Synthesis of Poly(2-aminothiazole)-Coated Polystyrene Particles and Their Excellent Hg(II) Adsorption Properties
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of PVP-Functionalized PS Latexes
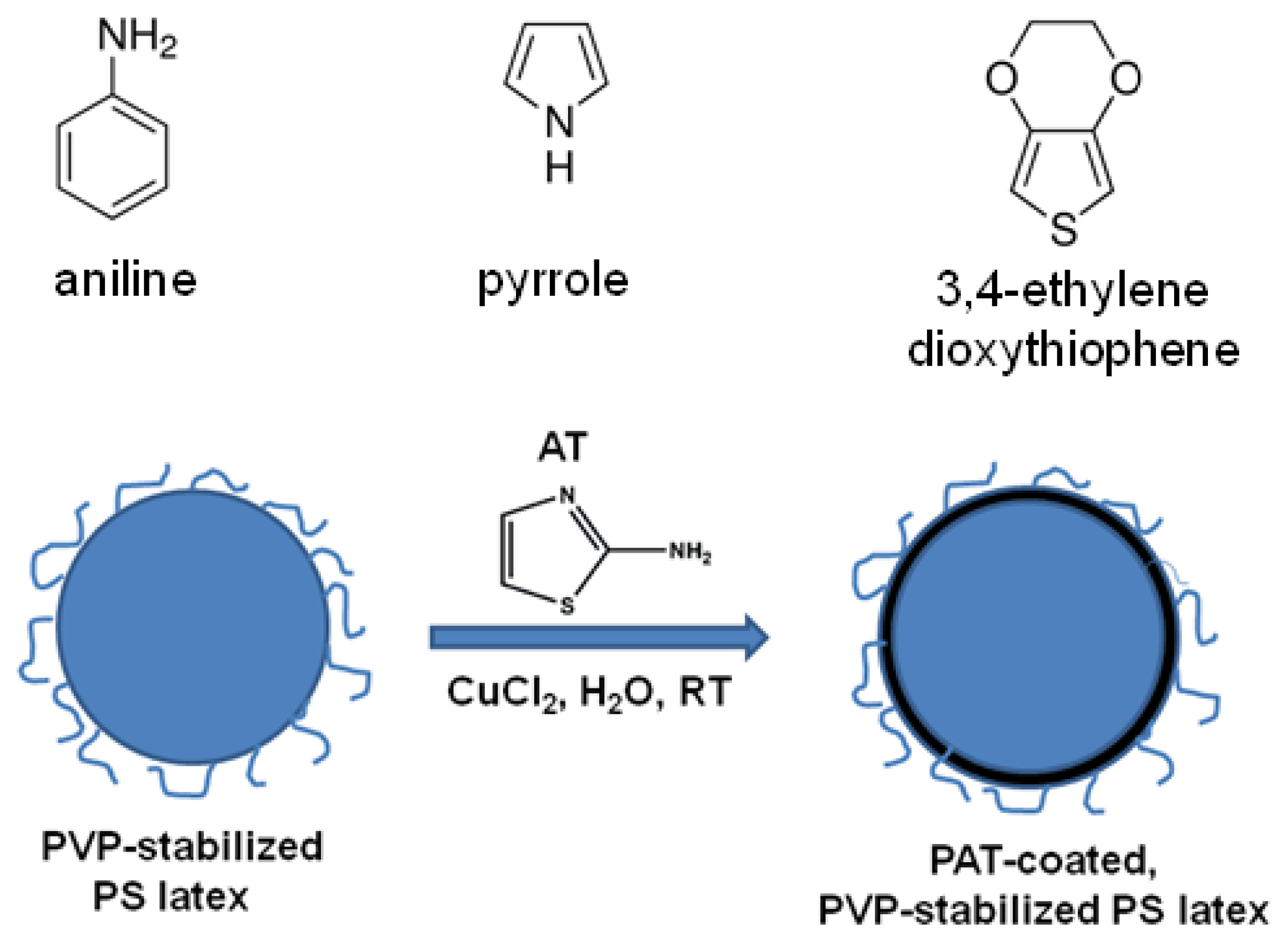
2.3. Preparation of PAT-PS Particles
2.4. Characterization
