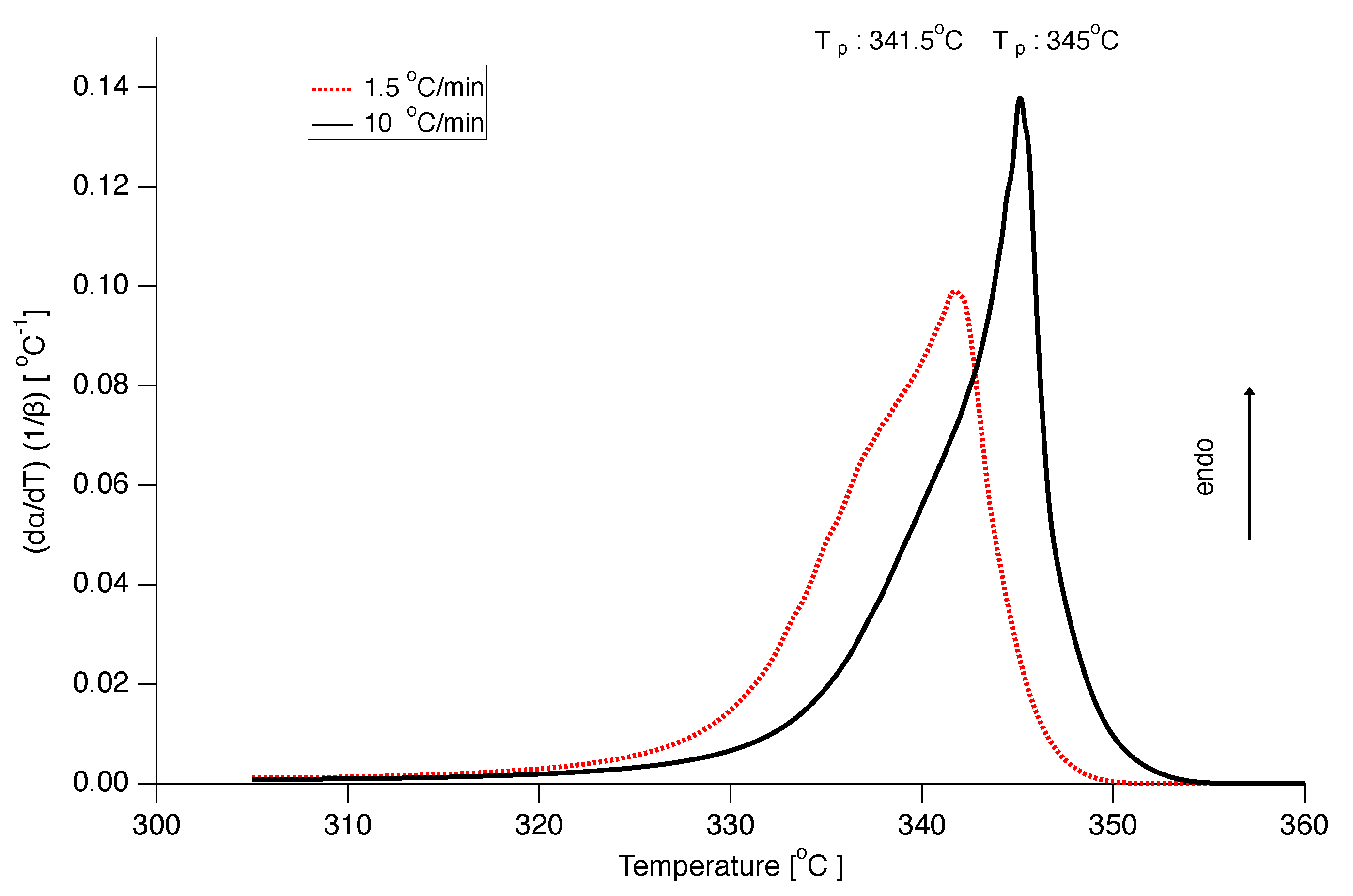
3.1. Material Characterization
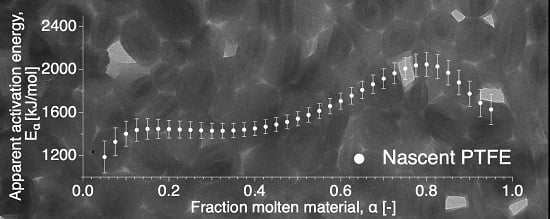
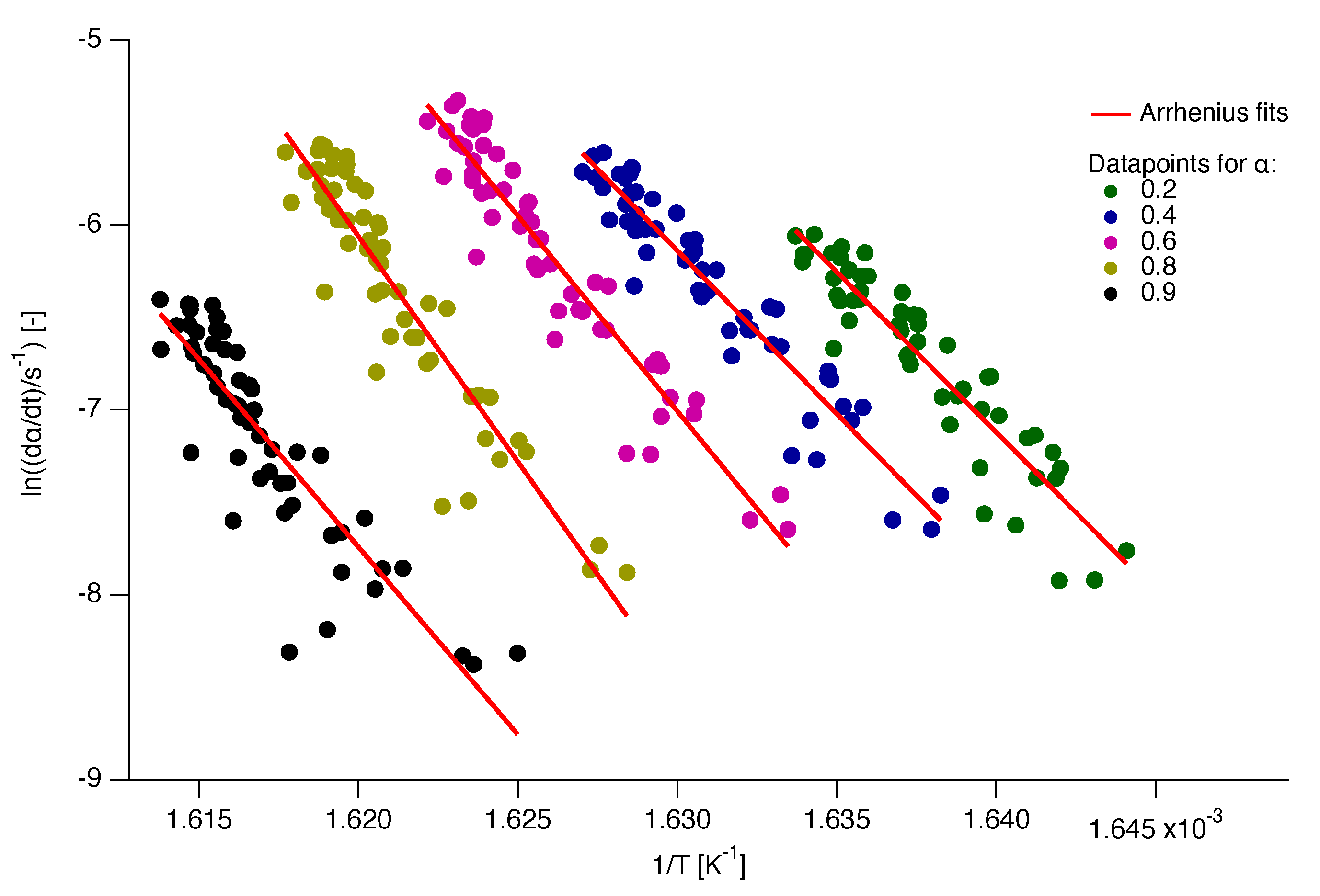
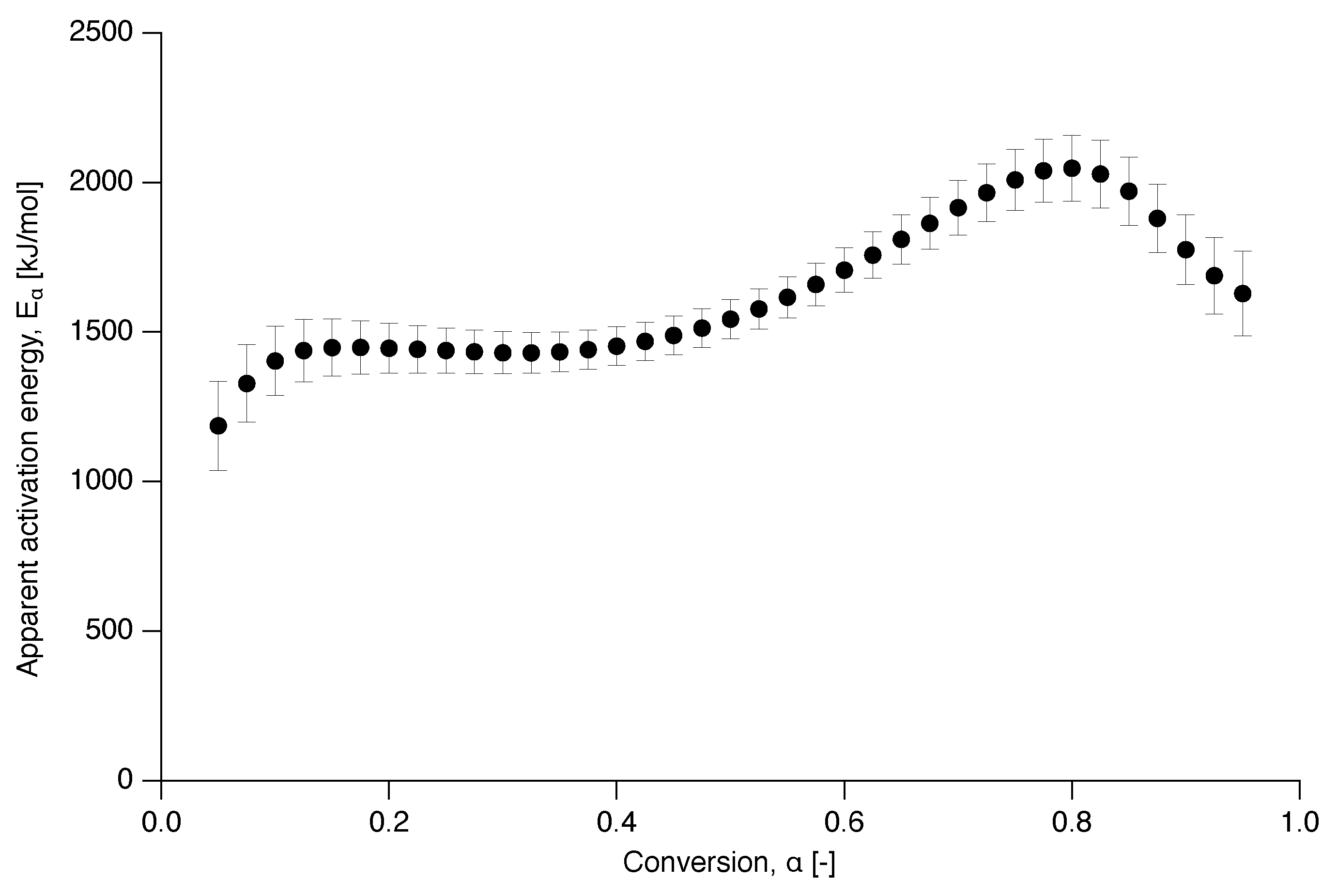
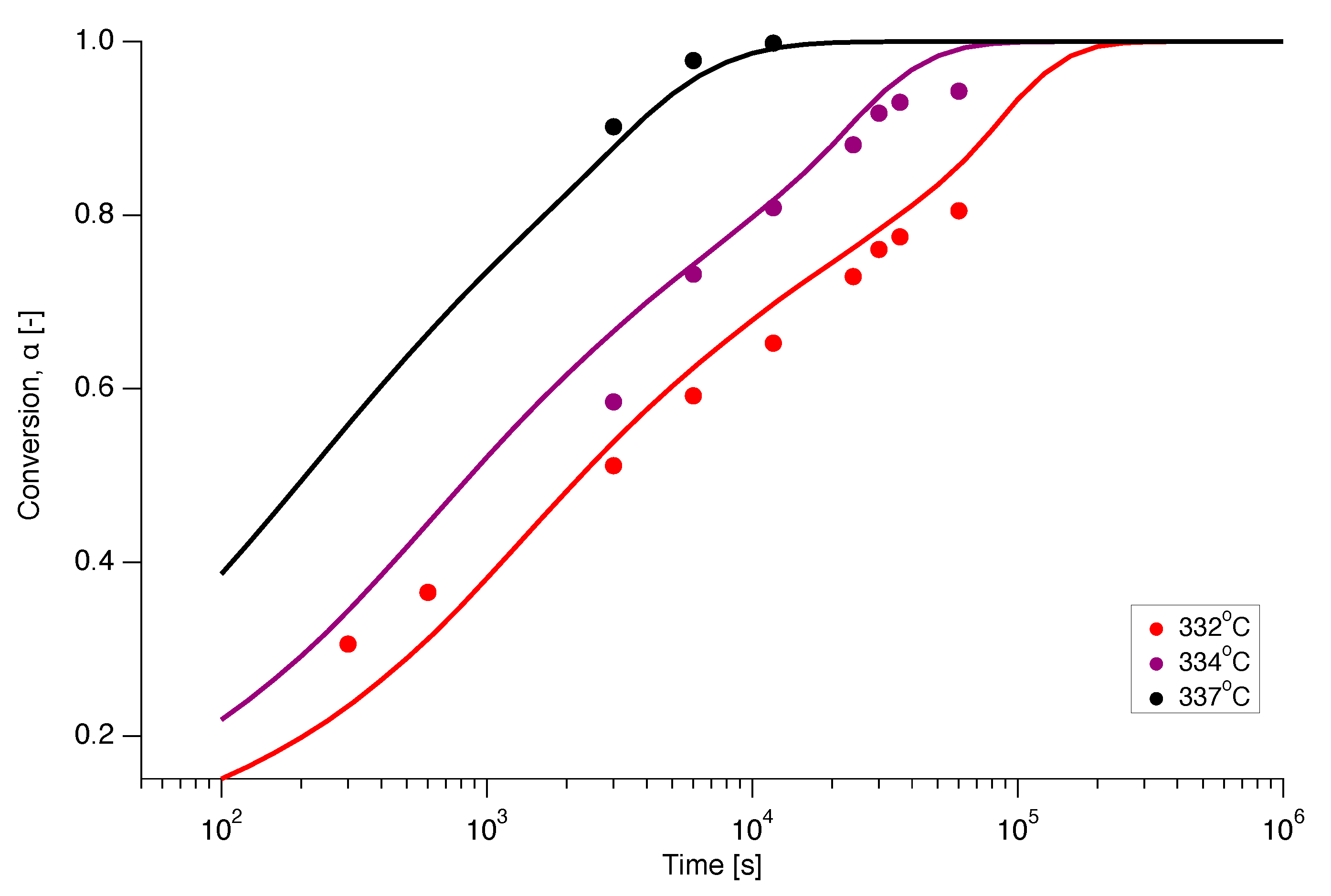
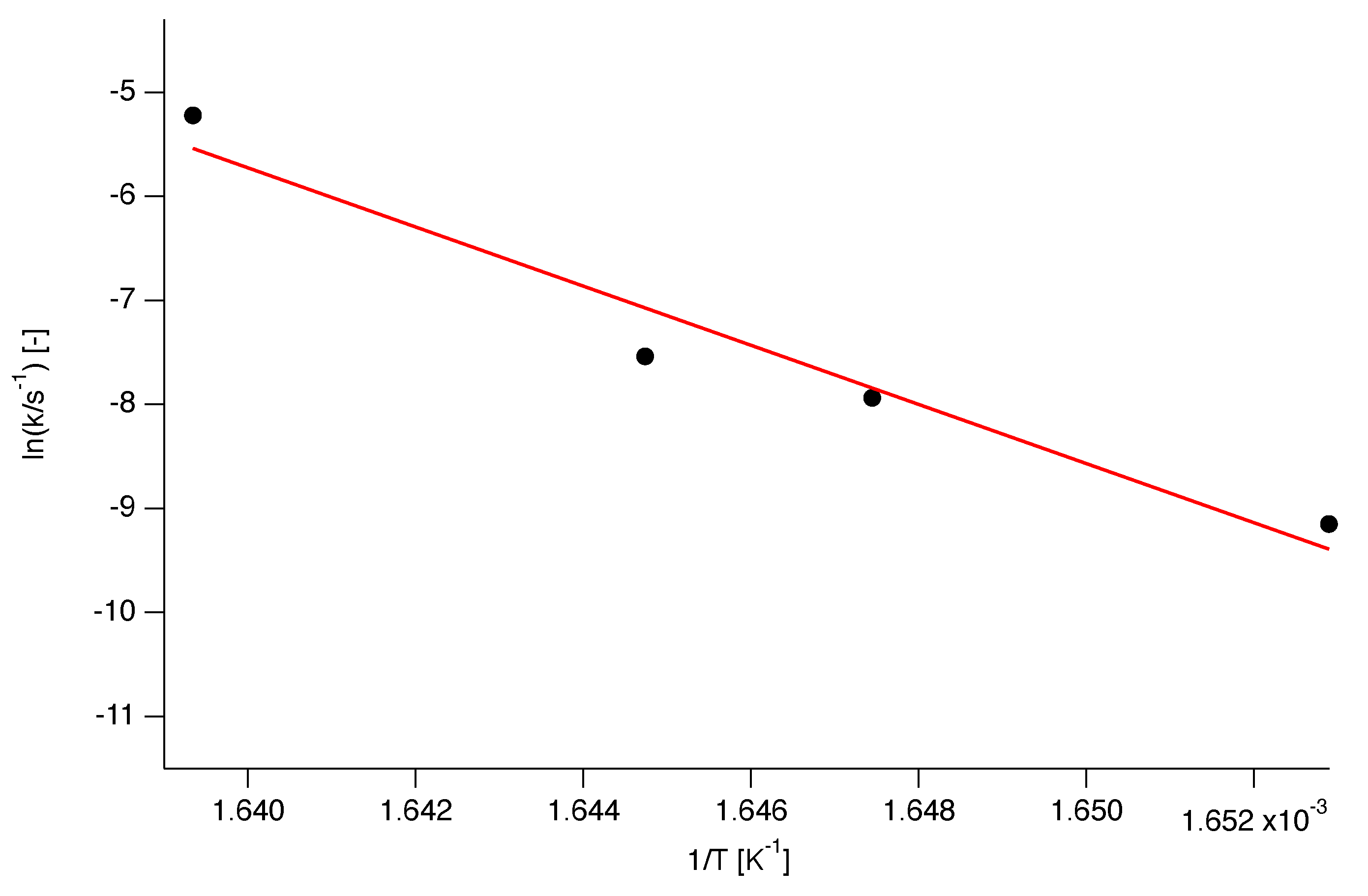
3.2. Apparent Activation Energy Calculation
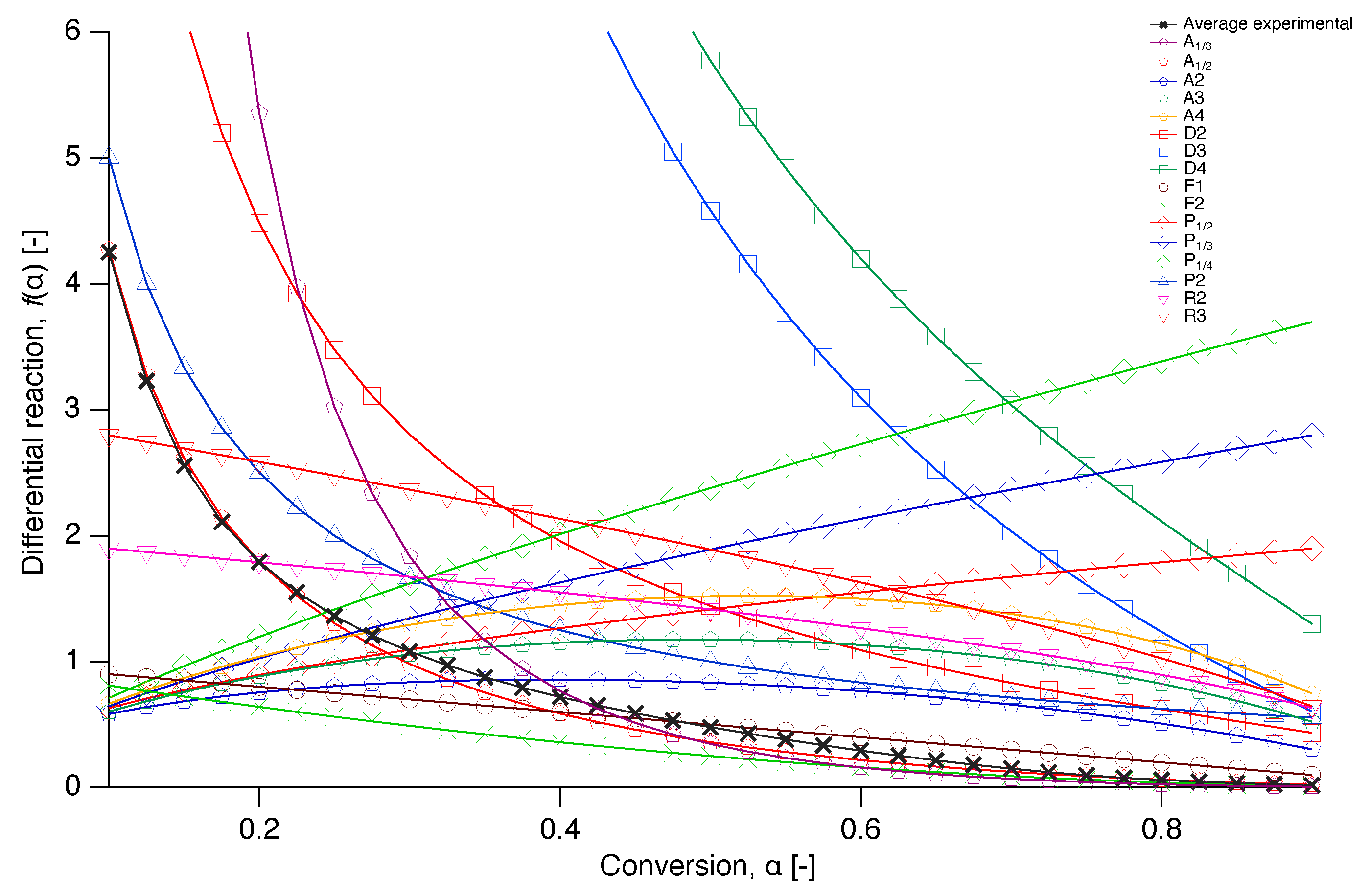
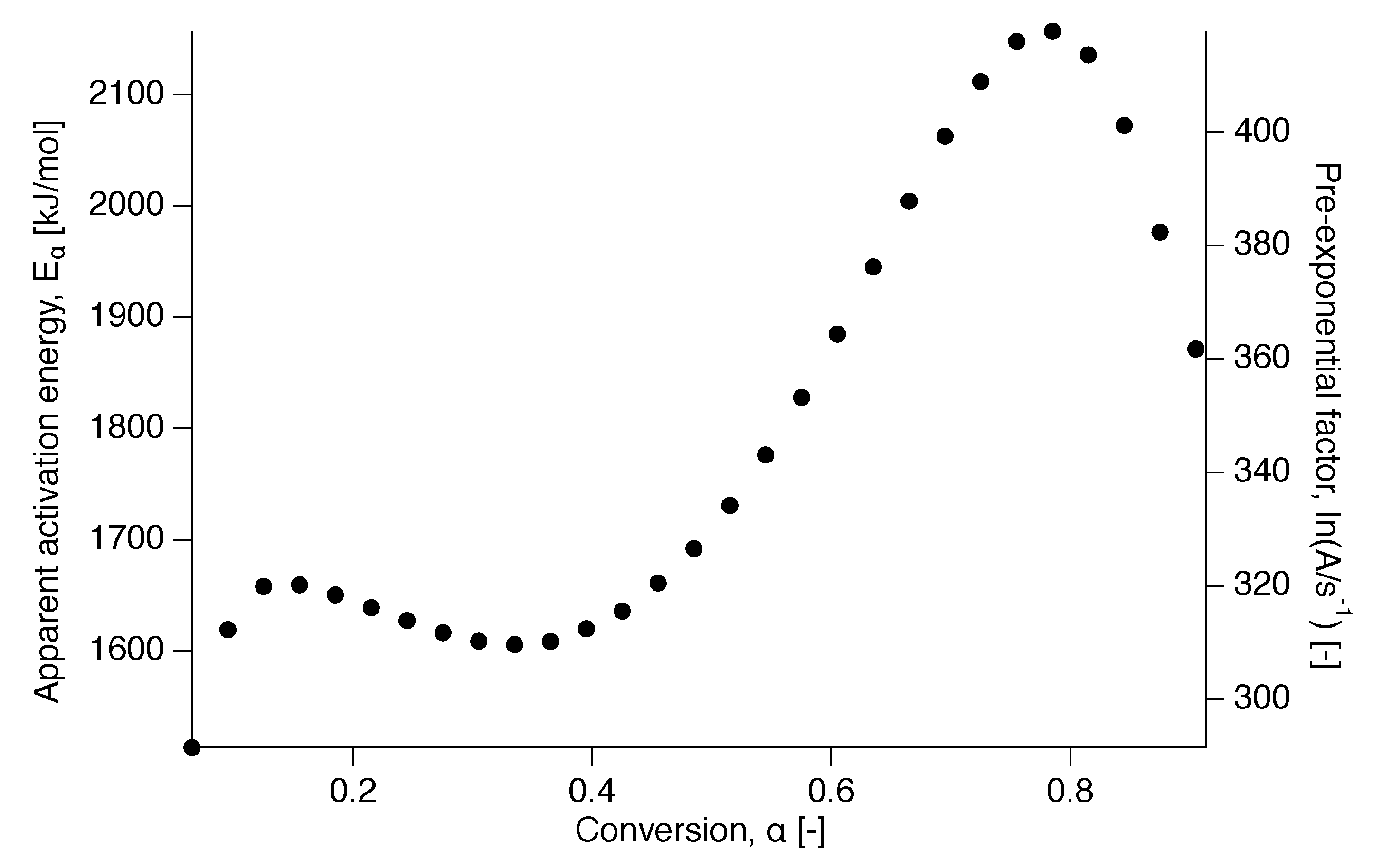
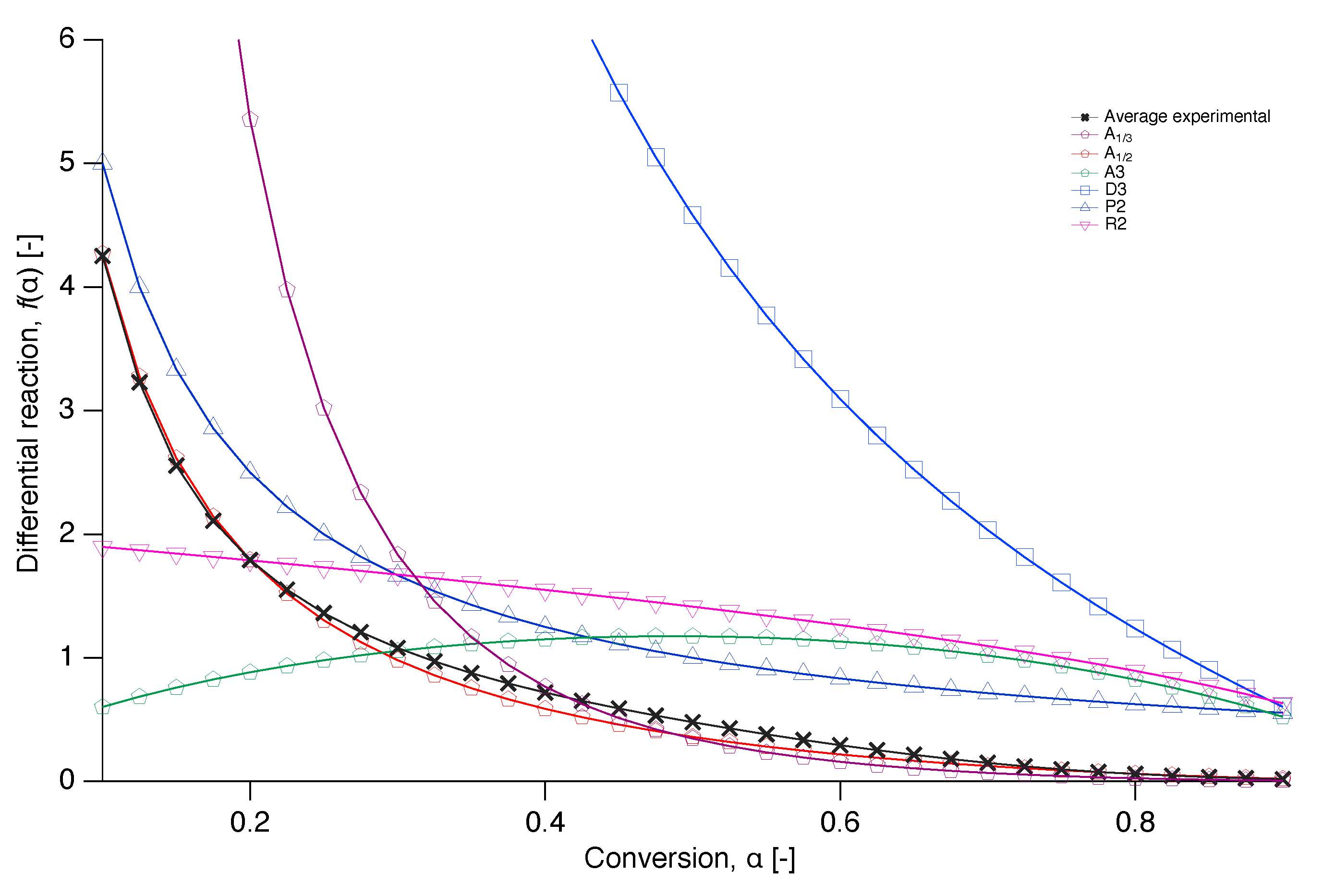
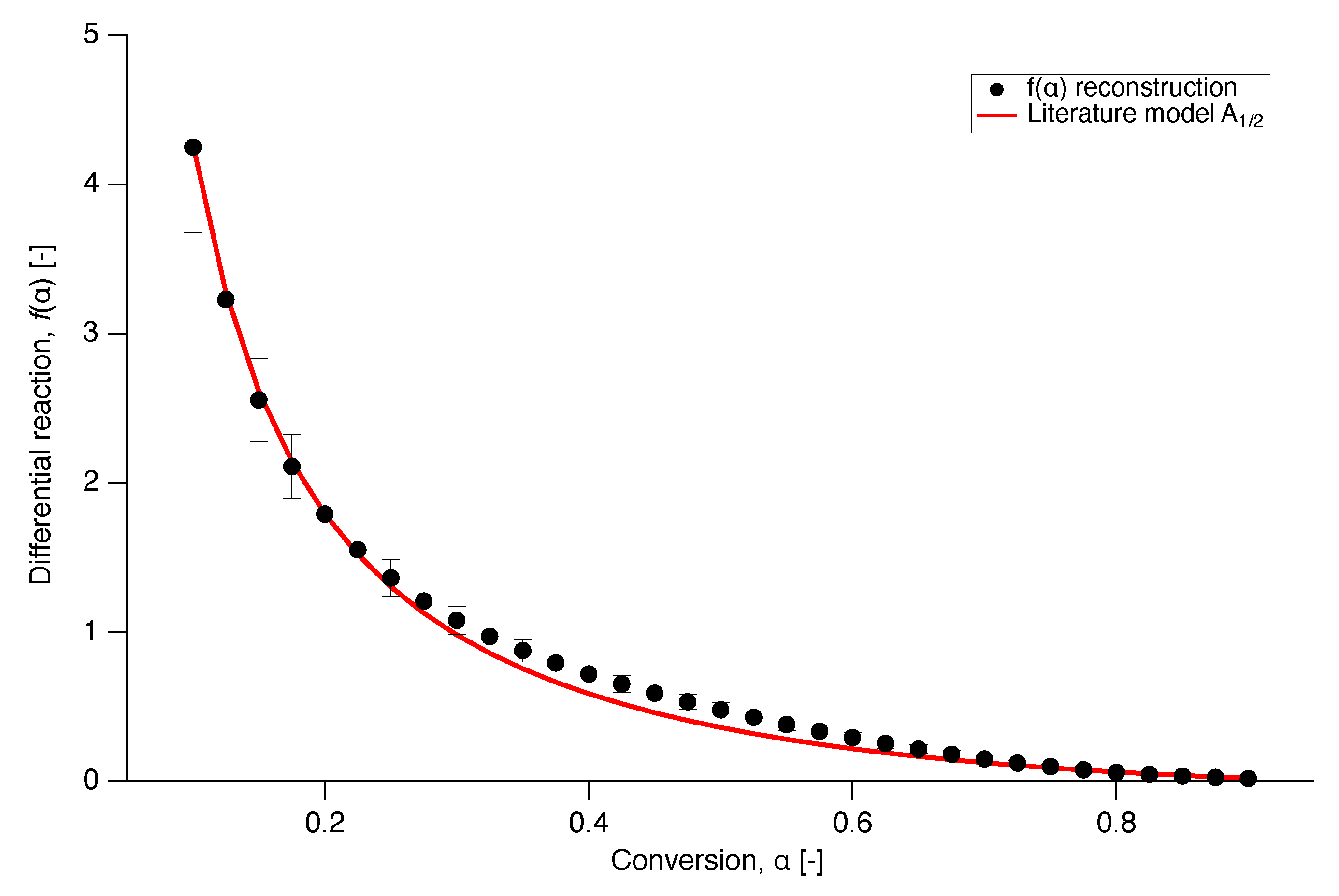
3.3. Pre-Exponential Factor and Reaction Model Calculation
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
Appendix A
References
- Ozawa, T. Kinetics of non-isothermal crystallization. Polymer 1971, 12, 150–158. [Google Scholar] [CrossRef]
- Mandelkern, L. Crystallization of Polymers Volume 2. Kinetics and Mechanisms; Cambridge University Press: Cambridge, UK, 2004; p. 478. [Google Scholar]
- Chuah Hoe, H. Crystallization kinetics of Poly(Trimethylene Terephthalate). Polym. Eng. Sci. 2001, 41, 308–313. [Google Scholar] [CrossRef]
- Hoffman, J.D.; Lauritzen, J.I. Crystallization of bulk polymers with chain folding: Theory of growth of lamellar spherulites. J. Res. Natl. Bur. Stand. Sect. A Phys. Chem. 1961, 65A, 297–336. [Google Scholar] [CrossRef] [PubMed]
- Vyazovkin, S.; Sbirrazzuoli, N. Isoconversional kinetic analysis of thermally stimulated processes in polymers. Macromol. Rapid Commun. 2006, 27, 1515–1532. [Google Scholar] [CrossRef]
