Design and Performance of Novel Self-Cleaning g-C3N4/PMMA/PUR Membranes
Abstract
1. Introduction
2. Experimental Section
2.1. Materials
2.2. Preparation of g-C3N4
2.3. Preparation of g-C3N4/PMMA/PUR
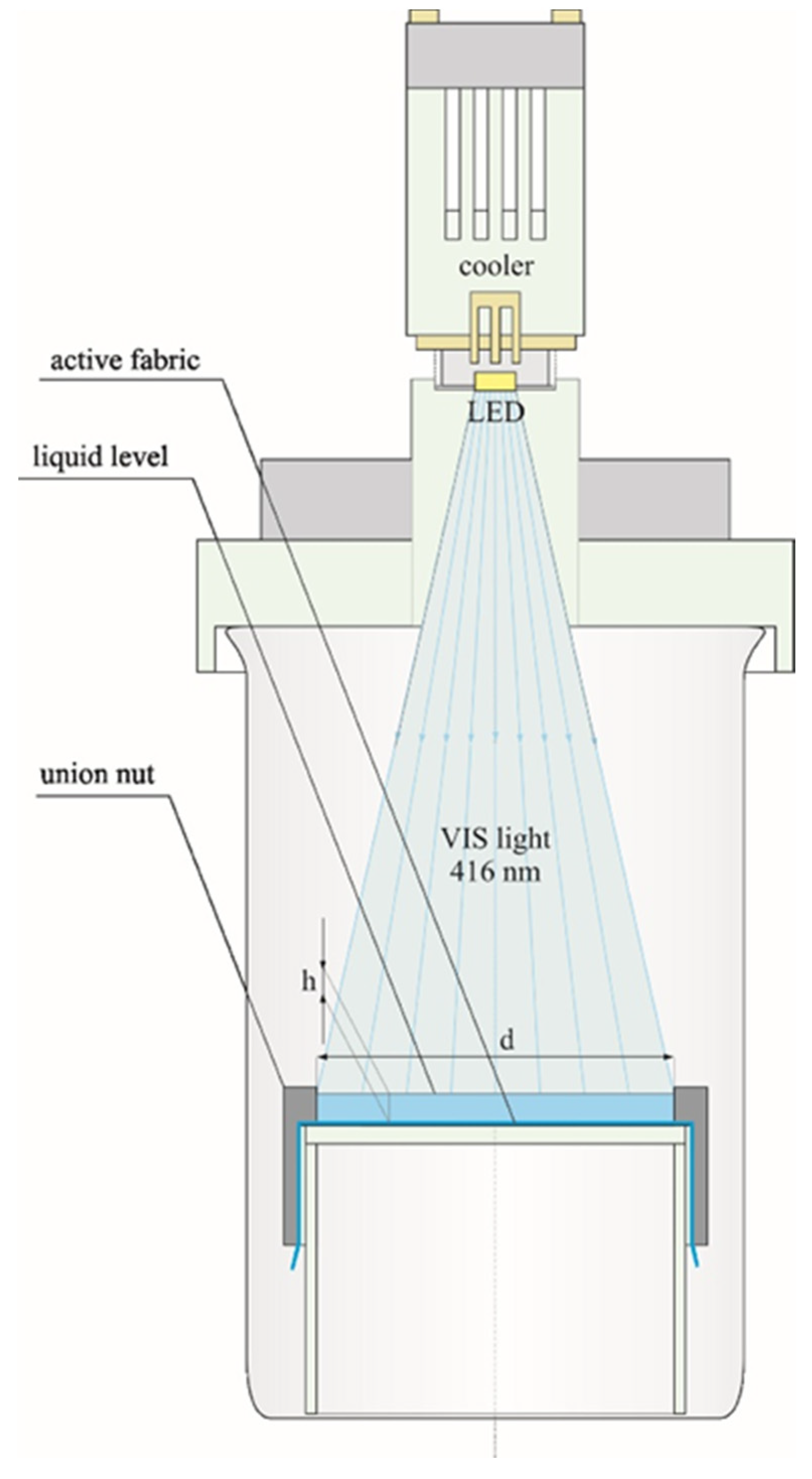
2.4. Material Characterization
2.5. Adsorption and Photocatalytic Experiments
2.6. Regeneration Studies
3. Results and Discussion
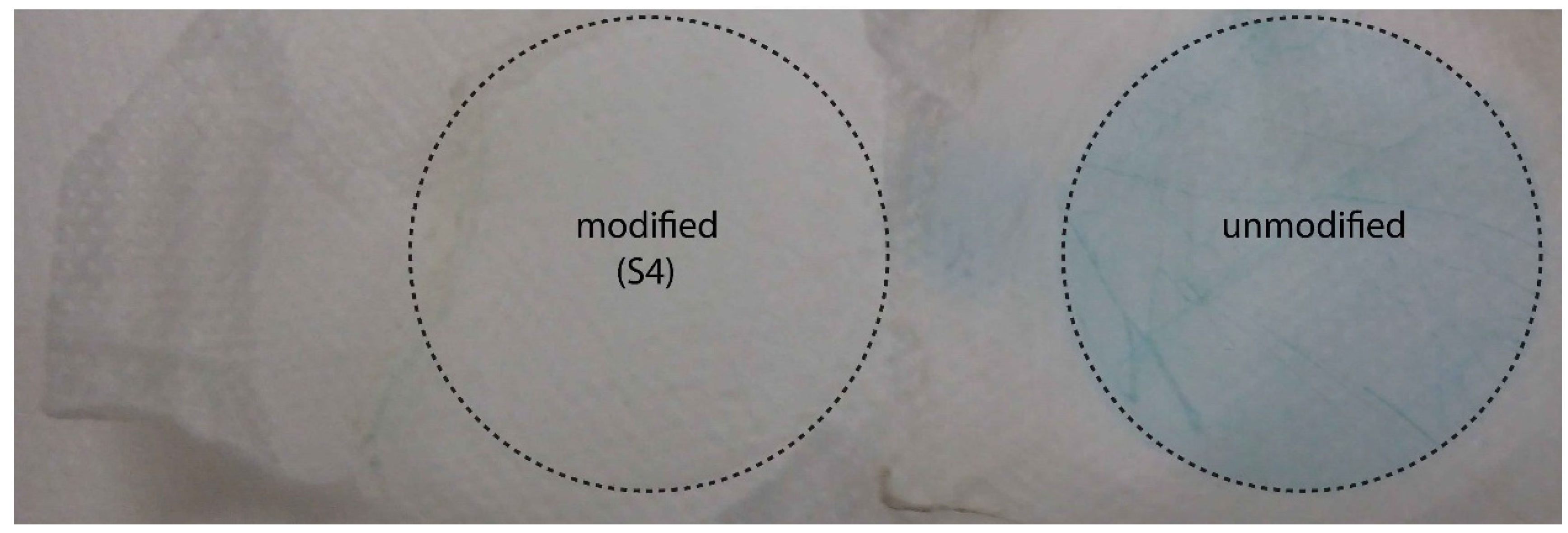
3.1. Preparation of the Membrane
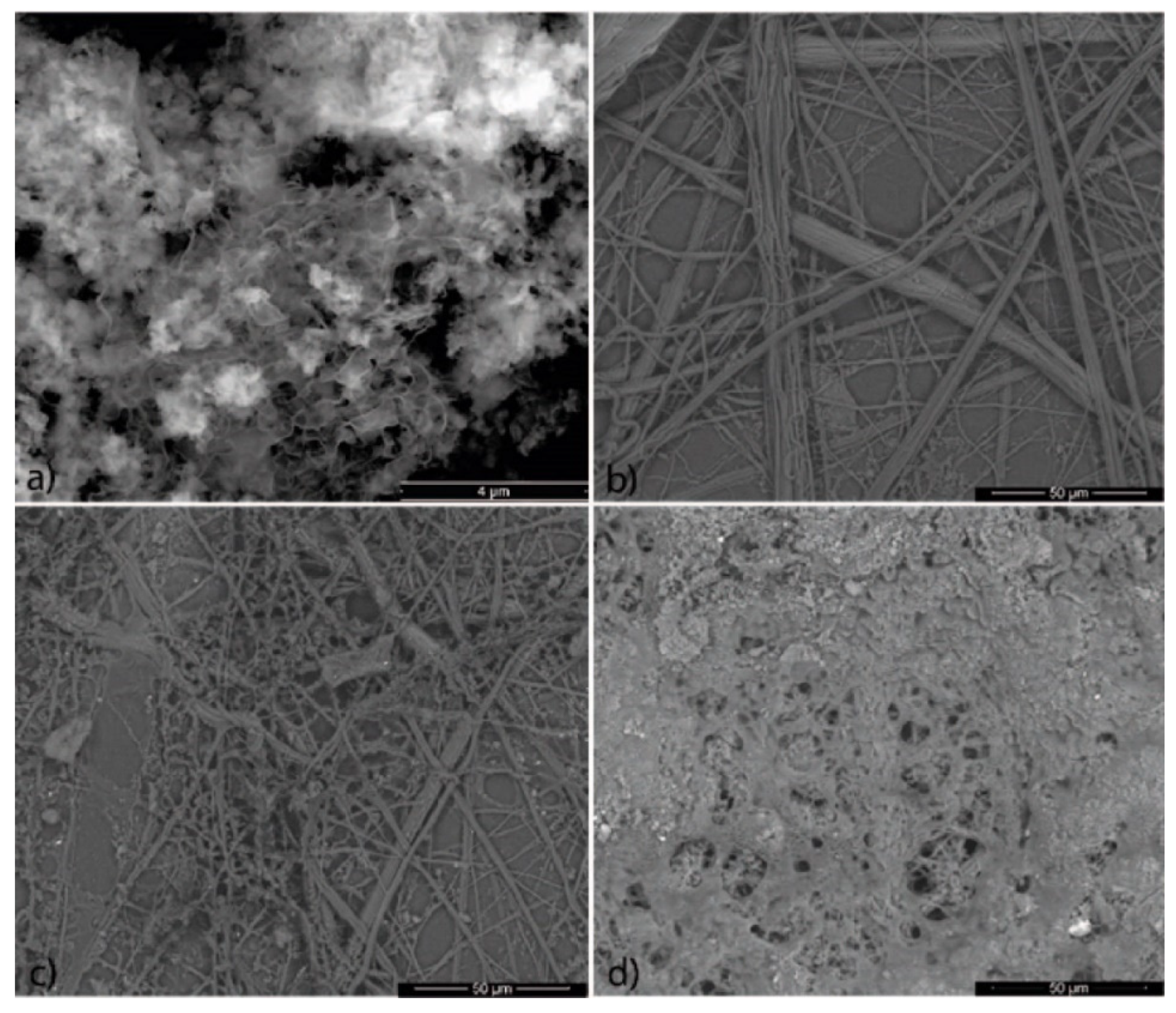
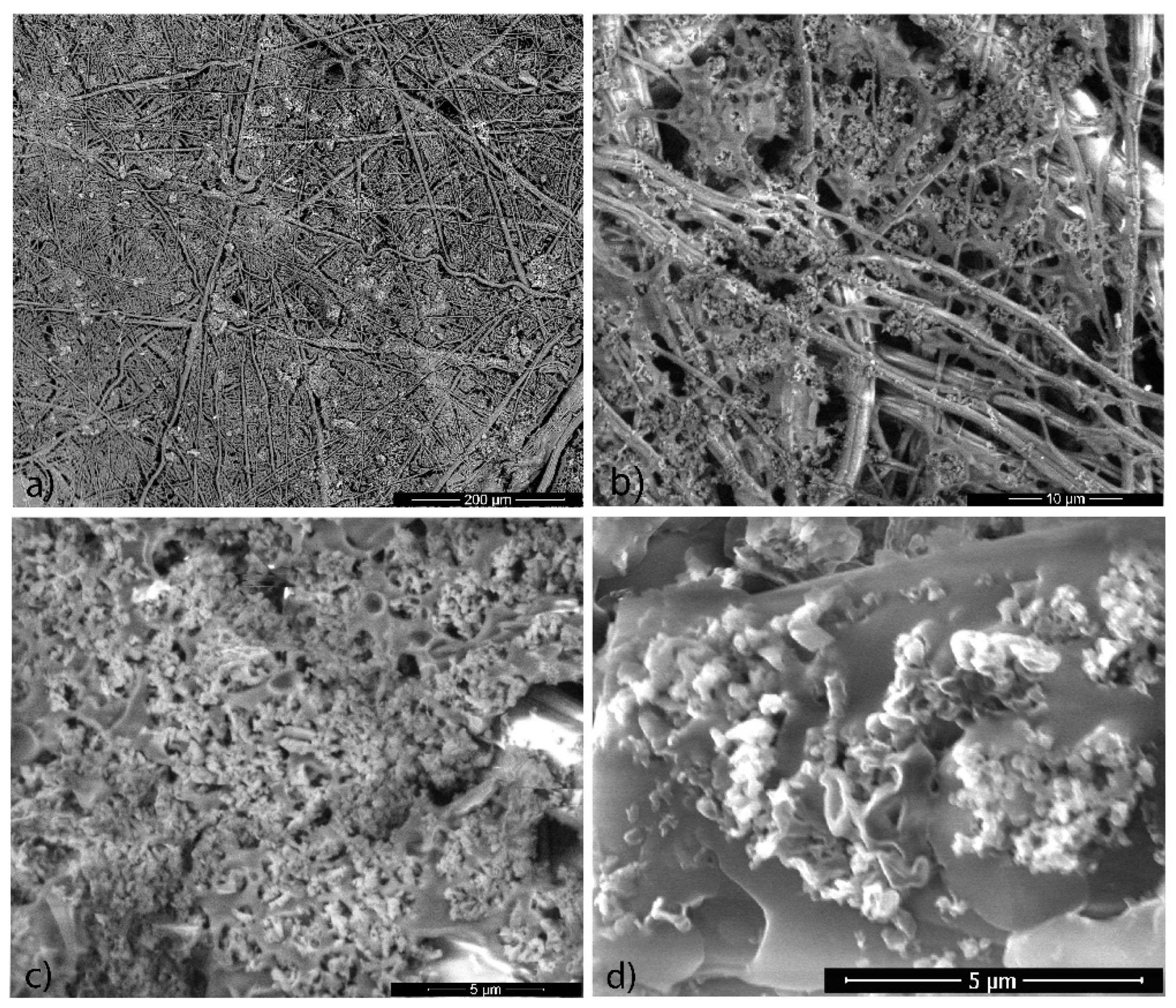
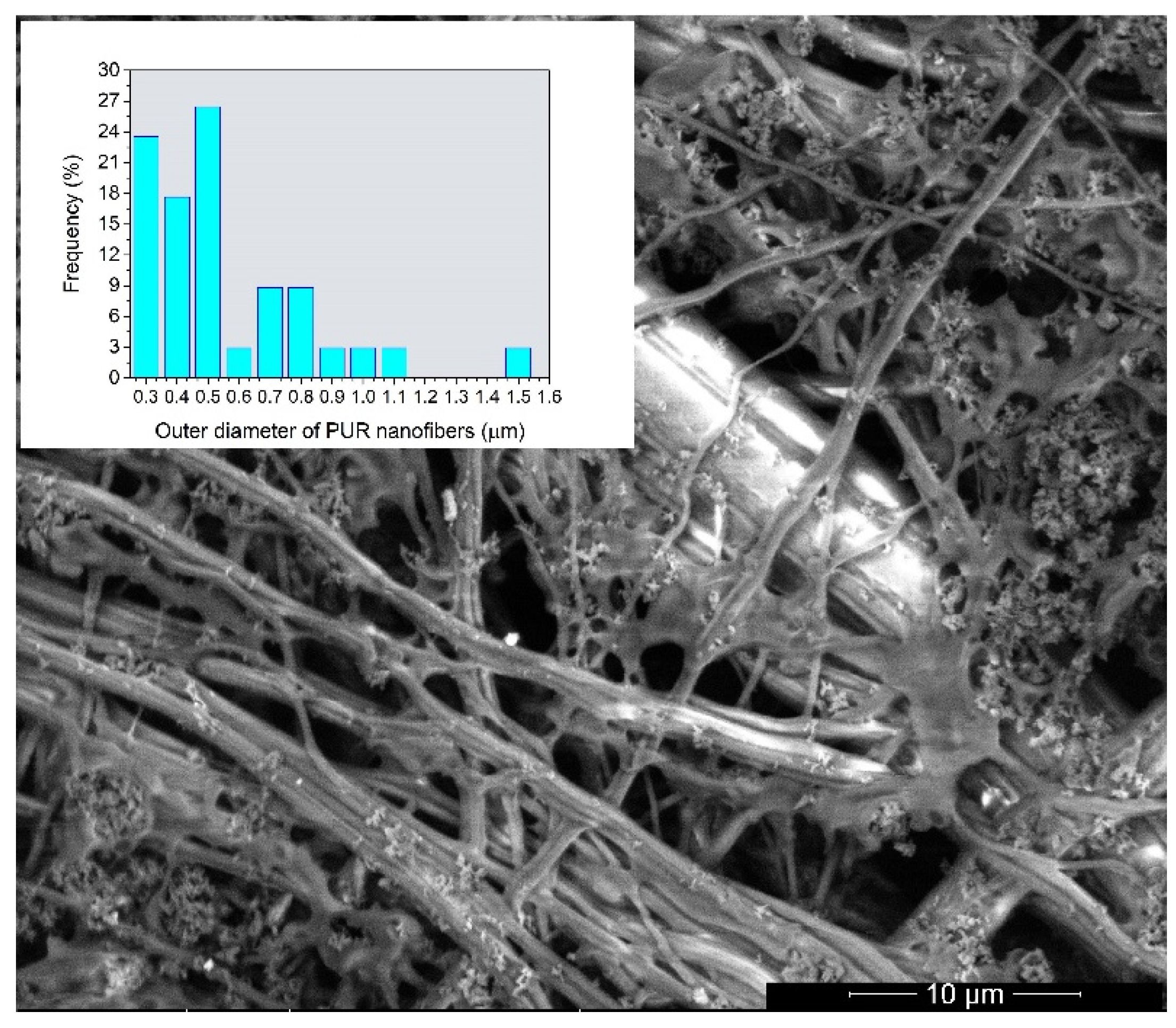
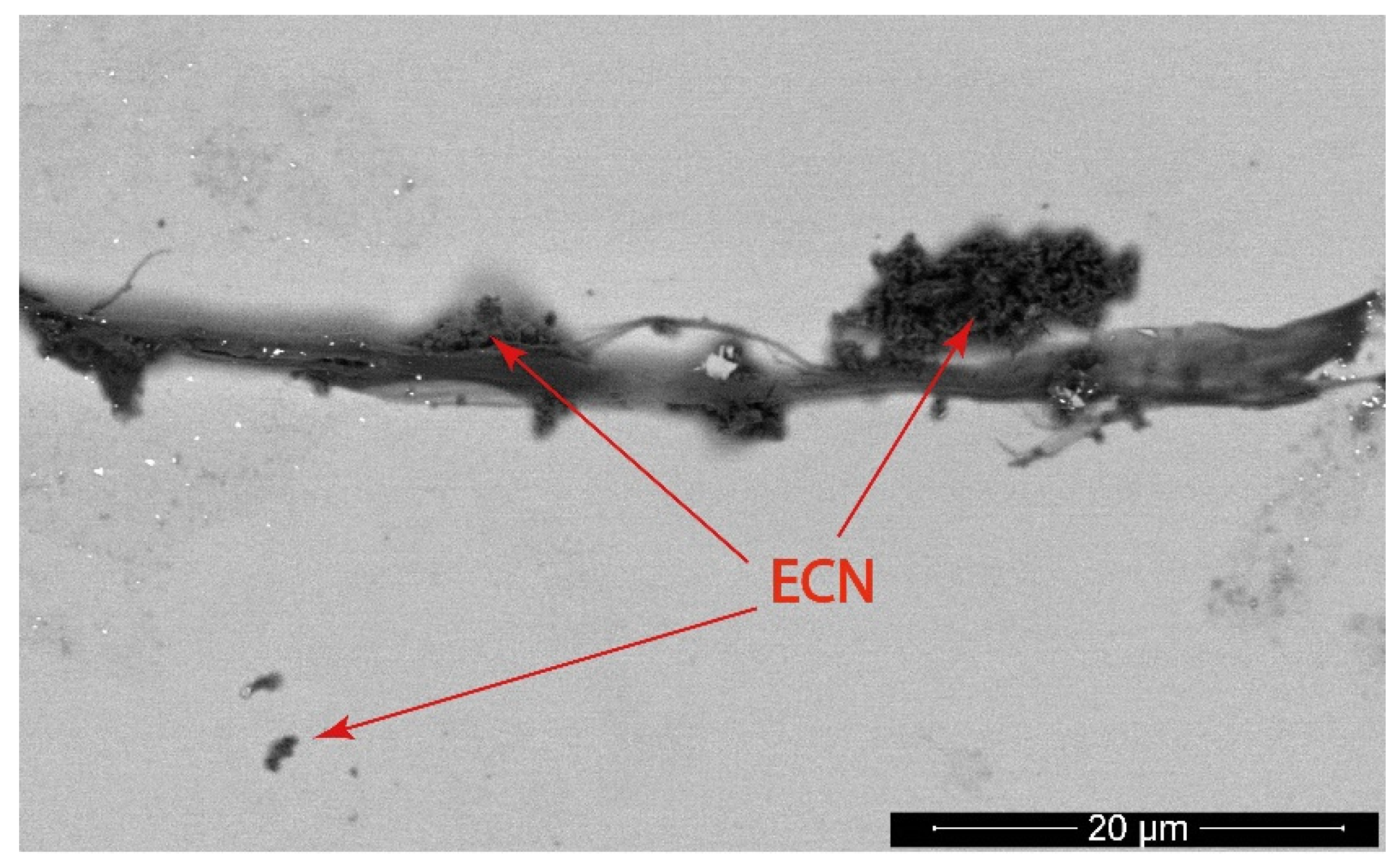
3.2. Microscopy Characterization
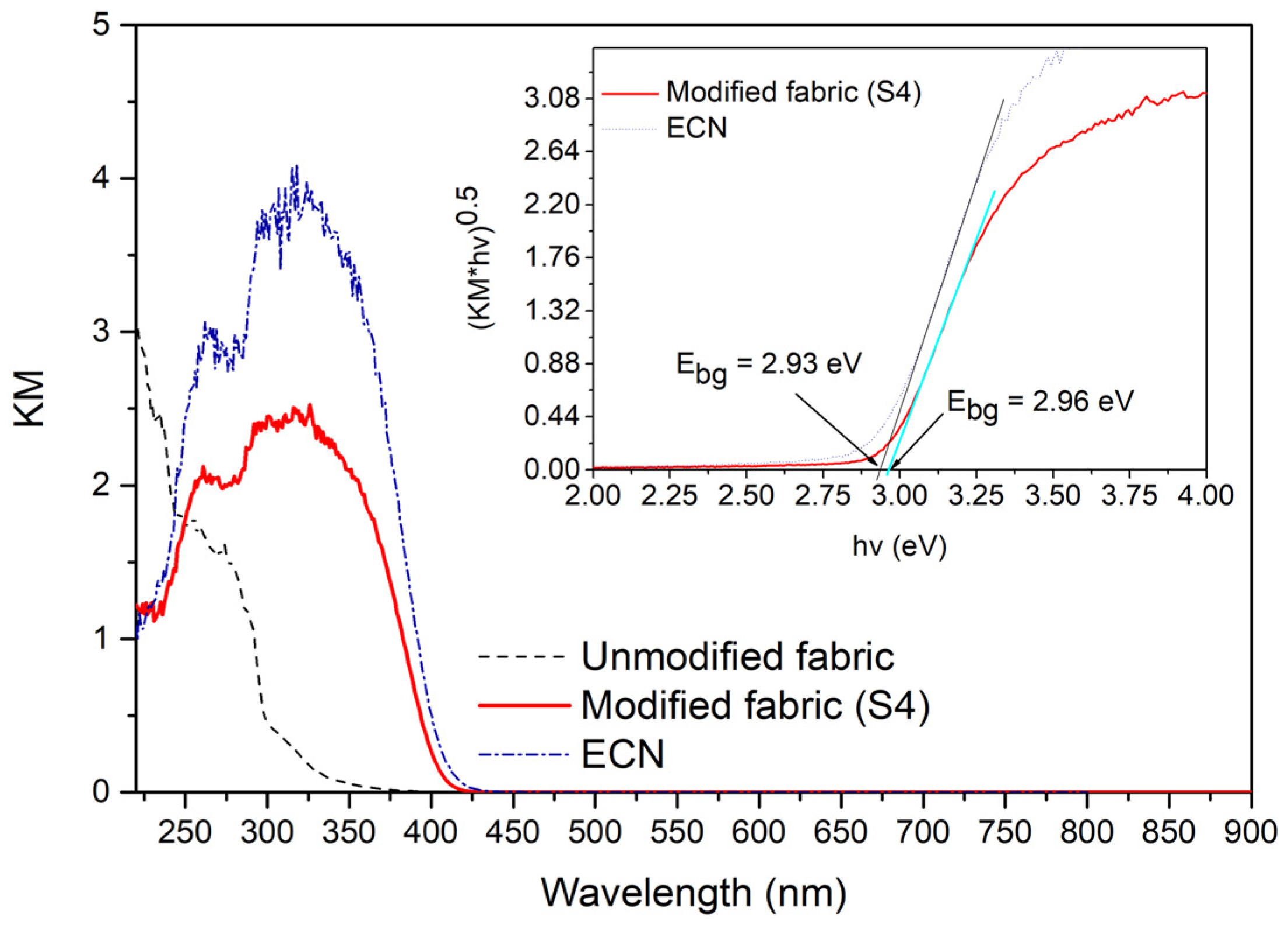
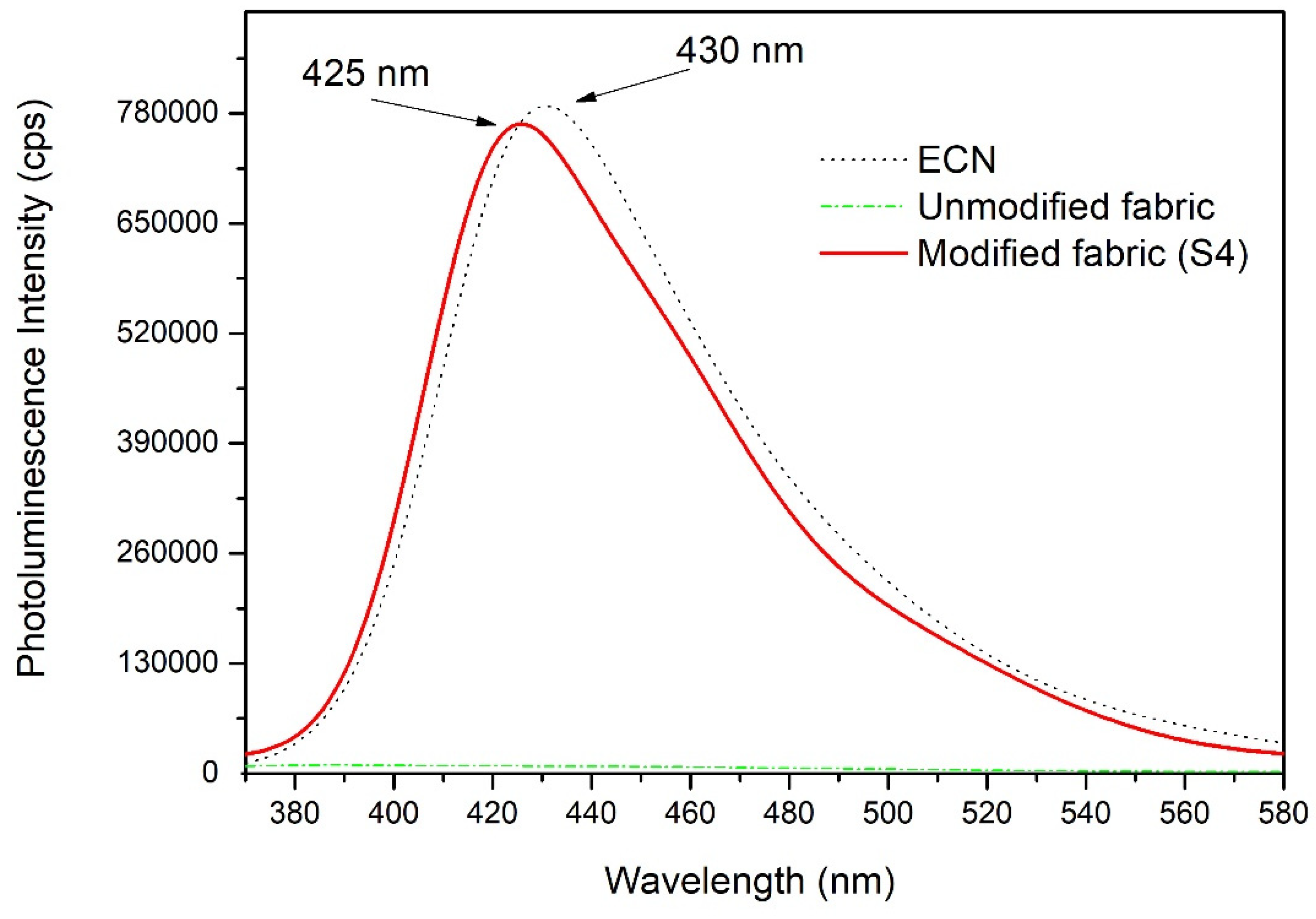
3.3. UV–Vis DRS and Photoluminescence Characterization
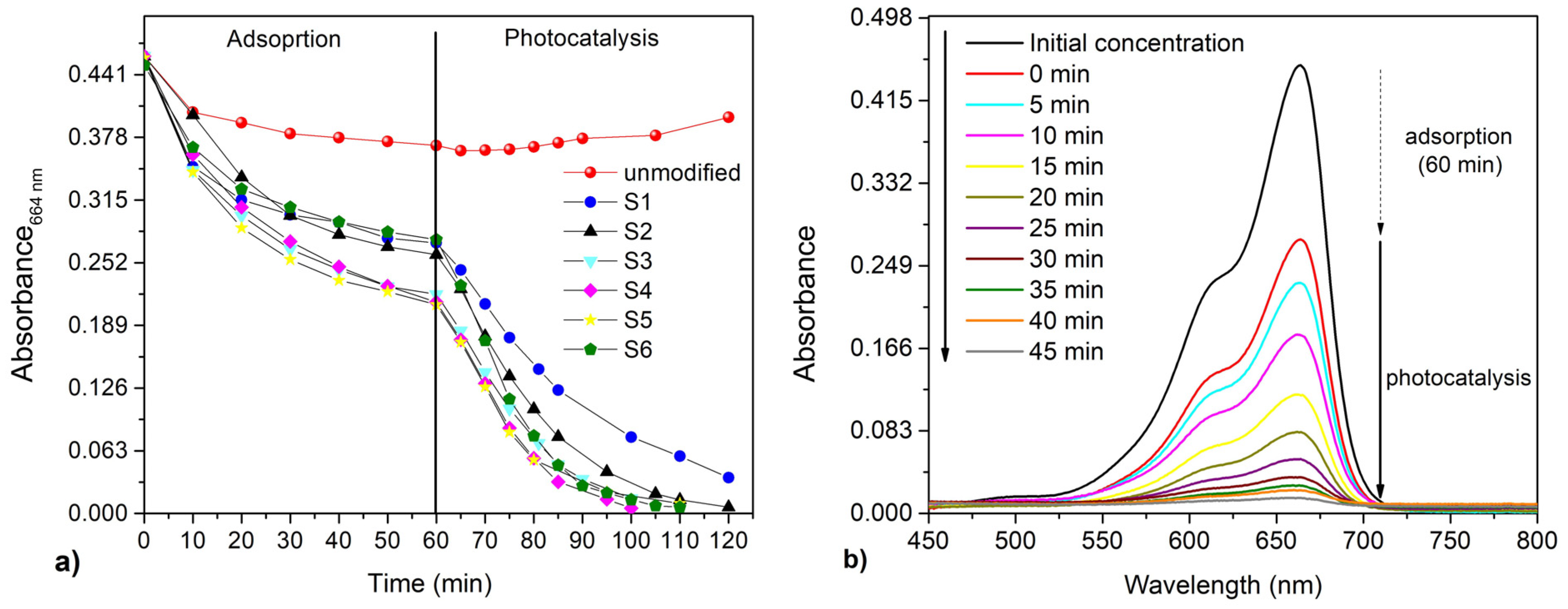
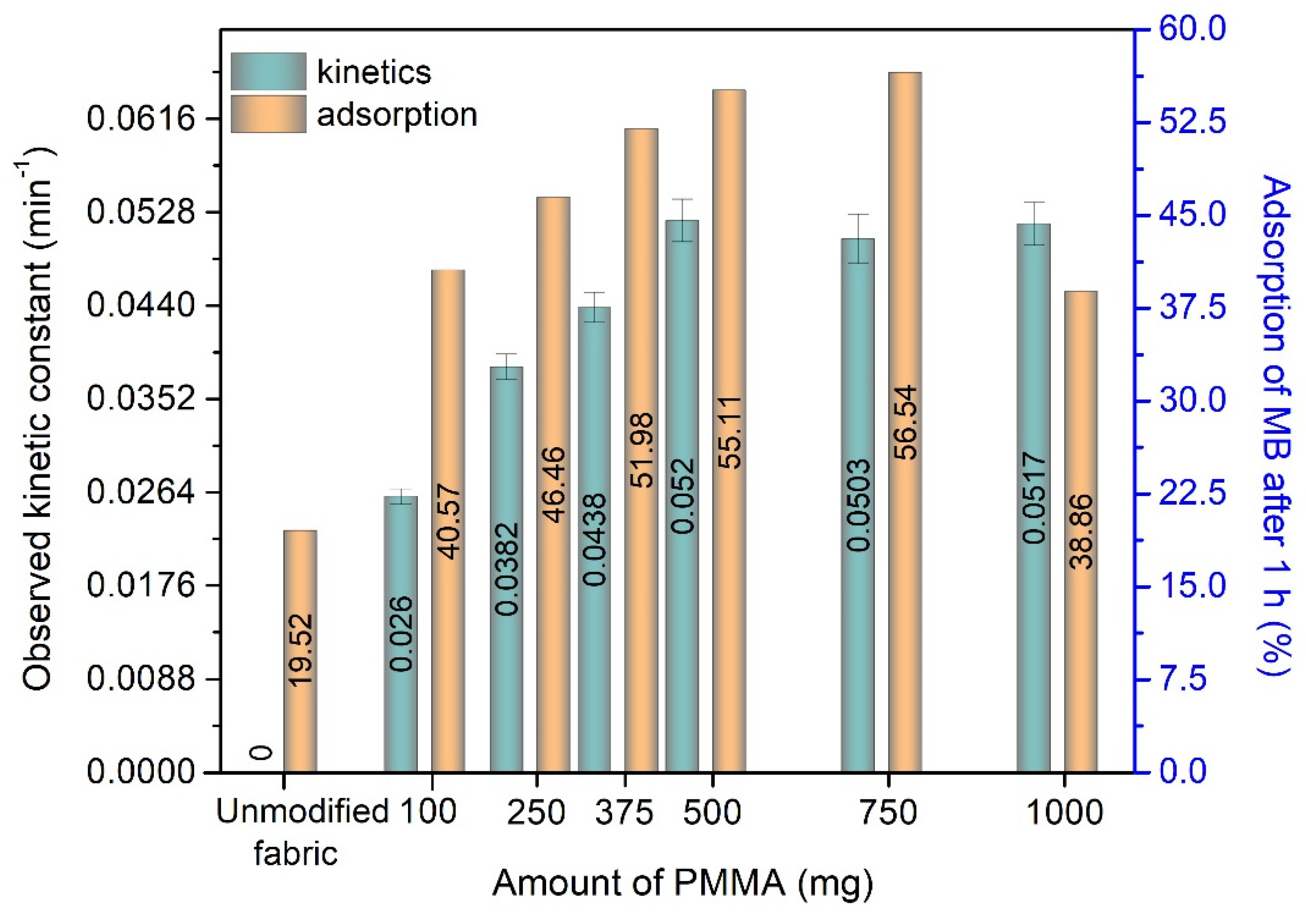
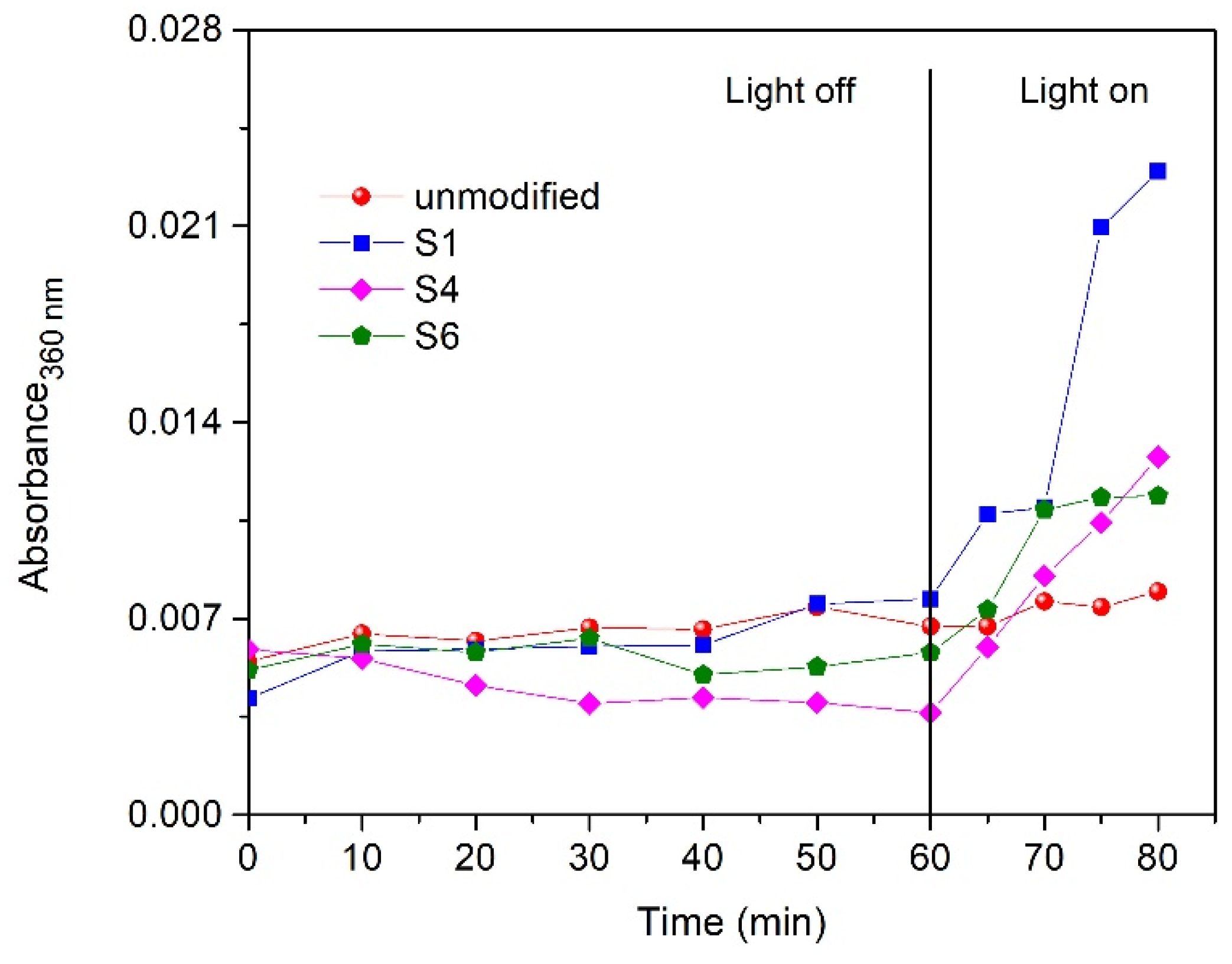
3.4. The Adsorption and Photocatalytic Degradation of Methylene Blue
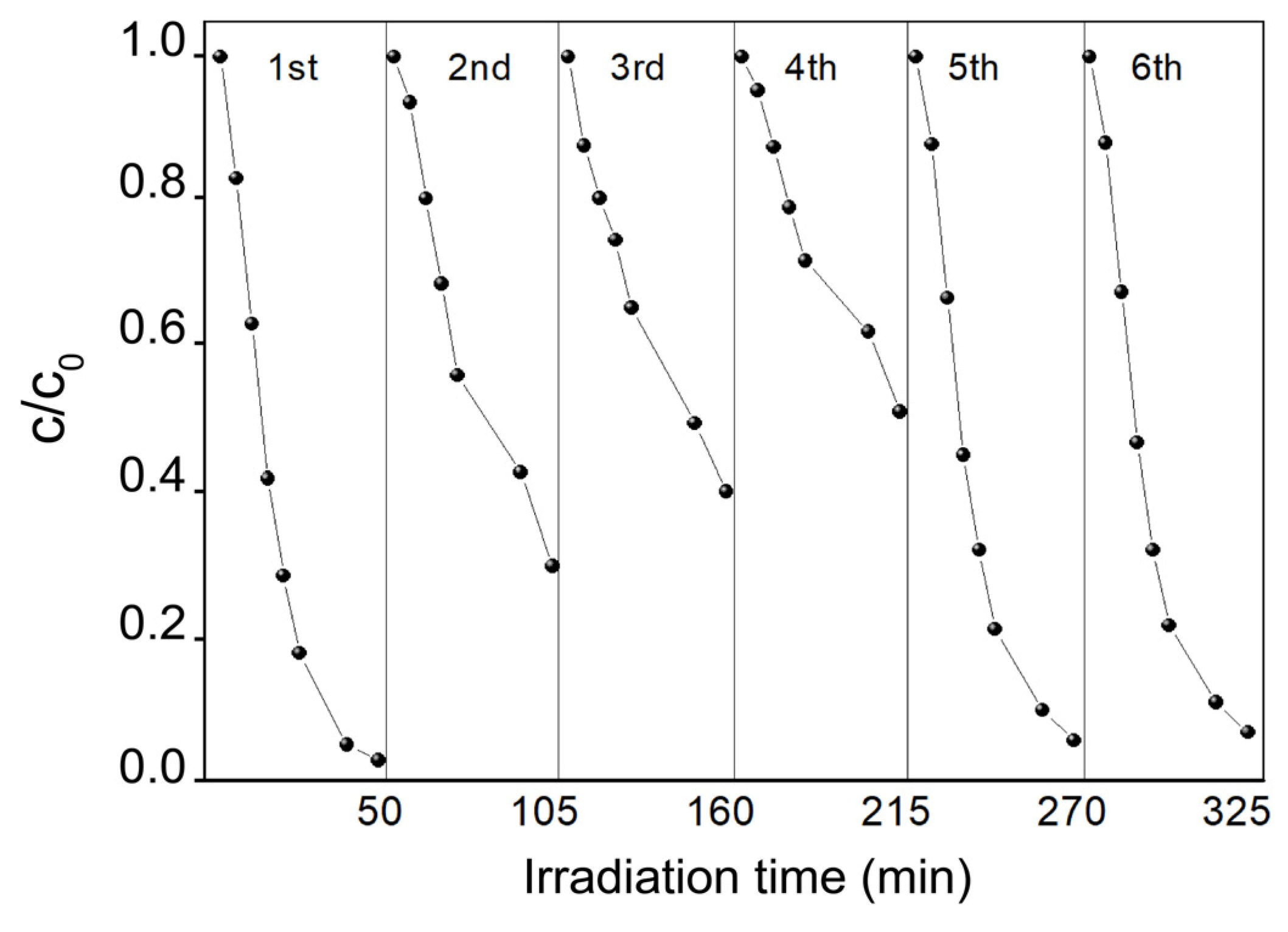
3.5. Recyclability Study
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Li, W.; Hua, F.; Yue, J.; Li, J. Ag@AgCl plasmon-induced sensitized ZnO particle for high-efficiency photocatalytic property under visible light. Appl. Surf. Sci. 2013, 285, 490–497. [Google Scholar] [CrossRef]
- Athanasekou, C.P.; Moustakas, N.G.; Morales-Torres, S.; Pastrana-Martínez, L.M.; Figueiredo, J.L.; Faria, J.L.; Silva, A.M.T.; Dona-Rodriguez, J.M.; Romanos, G.E.; Falaras, P. Ceramic photocatalytic membranes for water filtration under UV and visible light. Appl. Catal. B Environ. 2015, 178, 12–19. [Google Scholar] [CrossRef]
- Chew, C.M.; Aroua, M.K.; Hussain, M.A. Advanced process control for ultrafiltration membrane water treatment system. J. Clean. Prod. 2018, 179, 63–80. [Google Scholar] [CrossRef]
- Li, X.; Row, K.H. Development of deep eutectic solvents applied in extraction and separation. J. Sep. Sci. 2016, 39, 3505–3520. [Google Scholar] [CrossRef] [PubMed]
- Kazadi Mbamba, C.; Batstone, D.J.; Flores-Alsina, X.; Tait, S. A generalised chemical precipitation modelling approach in wastewater treatment applied to calcite. Water Res. 2015, 68, 342–353. [Google Scholar] [CrossRef]
- Särkkä, H.; Bhatnagar, A.; Sillanpää, M. Recent developments of electro-oxidation in water treatment—A review. J. Electroanal. Chem. 2015, 754, 46–56. [Google Scholar] [CrossRef]
- Chong, Y.T.; Mohd Ariffin, M.; Mohd Tahir, N.; Loh, S.H. A green solvent holder in electro-mediated microextraction for the extraction of phenols in water. Talanta 2018, 176, 558–564. [Google Scholar] [CrossRef]
- Phillips, R.B.; James, R.R.; Magnuson, M.L. Electrolyte selection and microbial toxicity for electrochemical oxidative water treatment using a boron-doped diamond anode to support site specific contamination incident response. Chemosphere 2018, 197, 135–141. [Google Scholar] [CrossRef]
- Subramani, A.; Jacangelo, J.G. Emerging desalination technologies for water treatment: A critical review. Water Res. 2015, 75, 164–187. [Google Scholar] [CrossRef]
