Preparation and Characterization of Condensed Tannin Non-Isocyanate Polyurethane (NIPU) Rigid Foams by Ambient Temperature Blowing
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
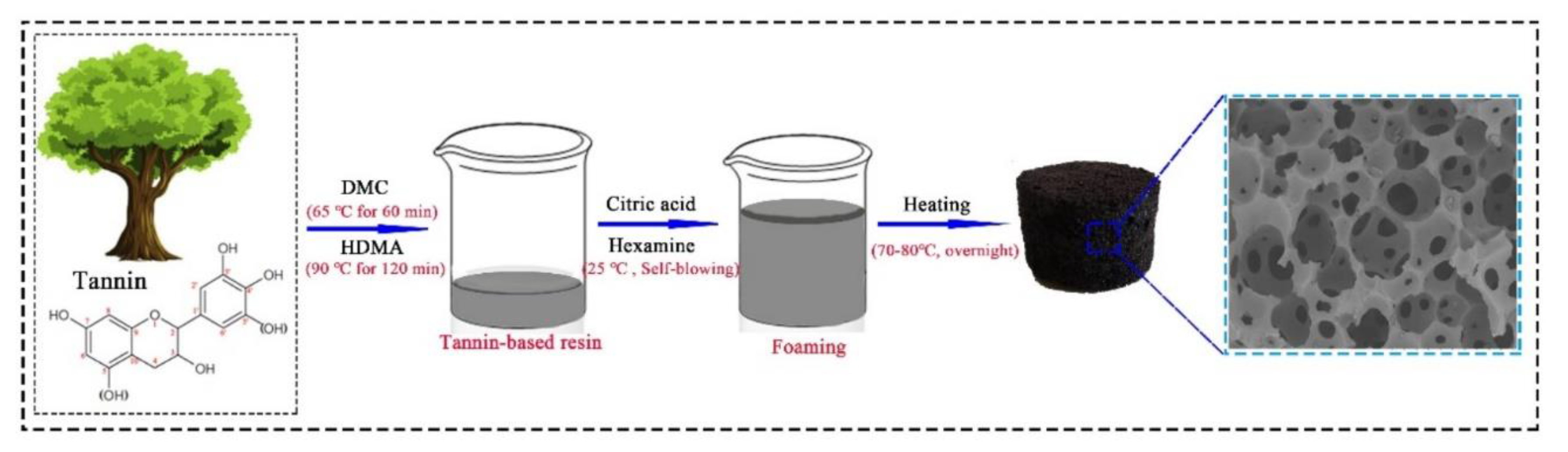
2.2. Preparation of the Tannin-Based NIPU Resins
2.3. Rigid Tannin-Based NIPU Foam Preparation
2.4. Apparent Density
2.5. Scanning Electron Microscopy (SEM) Analysis
2.6. Fourier Transform Infrared (FT-IR) Spectroscopy
2.7. MALDI-TOF
2.8. Solid State CP MAS 13C NMR
2.9. Compression Resistance
2.10. Thermogravimetric Analysis (TGA)
3. Results and Discussion
3.1. Preparation of Tannin-Based NIPU Foams
3.2. Apparent Density
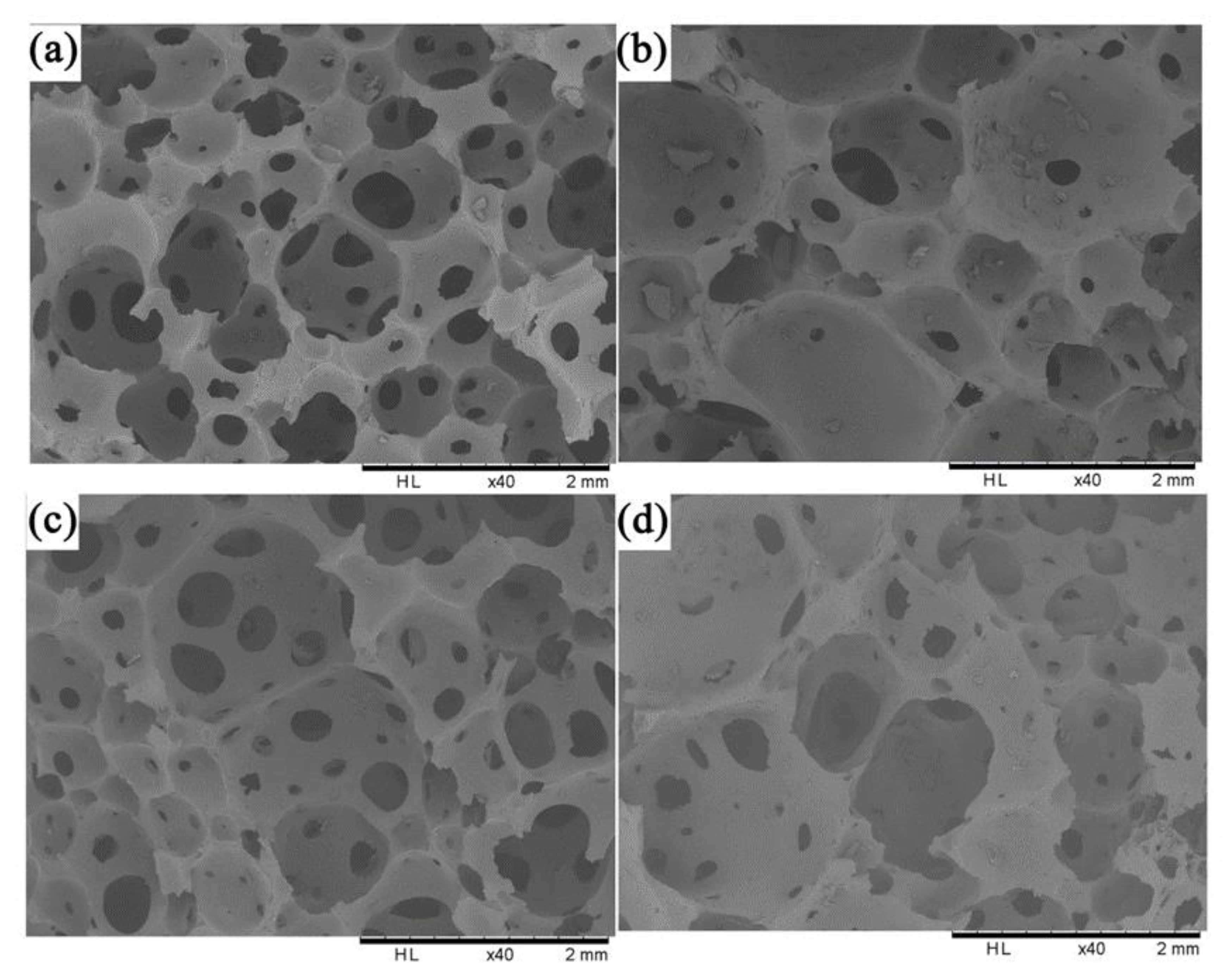
3.3. Scanning Electron Microscopy (SEM) Analysis
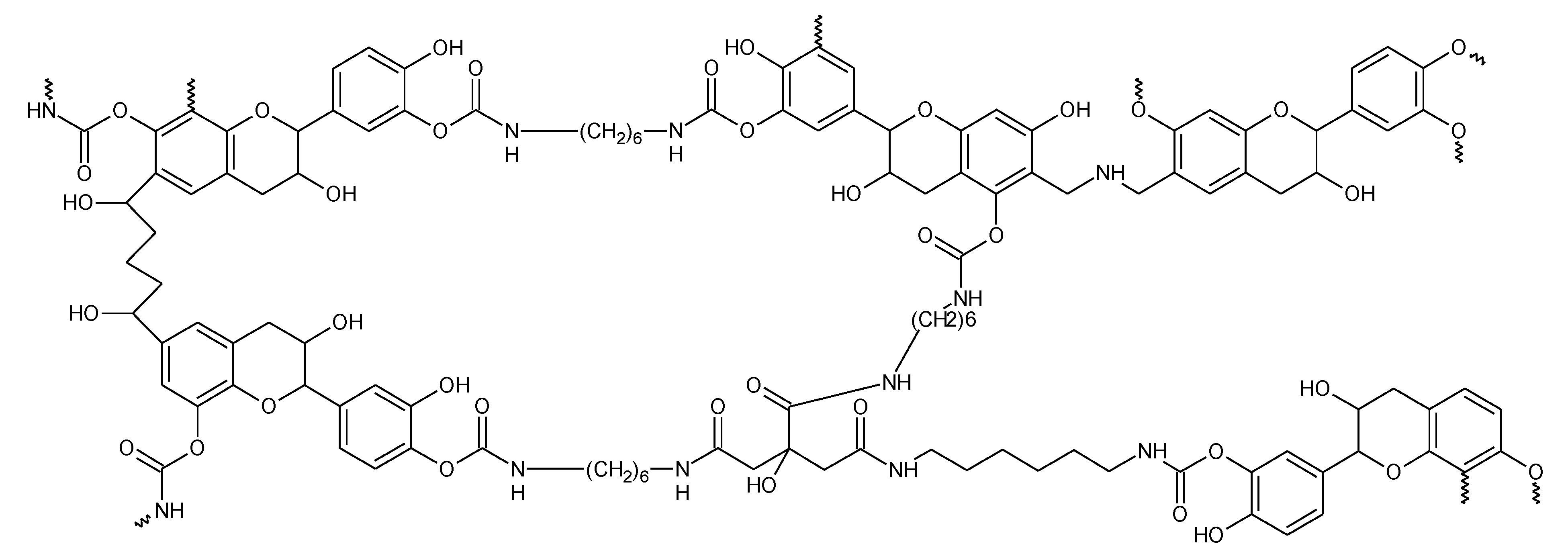
3.4. The Reaction Mechanism of Tannin-Based NIPU Foams
3.4.1. Fourier Transform Infrared (FT-IR) Spectroscopy
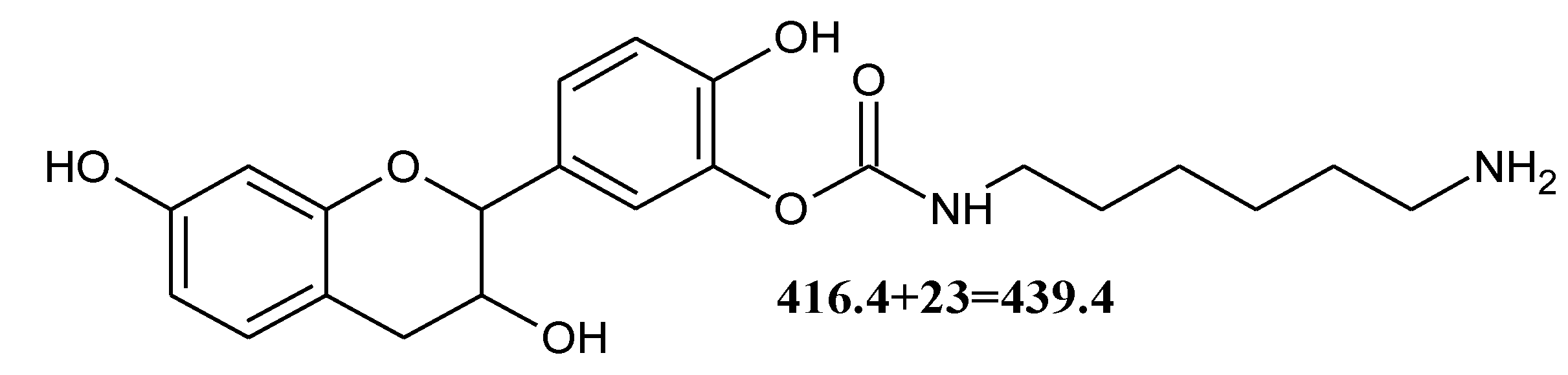
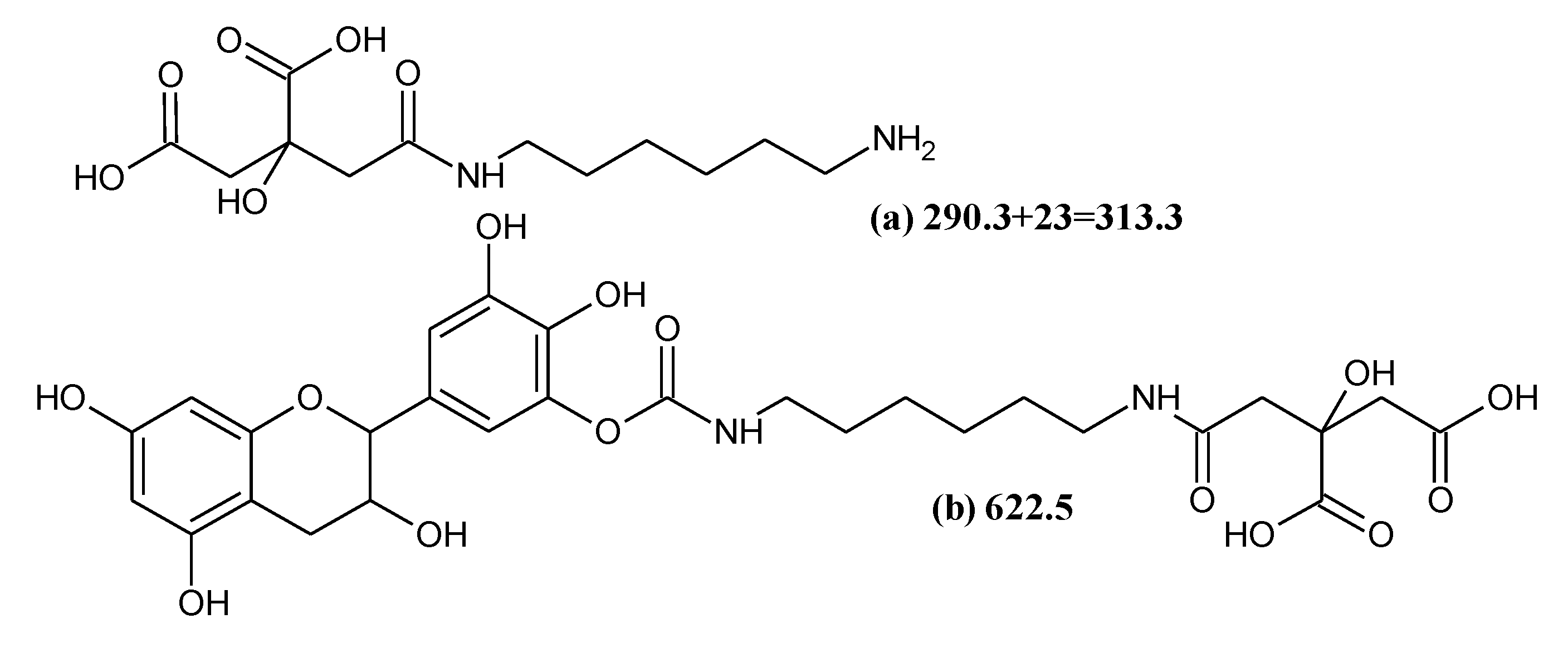
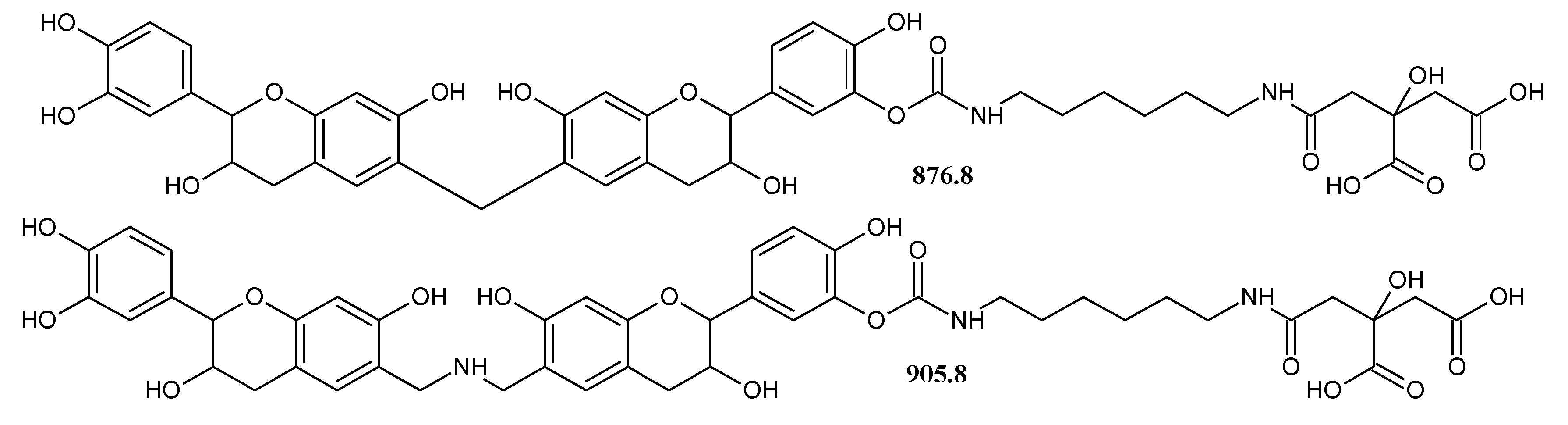
3.4.2. MALDI-TOF Analysis
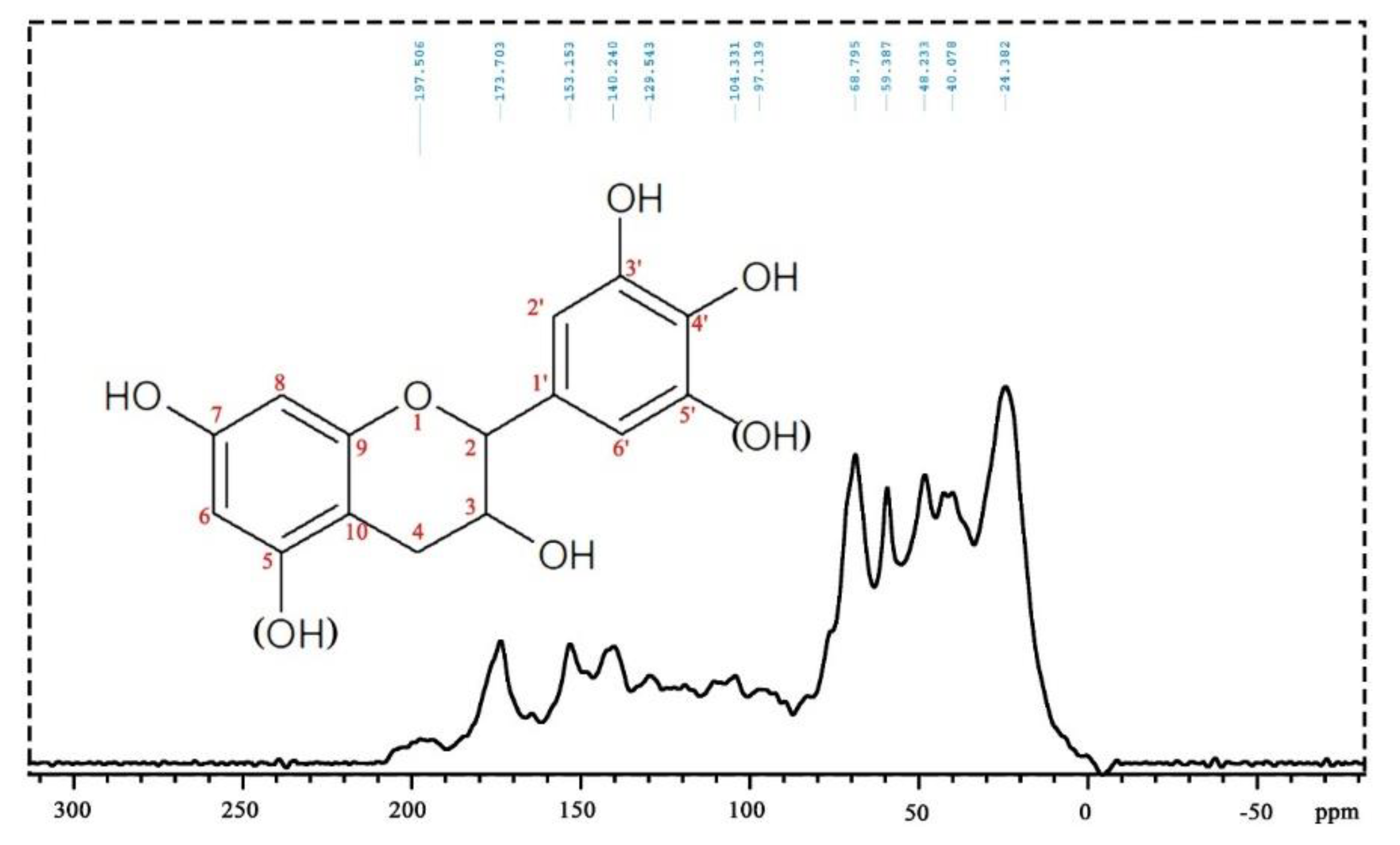
3.4.3. Solid State CP MAS 13C NMR Analysis
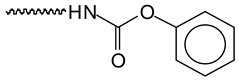 This supports the indication that urethane linkages on the aromatic tannin rings have formed and subsist in the final foam network. The peak at 48 ppm is assigned to the –CH2– of glutaraldehyde next to an unreacted aldehyde group. An overlapping peak does appear at 40 ppm and presents two little peaks that can be seen in this position. The peak at 40 ppm and the shoulder at 38 ppm belong to the –CH2– next to the aldehyde group that has reacted with the tannin aromatic ring site. The other one possible explanation is that it belongs to –CH2– on the unreacted heterocycle C4 site of the flavonoid [64]. This is, however, unlikely, considering the intensity of the signal in relation to the low intensity of all the other signals of the flavonoid carbons. The more likely explanation is that it is likely to belong to the inner –CH2– groups of glutaraldehyde or citric acid.
This supports the indication that urethane linkages on the aromatic tannin rings have formed and subsist in the final foam network. The peak at 48 ppm is assigned to the –CH2– of glutaraldehyde next to an unreacted aldehyde group. An overlapping peak does appear at 40 ppm and presents two little peaks that can be seen in this position. The peak at 40 ppm and the shoulder at 38 ppm belong to the –CH2– next to the aldehyde group that has reacted with the tannin aromatic ring site. The other one possible explanation is that it belongs to –CH2– on the unreacted heterocycle C4 site of the flavonoid [64]. This is, however, unlikely, considering the intensity of the signal in relation to the low intensity of all the other signals of the flavonoid carbons. The more likely explanation is that it is likely to belong to the inner –CH2– groups of glutaraldehyde or citric acid.3.5. Compression Mechanical Properties