- Vyazovkin, S.; Yancey, B.; Walker, K. Nucleation-Driven Kinetics of Poly (ethylene terephthalate) Melting. Macromol. Chem. Phys. 2013, 214, 2562–2566. [Google Scholar] [CrossRef]
- Toda, A.; Hikosaka, M.; Yamada, K. Superheating of the melting kinetics in polymer crystals: A possible nucleation mechanism. Polymer 2002, 43, 1667–1679. [Google Scholar] [CrossRef]
- Toda, A.; Kojima, I.; Hikosaka, M. Melting kinetics of polymer crystals with an entropic barrier. Macromolecules 2008, 41, 120–127. [Google Scholar] [CrossRef]
- Vyazovkin, S. Isoconversional Kinetics of Thermally Stimulated Processes; Springer: Berlin, Germany, 2015. [Google Scholar] [CrossRef]
- Vyazovkin, S. Isoconversional Kinetics of Polymers: The Decade Past. Macromol. Rapid Commun. 2017, 38, 1–21. [Google Scholar] [CrossRef]
- Toda, A.; Taguchi, K.; Nozaki, K.; Fukushima, T.; Kaji, H. Superheated Melting Kinetics of Metastable Chain-Folded Polymer Crystals. Cryst. Growth Des. 2018, 18, 3637–3643. [Google Scholar] [CrossRef]
- Starkweather, H.W. Effect of Heating Rate on the Melting of Polytetrafluoroethylene. J. Polym. Sci. Part A-2 Polym. Phys. 1985, 23, 1177–1185. [Google Scholar] [CrossRef]
