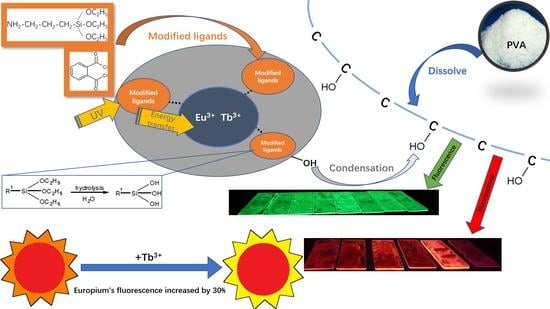
Preparation of PVA Fluorescent Gel and Luminescence of Europium Sensitized by Terbium (III)
Abstract
1. Introduction
2. Experimental Details
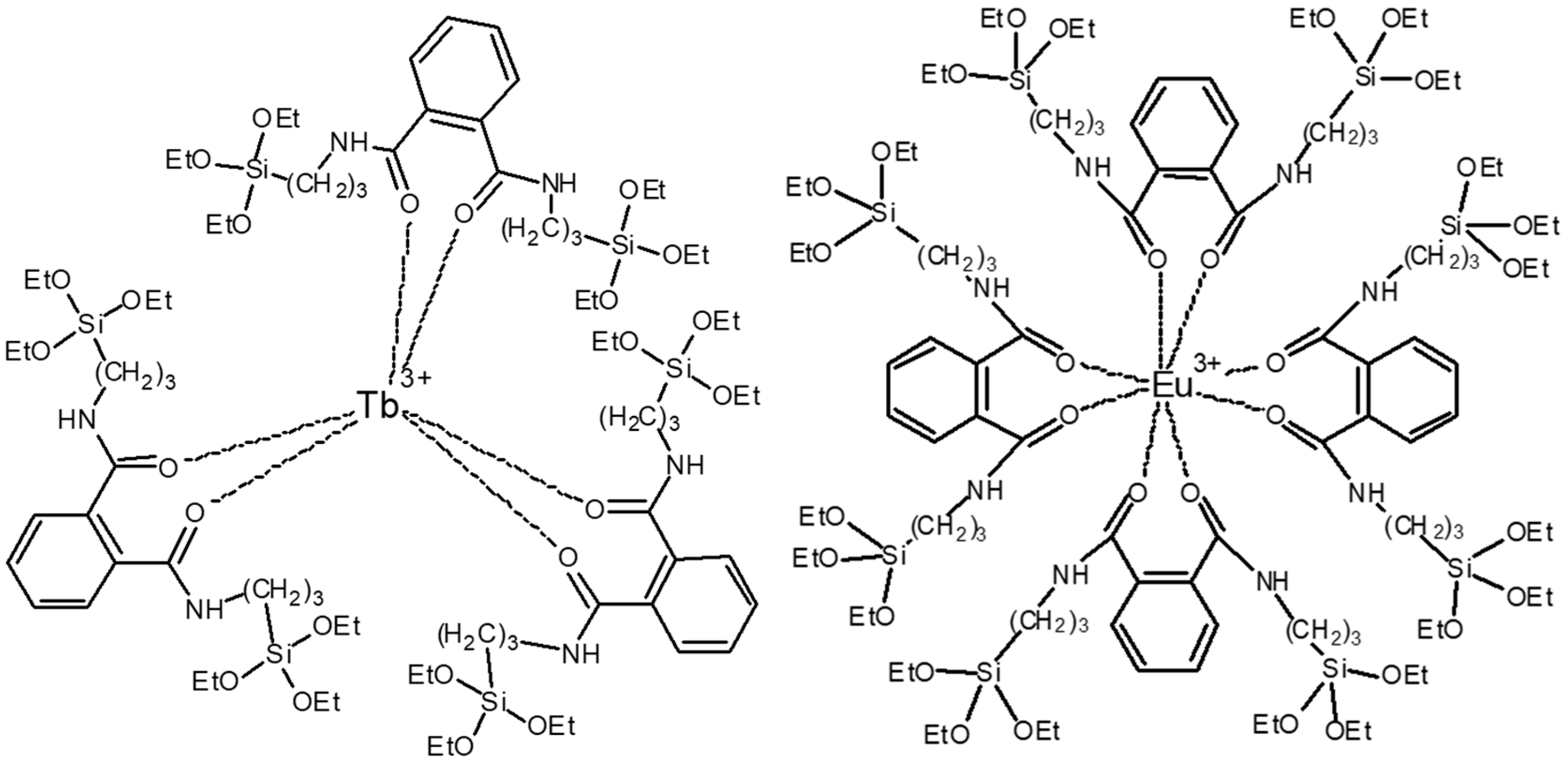
2.1. Preparation of Silane-Modified Eu/Tb Co-Doped Complexes
2.2. Preparation of PVA Fluorescent Gel
2.3. Structural Characterization and Performance Testing
3. Results and Discussion
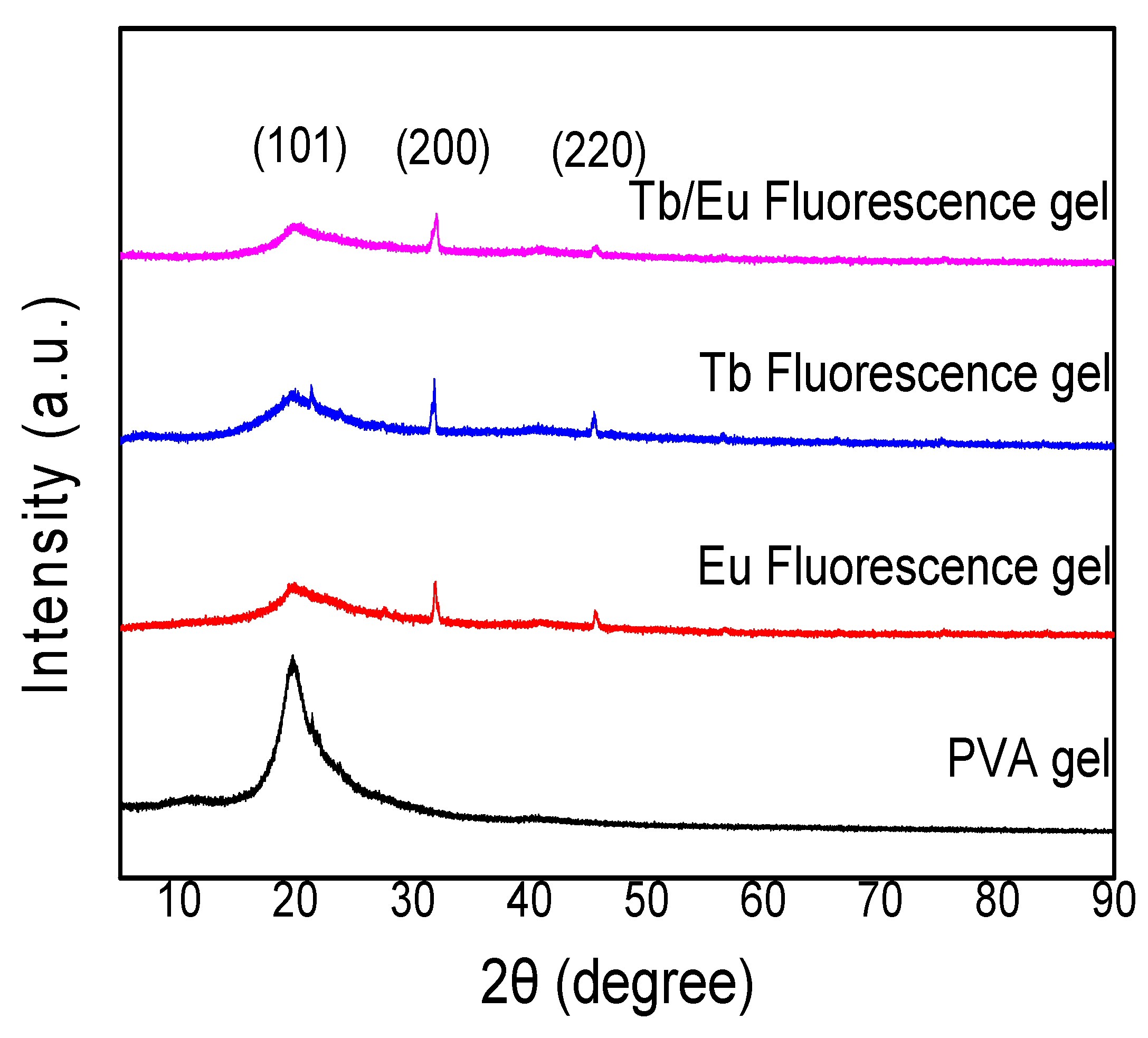
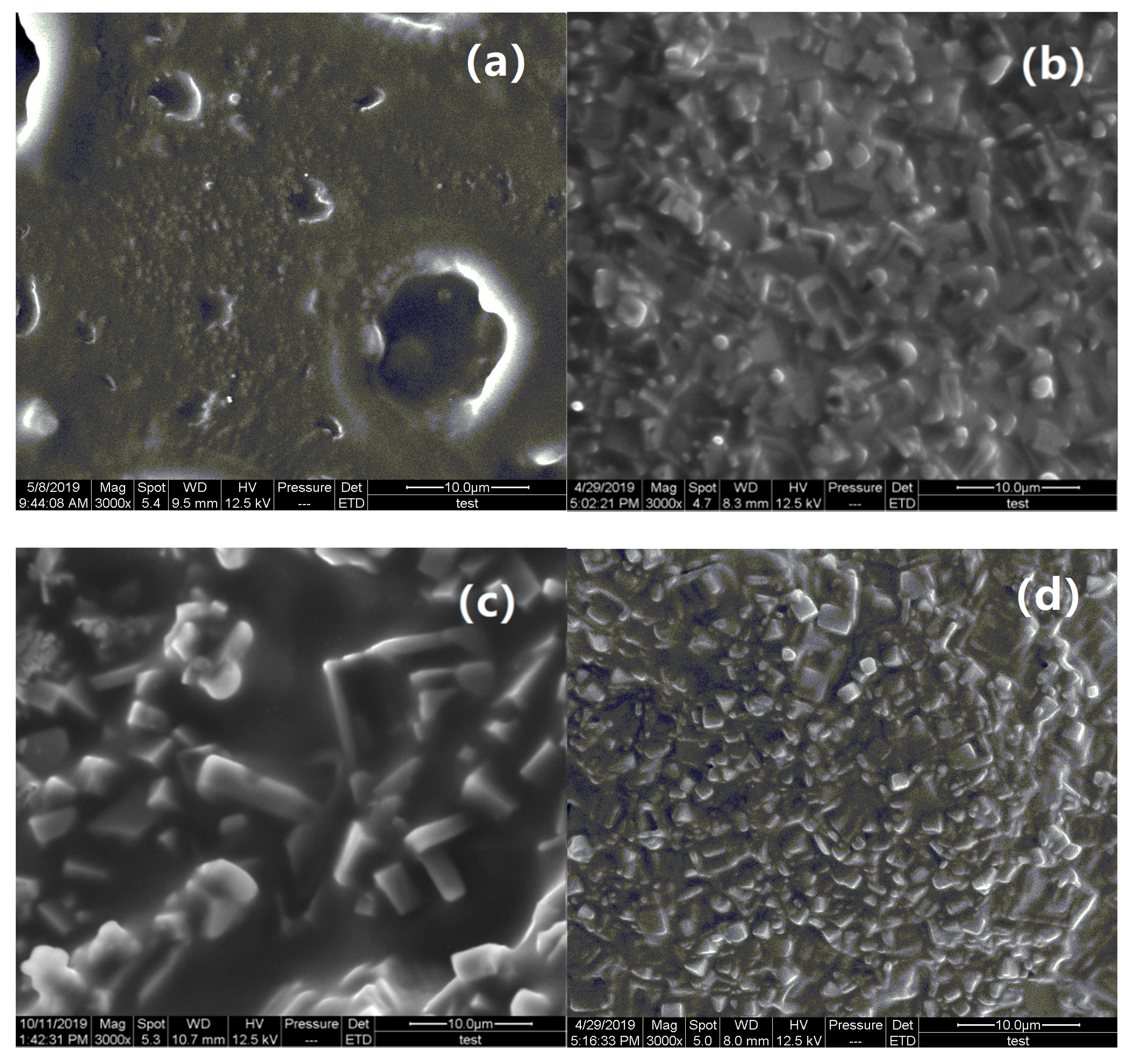
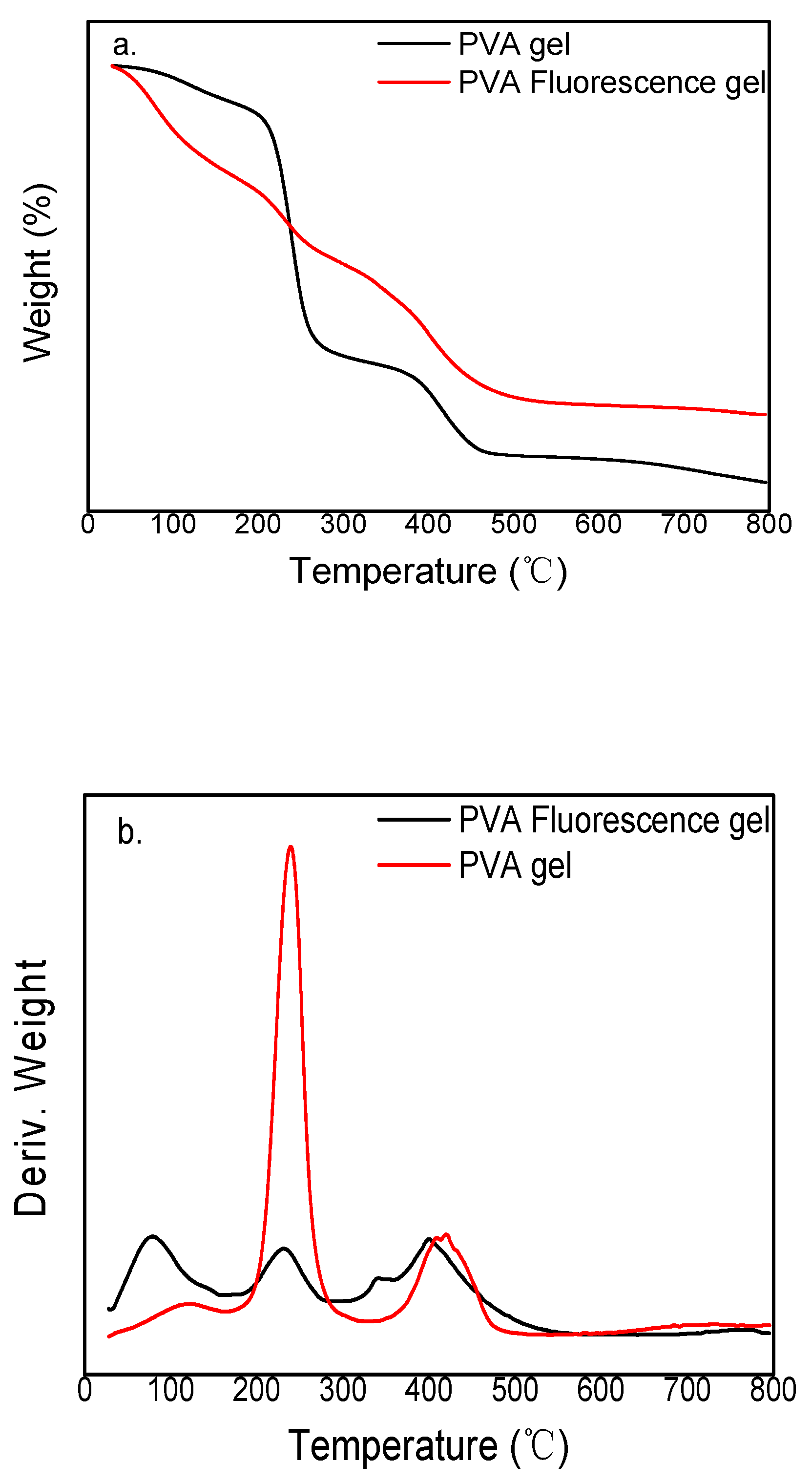
3.1. Structural Analysis
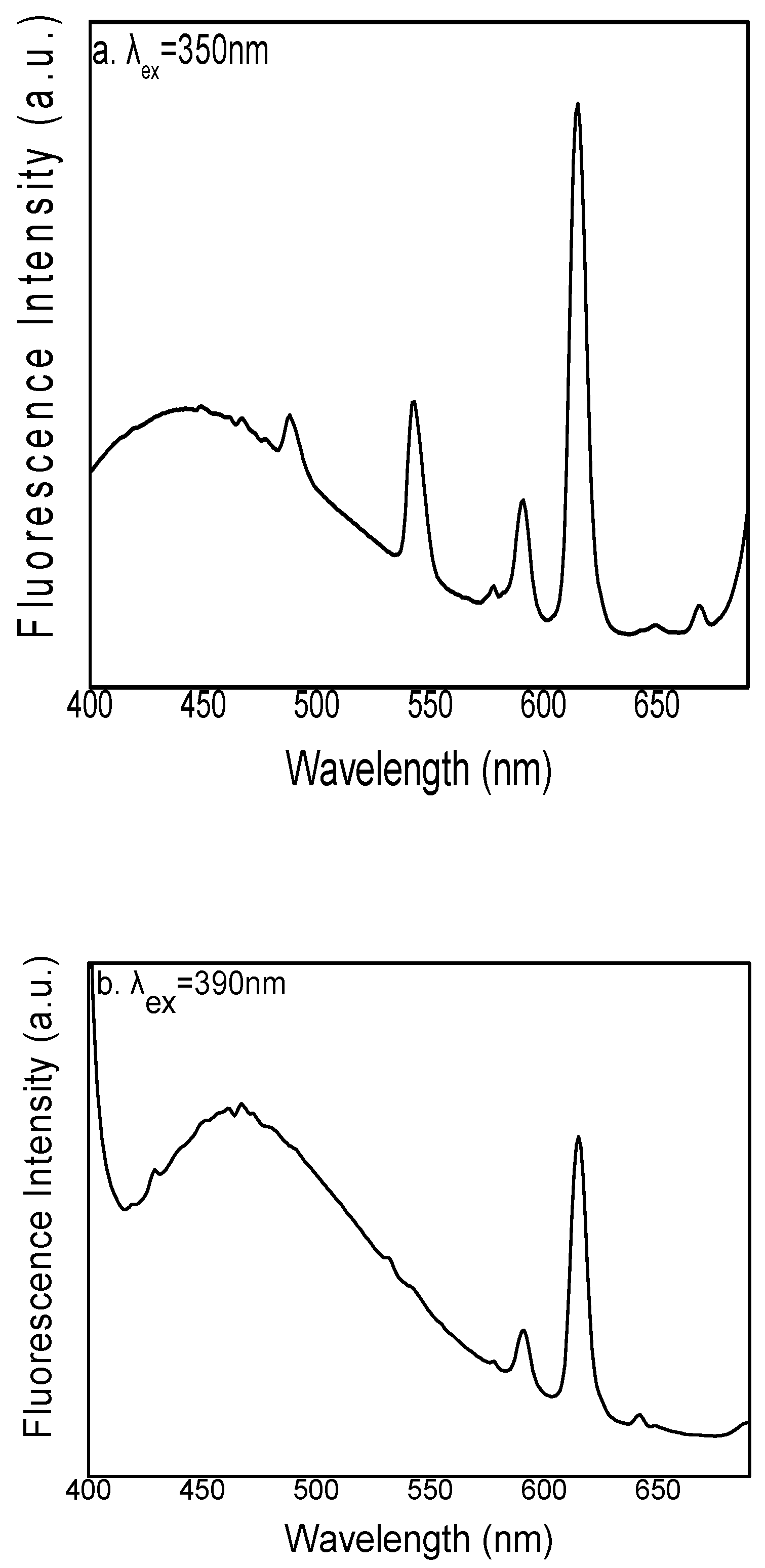
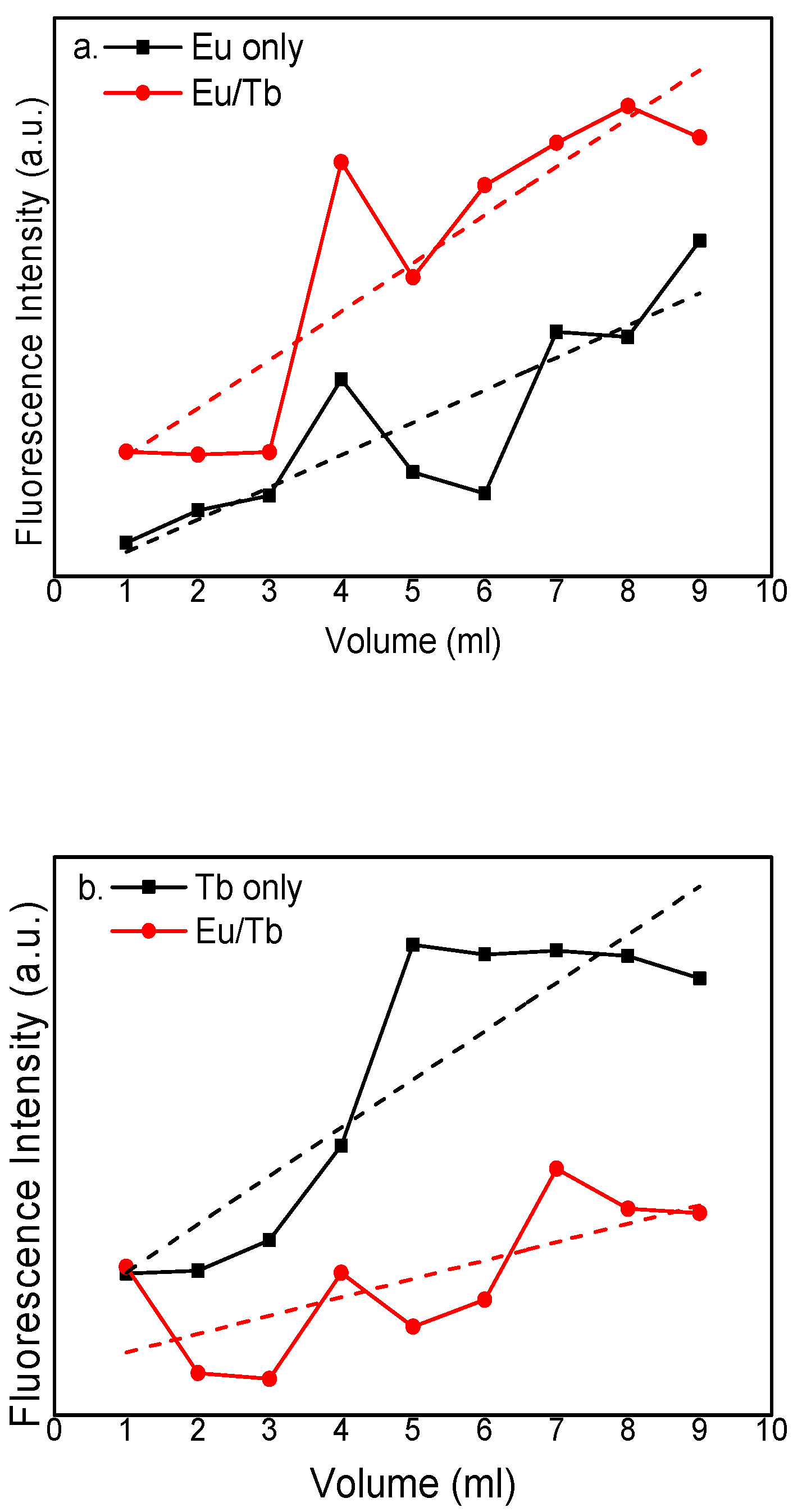
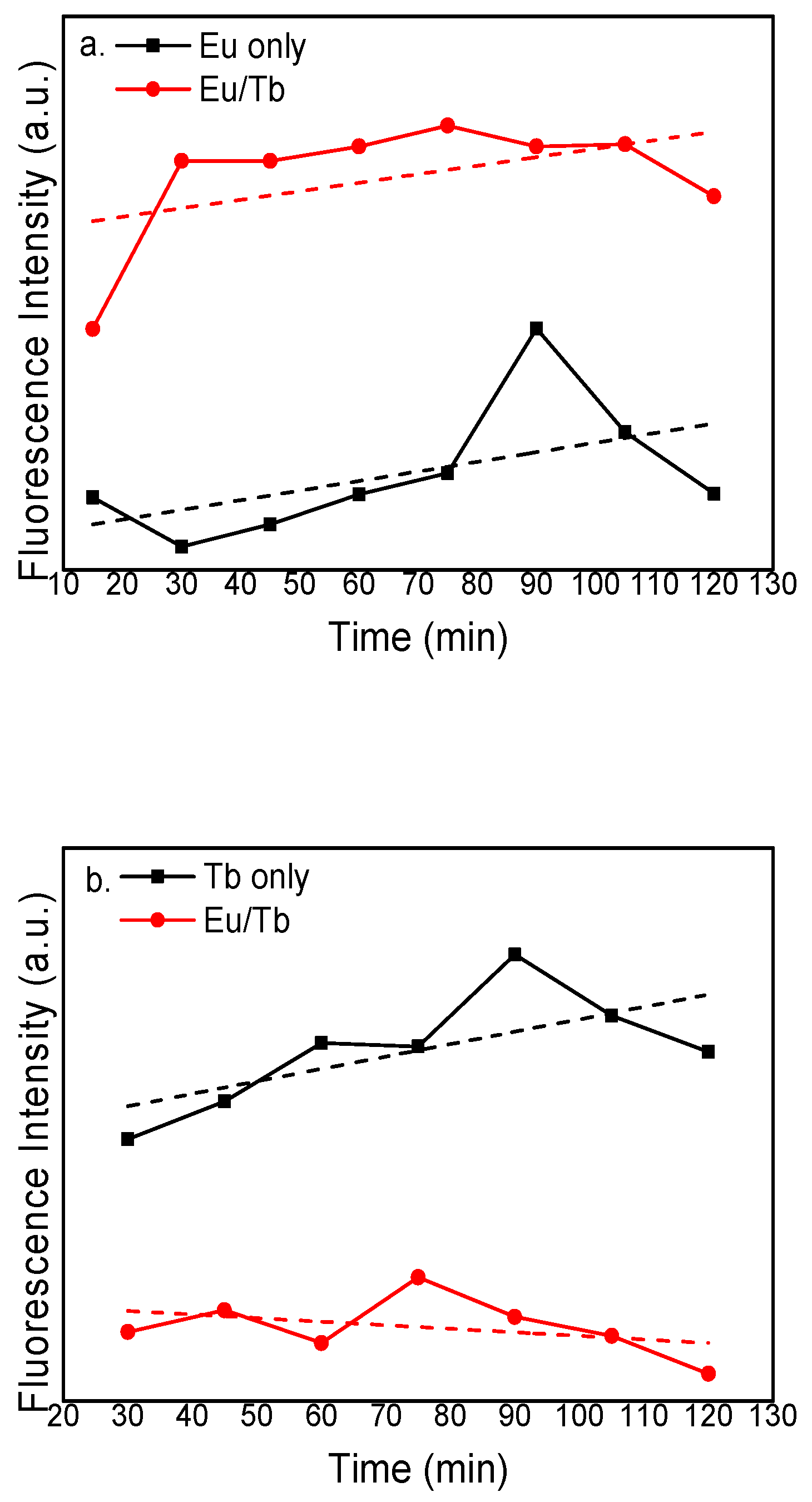
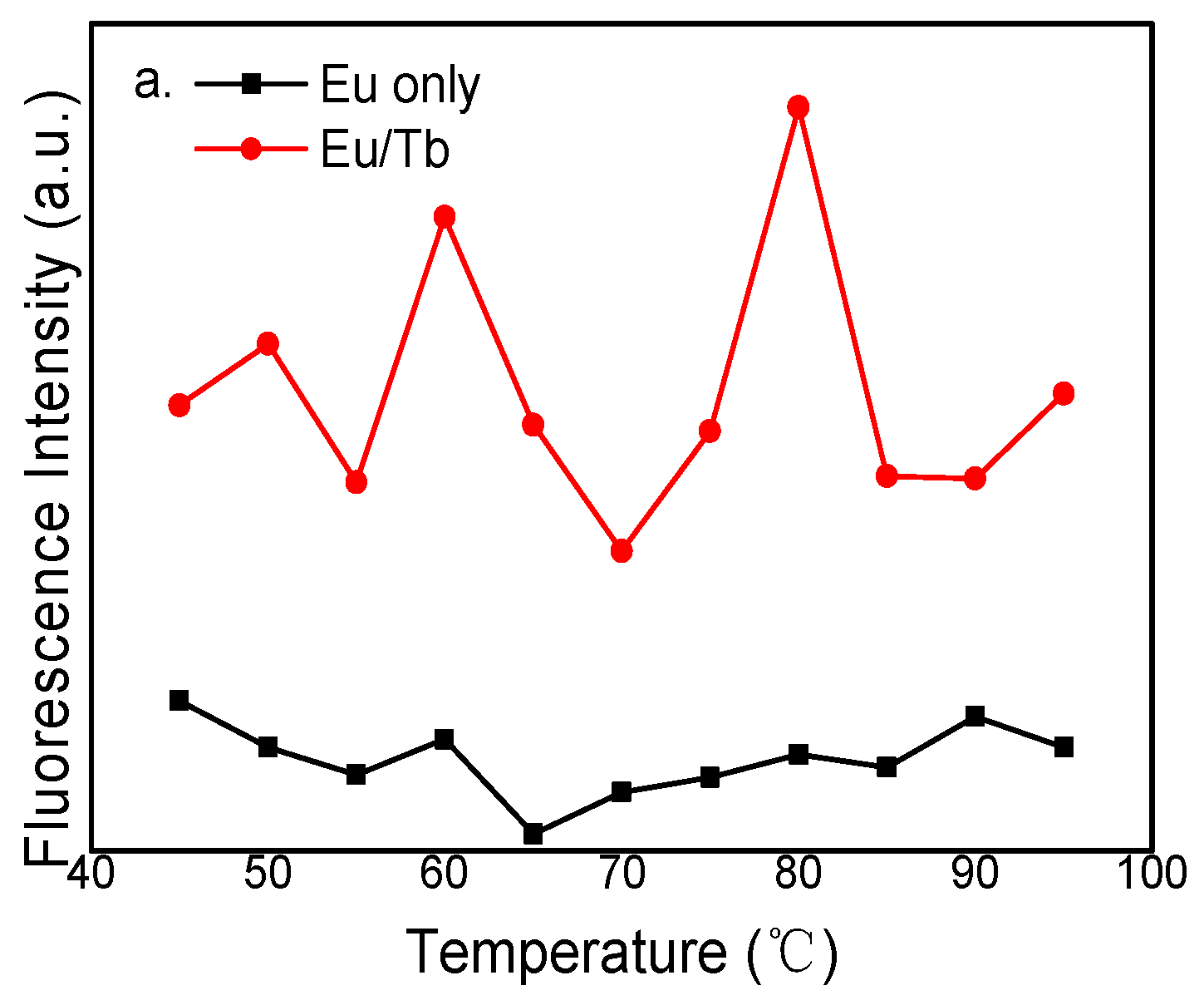
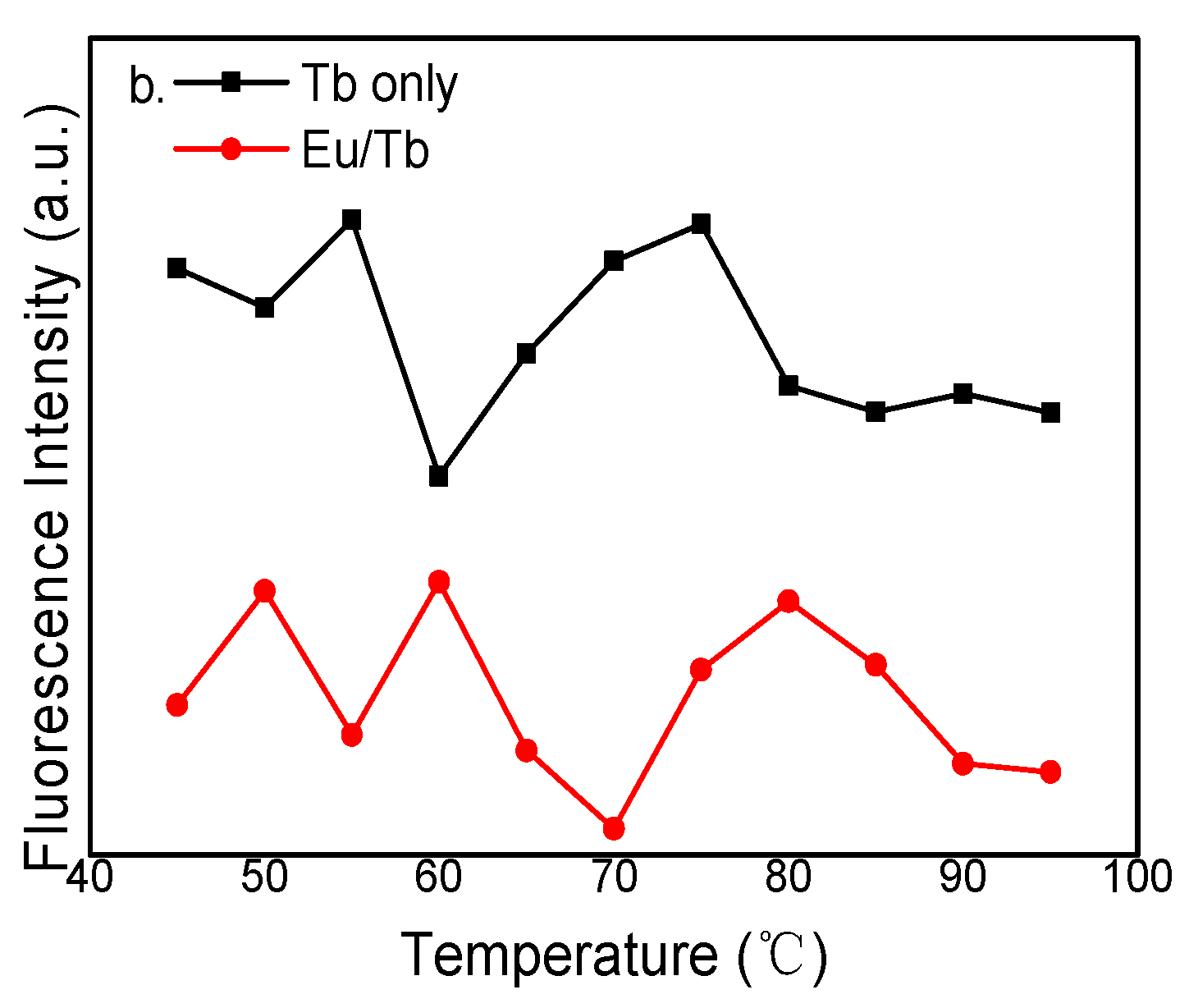
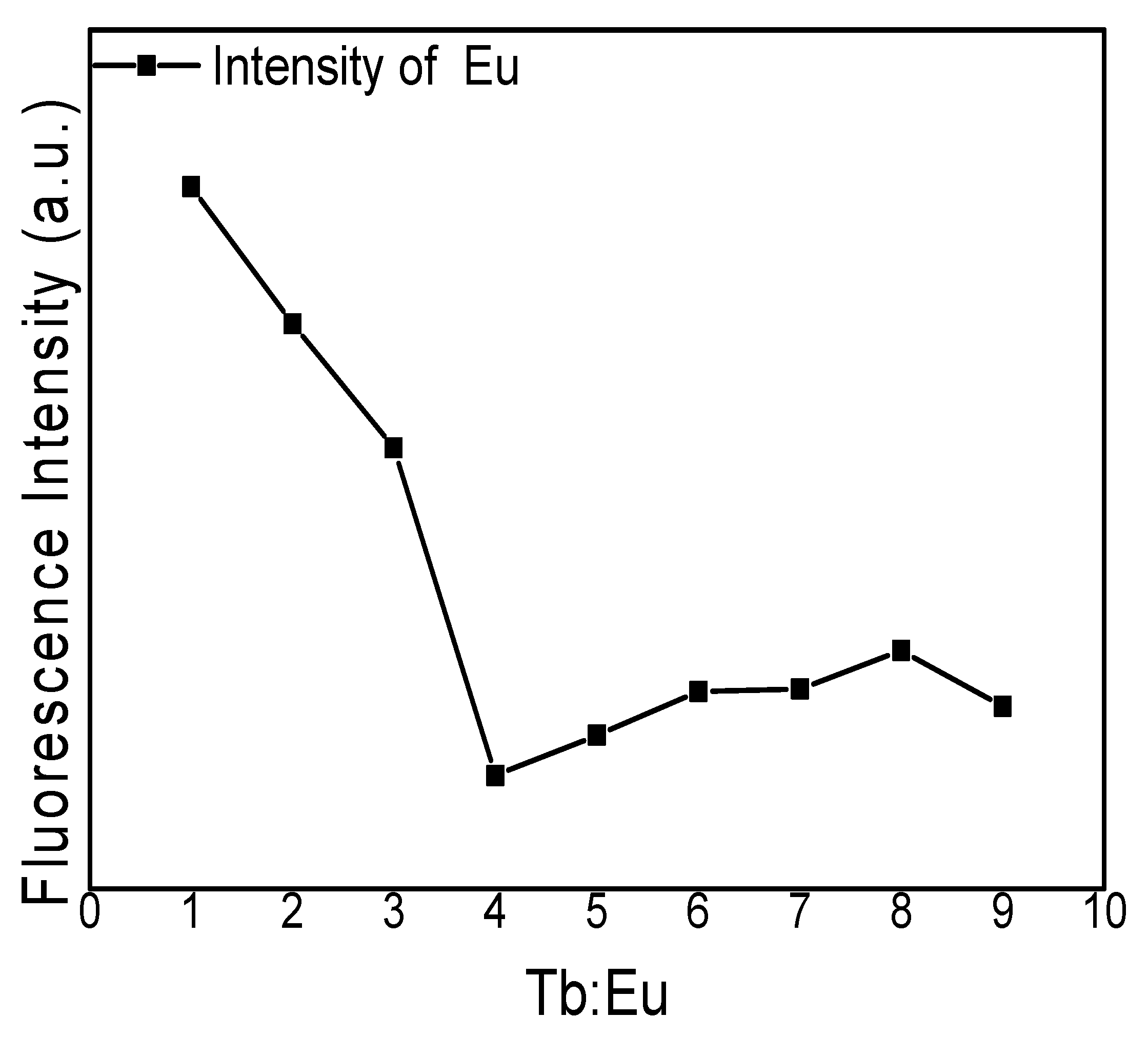
3.2. Fluorescence Spectra
4. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Wu, Z.Q.; Han, X.E.; Su, X.; Zhang, L.L. Recent advances in the luminescent materials of rare-earth complexes. J. Chem. Intermed. 2011, 8, 9–15. [Google Scholar]