2.5. Adsorption Experiments
3. Results and Discussion
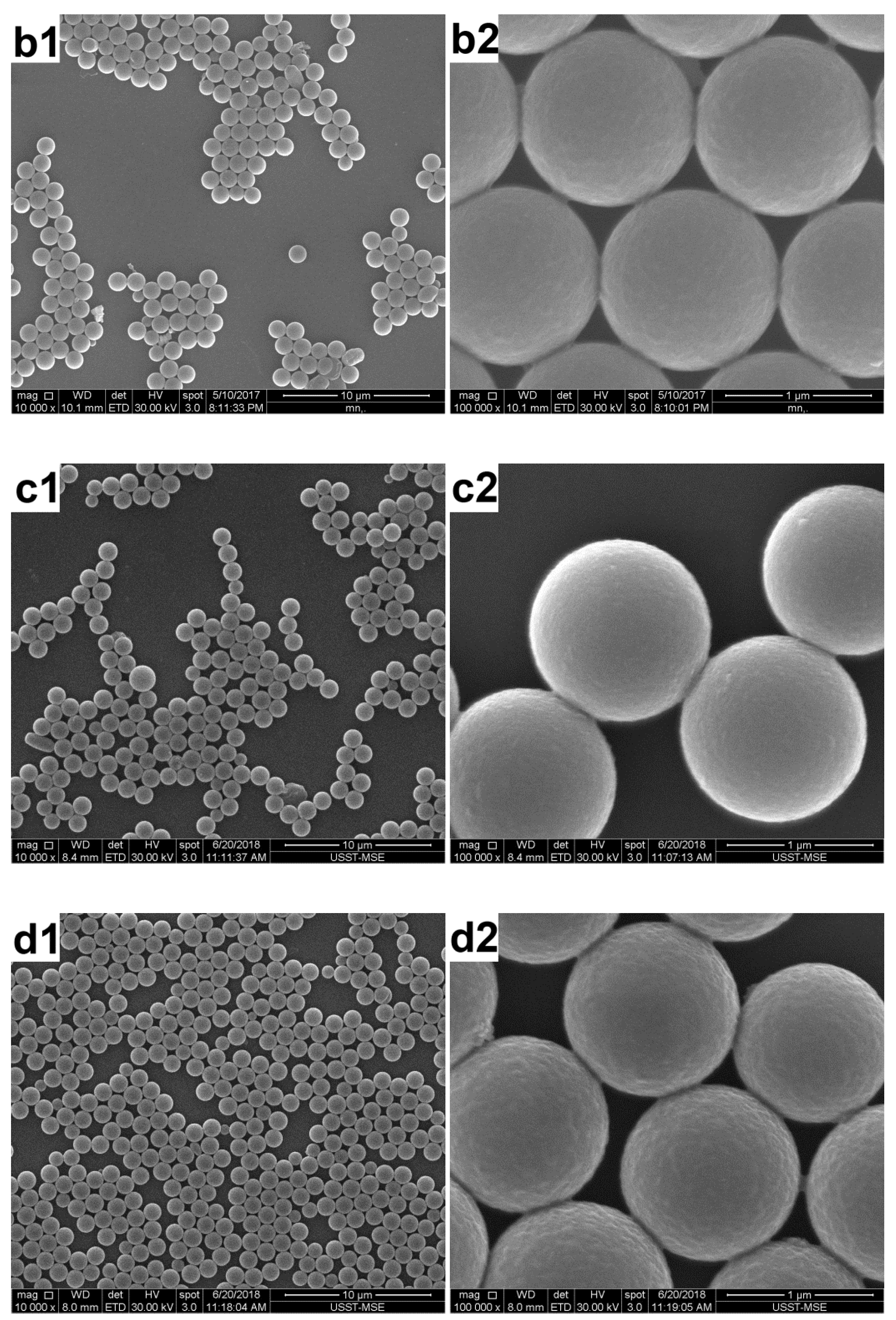