- Rastogi, S.; Lippits, D.R.; Peters, G.W.M.; Graf, R.; Yao, Y.; Spiess, H.W. Heterogeneity in polymer melts from melting of polymer crystals. Nat. Mater. 2005, 4, 635–641. [Google Scholar] [CrossRef] [PubMed]
- Hellmuth, E.; Wunderlich, B. Superheating of linear high-polymer polyethylene crystals. J. Appl. Phys. 1965, 36, 3039–3044. [Google Scholar] [CrossRef]
- Androsch, R.; Wunderlich, B.; Radusch, H.J. Analysis of reversible melting in polytetrafluoroethylene. J. Therm. Anal. Calorimet. 2005, 79, 615–622. [Google Scholar] [CrossRef]
- Wunderlich, B. One hundred years research on supercooling and superheating. Thermochim. Acta 2007, 461, 4–13. [Google Scholar] [CrossRef]
- Smith, P.; Chanzy, H.D.; Rotzinger, B.P. Drawing of virgin ultrahigh molecular weight polyethylene: An alternative route to high strength/high modulus materials—Part 2 Influence of polymerization temperature. J. Mater. Sci. 1987, 22, 523–531. [Google Scholar] [CrossRef]
- Rotzinger, B.P.; Chanzy, H.D.; Smith, P. High strength/high modulus polyethylene: Synthesis and processing of ultra-high molecular weight virgin powders. Polymer 1989, 30, 1814–1819. [Google Scholar] [CrossRef]
- Pandey, A.; Toda, A.; Rastogi, S. Influence of amorphous component on melting of semicrystalline polymers. Macromolecules 2011, 44, 8042–8055. [Google Scholar] [CrossRef]
