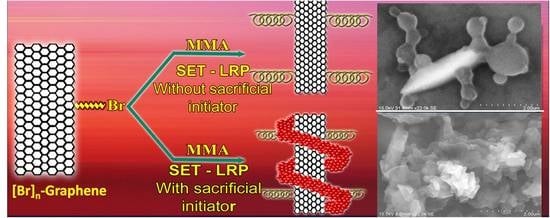
Copper (0) Mediated Single Electron Transfer-Living Radical Polymerization of Methyl Methacrylate: Functionalized Graphene as a Convenient Tool for Radical Initiator
Abstract
1. Introduction
2. Experimental Section
2.1. Material
2.2. Methods
2.3. Synthesis of [NO2]n-Graphene from Graphite
2.4. Synthesis of [OH]n-Graphene from [NO2]n-Graphene
2.5. Synthesis of [Br]n-Graphene
2.6. Synthesis of Graphene-g-PMMA
3. Results and Discussion
3.1. FT-IR and UV-Vis Spectroscopy Study
3.2. Structural Identification of Polymer Nanocomposite
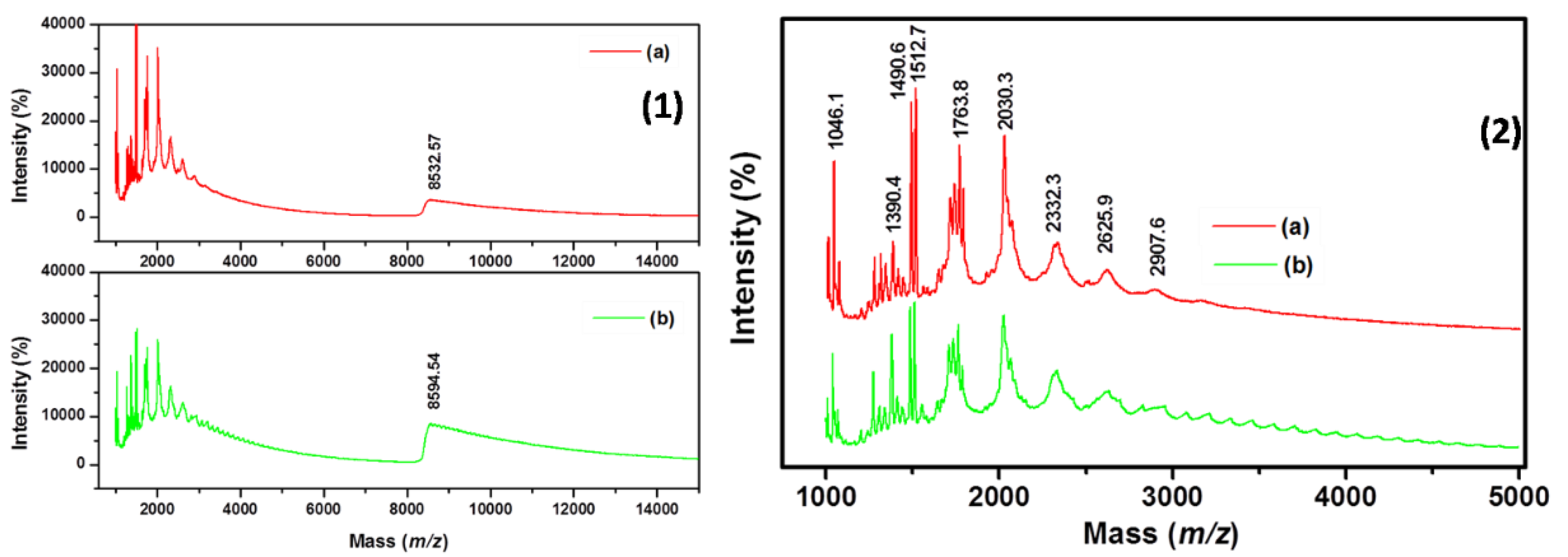
3.3. Matrix-Assisted Laser Desorption Ionization Time-Of-Flight Mass Spectroscopy (MALDI-TOF) Study
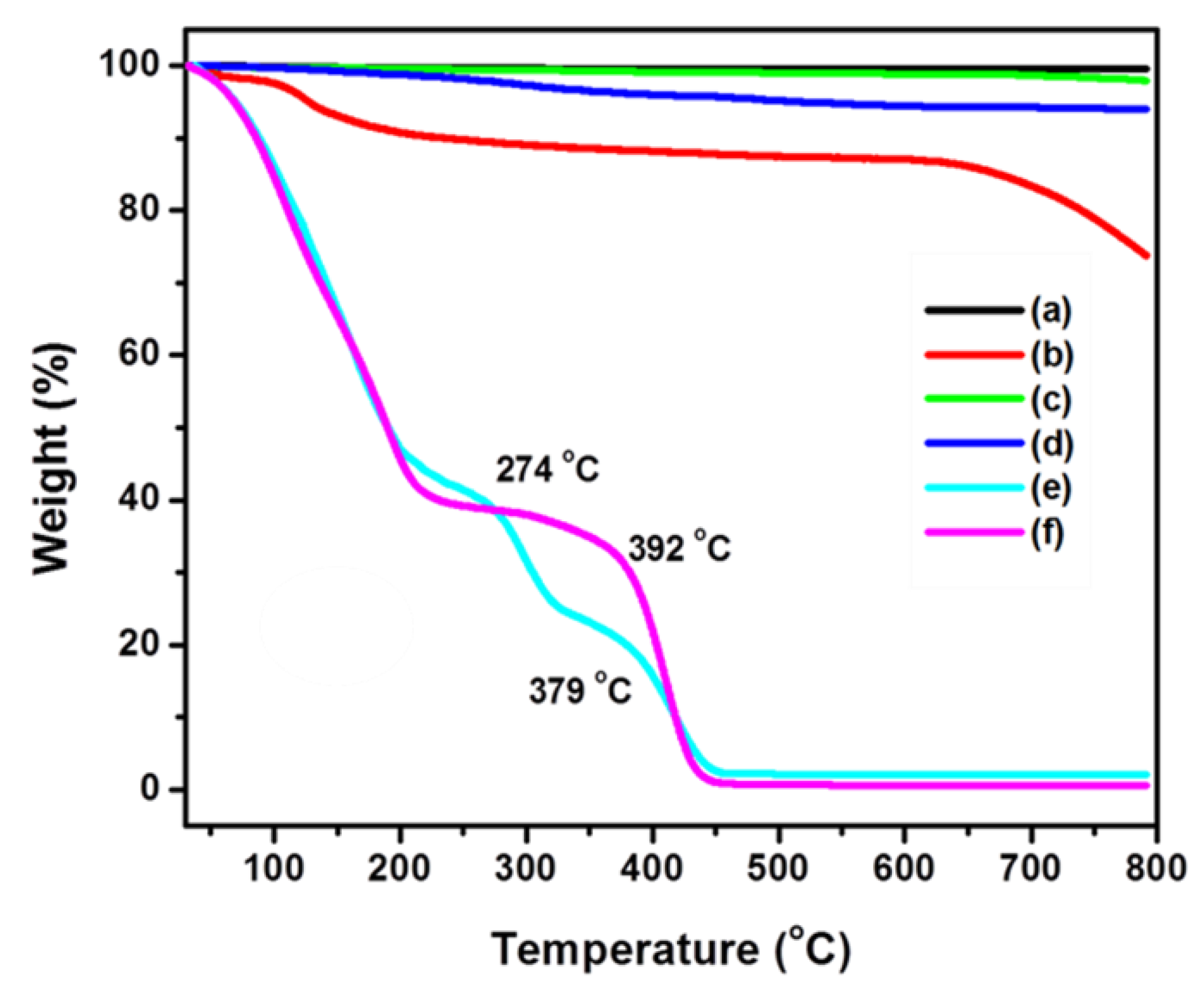
3.4. Surface and Thermal Properties of Polymer Nanocomposites
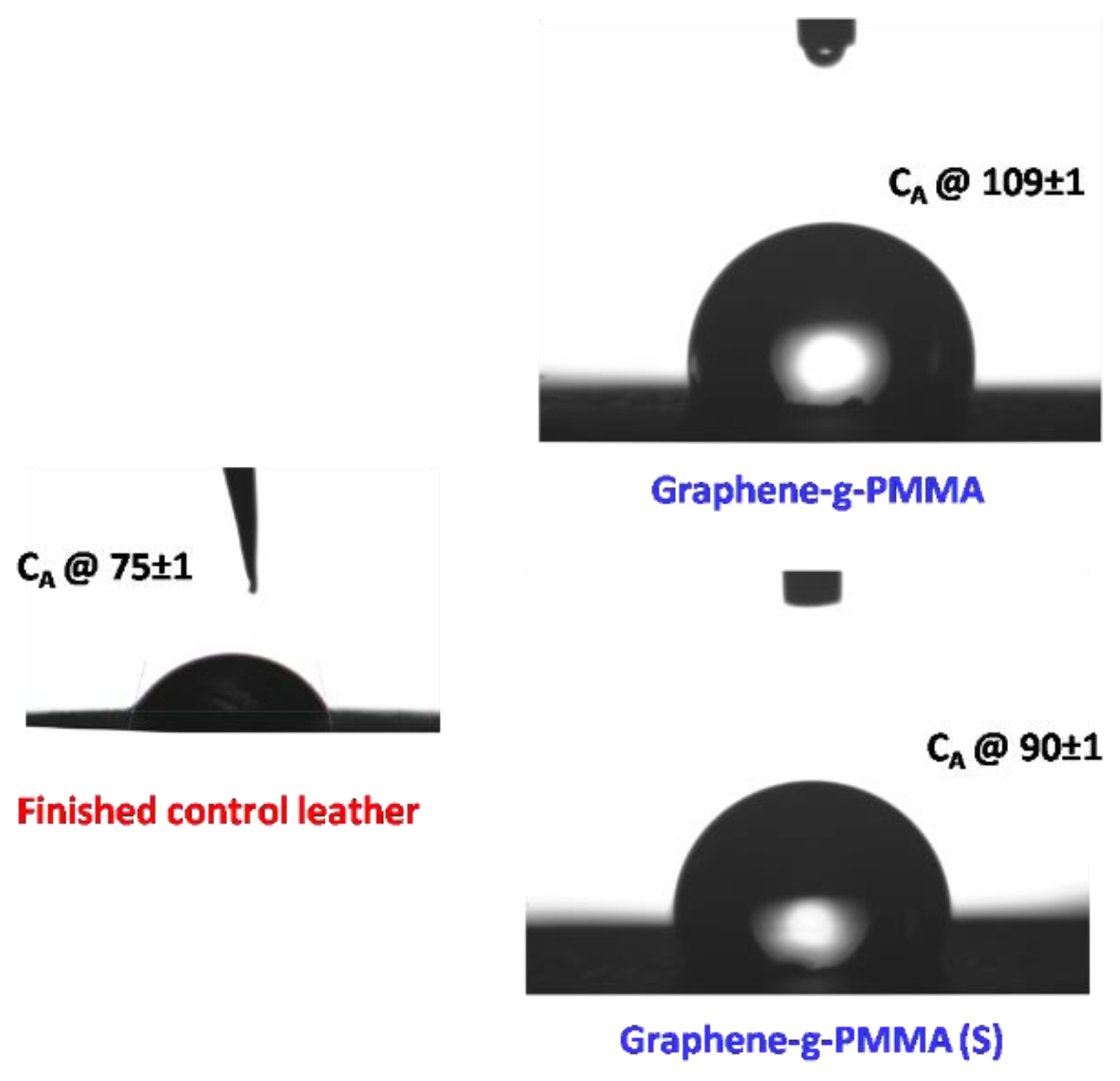
3.5. Surface Morphological and Hydrophobic Properties of Polymer Nanocomposite
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Srinivasan, S.; Sang, H.J.; Seoin, B.; Gokhan, B.; Onur, B.; Ruslan, G.; Yousung, J.; Ali, C. Ordered supramolecular gels based on graphene oxide and tetracationiccyclophanes. Adv. Mater. 2014, 26, 2725–2729. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Zhu, J.; Wu, X.; Han, Q.; Wang, X. Graphene Oxide−MnO2 Nanocomposites for Supercapacitors. ACS Nano 2010, 4, 2822–2830. [Google Scholar] [CrossRef] [PubMed]
- Christopher, A.; Dyke James, M.T. Covalent functionalization of single-walled carbon nanotubes for materials applications. Phys. Chem. A 2004, 108, 11151–11159. [Google Scholar]
- Sampath, S.; Palathingal, A.B.; Sankarapillai, M.; Ajayaghosh, A. Reversible self-assembly of entrapped fluorescent gelators in polymerized styrene gel matrix: Erasable thermal imaging via recreation of supramolecular architectures. J. Am. Chem. Soc. 2009, 42, 15122–15123. [Google Scholar]