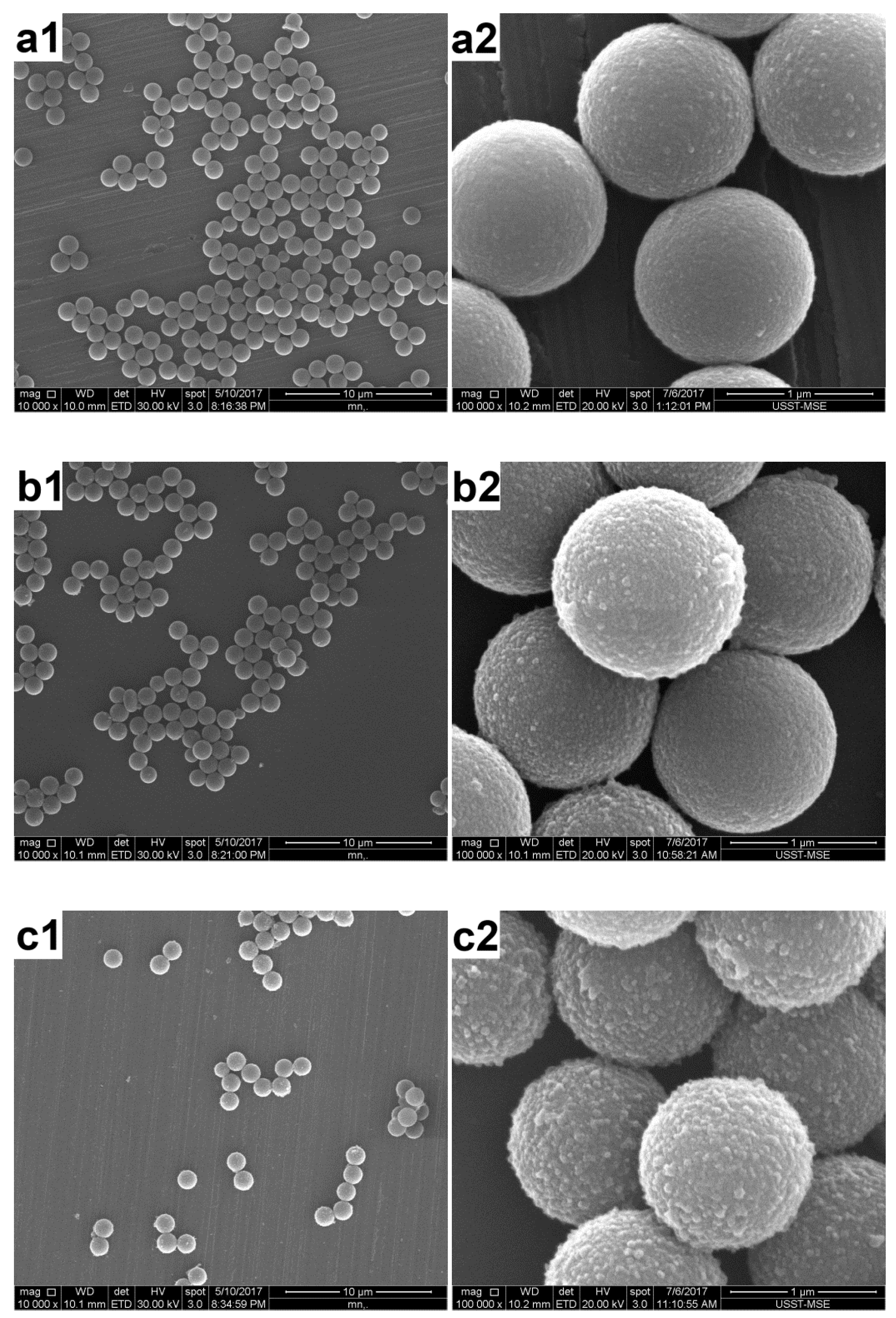
3.1. Effect of the AT Concentration
3.2. Effect of the PS Concentration