- Crini, G.; Lichtfouse, E.; Wilson, L.D.; Morin-Crini, N. Adsorption-oriented processes using conventional and non-conventional adsorbents for wastewater treatment. In Green Adsorbents for Pollutant Removal: Fundamentals and Design; Crini, G., Lichtfouse, E., Eds.; Springer International Publishing: Cham, Germany, 2018; pp. 23–71. ISBN 978-3-319-92111-2. [Google Scholar]
- Hasan, Z.; Jhung, S.H. Removal of hazardous organics from water using metal-organic frameworks (MOFs): Plausible mechanisms for selective adsorptions. J. Hazard. Mater. 2015, 283, 329–339. [Google Scholar] [CrossRef]
- Abbasi, Z.; Shamsaei, E.; Leong, S.K.; Ladewig, B.; Zhang, X.; Wang, H. Effect of carbonization temperature on adsorption property of ZIF-8 derived nanoporous carbon for water treatment. Microporous Mesoporous Mater. 2016, 236, 28–37. [Google Scholar] [CrossRef]
- Cui, L.; Wang, Y.; Gao, L.; Hu, L.; Yan, L.; Wei, Q.; Du, B. EDTA functionalized magnetic graphene oxide for removal of Pb(II), Hg(II) and Cu(II) in water treatment: Adsorption mechanism and separation property. Chem. Eng. J. 2015, 281, 1–10. [Google Scholar] [CrossRef]
- Burakov, A.E.; Galunin, E.V.; Burakova, I.V.; Kucherova, A.E.; Agarwal, S.; Tkachev, A.G.; Gupta, V.K. Adsorption of heavy metals on conventional and nanostructured materials for wastewater treatment purposes: A review. Ecotoxicol. Environ. Saf. 2018, 148, 702–712. [Google Scholar] [CrossRef] [PubMed]
- Giannakoudakis, D.A.; Kyzas, G.Z.; Avranas, A.; Lazaridis, N.K. Multi-parametric adsorption effects of the reactive dye removal with commercial activated carbons. J. Mol. Liq. 2016, 213, 381–389. [Google Scholar] [CrossRef]
- Suhas; Gupta, V.K.; Carrott, P.J.M.; Singh, R.; Chaudhary, M.; Kushwaha, S. Cellulose: A review as natural, modified and activated carbon adsorbent. Bioresour. Technol. 2016, 216, 1066–1076. [Google Scholar] [CrossRef]
- Robinson, T.; McMullan, G.; Marchant, R.; Nigam, P. Remediation of dyes in textile effluent: A critical review on current treatment technologies with a proposed alternative. Bioresour. Technol. 2001, 77, 247–255. [Google Scholar] [CrossRef]
- Zhu, B.; Zhang, L.; Cheng, B.; Yu, J. First-principle calculation study of tri-s-triazine-based g-C3N4: A review. Appl. Catal. B Environ. 2018, 224, 983–999. [Google Scholar] [CrossRef]
- Masih, D.; Ma, Y.; Rohani, S. Graphitic C3N4 based noble-metal-free photocatalyst systems: A review. Appl. Catal. B Environ. 2017, 206, 556–588. [Google Scholar] [CrossRef]
- Wen, J.; Xie, J.; Chen, X.; Li, X. A review on g-C3N4-based photocatalysts. Appl. Surf. Sci. 2017, 391, 72–123. [Google Scholar] [CrossRef]
- Lan, Z.-A.; Zhang, G.; Wang, X. A facile synthesis of Br-modified g-C3N4 semiconductors for photoredox water splitting. Appl. Catal. B Environ. 2016, 192, 116–125. [Google Scholar] [CrossRef]
- Qu, D.; Liu, J.; Miao, X.; Han, M.; Zhang, H.; Cui, Z.; Sun, S.; Kang, Z.; Fan, H.; Sun, Z. Peering into water splitting mechanism of g-C3N4-carbon dots metal-free photocatalyst. Appl. Catal. B Environ. 2018, 227, 418–424. [Google Scholar] [CrossRef]