- Dan, Q.; Min, Z.; Ligong, Z.; Haifeng, Z.; Zhigang, X.; Xiabin, J.; Raid, E.H.; Hongyou, F.; Zaicheng, S. Formation mechanism and optimization of highly luminescent N-doped graphene quantum dots. Sci. Rep. 2014, 4, 5294. [Google Scholar]
- Huang, C.; Bai, H.; Li, C.; Shi, G.A. A graphene oxide/hemoglobin composite hydrogel for enzymatic catalysis in organic solvents. Chem. Commun. 2011, 47, 4962–4964. [Google Scholar] [CrossRef]
- Tiller, J.C. Increasing the local concentration of drugs by hydrogel formation. Angew. Chem. Int. Ed. 2003, 42, 3072–3075. [Google Scholar] [CrossRef]
- Huang, X.; Qi, X.; Boey, F.; Zhang, H. Graphene-based composites. Chem. Soc. Rev. 2012, 41, 666–686. [Google Scholar] [CrossRef]
- Keith, E.W.J.; Jeremy, T.R.; Paul, E.S. Protection from Below: Stabilizing Hydrogenated Graphene Using Graphene Underlayers. Langmuir 2017, 33, 13749–13756. [Google Scholar]
- Xia, H.; Mo, S. Preparation and characterization of polyurethane–carbon nanotube composites. Soft. Matter. 2005, 1, 386–394. [Google Scholar] [CrossRef]
- Fan-Long, J.; Soo-Jin, P.A. A review of the preparation and properties of carbon nanotubes-reinforced polymer composites. Carbon Lett. 2011, 12, 57–69. [Google Scholar]
- Kudin, K.N.; Ozbas, B.; Schniepp, H.C.; Prud’homme, R.K.; Aksay, I.A.; Car, R. Raman spectra of graphite oxide and functionalized graphene sheets. Nano Lett. 2008, 8, 36–41. [Google Scholar] [CrossRef]
- Nandi, S.; Routh, P.; Layek, R.K.; Nandi, A.K. Ethidium bromide-adsorbed graphene templates as a platform for preferential sensing of DNA. Biomacromolecules 2012, 13, 3181–3188. [Google Scholar] [CrossRef]
- Deng, X.; Chen, M.; Fu, Q.; Smeets, N.M.B.; Xu, F.; Zhang, Z.; Filipe, C.D.M.; Hoare, T. A highly sensitive immunosorbent assay based on biotinylated graphene oxide and the quartz crystal microbalance. ACS Appl. Mater. Interfaces 2016, 8, 1893–1902. [Google Scholar] [CrossRef] [PubMed]