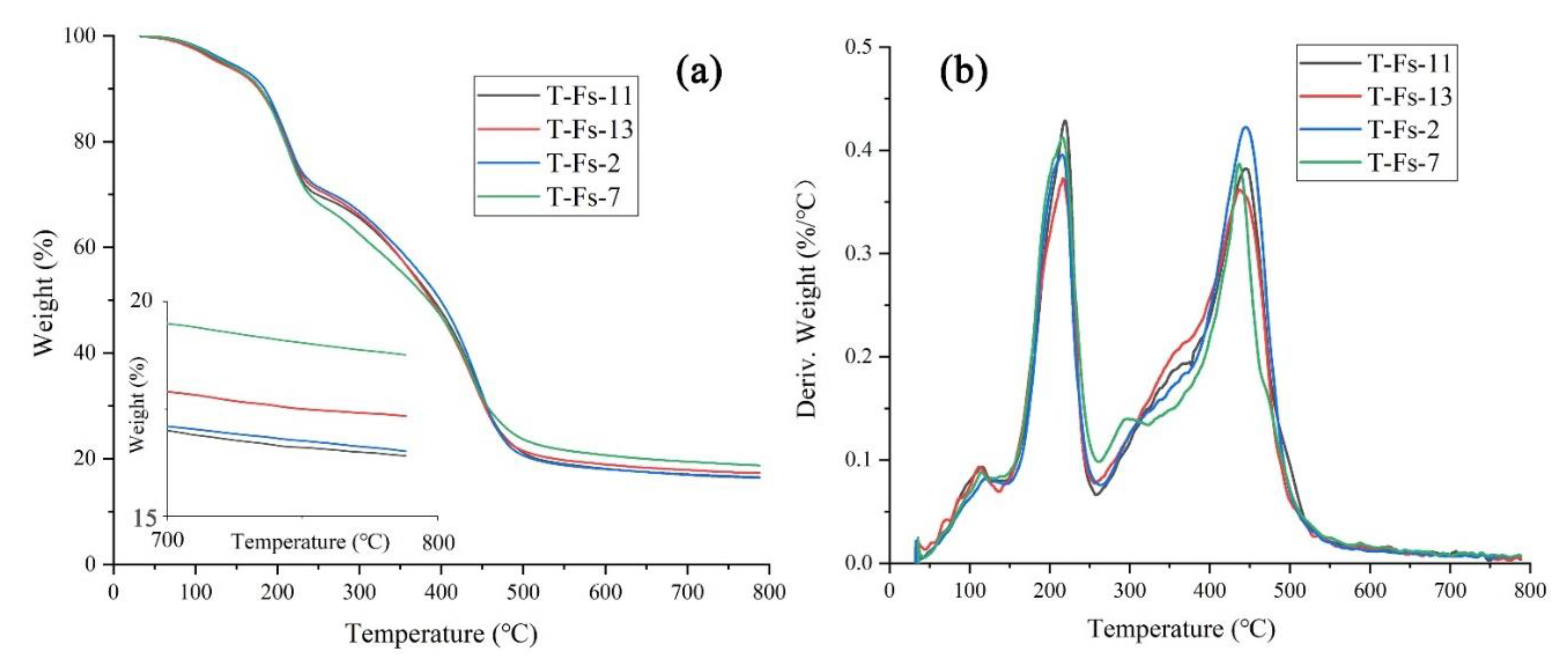
3.6. Thermogravimetric Analysis (TGA)
4. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Pizzi, A. Tannin-based biofoams-A review. J. Renew. Mater. 2019, 7, 474–489. [Google Scholar] [CrossRef]
- Guo, J.; Sun, W.; Kim, J.P.; Lu, X.; Li, Q.; Lin, M.; Mrowczynski, O.; Rizk, E.B.; Cheng, J.; Qian, G.; et al. Development of tannin-inspired antimicrobial bioadhesives. Acta Biomater. 2018, 72, 35–44. [Google Scholar] [CrossRef] [PubMed]
- Shin, M.; Kim, K.; Shim, W.; Yang, J.W.; Lee, H. Tannic acid as a degradable mucoadhesive compound. ACS Biomater. Sci. Eng. 2016, 2, 687–696. [Google Scholar] [CrossRef]
- Bacelo, H.A.; Santos, S.C.; Botelho, C.M. Tannin-based biosorbents for environmental applications-a review. Chem. Eng. J. 2016, 303, 575–587. [Google Scholar] [CrossRef]
- Sánchez-Martín, J.; Beltrán-Heredia, J.; Gibello-Pérez, P. Adsorbent biopolymers from tannin extracts for water treatment. Chem. Eng. J. 2011, 168, 1241–1247. [Google Scholar] [CrossRef]
- Morisada, S.; Rin, T.; Ogata, T.; Kim, Y.H.; Nakano, Y. Adsorption removal of boron in aqueous solutions by amine-modified tannin gel. Water Res. 2011, 45, 4028–4034. [Google Scholar] [CrossRef] [PubMed]
- Braghiroli, F.L.; Fierro, V.; Izquierdo, M.T.; Parmentier, J.; Pizzi, A.; Celzard, A. Nitrogen-doped carbon materials produced from hydrothermally treated tannin. Carbon 2012, 50, 5411–5420. [Google Scholar] [CrossRef]
- Selmi, T.; Sanchez-Sanchez, A.; Gadonneix, P.; Jagiello, J.; Seffen, M.; Sammouda, H.; Celzard, A.; Fierro, V. Tetracycline removal with activated carbons produced by hydrothermal carbonisation of Agave americana fibres and mimosa tannin. Ind. Crop. Prod. 2018, 115, 146–157. [Google Scholar] [CrossRef]
- Liu, C.; Zhang, Y.; Li, X.; Luo, J.; Gao, Q.; Li, J. A high-performance bio-adhesive derived from soy protein isolate and condensed tannins. Rsc Adv. 2017, 7, 21226–21233. [Google Scholar] [CrossRef]
- Xi, X.; Pizzi, A.; Frihart, C.R.; Lorenz, L.; Gerardin, C. Tannin plywood bioadhesives with non-volatile aldehydes generation by specific oxidation of mono-and disaccharides. Int. J. Adhes. Adhes. 2019, 98, 102499. [Google Scholar] [CrossRef]
- Ping, L.; Brosse, N.; Chrusciel, L.; Navarrete, P.; Pizzi, A. Extraction of condensed tannins from grape pomace for use as wood adhesives. Ind. Crop. Prod. 2011, 33, 253–257. [Google Scholar] [CrossRef]
- Li, J.; Zhu, W.; Zhang, S.; Gao, Q.; Xia, C.; Zhang, W.; Li, J. Depolymerization and characterization of Acacia mangium tannin for the preparation of mussel-inspired fast-curing tannin-based phenolic resins. Chem. Eng. J. 2019, 370, 420–431. [Google Scholar] [CrossRef]