3.3. Effect of the Polymerization Temperature
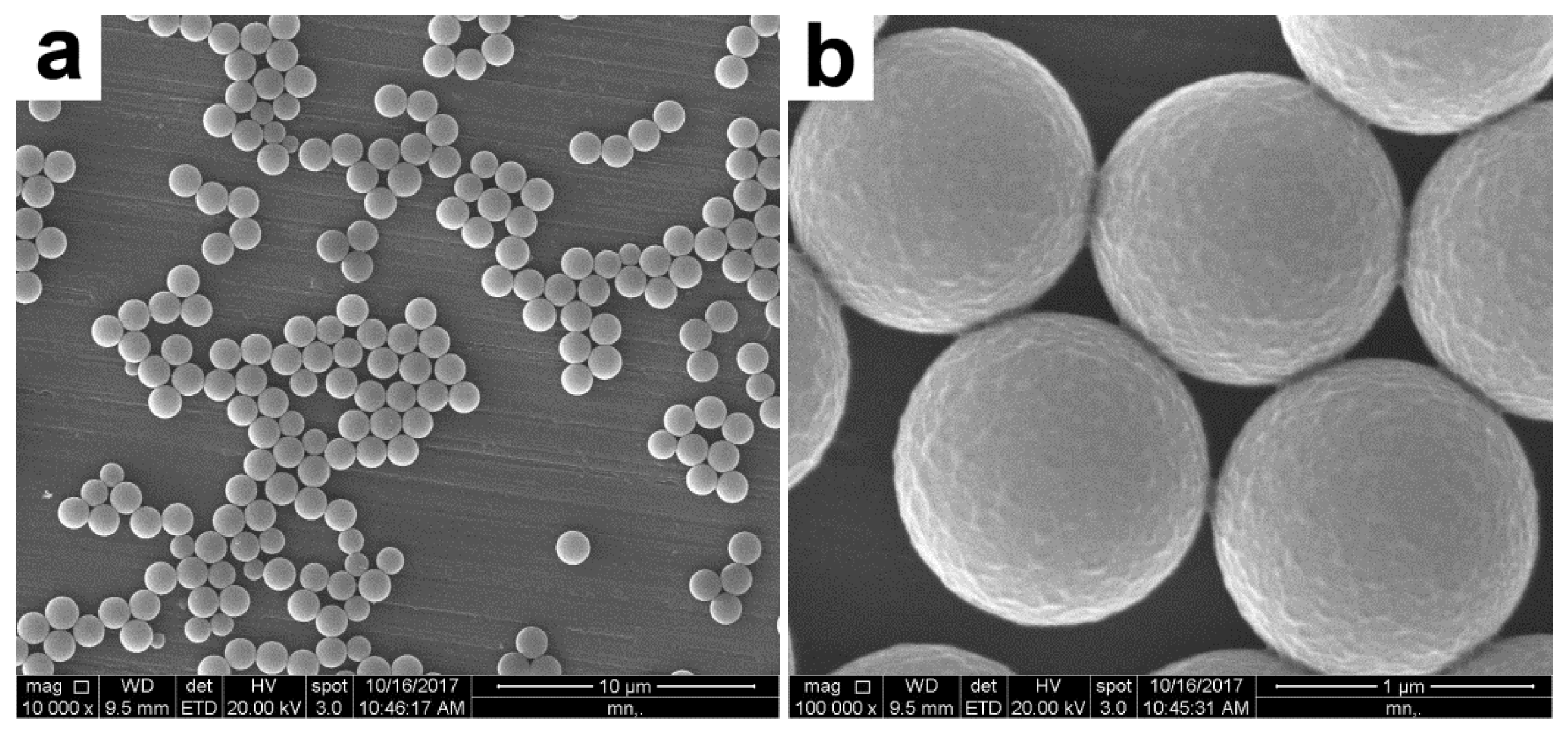