- Aliprandi, A.; Eredia, M.; Anichini, C.; Baaziz, W.; Ersen, O.; Ciesielski, A.; Samorì, P. Persian waxing of graphite: Towards green large-scale production of grapheme. Chem. Commun. 2019, 55, 5331–5334. [Google Scholar] [CrossRef] [PubMed]
- Kundu, A.; Layek, R.K.; Nandi, A.K. Enhanced fluorescent intensity of graphene oxide–methyl cellulose hybrid in acidic medium: Sensing of nitro-aromatics. J. Mater. Chem. 2012, 22, 8139. [Google Scholar] [CrossRef]
- Kundu, A.; Layek, R.K.; Kuila, A.; Nandi, A.K. Highly Fluorescent Graphene Oxide-Poly(vinyl alcohol) Hybrid: An Effective Material for Specific Au3+ Ion Sensors. ACS Appl. Mater. Interfaces 2012, 4, 5576–5582. [Google Scholar] [CrossRef]
- Muhammad, Z.I.; Saman, S.; Abbas, K.; Syed, S.H.; Mohammad, K. Honeycomb Carbon: A Review of Graphene. Mater. Res. Bull. 2020, 122, 110674. [Google Scholar]
- Rosen, B.M.; Percec, V. Single-electron transfer and single-electron transfer degenerative chain transfer living radical polymerization. Chem. Rev. 2009, 109, 5069–5119. [Google Scholar] [CrossRef]
- Nguyen, N.H.; Percec, V.J. Dramatic acceleration of SET-LRP of methyl acrylate during catalysis with activated Cu(0) wire. Polym Sci. Part A Polym. Chem. 2010, 48, 5109–5119. [Google Scholar] [CrossRef]
- Georgios, S.; Dimitrios, D. Surface-initiated polymerization from carbon nanotubes: Strategies and perspectives. Chem. Soc. Rev. 2013, 42, 677–704. [Google Scholar]
- Nguyen, N.H.; Rosen, B.M.; Jiang, X.; Fleischmann, S.; Percec, V. New efficient reaction media for SET-LRP produced from binary mixtures of organic solvents and H2O. J. Polym. Sci. Part A Polym. Chem. 2009, 47, 5577–5590. [Google Scholar] [CrossRef]
- Konkolewicz, D.; Wang, Y.; Mingjiang, Z.; Pawel, K.; Abdirisak, A.I.; Armando, G.; Matyjaszewski, K. Reversible-Deactivation Radical Polymerization in the Presence of Metallic Copper. A Critical Assessment of the SARA ATRP and SET-LRP Mechanisms. Macromolecules 2013, 46, 8749–8772. [Google Scholar] [CrossRef]