- Franville, A.C.; Zambon, D. Synthesis and optical features of a europium organic-inorganic silicate hybrid. J. Alloy. Compd. 1998, 275, 831–834. [Google Scholar] [CrossRef]
- Tsaryuk, V.; Zolin, V.; Legendziewicz, J. The structure of ligand and effects of the europium luminescence excitation. J. Lumin. 2003, 102, 744–750. [Google Scholar] [CrossRef]
- Wu, Y.C.; Zhu, L.; Wang, H.S. Synthesis of rare earth complexes of Eu3+ and Dy3+ ions and their luminescent properties. J. Chem. Res. 2014, 25, 29–32. [Google Scholar]
- Stammen, J.A.; Williams, S.; Ku, D.N. Mechanical properties of a novel PVA hydrogel in shear and unconfined compression. Biomaterials 2001, 22, 799–806. [Google Scholar] [CrossRef]
- Myung, D.; Duhamel, P.E.; Cochran, J.R. Development of hydrogel-based keratoprostheses: A materials perspective. Biotechnol. Prog. 2010, 3, 735–741. [Google Scholar] [CrossRef]
- Wang, J.; Gao, C.; Zhang, Y. Preparation and in vitro characterization of BC/PVA hydrogel composite for its potential use as artificial cornea biomaterial. Mater. Sci. Eng. 2010, 30, 214–218. [Google Scholar] [CrossRef]