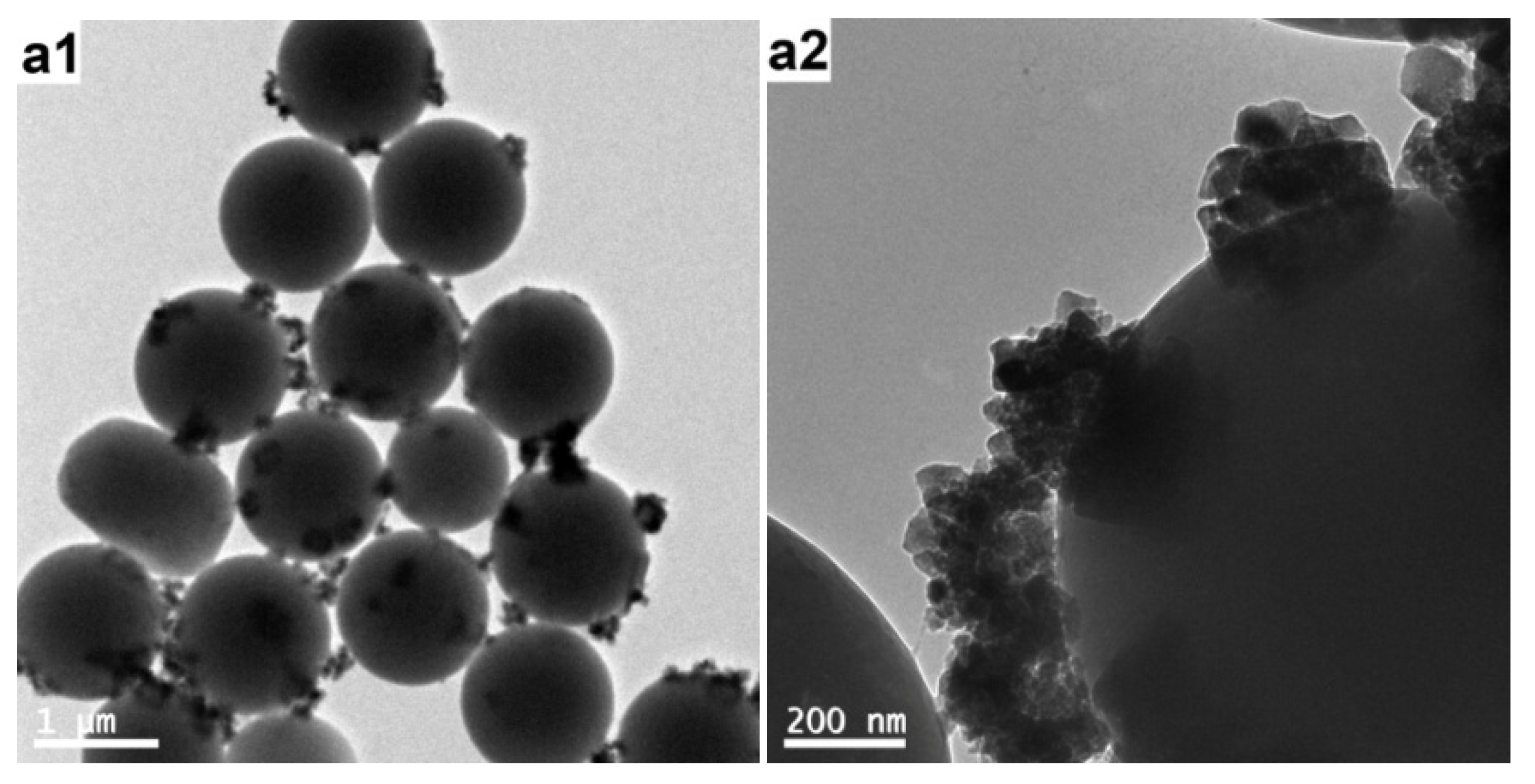
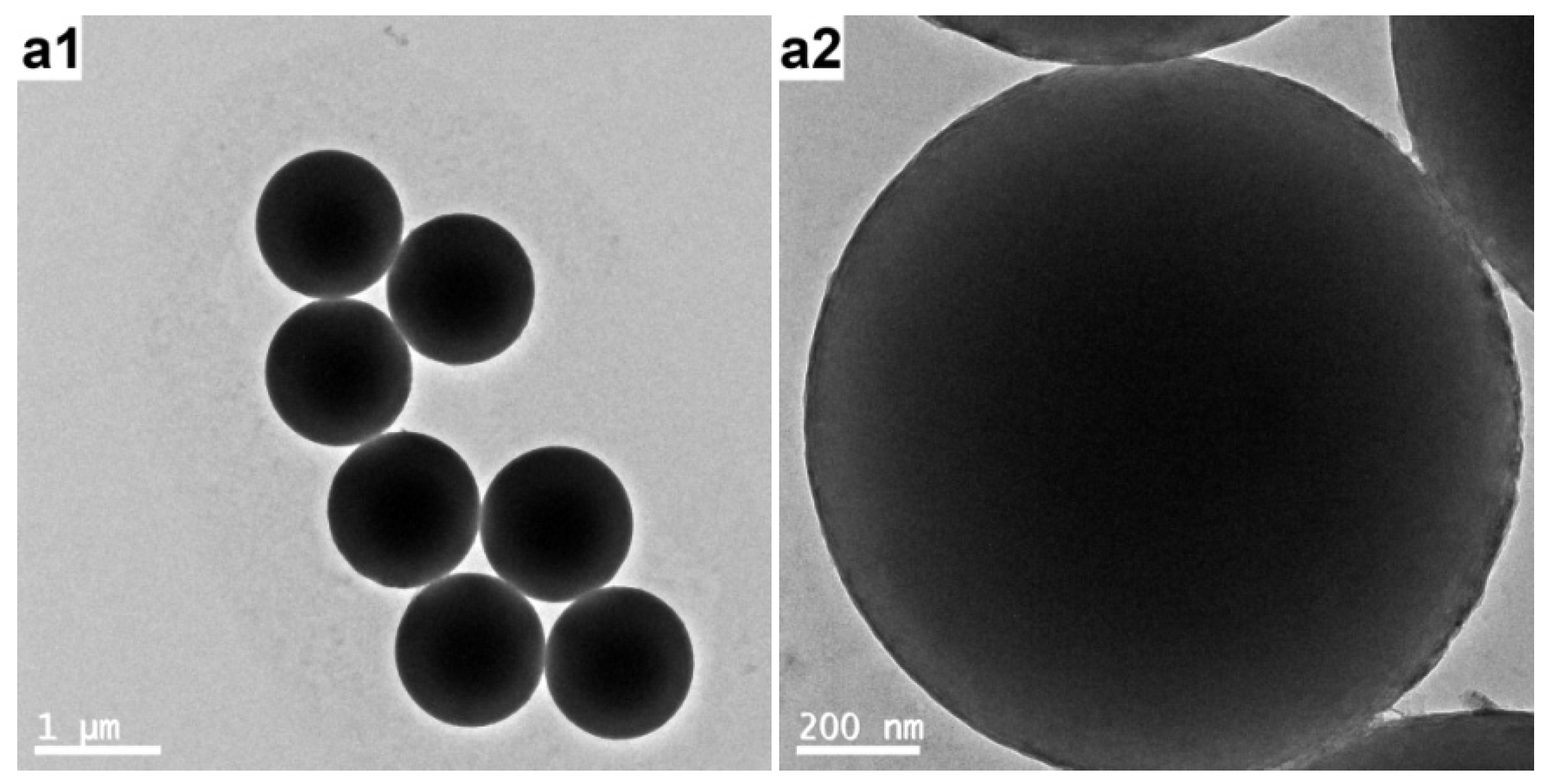
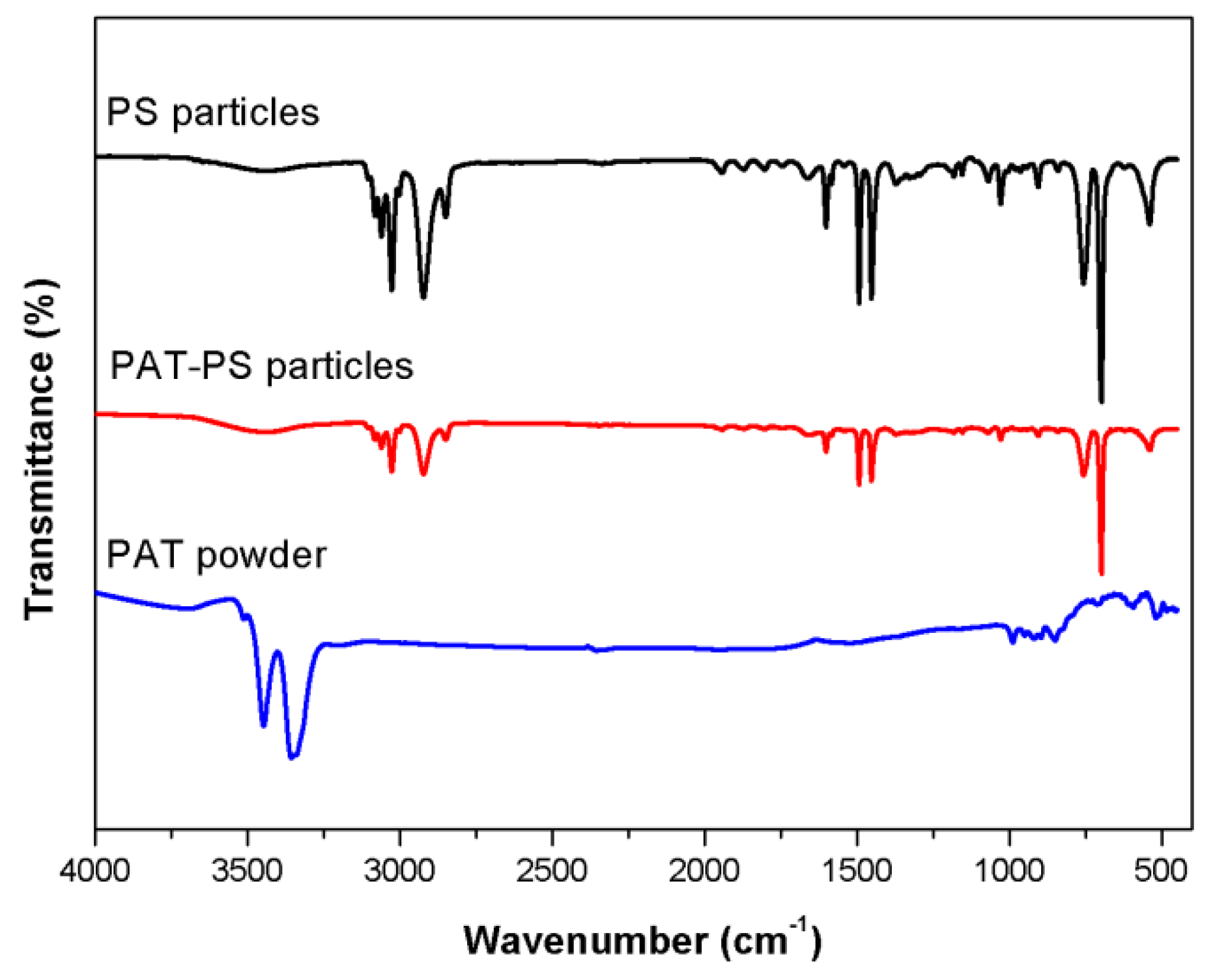