- Dominik, K.; Yu, W.; Pawel, K.; Mingjiang, Z.; Abdirisak, A.I.; Armando, G.; Matyjaszewski, K. SARA ATRP or SET-LRP. End of controversy? Polym. Chem. 2014, 5, 4396–4417. [Google Scholar]
- Sellamuthu, N.J.; Neelamegan, H.; Murali, A.; Ponyrko, S.; Milena, S.; Asit, B.M.; Libor, M. Single-electron transfer living radical copolymerization of SWCNT-g-PMMA via graft from approach. Polymer 2014, 55, 2959–2966. [Google Scholar]
- Krishna, P.S.; Marlin, B.; Murali, A.; Sellamuthu, N.J. Carbon Nanotube Reinforced Polymer-Stabilized Liquid Crystal Device: Lowered and Thermally Invariant Threshold with Accelerated Dynamics. ACS Appl. Mater. Interfaces 2017, 31, 26622–26629. [Google Scholar] [CrossRef] [PubMed]
- Anna, V.P.; Liubov, A.B.; Lin, J.; Lia, M.C.; Grégory, F.S. Contact angle measurement of free-standing square-millimeter single-layer graphene. Nat. Commun. 2018, 9, 4185. [Google Scholar]
- Rishi RajShalabh, C.M.; Evelyn, N.W. Wettability of grapheme. Nano Lett. 2013, 13, 1509–1515. [Google Scholar]
- Nurul, H.O.; Mokhtar, C.I.; Mazli, M.; Nabihah, S.; Kok, E.K.; Rafida, A.J. Graphene based polymer nanocomposites as barrier coatings for corrosion protection. Prog. Org. Coat. 2019, 135, 82–99. [Google Scholar]
- Murali, A.; Senthil, A.G.T.; Jaisankar, S.N.; Mandal, A.B. Augmentation of properties on sparingly loaded nanocomposites via functionalized single-walled carbon nanotubes using a covalent approach. RSC Adv. 2014, 4, 62947–62950. [Google Scholar] [CrossRef]
- Moumita, M.; Senthil, A.G.T.; Dinesh, K.C.; Ananthan, A.; Bhabendra, N.D.; Jaisankar, S.N.; Mandal, A.B. Biodegradable polyurethane foam as shoe insole to reduce footwear waste: Optimization by morphological physicochemical and mechanical properties. Appl. Surf. Sci. 2020, 499, 143966. [Google Scholar]