- Mansur, H.S.; Oréfice, R.L.; Mansur, A.A. Characterization of poly (vinyl alcohol)/poly (ethylene glycol) hydrogels and PVA-derived hybrids by small-angle X-ray scattering and FTIR spectroscopy. Polymer 2014, 45, 7193–7202. [Google Scholar] [CrossRef]
- Hämmer, M.; Gassmann, A.; Reller, A.; von Seggern, H.; Gutfleisch, O.; Stauber, R.; Zimmermann, J. Recyclable Phosphor Films: Three Water-Soluble Binder Systems Enabling the Recovery of Phosphor Powders in White LEDs. J. Electron. Mater. 2019, 48, 2294–2300. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, H.; Li, H. Preparation and characterization of modified polyvinyl alcohol ultrafiltration membranes. Desalination 2006, 192, 214–223. [Google Scholar] [CrossRef]
- Li, Y.; Lin, X. Simultaneous electroanalysis of dopamine, ascorbic acid and uric acid by poly (vinyl alcohol) covalently modified glassy carbon electrode. Sens. Actuators B 2006, 115, 134–139. [Google Scholar] [CrossRef]
- Zhang, X.; Tang, K.; Zheng, X. Electrospinning and Crosslinking of COL/PVA Nanofiber-microsphere Containing Salicylic Acid for Drug Delivery. J. Bionic Eng. 2016, 13, 143–149. [Google Scholar] [CrossRef]