- Xia, Z.; Kiratitanavit, W.; Facendola, P.; Thota, S.; Yu, S.; Kumar, J.; Mosurkal, R.; Nagarajan, R. Fire resistant polyphenols based on chemical modification of bio-derived tannic acid. Polym. Degrad. Stab. 2018, 153, 227–243. [Google Scholar] [CrossRef]
- Laoutid, F.; Karaseva, V.; Costes, L.; Brohez, S.; Mincheva, R.; Dubois, P. Novel bio-based flame retardant systems derived from tannic acid. J. Renew. Mater. 2018, 6, 559–572. [Google Scholar] [CrossRef]
- Nardeli, J.V.; Fugivara, C.S.; Pinto, E.R.P.; Polito, W.L.; Messaddeq, Y.; José Lima Ribeiro, S.; Benedetti, A.V. Preparation of Polyurethane Monolithic Resins and Modification with a Condensed Tannin-Yielding Self-Healing Property. Polymers 2019, 11, 1890. [Google Scholar] [CrossRef] [PubMed]
- Nardeli, J.V.; Fugivara, C.S.; Taryba, M.; Montemor, M.F.; Ribeiro, S.J.; Benedetti, A.V. Novel healing coatings based on natural-derived polyurethane modified with tannins for corrosion protection of AA2024-T3. Corros. Sci. 2020, 162, 108213. [Google Scholar] [CrossRef]
- Nardeli, J.V.; Fugivara, C.S.; Taryba, M.; Pinto, E.R.; Montemor, M.F.; Benedetti, A.V. Tannin: A natural corrosion inhibitor for aluminum alloys. Prog. Org. Coat. 2019, 135, 368–381. [Google Scholar] [CrossRef]
- Tondi, G.; Zhao, W.; Pizzi, A.; Du, G.; Fierro, V.; Celzard, A. Tannin-based rigid foams: A survey of chemical and physical properties. Bioresour. Technol. 2009, 100, 5162–5169. [Google Scholar] [CrossRef]
- Li, X.; Pizzi, A.; Cangemi, M.; Fierro, V.; Celzard, A. Flexible natural tannin-based and protein-based biosourced foams. Ind. Crop. Prod. 2012, 37, 389–393. [Google Scholar] [CrossRef]
- Grishechko, L.I.; Amaral-Labat, G.; Szczurek, A.; Fierro, V.; Kuznetsov, B.N.; Pizzi, A.; Celzard, A. New tannin-lignin aerogels. Ind. Crop. Prod. 2013, 41, 347–355. [Google Scholar] [CrossRef]
- Santiago-Medina, F.J.; Tenorio-Alfonso, A.; Delgado-Sánchez, C.; Basso, M.C.; Pizzi, A.; Celzard, A.; Fierro, V.; Sánchez, M.C.; Franco, J.M. Projectable tannin foams by mechanical and chemical expansion. Ind. Crop. Prod. 2018, 120, 90–96. [Google Scholar] [CrossRef]
- Zhao, W.; Pizzi, A.; Fierro, V.; Du, G.; Celzard, A. Effect of composition and processing parameters on the characteristics of tannin-based rigid foams. Part I Cell Struct. Mater. Chem. Phys. 2010, 122, 175–182. [Google Scholar] [CrossRef]
- Lacoste, C.; Basso, M.C.; Pizzi, A.; Laborie, M.P.; Garcia, D.; Celzard, A. Bioresourced pine tannin/furanic foams with glyoxal and glutaraldehyde. Ind. Crop. Prod. 2013, 45, 401–405. [Google Scholar] [CrossRef]
- Basso, M.C.; Pizzi, A.; Lacoste, C.; Delmotte, L.; Al-Marzouki, F.M.; Abdalla, S.; Celzard, A. MALDI-TOF and 13C NMR analysis of tannin-furanic-polyurethane foams adapted for industrial continuous lines application. Polymers 2014, 6, 2985–3004. [Google Scholar] [CrossRef]
- Lacoste, C.; Pizzi, A.; Basso, M.C.; Laborie, M.P.; Celzard, A. Pinus pinaster tannin/furanic foams: PART I. Formulation. Ind. Crop. Prod. 2014, 52, 450–456. [Google Scholar] [CrossRef]