- Sun, Y.; Xiong, T.; Ni, Z.; Liu, J.; Dong, F.; Zhang, W.; Ho, W.-K. Improving g-C3N4 photocatalysis for NOx removal by Ag nanoparticles decoration. Appl. Surf. Sci. 2015, 358, 356–362. [Google Scholar] [CrossRef]
- Reli, M.; Svoboda, L.; Šihor, M.; Troppová, I.; Pavlovský, J.; Praus, P.; Kočí, K. Photocatalytic decomposition of N2O over g-C3N4/WO3 photocatalysts. Environ. Sci. Pollut. Res. 2018, 25, 34839–34850. [Google Scholar] [CrossRef]
- Yu, Y.; Wang, C.; Luo, L.; Wang, J.; Meng, J. An environment-friendly route to synthesize pyramid-like g-C3N4 arrays for efficient degradation of rhodamine B under visible-light irradiation. Chem. Eng. J. 2018, 334, 1869–1877. [Google Scholar] [CrossRef]
- Fang, S.; Lv, K.; Li, Q.; Ye, H.; Du, D.; Li, M. Effect of acid on the photocatalytic degradation of rhodamine B over g-C3N4. Appl. Surf. Sci. 2015, 358, 336–342. [Google Scholar] [CrossRef]
- Praus, P.; Svoboda, L.; Dvorský, R.; Reli, M. Nanocomposites of SnO2 and g-C3N4: Preparation, characterization and photocatalysis under visible LED irradiation. Ceram. Int. 2018, 44, 3837–3846. [Google Scholar] [CrossRef]
- Shan, W.; Hu, Y.; Bai, Z.; Zheng, M.; Wei, C. In situ preparation of g-C3N4/bismuth-based oxide nanocomposites with enhanced photocatalytic activity. Appl. Catal. B Environ. 2016, 188, 1–12. [Google Scholar] [CrossRef]
- Meng, Y.; Shen, J.; Chen, D.; Xin, G. Photodegradation performance of methylene blue aqueous solution on Ag/g-C3N4 catalyst. Rare Met. 2011, 30, 276–279. [Google Scholar] [CrossRef]
- Wu, F.; Li, X.; Liu, W.; Zhang, S. Highly enhanced photocatalytic degradation of methylene blue over the indirect all-solid-state Z-scheme g-C3N4-RGO-TiO2 nanoheterojunctions. Appl. Surf. Sci. 2017, 405, 60–70. [Google Scholar] [CrossRef]
- Vadivel, S.; Maruthamani, D.; Habibi-Yangjeh, A.; Paul, B.; Dhar, S.S.; Selvam, K. Facile synthesis of novel CaFe2O4/g-C3N4 nanocomposites for degradation of methylene blue under visible-light irradiation. J. Colloid Interface Sci. 2016, 480, 126–136. [Google Scholar] [CrossRef]
- Wang, F.; Feng, Y.; Chen, P.; Wang, Y.; Su, Y.; Zhang, Q.; Zeng, Y.; Xie, Z.; Liu, H.; Liu, Y.; et al. Photocatalytic degradation of fluoroquinolone antibiotics using ordered mesoporous g-C3N4 under simulated sunlight irradiation: Kinetics, mechanism, and antibacterial activity elimination. Appl. Catal. B Environ. 2018, 227, 114–122. [Google Scholar] [CrossRef]
- Zhang, M.; Jiang, W.; Liu, D.; Wang, J.; Liu, Y.; Zhu, Y.; Zhu, Y. Photodegradation of phenol via C3N4-agar hybrid hydrogel 3D photocatalysts with free separation. Appl. Catal. B Environ. 2016, 183, 263–268. [Google Scholar] [CrossRef]
- Li, Y.; Ruan, Z.; He, Y.; Li, J.; Li, K.; Yang, Y.; Xia, D.; Lin, K.; Yuan, Y. Enhanced photocatalytic H2 evolution and phenol degradation over sulfur doped meso/macroporous g-C3N4 spheres with continuous channels. Int. J. Hydrogen Energy 2019, 44, 707–719. [Google Scholar] [CrossRef]
- Praus, P.; Svoboda, L.; Dvorský, R.; Reli, M.; Kormunda, M.; Mančík, P. Synthesis and properties of nanocomposites of WO3 and exfoliated g-C3N4. Ceram. Int. 2017, 43, 13581–13591. [Google Scholar] [CrossRef]