- Habiba, U.; Islam, M.S.; Siddique, T.A. Adsorption and photocatalytic degradation of anionic dyes on Chitosan/PVA/Na–Titanate/TiO2 composites synthesized by solution casting method. Carbohydr. Polym. 2016, 149, 317–331. [Google Scholar] [CrossRef] [PubMed]
- Pisuchpen, T.; Chaim-Ngoen, N.; Intasanta, N. Tuning Hydrophobicity and Water Adhesion by Electrospinning and Silanization. Langmuir 2011, 27, 3654–3661. [Google Scholar] [CrossRef]
- Xie, Y.; Hill, C.A.S.; Xiao, Z. Silane coupling agents used for natural fiber/polymer composites: A review. Compos. Part A Appl. Sci. Manuf. Inc. Compos. Compos. Manuf. 2010, 41, 806–819. [Google Scholar] [CrossRef]
- Herrera-Franco, P.; Valadez-Gonzalez, A. A study of the mechanical properties of short natural-fiber reinforced composites. Compos. Part B Eng. 2005, 36, 597–608. [Google Scholar] [CrossRef]
- Ion, A.; Parvulescu, V.; Jacobs, P.; De Vos, D. Sc and Zn-catalyzed synthesis of cyclic carbonates from CO2 and epoxides. Appl. Catal. A Gen. 2009, 363, 40–44. [Google Scholar] [CrossRef]
- Li, Y.Y.; Yan, B.; Guo, L.; Li, Y.J. Ternar rare earth sulfoxide-functionalized mesoporous hybrids Phen-RE(OBDS(BSAB))3-SBA-15(RE=Eu, Tb): Coordination bonding assembly, characterization, and photoluminescence. Microporous Mesoporous Mater. 2012, 148, 73–79. [Google Scholar] [CrossRef]