- Lacoste, C.; Basso, M.C.; Pizzi, A.; Celzard, A.; Ebang, E.E.; Gallon, N.; Charrier, B. Pine (P. pinaster) and quebracho (S. lorentzii) tannin-based foams as green acoustic absorbers. Ind. Crop. Prod. 2015, 67, 70–73. [Google Scholar] [CrossRef]
- Amaral-Labat, G.; Grishechko, L.; Szczurek, A.; Fierro, V.; Pizzi, A.; Kuznetsov, B.; Celzard, A. Highly mesoporous organic aerogels derived from soy and tannin. Green Chem. 2012, 14, 3099–3106. [Google Scholar] [CrossRef]
- Merle, J.; Birot, M.; Deleuze, H.; Mitterer, C.; Carré, H.; Charrier-El Bouhtoury, F. New biobased foams from wood byproducts. Mater. Des. 2016, 91, 186–192. [Google Scholar] [CrossRef]
- Basso, M.C.; Giovando, S.; Pizzi, A.; Pasch, H.; Pretorius, N.; Delmotte, L.; Celzard, A. Flexible-elastic copolymerized polyurethane-tannin foams. J. Appl. Polym. Sci. 2014, 131, 40499. [Google Scholar] [CrossRef]
- Li, X.; Essawy, H.A.; Pizzi, A.; Delmotte, L.; Rode, K.; Le Nouen, D.; Fierro, V.; Celzard, A. Modification of tannin based rigid foams using oligomers of a hyperbranched poly (amine-ester). J. Polym. Res. 2012, 19, 21. [Google Scholar] [CrossRef]
- Santiago-Medina, F.J.; Delgado-Sánchez, C.; Basso, M.C.; Pizzi, A.; Fierro, V.; Celzard, A. Mechanically blown wall-projected tannin-based foams. Ind. Crop. Prod. 2018, 113, 316–323. [Google Scholar] [CrossRef]
- Szczurek, A.; Fierro, V.; Pizzi, A.; Stauber, M.; Celzard, A. A new method for preparing tannin-based foams. Ind. Crop. Prod. 2014, 54, 40–53. [Google Scholar] [CrossRef]
- Kihara, N.; Endo, T. Synthesis and properties of poly (hydroxyurethane)s. J. Polym. Sci. Part A Polym. Chem. 1993, 31, 2765–2773. [Google Scholar] [CrossRef]
- Kihara, N.; Kushida, Y.; Endo, T. Optically active poly (hydroxyurethane)s derived from cyclic carbonate and L-lysine derivatives. J. Polym. Sci. Part A Polym. Chem. 1996, 34, 2173–2179. [Google Scholar] [CrossRef]
- Kim, M.R.; Kim, H.S.; Ha, C.S.; Park, D.W.; Lee, J.K. Syntheses and thermal properties of poly (hydroxy) urethanes by polyaddition reaction of bis (cyclic carbonate) and diamines. J. Appl. Polym. Sci. 2001, 81, 2735–2743. [Google Scholar] [CrossRef]
- Tomita, H.; Sanda, F.; Endo, T. Model reaction for the synthesis of polyhydroxyurethanes from cyclic carbonates with amines: Substituent effect on the reactivity and selectivity of ring-opening direction in the reaction of five-membered cyclic carbonates with amine. J. Polym. Sci. Part A Polym. Chem. 2001, 39, 3678–3685. [Google Scholar] [CrossRef]
- Rokicki, G.; Piotrowska, A. A new route to polyurethanes from ethylene carbonate, diamines and diols. Polymer 2002, 43, 2927–2935. [Google Scholar] [CrossRef]
- Ubaghs, L.; Fricke, N.; Keul, H.; Höcker, H. Polyurethanes with pendant hydroxyl groups: Synthesis and characterization. Macromol. Rapid Commun. 2004, 25, 517–521. [Google Scholar] [CrossRef]