- Romano, D.; Tops, N.; Andablo-Reyes, E.; Ronca, S.; Rastogi, S. Influence of polymerization conditions on melting kinetics of low entangled uhmwpe and its implications on mechanical properties. Macromolecules 2014, 47, 4750–4760. [Google Scholar] [CrossRef]
- Keller, A.; Willmouth, F.M. On the morphology and orgin of the fibres observed in Nascent Ziegler polyethylene. Die Makromolekulare Chemie 1969, 121, 42–50. [Google Scholar] [CrossRef]
- Tervoort-Engelen, Y.M.T.; Lemstra, P.J. Morphology of nascent ultra-high molecular weight polyethylene reactor powder: Chain-extended versus chain-folded crystals. Polym. Commun. 1991, 32, 343–345. [Google Scholar]
- Phillips, R.A. Morphology and melting behavior of nascent ultra-high molecular weight polyethylene. J. Polym. Sci. Part B Polym. Phys. 1998, 36, 495–517. [Google Scholar] [CrossRef]
- Starkweather, H.W.; Zoller, P.; Jones, G.A.; Vega, A.J. The heat of fusion of polytetrafluoroethylene. J. Polym. Sci. Polym. Phys. Ed. 1982, 20, 751–761. [Google Scholar] [CrossRef]
- Menczel, J.D.; Prime, R.B. Thermal Analysis of Polymers: Fundamentals and Applications; John Wiley & Sons: Hoboken, NJ, USA, 2008; pp. 1–688. [Google Scholar] [CrossRef]
- Vyazovkin, S. A Unified Approach to Nonisothermal Data. Unified Kinet. Process. 1995, 28, 95–101. [Google Scholar]
- Friedman, H.L. Kinetics of thermal degradation of char-forming plastics from thermogravimetry. Application to a phenolic plastic. J. Polym. Sci. Part C Polym. Symp. 1964, 6, 183–195. [Google Scholar] [CrossRef]