- Zhou, C.; Zeng, Z.; Zeng, G.; Huang, D.; Xiao, R.; Cheng, M.; Zhang, C.; Xiong, W.; Lai, C.; Yang, Y.; et al. Visible-light-driven photocatalytic degradation of sulfamethazine by surface engineering of carbon nitride: Properties, degradation pathway and mechanisms. J. Hazard. Mater. 2019, 380, 120815. [Google Scholar] [CrossRef]
- Pattnaik, S.P.; Behera, A.; Martha, S.; Acharya, R.; Parida, K. Facile synthesis of exfoliated graphitic carbon nitride for photocatalytic degradation of ciprofloxacin under solar irradiation. J. Mater. Sci. 2019, 54, 5726–5742. [Google Scholar] [CrossRef]
- Svoboda, L.; Škuta, R.; Matějka, V.; Dvorský, R.; Matýsek, D.; Henych, J.; Mančík, P.; Praus, P. Graphene oxide and graphitic carbon nitride nanocomposites assembled by electrostatic attraction forces: Synthesis and characterization. Mater. Chem. Phys. 2019, 228, 228–236. [Google Scholar] [CrossRef]
- Svoboda, L.; Praus, P.; Lima, M.J.; Sampaio, M.J.; Matýsek, D.; Ritz, M.; Dvorský, R.; Faria, J.L.; Silva, C.G. Graphitic carbon nitride nanosheets as highly efficient photocatalysts for phenol degradation under high-power visible LED irradiation. Mater. Res. Bull. 2018, 100, 322–332. [Google Scholar] [CrossRef]
- Hu, Z.; Cai, X.; Wang, Z.; Li, S.; Wang, Z.; Xie, X. Construction of carbon-doped supramolecule-based g-C3N4/TiO2 composites for removal of diclofenac and carbamazepine: A comparative study of operating parameters, mechanisms, degradation pathways. J. Hazard. Mater. 2019, 380, 120812. [Google Scholar] [CrossRef]
- Zhu, Q.; Qiu, B.; Duan, H.; Gong, Y.; Qin, Z.; Shen, B.; Xing, M.; Zhang, J. Electron directed migration cooperated with thermodynamic regulation over bimetallic NiFeP/g-C3N4 for enhanced photocatalytic hydrogen evolution. Appl. Catal. B Environ. 2019, 259, 118078. [Google Scholar] [CrossRef]
- Wu, B.; Li, Y.; Su, K.; Tan, L.; Liu, X.; Cui, Z.; Yang, X.; Liang, Y.; Li, Z.; Zhu, S.; et al. The enhanced photocatalytic properties of MnO2/g-C3N4 heterostructure for rapid sterilization under visible light. J. Hazard. Mater. 2019, 377, 227–236. [Google Scholar] [CrossRef] [PubMed]
- Cao, L.; Li, Y.-F.; Tong, Y.; Yang, R.; Sun, L.; Cao, Q.; Chen, R. A novel Bi12TiO20/g-C3N4 hybrid catalyst with a bionic granum configuration for enhanced photocatalytic degradation of organic pollutants. J. Hazard. Mater. 2019, 379, 120808. [Google Scholar] [CrossRef]
- Olabarrieta, J.; Monzón, O.; Belaustegui, Y.; Alvarez, J.-I.; Zorita, S. Removal of TiO2 nanoparticles from water by low pressure pilot plant filtration. Sci. Total Environ. 2018, 618, 551–560. [Google Scholar] [CrossRef] [PubMed]
- McCullagh, C.; Robertson, J.M.C.; Bahnemann, D.W.; Robertson, P.K.J. The application of TiO2 photocatalysis for disinfection of water contaminated with pathogenic micro-organisms: A review. Res. Chem. Intermed. 2007, 33, 359–375. [Google Scholar] [CrossRef]
- Geyer, F.; D’Acunzi, M.; Sharifi-Aghili, A.; Saal, A.; Gao, N.; Kaltbeitzel, A.; Sloot, T.-F.; Berger, R.; Butt, H.-J.; Vollmer, D. When and how self-cleaning of superhydrophobic surfaces works. Sci. Adv. 2020, 6, eaaw9727. [Google Scholar] [CrossRef]