- Ochiai, B.; Inoue, S.; Endo, T. Salt effect on polyaddition of bifunctional cyclic carbonate and diamine. J. Polym. Sci. Part A Polym. Chem. 2005, 43, 6282–6286. [Google Scholar] [CrossRef]
- Birukov, O.; Potashnikova, R.; Leykin, A.; Figovsky, O.; Shapovalov, L. Advantages in chemistry and technology of non-isocyanate polyurethane. J. Sci. Isr. Technol. Adv. 2014, 11, 160–167. [Google Scholar]
- Boyer, A.; Cloutet, E.; Tassaing, T.; Gadenne, B.; Alfos, C.; Cramail, H. Solubility in CO2 and carbonation studies of epoxidized fatty acid diesters: towards novel precursors for polyurethane synthesis. Green Chem. 2010, 12, 2205–2213. [Google Scholar] [CrossRef]
- Besse, V.; Auvergne, R.; Carlotti, S.; Boutevin, G.; Otazaghine, B.; Caillol, S.; Pascault, J.-P.; Boutevin, B. Synthesis of isosorbide based polyurethanes: An isocyanate free method. React. Funct. Polym. 2013, 73, 588–594. [Google Scholar] [CrossRef]
- Fleischer, M.; Blattmann, H.; Mülhaupt, R. Glycerol-, pentaerythritol-and trimethylolpropane-based polyurethanes and their cellulose carbonate composites prepared via the non-isocyanate route with catalytic carbon dioxide fixation. Green Chem. 2013, 15, 934–942. [Google Scholar] [CrossRef]
- Nohra, B.; Candy, L.; Blanco, J.F.; Guerin, C.; Raoul, Y.; Mouloungui, Z. From petrochemical polyurethanes to biobased polyhydroxyurethanes. Macromolecules 2013, 46, 3771–3792. [Google Scholar] [CrossRef]
- Annunziata, L.; Diallo, A.K.; Fouquay, S.; Michaud, G.; Simon, F.; Brusson, J.M.; Carpentier, J.F.; Guillaume, S.M. α, ω-Di (glycerol carbonate) telechelic polyesters and polyolefins as precursors to polyhydroxyurethanes: An isocyanate-free approach. Green Chem. 2014, 16, 1947–1956. [Google Scholar] [CrossRef]
- Blattmann, H.; Fleischer, M.; Bähr, M.; Mülhaupt, R. Isocyanate-and phosgene-free routes to polyfunctional cyclic carbonates and green polyurethanes by fixation of carbon dioxide. Macromol. Rapid Commun. 2014, 35, 1238–1254. [Google Scholar] [CrossRef]
- Camara, F.; Benyahya, S.; Besse, V.; Boutevin, G.; Auvergne, R.; Boutevin, B.; Caillol, S. Reactivity of secondary amines for the synthesis of non-isocyanate polyurethanes. Eur. Polym. J. 2014, 55, 17–26. [Google Scholar] [CrossRef]
- Cornille, A.; Auvergne, R.; Figovsky, O.; Boutevin, B.; Caillol, S. A perspective approach to sustainable routes for non-isocyanate polyurethanes. Eur. Polym. J. 2017, 87, 535–552. [Google Scholar] [CrossRef]
- Thébault, M.; Pizzi, A.; Dumarçay, S.; Gerardin, P.; Fredon, E.; Delmotte, L. Polyurethanes from hydrolysable tannins obtained without using isocyanates. Ind. Crop. Prod. 2014, 59, 329–336. [Google Scholar] [CrossRef]
- Thébault, M.; Pizzi, A.; Essawy, H.A.; Barhoum, A.; Van Assche, G. Isocyanate free condensed tannin-based polyurethanes. Eur. Polym. J. 2015, 67, 513–526. [Google Scholar] [CrossRef]