- Kissinger, H.E. Reaction Kinetics in Differential Thermal Analysis. Anal. Chem. 1957, 29, 1702–1706. [Google Scholar] [CrossRef]
- Vyazovkin, S.; Burnham, A.K.; Criado, J.M.; Pérez-Maqueda, L.A.; Popescu, C.; Sbirrazzuoli, N. ICTAC Kinetics Committee recommendations for performing kinetic computations on thermal analysis data. Thermochim. Acta 2011, 520, 1–19. [Google Scholar] [CrossRef]
- Vyazovkin, S.; Wight, C.A. Isothermal and non-isothermal kinetics of thermally stimulated reactions of solids. Int. Rev. Phys.Chem. 1998, 17, 407–433. [Google Scholar] [CrossRef]
- Arrhenius, S. Über die Reaktionsgeschwindigkeit bei der Inversion von Rohrzucker durch Säuren. Zeitschrift für Physikalische Chemie 1889, 4. [Google Scholar] [CrossRef]
- Sbirrazzuoli, N. Determination of pre-exponential factors and of the mathematical functions f(α) or G(α) that describe the reaction mechanism in a model-free way. Thermochim. Acta 2013, 564, 59–69. [Google Scholar] [CrossRef]
- Vyazovkin, S.; Gang Linert, W. Thermally induced reactions of solids: Isokinetic relationships of non-isothermal systems. Int. Rev. Phys. Chem. 1995, 14, 355–369. [Google Scholar] [CrossRef]
- Trache, D.; Abdelaziz, A.; Siouani, B. A simple and linear isoconversional method to determine the pre-exponential factors and the mathematical reaction mechanism functions. J. Therm. Anal. Calorim. 2017, 128, 335–348. [Google Scholar] [CrossRef]