- Samira, H.; Ibrahim, F.; Djordjevic, I.; Koole, L.H. Polymethyl methacrylate-co-methacrylic acid coatings with controllable concentration of surface carboxyl groups: A novel approach in fabrication of polymeric platforms for potential bio-diagnostic devices. Appl. Surf. Sci. 2014, 300, 43–50. [Google Scholar]
- Justine, C.; Helmut, K.; Martin, M. MALDI-TOF Analysis of Halogen Telechelic Poly(methyl methacrylate)s and Poly(methyl acrylate)s Prepared by Atom Transfer Radical Polymerization (ATRP) or Single Electron Transfer-Living Radical Polymerization (SET-LRP). Macromol. Chem. Phys. 2015, 216, 1791–1800. [Google Scholar]
- Ilčíková, M.; Mrlík, M.; Špitalský, Z.; Mičušík, M.; Csomorová, K.; Sasinková, V.; Kleinová, A.; Mosnáček, J. A tertiary amine in two competitive processes: Reduction of graphene oxide vs. Catalysis of Atom Transfer Radical Polymerization. RSC Adv. 2015, 5, 3370–3376. [Google Scholar]
- Fernández-Merino, M.J.; Guardia, L.; Paredes, J.I.; Villar-Rodil, S.; Solís-Fernández, P.; Martínez-Alonso, A.; Tascón, J.M.D. Vitamin C Is an Ideal Substitute for Hydrazine in the Reduction of Graphene Oxide Suspensions. J. Phys. Chem. C 2010, 114, 6426–6432. [Google Scholar] [CrossRef]
- Zhang, W.; Pan-Pan, H.; Sheng, W.; Jie, X.; Yongxin, L.; Gen Zhang, W. Graphene oxide grafted hydroxyl-functionalized ionic liquid: A highly efficient catalyst for cycloaddition of CO2 with epoxides. Appl. Catal. A 2016, 5, 111–117. [Google Scholar] [CrossRef]
- Chao, B.; Yin-Ning, Z.; Joshua, D.D.; Zheng-Hong, L. Visible-Light-Induced Atom-Transfer-Radical Polymerization with a ppm-Level Iron Catalyst. Chem. Eng. J. 2019, 362, 721–730. [Google Scholar]
- Senthil, A.G.T.; Murali, A.; Sharanya, M.; Jaisankar, S.N.; Mandal, A.B. Studies on biodegradable polyurethane-SWCNTs nanocomposite films by covalent approach: Physicochemical, electric and mechanical properties. Appl. Surf. Sci. 2018, 449, 745–754. [Google Scholar]