- Thébault, M.; Pizzi, A.; Santiago-Medina, F.J.; Al-Marzouki, F.M.; Abdalla, S. Isocyanate-free polyurethanes by coreaction of condensed tannins with aminated tannins. J. Renew. Mater. 2017, 5, 21–29. [Google Scholar] [CrossRef]
- Xi, X.; Pizzi, A.; Delmotte, L. Isocyanate-free polyurethane coatings and adhesives from mono-and di-saccharides. Polymers 2018, 10, 402. [Google Scholar] [CrossRef] [PubMed]
- Xi, X.; Pizzi, A.; Gerardin, C.; Lei, H.; Chen, X.; Amirou, S. Preparation and Evaluation of Glucose Based Non-Isocyanate Polyurethane Self-Blowing Rigid Foams. Polymers 2019, 11, 1802. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Zhang, A.; Zhang, S.; Gao, Q.; Zhang, W.; Li, J. Larch tannin-based rigid phenolic foam with high compressive strength, low friability, and low thermal conductivity reinforced by cork powder. Compos. Part B Eng. 2019, 156, 368–377. [Google Scholar] [CrossRef]
- Santos, O.S.H.; da Silva, M.C.; Silva, V.R.; Mussel, W.N.; Yoshida, M.I. Polyurethane foam impregnated with lignin as a filler for the removal of crude oil from contaminated water. J. Hazard. Mater. 2017, 324, 406–413. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Qi, Y.; Yue, G.; Wu, Q.; Li, Y.; Zhang, M.; Guo, X.; Li, X.; Ma, L.; Li, S. Solvent-and catalyst-free synthesis of an azine-linked covalent organic framework and the induced tautomerization in the adsorption of U(VI) and Hg(II). Green Chem. 2019, 21, 649–657. [Google Scholar] [CrossRef]
- Zhou, F.; Zhang, T.; Zou, B.; Hu, W.; Wang, B.; Zhan, J.; Ma, C.; Hu, Y. Synthesis of a novel liquid phosphorus-containing flame retardant for flexible polyurethane foam: Combustion behaviors and thermal properties. Polym. Degrad. Stab. 2020, 171, 109029. [Google Scholar] [CrossRef]
- Lacoste, C.; Čop, M.; Kemppainen, K.; Giovando, S.; Pizzi, A.; Laborie, M.P.; Sernek, M.; Celzard, A. Biobased foams from condensed tannin extracts from Norway spruce (Picea abies) bark. Ind. Crop. Prod. 2015, 73, 144–153. [Google Scholar] [CrossRef]
- Pizzi, A.; Tekely, P. Mechanism of polyphenolic tannin resin hardening by hexamethylenetetramine: CP-MAS 13C-NMR. J. Appl. Polym. Sci. 1995, 56, 1645–1650. [Google Scholar] [CrossRef]
- Pichelin, F.; Kamoun, C.; Pizzi, A. Hexamine hardener behaviour: effects on wood glueing, tannin and other wood adhesives. Holz Als Roh-Und Werkst. 1999, 57, 305–317. [Google Scholar] [CrossRef]
- Kamoun, C.; Pizzi, A. Mechanism of hexamine as a non-aldehyde polycondensation resins hardener. Part 1: Hexamine decomposition and reactive intermediates. Holzforsch. Holzverwert. 2000, 52, 16–19. [Google Scholar]
- Kamoun, C.; Pizzi, A. Mechanism of hexamine as a non-aldehyde polycondensation hardener. Part 2: Recomposition of intermediate reactive compounds. Holzforsch. Holzverwert. 2000, 52, 66–67. [Google Scholar]