- Lau, S.F.; Suzuki, H.; Wunderlich, B. Thermodynamic Properties of Polytetrafluoroethylene. J. Polym. Sci. Part A-2 Polym. Phys. 1984, 22, 379–405. [Google Scholar] [CrossRef]
- Illers, K.H. Die ermittlung des schmelzpunktes von kristallinen polymeren mittels wärmeflusskalorimetrie (DSC). Eur. Polym. J. 1974, 10, 911–916. [Google Scholar] [CrossRef]
- Liavitskaya, T.; Birx, L.; Vyazovkin, S. Melting kinetics of superheated crystals of glucose and fructose. Phys. Chem. Chem. Phys. 2017, 19, 26056–26064. [Google Scholar] [CrossRef]
- De Bruijn, T.J.W.; De Jong, W.A.; Van Den Berg, P.J. Kinetic parameters in Avrami-Erofeev type reactions from isothermal and non-isothermal experiments. Thermochim. Acta 1981, 45, 315–325. [Google Scholar] [CrossRef]
- Vyazovkin, S. A time to search: Finding the meaning of variable activation energy. Phys. Chem. Chem. Phys. 2016, 18, 18643–18656. [Google Scholar] [CrossRef]
- Liu, F.; Sommer, F.; Bos, C.; Mittemeijer, E.J. Analysis of solid state phase transformation kinetics: Models and recipes. Int. Mater. Rev. 2007, 52, 193–212. [Google Scholar] [CrossRef]
- Grapes, M.D.; Santala, M.K.; Campbell, G.H.; LaVan, D.A.; Weihs, T.P. A detailed study of the Al3Ni formation reaction using nanocalorimetry. Thermochim. Acta 2017, 658, 72–83. [Google Scholar] [CrossRef]