- Binnemans, K. ChemInform Abstract: Lanthanide-Based Luminescent Hybrid Materials. Cheminform 2009, 40, 4283–4374. [Google Scholar] [CrossRef]
- Fadeyev, E.; Smola, S.; Snurnikova, O.; Korovin, O.; Rusakova, N. Luminescent sol-gel materials based on lanthanide aminopolycarboxylates (Ln = Nd, Eu, Tb, Yb). J. Sol Gel Sci. Technol. 2013, 68, 479–487. [Google Scholar] [CrossRef]
- Xu, Q.J.; Wang, D.; Ren, S.X.; Fang, G.Z. Investigation of preparation and luminescent of europium complexes modified by silane. J. Funct. Mater. 2015, 46, 139–143. [Google Scholar]
- Wang, Y.; Zhang, W.J.; Li, J.L.; Fu, J. A novel LEuH/PVA luminescent hydrogel with ammonia response and self-recovery luminescence behavior. Arab. J. Chem. 2019, 43, 5133–5138. [Google Scholar] [CrossRef]
- Shao, C.; Kim, H.Y.; Gong, J. Fiber mats of poly (vinyl alcohol)/silica composite via electrospinning. Mater. Lett. 2003, 57, 1579–1584. [Google Scholar] [CrossRef]