- Byun, D.; Hong, J.; Saputra; Ko, J.H.; Lee, Y.J.; Park, H.C.; Byun, B.-K.; Lukes, J.R. Wetting characteristics of insect wing surfaces. J. Bionic Eng. 2009, 6, 63–70. [Google Scholar] [CrossRef]
- Ganesh, V.A.; Raut, H.K.; Nair, A.S.; Ramakrishna, S. A review on self-cleaning coatings. J. Mater. Chem. 2011, 21, 16304–16322. [Google Scholar] [CrossRef]
- Wang, S.; Ajji, A.; Guo, S.; Xiong, C. Preparation of microporous polypropylene/titanium dioxide composite membranes with enhanced electrolyte uptake capability via melt extruding and stretching. Polymers 2017, 9, 110. [Google Scholar] [CrossRef]
- Han, H.; Bai, R. Highly effective buoyant photocatalyst prepared with a novel layered-TiO2 configuration on polypropylene fabric and the degradation performance for methyl orange dye under UV–Vis and Vis lights. Sep. Purif. Technol. 2010, 73, 142–150. [Google Scholar] [CrossRef]
- Dong, P.; Nie, X.; Jin, Z.; Huang, Z.; Wang, X.; Zhang, X. Dual dielectric barrier discharge plasma treatments for synthesis of Ag–TiO2 functionalized polypropylene fabrics. Ind. Eng. Chem. Res. 2019, 58, 7734–7741. [Google Scholar] [CrossRef]
- Zhang, H.; Li, X.; Han, B.; Wu, H.; Mao, N. Simultaneous reactive dyeing and surface modification of polyamide fabric with TiO2 precursor finish using a one-step hydrothermal process. Text. Res. J. 2018, 88, 2611–2623. [Google Scholar] [CrossRef]
- Zhang, H.; Zhu, H. Preparation of Fe-doped TiO2 nanoparticles immobilized on polyamide fabric. Appl. Surf. Sci. 2012, 258, 10034–10041. [Google Scholar] [CrossRef]
- Ghaffari, S.; Mojtahedi, M.R.M.; Dastjerdi, R. Comparison of the morphological, mechanical, and UV protection properties of TiO2/polyamide 6 (PA6), and ZnO/PA6 nanocomposite multifilament yarns. J. Macromol. Sci. Part B 2015, 54, 783–798. [Google Scholar] [CrossRef]
- Blanco, M.; Monteserín, C.; Angulo, A.; Pérez-Márquez, A.; Maudes, J.; Murillo, N.; Aranzabe, E.; Ruiz-Rubio, L.; Vilas, J.L. TiO2-doped electrospun nanofibrous membrane for photocatalytic water treatment. Polymers 2019, 11, 747. [Google Scholar] [CrossRef] [PubMed]
- Khaled, S.M.; Sui, R.; Charpentier, P.A.; Rizkalla, A.S. Synthesis of TiO 2—PMMA nanocomposite: Using methacrylic acid as a coupling agent. Langmuir 2007, 23, 3988–3995. [Google Scholar] [CrossRef]
- Teixeira, S.; Magalhães, B.; Martins, P.M.; Kühn, K.; Soler, L.; Lanceros-Méndez, S.; Cuniberti, G. Reusable photocatalytic optical fibers for underground, deep-sea, and turbid water remediation. Glob. Chall. 2018, 2, 1700124. [Google Scholar] [CrossRef]
- Galiano, F.; Song, X.; Marino, T.; Boerrigter, M.; Saoncella, O.; Simone, S.; Faccini, M.; Chaumette, C.; Drioli, E.; Figoli, A. Novel photocatalytic PVDF/Nano-TiO2 hollow fibers for environmental remediation. Polymers 2018, 10, 1134. [Google Scholar] [CrossRef]
- Tan, B.; Gao, B.; Guo, J.; Guo, X.; Long, M. A comparison of TiO2 coated self-cleaning cotton by the sols from peptizing and hydrothermal routes. Surf. Coat. Technol. 2013, 232, 26–32. [Google Scholar] [CrossRef]
- Wu, D.; Wang, H.; Li, C.; Xia, J.; Song, X.; Huang, W. Photocatalytic self-cleaning properties of cotton fabrics functionalized with p-BiOI/n-TiO 2 heterojunction. Surf. Coat. Technol. 2014, 258, 672–676. [Google Scholar] [CrossRef]