- Torrens-Serra, J.; Venkataraman, S.; Stoica, M.; Kuehn, U.; Roth, S.; Eckert, J. Non-isothermal kinetic analysis of the crystallization of metallic glasses using the master curve method. Materials 2011, 4, 2231–2243. [Google Scholar] [CrossRef]
- Ebnesajjad, S. FLUOROPLASTICS Volume 1: Non-Melt Processible Fluoropolymers—The Definitive User’s Guide and Data Book; Elsevier: Amsterdam, The Netherlands, 2015. [Google Scholar]




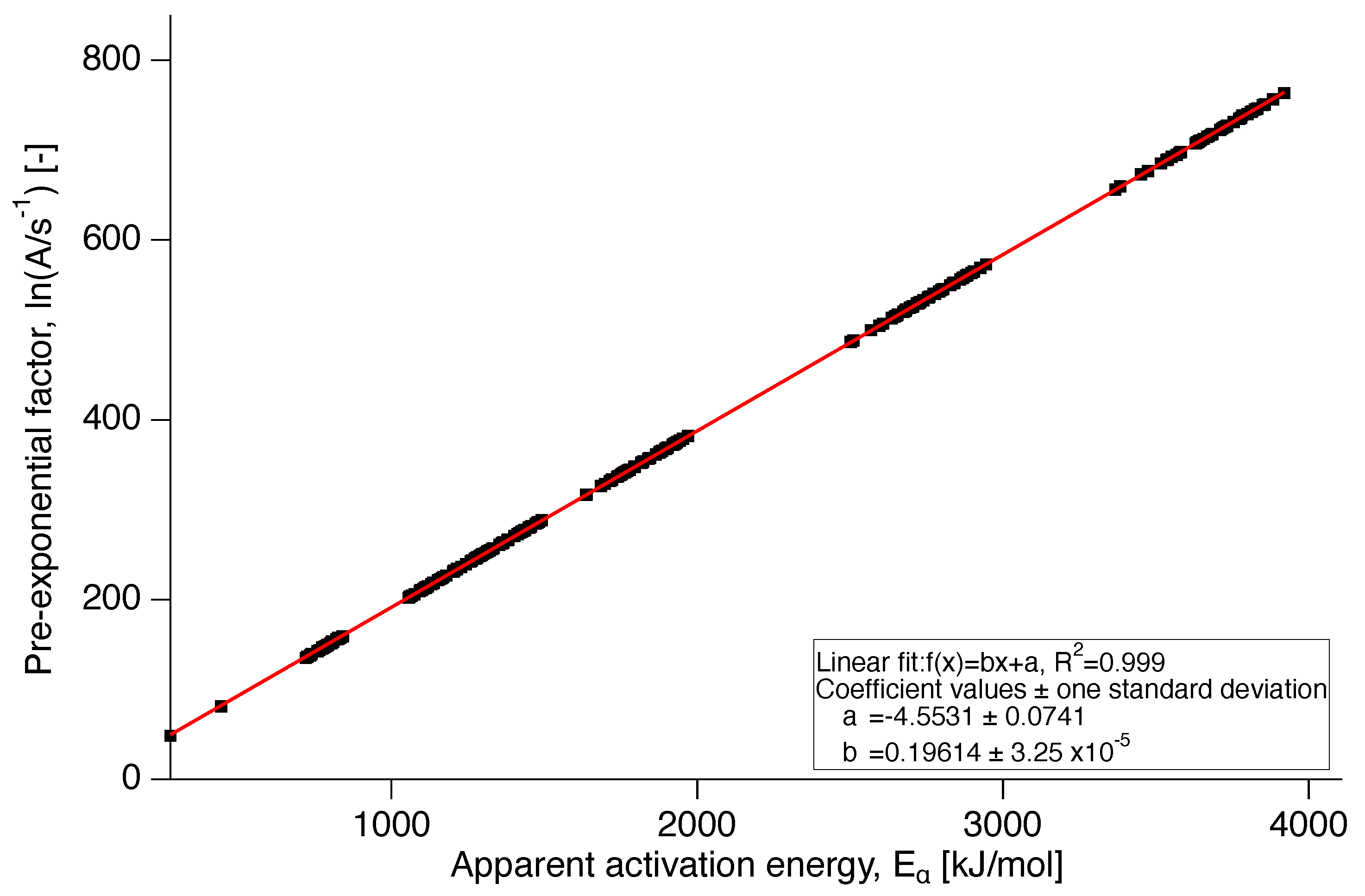


| Number | Model | Rate-Determining Mechanism | |
|---|---|---|---|
| 1 | Chemical reaction | ||
| 2 | Chemical reaction | ||
| 3 | Chemical reaction | ||
| 4 | Chemical reaction | ||
| 5 | Chemical reaction | ||
| 6 | Chemical reaction | ||
| 7 | Chemical reaction | ||
| 8 | Chemical reaction | ||
| 9 | Chemical reaction | ||
| 10 | Nucleation (power law) | ||
| 11 | Nucleation (power law) | ||
| 12 | Nucleation (power law) | ||
| 13 | Nucleation (power law) | ||
| 14 | Nucleation (parabolic law) | ||
| 15 | Nucleation (exponential law) | ||
| 16 | Nucleation (exponential law) | ||
| 17 | Random nucleation/ first order (Mampel) | ||
| 18 | Random nucleation (Avrami-Erofeev) | ||
| 19 | Random nucleation (Avrami-Erofeev) | ||
| 20 | Random nucleation (Avrami-Erofeev) | ||
| 21 | Random nucleation (Avrami-Erofeev) | ||