- Kamoun, C.; Pizzi, A.; Zanetti, M. Upgrading of MUF resins by buffering additives-Part 1: Hexamine sulphate effect and its limits. J. Appl. Polym. Sci. 2003, 90, 203–214. [Google Scholar] [CrossRef]
- Tondi, G. Tannin-based copolymer resins: Synthesis and characterization by solid state 13C NMR and FT-IR spectroscopy. Polymers 2017, 9, 223. [Google Scholar] [CrossRef]
- Basso, M.C.; Pizzi, A.; Maris, J.P.; Delmotte, L.; Colin, B.; Rogaume, Y. MALDI-TOF, 13C NMR and FTIR analysis of the cross-linking reaction of condensed tannins by triethyl phosphate. Ind. Crop. Prod. 2017, 95, 621–631. [Google Scholar] [CrossRef]
- Zhou, X.; Li, B.; Xu, Y.; Essawy, H.; Wu, Z.; Du, G. Tannin-furanic resin foam reinforced with cellulose nanofibers (CNF). Ind. Crop. Prod. 2019, 134, 107–112. [Google Scholar] [CrossRef]
- Zhang, A.; Li, J.; Zhang, S.; Mu, Y.; Zhang, W.; Li, J. Characterization and acid-catalysed depolymerization of condensed tannins derived from larch bark. Rsc. Adv. 2017, 7, 35135–35146. [Google Scholar] [CrossRef]

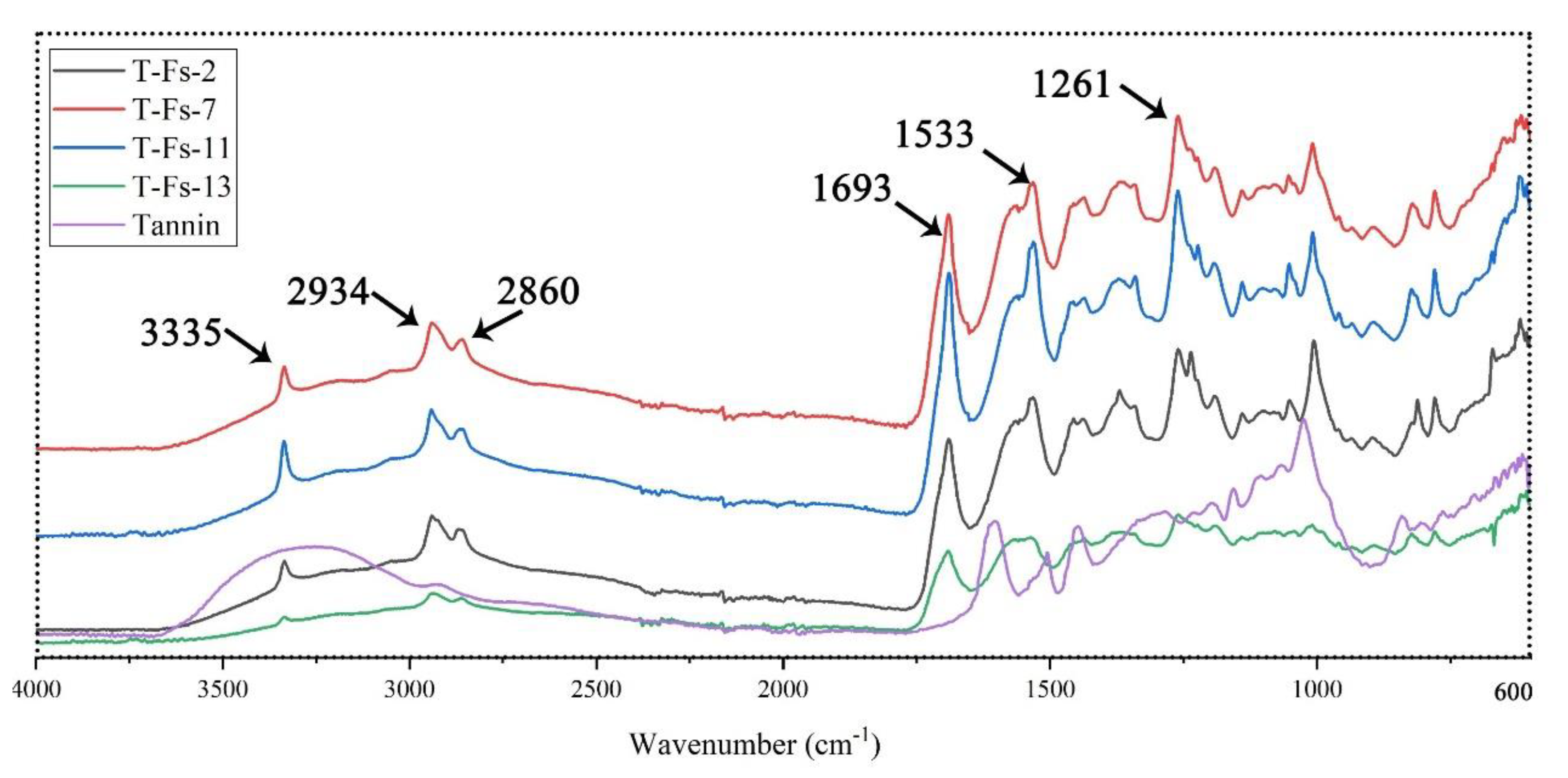
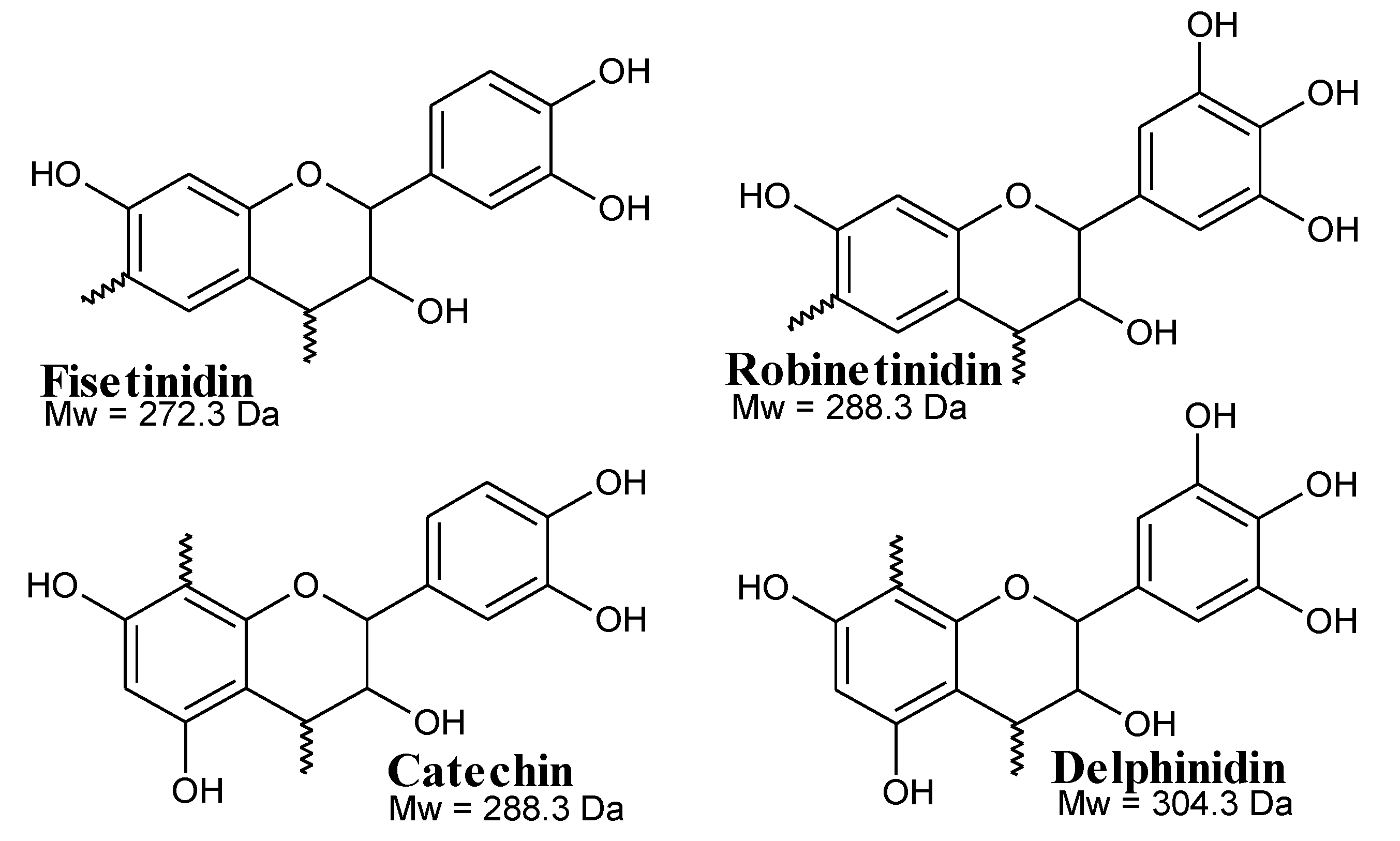
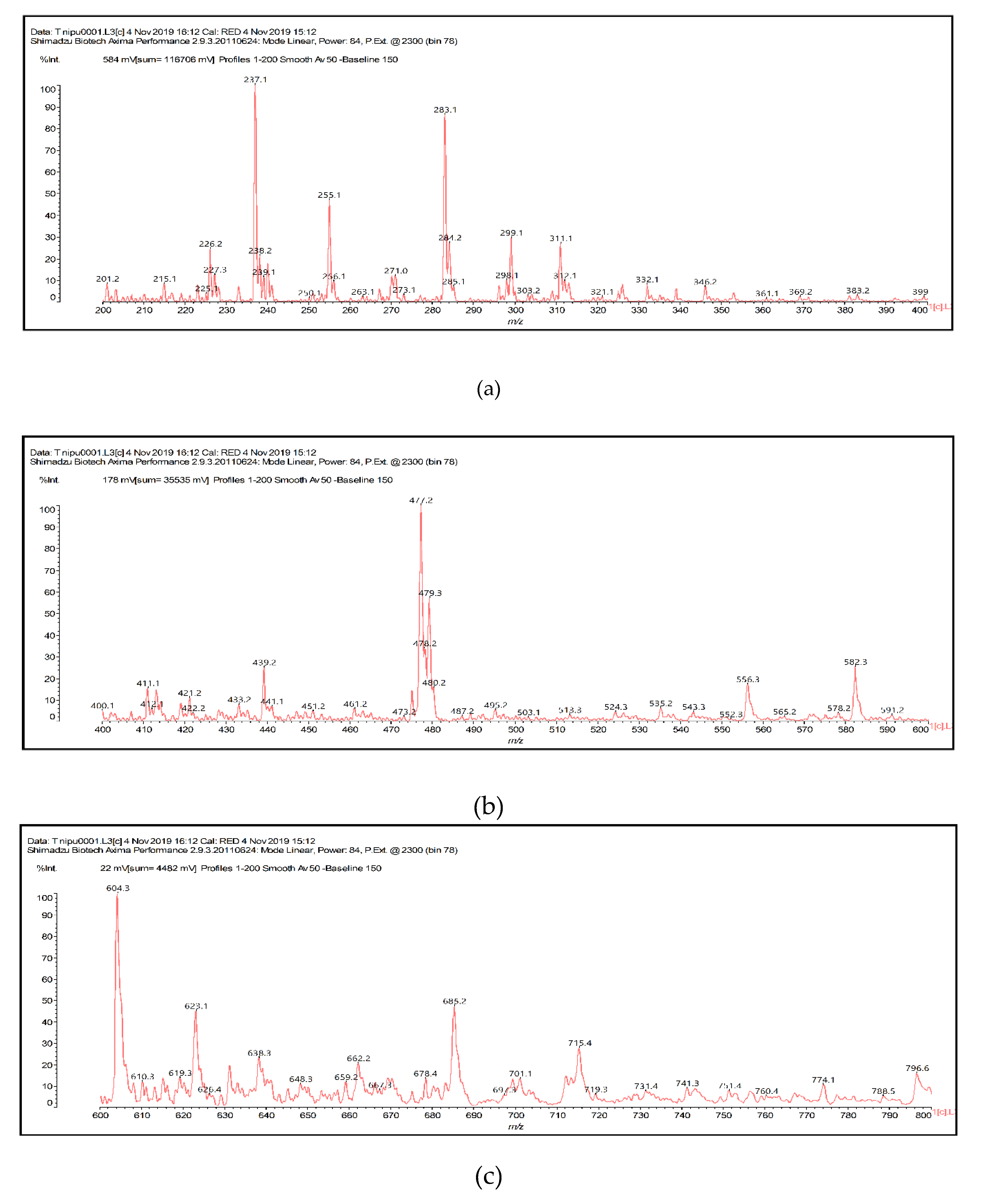
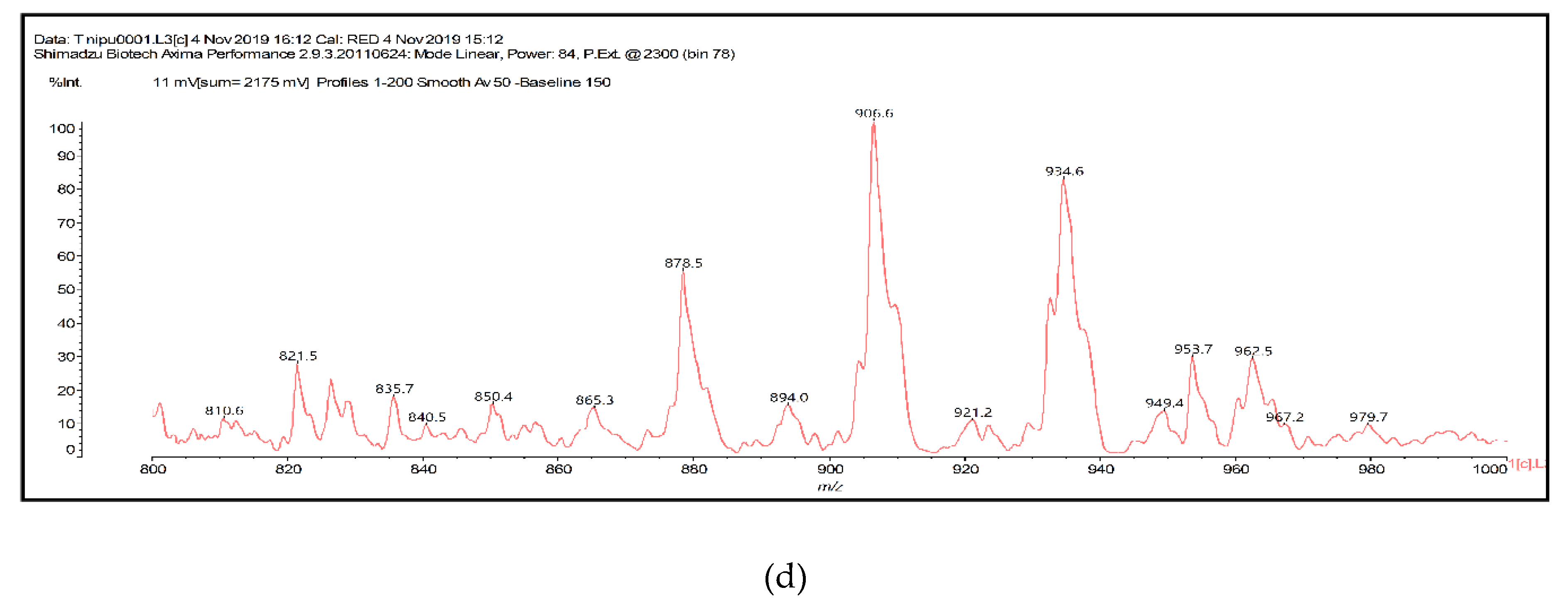


| Foams | Resins (g) | Hexamine (g) | Citric Acid (g) | Glutaraldehyde (g) |
|---|---|---|---|---|
| T-Fs-2 | 10 | 2 | 6 | 2 |
| T-Fs-5 | 10 | 0 | 6 | 2 |
| T-Fs-7 | 10 | 2 | 6 | 4 |
| T-Fs-9 | 10 | 2 | 4 | 0 |
| T-Fs-11 | 10 | 2 | 8 | 2 |
| T-Fs-13 | 10 | 2 | 9 | 3 |
| Foams | Apparent Density (g/cm3) | Compressive Strength (KN) | Specific Compressive Strength (kPa/kg·m−3) |
|---|---|---|---|
| T-Fs-2 | 0.15 ± 0.02 | 0.15 ± 0.02 | 1.62 ± 0.33 |
| T-Fs-5 | - | - | - |
| T-Fs-7 | 0.26 ± 0.04 | 0.57 ± 0.03 | 3.47 ± 0.38 |
| T-Fs-9 | - | - | - |
| T-Fs-11 | 0.12 ± 0.03 | 0.13 ± 0.01 | 1.63 ± 0.24 |
| T-Fs-13 | 0.22 ± 0.02 | 0.32 ± 0.03 | 2.31 ± 0.18 |
| Peak (Da) | Experimental Peak (Da) | Species |
|---|---|---|
| 299.1 | 274.2 | Fi |
| 311.1 | 290.2 + 23 = 313.2 | Ro with Na+ |
| 313.1 | 290.3 + 23 = 313.3 | Citric acid-HDMA |
| 326.2 | 306.2 + 23 = 329.2 | De with Na+ |
| 433.2 | 432.4 | Ro-DMC-HDMA |
| 411.1 | 388.5 + 23 = 411.5 | Citric acid-(HDMA)2 with Na+ |
| 439.3 | 416.4 + 23 = 439.4 | Fi-DMC-HDMA with Na+ |
| 451.2 | 448.4 | De-DMC-HDMA |
| 556.3 | 558.6 | Fi-(DMC)2-2HDMA |
| 578.3 | 576.5 | Fi-CH2-Ro |
| 582.4 | 560.5 + 23 = 583.5 | Fi-CH2-Fi with Na+ |
| 591.3 | 592.5 | Ro-CH2-Ro |
| 604.3 | 606.5 and/or 605.5 | Ro-DMC-HDMA-Citric and/or Ro-CH2NHCH2-Fi |
| 615.4 | 590.6 + 23 = 613.6 and/or 590.5 + 23 = 612.5 and/or 592.5 + 23 = 615.5 | De-(DMC)2-(HDMA)2 and/or Fi-DMC-HDMA-Citric and/or Ro-CH2-Ro with Na+ |
| 611.3 | 589.5 + 23 = 612.5 | Fi-CH2NHCH2-Fi with Na+ |
| 619.5 | 621.5 and/or 622.5 | De-DMC-HDMA-Citric and/or Ro-CH2NHCH2-Ro |
| 623.2 | 624.5 | De-CH2-De |
| 631.3 | 608.5 + 23 = 631.5 | Ro-CH2-De with Na+ |
| 638.4 | 637.5 | Ro-CH2NHCH2-De |