- Xu, B.; Ding, J.; Feng, L.; Ding, Y.; Ge, F.; Cai, Z. Self-cleaning cotton fabrics via combination of photocatalytic TiO2 and superhydrophobic SiO2. Surf. Coat. Technol. 2015, 262, 70–76. [Google Scholar] [CrossRef]
- Iavicoli, I.; Leso, V.; Bergamaschi, A. Toxicological effects of titanium dioxide nanoparticles: A review of in vivo studies. J. Nanomater. 2012, 2012, 964381. [Google Scholar] [CrossRef]
- Ze, Y.; Sheng, L.; Zhao, X.; Hong, J.; Ze, X.; Yu, X.; Pan, X.; Lin, A.; Zhao, Y.; Zhang, C.; et al. TiO2 nanoparticles induced hippocampal neuroinflammation in mice. PLoS ONE 2014, 9, e92230. [Google Scholar] [CrossRef] [PubMed]
- Park, E.-J.; Yi, J.; Chung, K.-H.; Ryu, D.-Y.; Choi, J.; Park, K. Oxidative stress and apoptosis induced by titanium dioxide nanoparticles in cultured BEAS-2B cells. Toxicol. Lett. 2008, 180, 222–229. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Xie, X.; Wang, H.; Zhang, J.; Pan, B.; Xie, Y. Enhanced photoresponsive ultrathin graphitic-phase C3N4 nanosheets for bioimaging. J. Am. Chem. Soc. 2013, 135, 18–21. [Google Scholar] [CrossRef]
- Yang, Y.; Ji, T.; Su, W.; Yang, B.; Zhang, Y.; Yang, Z. Photocatalytic NOx abatement and self-cleaning performance of cementitious composites with g-C3N4 nanosheets under visible light. Constr. Build. Mater. 2019, 225, 120–131. [Google Scholar] [CrossRef]
- Dong, Y.; Ji, X.; Li, F.; Nguyen, T.T.; Huang, Z.; Guo, M. A self-cleaning surface based on heat treatment of g-C3N4-coated wood prepared by a rapid and eco-friendly method. Holzforschung 2019, 73, 393–399. [Google Scholar] [CrossRef]
- Dong, F.; Wang, Z.; Li, Y.; Ho, W.-K.; Lee, S.C. Immobilization of polymeric g-C3N4 on structured ceramic foam for efficient visible light photocatalytic air purification with real indoor illumination. Environ. Sci. Technol. 2014, 48, 10345–10353. [Google Scholar] [CrossRef]
- Wang, X.; Wang, H.; Yu, K.; Hu, X. Immobilization of 2D/2D structured g-C3N4 nanosheet/reduced graphene oxide hybrids on 3D nickel foam and its photocatalytic performance. Mater. Res. Bull. 2018, 97, 306–313. [Google Scholar] [CrossRef]
- Hu, X.; Deng, L.; Ouyang, H.; Wang, H. Immobilization of g-C3N4 nanosheets on diatomite via electrostatic adsorption and their photocatalytic activity. RSC Adv. 2018, 8, 28032–28040. [Google Scholar] [CrossRef]
- Fan, Y.; Zhou, J.; Zhang, J.; Lou, Y.; Huang, Z.; Ye, Y.; Jia, L.; Tang, B. Photocatalysis and self-cleaning from g-C3N4 coated cotton fabrics under sunlight irradiation. Chem. Phys. Lett. 2018, 699, 146–154. [Google Scholar] [CrossRef]
- Lin, H.; Day, D.E.; Stoffer, J.O. Optical and mechanical properties of optically transparent poly(methyl methacrylate) composites. Polym. Eng. Sci. 2004, 32, 344–350. [Google Scholar] [CrossRef]
- Mahmood Raouf, R.; Abdul Wahab, Z.; Azowa Ibrahim, N.; Abidin Talib, Z.; Chieng, B. Transparent blend of poly(methylmethacrylate)/cellulose acetate butyrate for the protection from ultraviolet. Polymers 2016, 8, 128. [Google Scholar] [CrossRef] [PubMed]