- Jianfeng, W.; Xiuxiu, J.C.L.; Wanjie, W.; Hong, W.; Shaoyun, G. Graphen and graphene derivatives toughening polymers: Toward high toughness and strength. Chem. Eng. J. 2019, 370, 831–854. [Google Scholar]




© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Murali, A.; Sampath, S.; Appukutti Achuthan, B.; Sakar, M.; Chandrasekaran, S.; Suthanthira Vanitha, N.; Joseph Bensingh, R.; Abdul Kader, M.; Jaisankar, S.N. Copper (0) Mediated Single Electron Transfer-Living Radical Polymerization of Methyl Methacrylate: Functionalized Graphene as a Convenient Tool for Radical Initiator. Polymers 2020, 12, 874. https://doi.org/10.3390/polym12040874
Murali A, Sampath S, Appukutti Achuthan B, Sakar M, Chandrasekaran S, Suthanthira Vanitha N, Joseph Bensingh R, Abdul Kader M, Jaisankar SN. Copper (0) Mediated Single Electron Transfer-Living Radical Polymerization of Methyl Methacrylate: Functionalized Graphene as a Convenient Tool for Radical Initiator. Polymers. 2020; 12(4):874. https://doi.org/10.3390/polym12040874
Chicago/Turabian StyleMurali, Adhigan, Srinivasan Sampath, Boopathi Appukutti Achuthan, Mohan Sakar, Suryanarayanan Chandrasekaran, N. Suthanthira Vanitha, R. Joseph Bensingh, M. Abdul Kader, and Sellamuthu N. Jaisankar. 2020. "Copper (0) Mediated Single Electron Transfer-Living Radical Polymerization of Methyl Methacrylate: Functionalized Graphene as a Convenient Tool for Radical Initiator" Polymers 12, no. 4: 874. https://doi.org/10.3390/polym12040874
APA StyleMurali, A., Sampath, S., Appukutti Achuthan, B., Sakar, M., Chandrasekaran, S., Suthanthira Vanitha, N., Joseph Bensingh, R., Abdul Kader, M., & Jaisankar, S. N. (2020). Copper (0) Mediated Single Electron Transfer-Living Radical Polymerization of Methyl Methacrylate: Functionalized Graphene as a Convenient Tool for Radical Initiator. Polymers, 12(4), 874. https://doi.org/10.3390/polym12040874