| 22 | Random nucleation (Avrami-Erofeev) | ||
| 23 | Random nucleation (Avrami-Erofeev) | ||
| 24 | Random nucleation (Avrami-Erofeev) | ||
| 25 | Random nucleation (Avrami-Erofeev) | ||
| 26 | Random nucleation (Avrami-Erofeev) | ||
| 27 | Random nucleation (Avrami-Erofeev) | ||
| 28 | 1 | Contracting disc | |
| 29 | Contracting cylinder | ||
| 30 | Contracting sphere | ||
| 31 | One-dimensional diffusion | ||
| 32 | Three-dimensional diffusion | ||
| 33 | Three-dimensional diffusion (Jander) | ||
| 34 | Three-dimensional diffusion (Ginstling-Brounshtein) |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Christakopoulos, F.; Troisi, E.; Tervoort, T.A. Melting Kinetics of Nascent Poly(tetrafluoroethylene) Powder. Polymers 2020, 12, 791. https://doi.org/10.3390/polym12040791
Christakopoulos F, Troisi E, Tervoort TA. Melting Kinetics of Nascent Poly(tetrafluoroethylene) Powder. Polymers. 2020; 12(4):791. https://doi.org/10.3390/polym12040791
Chicago/Turabian StyleChristakopoulos, Fotis, Enrico Troisi, and Theo A. Tervoort. 2020. "Melting Kinetics of Nascent Poly(tetrafluoroethylene) Powder" Polymers 12, no. 4: 791. https://doi.org/10.3390/polym12040791
APA StyleChristakopoulos, F., Troisi, E., & Tervoort, T. A. (2020). Melting Kinetics of Nascent Poly(tetrafluoroethylene) Powder. Polymers, 12(4), 791. https://doi.org/10.3390/polym12040791