- Soumya, S.; Kumar, S.N.; Mohamed, A.P.; Ananthakumar, S. Silanated nano ZnO hybrid embedded PMMA polymer coatings on cotton fabrics for near-IR reflective, antifungal cool-textiles. New J. Chem. 2016, 40, 7210–7221. [Google Scholar] [CrossRef]
- Karim, K.J.B.A.; Buang, N.A. A review of the properties and applications of poly (methyl methacrylate) (PMMA). Polym. Rev. 2015, 55, 678–705. [Google Scholar]
- Zidan, H.M.; Abu-Elnader, M. Structural and optical properties of pure PMMA and metal chloride-doped PMMA films. Phys. B Condens. Matter 2005, 355, 308–317. [Google Scholar] [CrossRef]
- Wochnowski, C.; Metev, S.; Sepold, G. UV–laser-assisted modification of the optical properties of polymethylmethacrylate. Appl. Surf. Sci. 2000, 154–155, 706–711. [Google Scholar] [CrossRef]
- Abd El-Ghani, W.M.A. Cranioplasty with polymethyl methacrylate implant: Solutions of pitfalls. Egypt. J. Neurosurg. 2018, 33, 7. [Google Scholar] [CrossRef][Green Version]
- Kalteis, T.; Lüring, C.; Gugler, G.; Zysk, S.; Caro, W.; Handel, M.; Grifka, J. Acute tissue toxicity of PMMA bone cements. Z. Orthop. Ihre Grenzgeb. 2004, 142, 666–672. [Google Scholar] [CrossRef]
- Frazer, R.Q.; Byron, R.T.; Osborne, P.B.; West, K.P. PMMA: An essential material in medicine and dentistry. J. Long Term Eff. Med. Implants 2005, 15, 629–639. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhuang, S.; Xu, X.; Hu, J. Transparent and UV-shielding ZnO@PMMA nanocomposite films. Opt. Mater. (Amsterdam) 2013, 36, 169–172. [Google Scholar] [CrossRef]
- Rafatullah, M.; Sulaiman, O.; Hashim, R.; Ahmad, A. Adsorption of methylene blue on low-cost adsorbents: A review. J. Hazard. Mater. 2010, 177, 70–80. [Google Scholar] [CrossRef] [PubMed]
- Chang, F.; Xie, Y.; Li, C.; Chen, J.; Luo, J.; Hu, X.; Shen, J. A facile modification of g-C3N4 with enhanced photocatalytic activity for degradation of methylene blue. Appl. Surf. Sci. 2013, 280, 967–974. [Google Scholar] [CrossRef]
- Svoboda, L.; Dvorský, R.; Praus, P.; Matýsek, D.; Bednář, J. Synthesis of ZnO nanocoatings by decomposition of zinc acetate induced by electrons emitted by indium. Appl. Surf. Sci. 2016, 388. [Google Scholar] [CrossRef]
- Liu, X.; Xu, J.; Ni, Z.; Wang, R.; You, J.; Guo, R. Adsorption and visible-light-driven photocatalytic properties of Ag3PO4/WO3 composites: A discussion of the mechanism. Chem. Eng. J. 2019, 356, 22–33. [Google Scholar] [CrossRef]
- Lakshmi, S.; Renganathan, R.; Fujita, S. Study on TiO2-mediated photocatalytic degradation of methylene blue. J. Photochem. Photobiol. A Chem. 1995, 88, 163–167. [Google Scholar] [CrossRef]
- Dvorsky, R.; Svoboda, L.; Bednář, J.; Mančík, P.; Matýsek, D.; Pomiklová, M. Deposition of sorption and photocatalytic material on nanofibers and fabric by controlled sublimation. Mater. Sci. Forum 2018, 936, 63–67. [Google Scholar] [CrossRef]
- Miller-Chou, B.A.; Koenig, J.L. A review of polymer dissolution. Prog. Polym. Sci. 2003, 28, 1223–1270. [Google Scholar] [CrossRef]