- Nagaoka, Y.; Adachi, S. Photoluminescent properties of NaCl: Ce3+ phosphor synthesized using antisolvent crystallization. J. Lumin. 2014, 145, 797–802. [Google Scholar] [CrossRef]
- Bünzli, J.C.G. Lanthanide Luminescence for Biomedical Analyses and Imaging. Chem. Rev. 2010, 110, 2729–2755. [Google Scholar] [CrossRef]
- Zhao, B.; Chen, X.Y.; Cheng, P. Coordination Polymers Containing 1D Channels as Selective Luminescent Probes. J. Am. Chem. Soc. 2004, 126, 15394–15395. [Google Scholar] [CrossRef]
- Moore, E.G.; Samuel, A.P.S.; Raymond, K.N. From Antenna to Assay: Lessons Learned in Lanthanide Luminescence. Acc. Chem. Res. 2009, 42, 542–552. [Google Scholar] [CrossRef]
- Craig, P.M.; Benjamin, S.M.; Robert, P.; David, P. Cell-Penetrating Metal Complex Optical Probes: Targeted and Responsive Systems Based on Lanthanide Luminescence. Acc. Chem. Res. 2009, 42, 925–937. [Google Scholar]
- Petoud, S.; Cohen, S.M.; Bünzli, J.C.G.; Raymond, K.N. Stable Lanthanide Luminescence Agents Highly Emissive in Aqueous Solution Multidentate 2-Hydroxyisophthalamide Complexes of Sm3+, Eu3+, Tb3+, Dy3+. J. Am. Chem. Soc. 2003, 125, 13324–13325. [Google Scholar] [CrossRef]