| 649.3 | 648.6 | Fi-CH(–OH)–(CH2)3–CH(–OH)-Fi |
| 662.2 | 664.6 | Ro-CH(–OH)–(CH2)3–CH(–OH)-Fi |
| 683.4 | 680.6 | Ro-CH(–OH)–(CH2)3–CH(–OH)-Ro |
| 699.4 | 696.6 | De-CH(–OH)–(CH2)3–CH(–OH)-Ro |
| 715.4 | 712.6 | De-CH(–OH)–(CH2)3–CH(–OH)-De |
| 741.5 | 720.7 + 23 = 743.7 | De-DMC-(HDMA)2-Citric with Na+ |
| 768.7 | 747.7 + 23 = 770.7 | Fi-CH2NHCH2-Ro-DMC-HDMA with Na+ |
| 796.5 | 795.7 | De-CH2NHCH2-Ro-DMC-HDMA |
| 828.6 | 830.9 | Fi-(DMC)2-(HDMA)3-Citric |
| 821.6 | 822.8 | Ro-CH(–OH)(CH2)3CH(–OH)-Ro-DMC-HDMA |
| 830.1 | 806.8 + 23 = 829.8 | Fi-CH(–OH)(CH2)3CH(–OH)-Ro-DMC-HDMA with Na+ |
| 865.2 | 862.9 | De-(DMC)2-(HDMA)3-Citric |
| 878.6 | 854.8 + 23 = 877.8 and/or 876.8 | De-CH(–OH)(CH2)3CH(–OH)-De-DMC-HDMA with Na+, and/or Fi-CH2-Fi-DMC-HDMA-Citric |
| 894.4 (921.5) | 897 and/or 897 + 23 = 920 | Fi-DMC-HDMA-CH(–OH)(CH2)3CH(–OH)-Fi without and/or with Na+ (920 Da) |
| 906.6 | 905.8 | Fi-CH2NHCH2-Fi-DMC-HDMA-Citric and/or Ro-CH2NHCH2-Ro-CH2-Fi |
| 921.5 | 921.8 | Ro-CH2NHCH2-Fi-DMC-HDMA-Citric and/or Ro-CH2NHCH2-Ro-CH2-Ro and/or De-CH2NHCH2-Ro-CH2-Fi |
| 934.9 | 913 + 23 = 936 | Ro-DMC-HDMA-CH(–OH)(CH2)3CH(–OH)-Fi-DMC-HDMA with Na+ |
| 951 | 829 + 23 = 952 | Ro-DMC-HDMA-CH(–OH)(CH2)3CH(–OH)-Ro-DMC-HDMA with Na+ |
| 954.5 | 953.8 | De-CH2NHCH2-Ro-DMC-HDMA-Citric |
| 963.4 | 937.8 + 23 = 960.8 | Ro-CH2NHCH2-Ro-DMC-HDMA-Citric with Na+ |
| Samples | Tmax (°C) | Residual Mass at 790 °C (%) | ||
|---|---|---|---|---|
| Step Ⅰ | Step Ⅱ | Step Ⅲ | ||
| T-Fs-2 | 119.2 | 214.8 | 444.8 | 16.5 |
| T-Fs-7 | 115.1 | 215.4 | 436.2 | 18.7 |
| T-Fs-11 | 114.8 | 218.6 | 445.4 | 16.4 |
| T-Fs-13 | 112.2 | 216.1 | 437.1 | 17.3 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, X.; Xi, X.; Pizzi, A.; Fredon, E.; Zhou, X.; Li, J.; Gerardin, C.; Du, G. Preparation and Characterization of Condensed Tannin Non-Isocyanate Polyurethane (NIPU) Rigid Foams by Ambient Temperature Blowing. Polymers 2020, 12, 750. https://doi.org/10.3390/polym12040750
Chen X, Xi X, Pizzi A, Fredon E, Zhou X, Li J, Gerardin C, Du G. Preparation and Characterization of Condensed Tannin Non-Isocyanate Polyurethane (NIPU) Rigid Foams by Ambient Temperature Blowing. Polymers. 2020; 12(4):750. https://doi.org/10.3390/polym12040750
Chicago/Turabian StyleChen, Xinyi, Xuedong Xi, Antonio Pizzi, Emmanuel Fredon, Xiaojian Zhou, Jinxing Li, Christine Gerardin, and Guanben Du. 2020. "Preparation and Characterization of Condensed Tannin Non-Isocyanate Polyurethane (NIPU) Rigid Foams by Ambient Temperature Blowing" Polymers 12, no. 4: 750. https://doi.org/10.3390/polym12040750
APA StyleChen, X., Xi, X., Pizzi, A., Fredon, E., Zhou, X., Li, J., Gerardin, C., & Du, G. (2020). Preparation and Characterization of Condensed Tannin Non-Isocyanate Polyurethane (NIPU) Rigid Foams by Ambient Temperature Blowing. Polymers, 12(4), 750. https://doi.org/10.3390/polym12040750