- Wang, Z.; Liang, H.; Yang, H.; Xiong, L.; Zhou, J.; Huang, S.; Zhao, C.; Zhong, J.; Fan, X. UV-curable self-healing polyurethane coating based on thiol-ene and Diels-Alder double click reactions. Prog. Org. Coat. 2019, 137, 105282. [Google Scholar] [CrossRef]
- Yang, S.; Gong, Y.; Zhang, J.; Zhan, L.; Ma, L.; Fang, Z.; Vajtai, R.; Wang, X.; Ajayan, P.M. Exfoliated graphitic carbon nitride nanosheets as efficient catalysts for hydrogen evolution under visible light. Adv. Mater. 2013, 25, 2452–2456. [Google Scholar] [CrossRef]
- Praus, P.; Svoboda, L.; Ritz, M.; Troppová, I.; Šihor, M.; Kočí, K. Graphitic carbon nitride: Synthesis, characterization and photocatalytic decomposition of nitrous oxide. Mater. Chem. Phys. 2017, 193, 438–446. [Google Scholar] [CrossRef]
- Kumar, K.V.; Porkodi, K.; Selvaganapathi, A. Constrain in solving langmuir–hinshelwood kinetic expression for the photocatalytic degradation of Auramine O aqueous solutions by ZnO catalyst. Dye. Pigment. 2007, 75, 246–249. [Google Scholar]
- Khademi, M.; Wang, W.; Reitinger, W.; Barz, D.P.J. Zeta potential of poly(methyl methacrylate) (PMMA) in contact with aqueous electrolyte-surfactant solutions. Langmuir 2017, 33, 10473–10482. [Google Scholar] [CrossRef] [PubMed]
- Mamba, G.; Mishra, A.K. Graphitic carbon nitride (g-C3N4) nanocomposites: A new and exciting generation of visible light driven photocatalysts for environmental pollution remediation. Appl. Catal. B Environ. 2016, 198, 347–377. [Google Scholar] [CrossRef]
- Zhen, W.; Ning, X.; Yang, B.; Wu, Y.; Li, Z.; Lu, G. The enhancement of CdS photocatalytic activity for water splitting via anti-photocorrosion by coating Ni2P shell and removing nascent formed oxygen with artificial gill. Appl. Catal. B Environ. 2018, 221, 243–257. [Google Scholar] [CrossRef]
- Ma, H.; Han, J.; Fu, Y.; Song, Y.; Yu, C.; Dong, X. Synthesis of visible light responsive ZnO–ZnS/C photocatalyst by simple carbothermal reduction. Appl. Catal. B Environ. 2011, 102, 417–423. [Google Scholar] [CrossRef]
| Sample | PMMA (mg) | ECN (mg) | Acetone (mL) |
|---|---|---|---|
| S1 | 100 | 30 | 10 |
| S2 | 250 | 30 | 10 |
| S3 | 375 | 30 | 10 |
| S4 | 500 | 30 | 10 |
| S5 | 750 | 30 | 10 |
| S6 | 1000 | 30 | 10 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Svoboda, L.; Licciardello, N.; Dvorský, R.; Bednář, J.; Henych, J.; Cuniberti, G. Design and Performance of Novel Self-Cleaning g-C3N4/PMMA/PUR Membranes. Polymers 2020, 12, 850. https://doi.org/10.3390/polym12040850
Svoboda L, Licciardello N, Dvorský R, Bednář J, Henych J, Cuniberti G. Design and Performance of Novel Self-Cleaning g-C3N4/PMMA/PUR Membranes. Polymers. 2020; 12(4):850. https://doi.org/10.3390/polym12040850
Chicago/Turabian StyleSvoboda, Ladislav, Nadia Licciardello, Richard Dvorský, Jiří Bednář, Jiří Henych, and Gianaurelio Cuniberti. 2020. "Design and Performance of Novel Self-Cleaning g-C3N4/PMMA/PUR Membranes" Polymers 12, no. 4: 850. https://doi.org/10.3390/polym12040850
APA StyleSvoboda, L., Licciardello, N., Dvorský, R., Bednář, J., Henych, J., & Cuniberti, G. (2020). Design and Performance of Novel Self-Cleaning g-C3N4/PMMA/PUR Membranes. Polymers, 12(4), 850. https://doi.org/10.3390/polym12040850