- Chi, Y.; Fu, L.M.; Wang, Y.; Zhang, J.P. A Highly Luminescent Europium Complex Showing Visible-Light-Sensitized Red Emission: Direct Observation of the Singlet Pathway. Angew. Chem. 2004, 116, 5120–5123. [Google Scholar]
- Min, L.; Paul, R.S. Luminescent Polyaminocarboxylate Chelates of Terbium and Europium: The Effect of Chelate Structure. J. Am. Chem. Soc. 1995, 117, 8132–8138. [Google Scholar]
- Gao, J.; Li, Q.; Wang, C.H.; Tan, H.L. Ratiometric detection of hydroxy radicals based on functionalized europium (III) coordination polymers. Mikrochim. Acta Int. J. Phys. Chem. Methods Anal. 2017, 185, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Li, T.; Wu, J. Luminescence of Europium (III) and Terbium (III) Complexes Incorporated in Poly (Vinyl Pyrrolidone) Matrix. J. Phys. Chem. B 2001, 105, 12293–12296. [Google Scholar] [CrossRef]
- Topilova, Z.M.; Meshkova, S.B.; Dotsenko, V.P. Sensitization of Luminescence of Europium Compounds on Solid Matrices by Terbium (III) Ions. J. Appl. Spectrosc. 2004, 71, 253–256. [Google Scholar] [CrossRef]

| Element | wt.% | at. % |
|---|---|---|
| C | 51.50 | 66.85 |
| N | 10.96 | 12.20 |
| O | 12.73 | 12.40 |
| Na | 03.68 | 02.50 |
| Si | 03.33 | 01.85 |
| Cl | 07.15 | 03.14 |
| Eu | 03.90 | 00.40 |
| Tb | 06.75 | 00.66 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wei, Y.; Fu, Z.; Zhao, H.; Liang, R.; Wang, C.; Wang, D.; Li, J. Preparation of PVA Fluorescent Gel and Luminescence of Europium Sensitized by Terbium (III). Polymers 2020, 12, 893. https://doi.org/10.3390/polym12040893
Wei Y, Fu Z, Zhao H, Liang R, Wang C, Wang D, Li J. Preparation of PVA Fluorescent Gel and Luminescence of Europium Sensitized by Terbium (III). Polymers. 2020; 12(4):893. https://doi.org/10.3390/polym12040893
Chicago/Turabian StyleWei, Yifan, Zhengquan Fu, Hao Zhao, Ruiqi Liang, Chengyu Wang, Di Wang, and Jian Li. 2020. "Preparation of PVA Fluorescent Gel and Luminescence of Europium Sensitized by Terbium (III)" Polymers 12, no. 4: 893. https://doi.org/10.3390/polym12040893
APA StyleWei, Y., Fu, Z., Zhao, H., Liang, R., Wang, C., Wang, D., & Li, J. (2020). Preparation of PVA Fluorescent Gel and Luminescence of Europium Sensitized by Terbium (III). Polymers, 12(4), 893. https://doi.org/10.3390/polym12040893