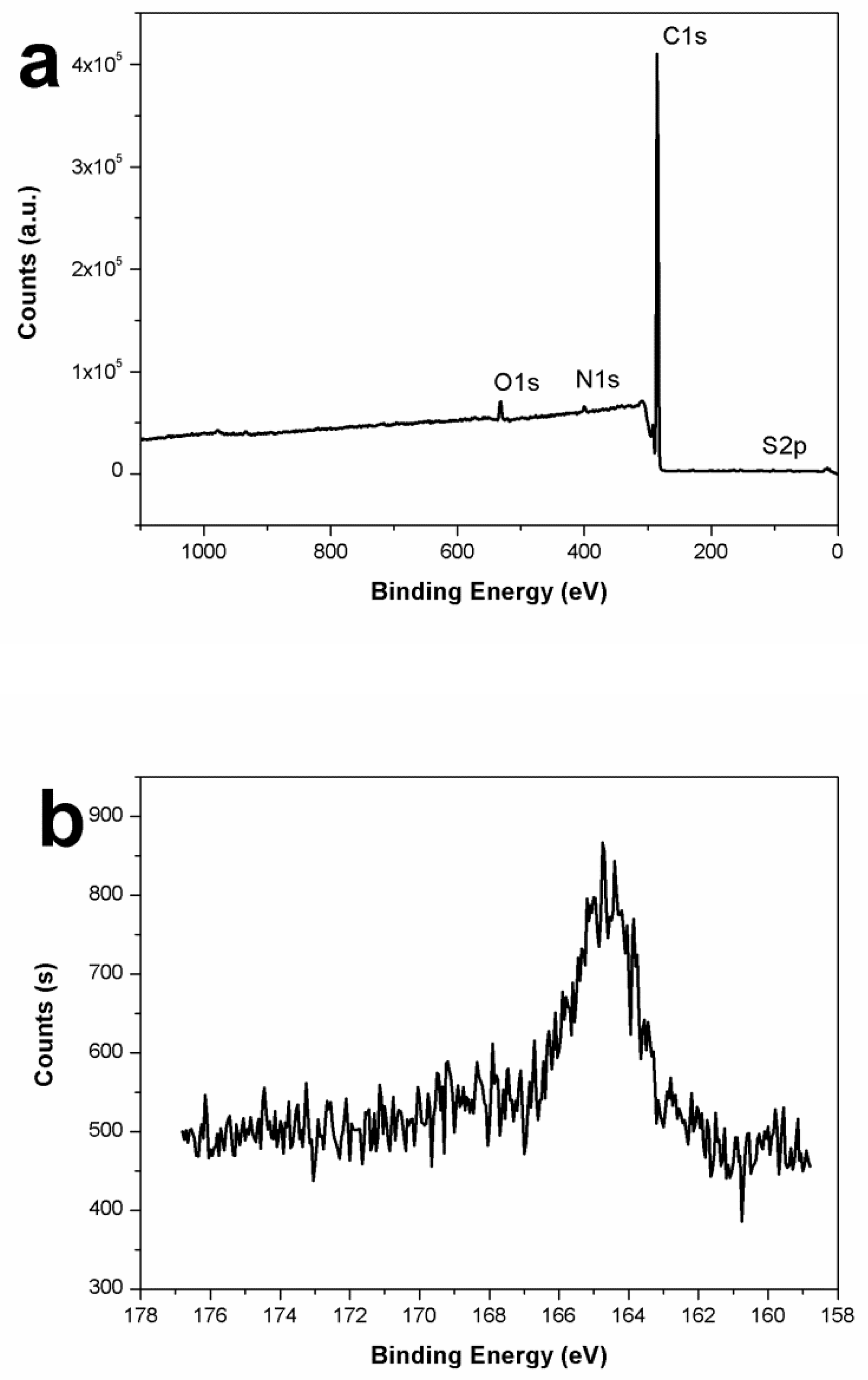
3.4. Characterization of the PAT-PS Particles
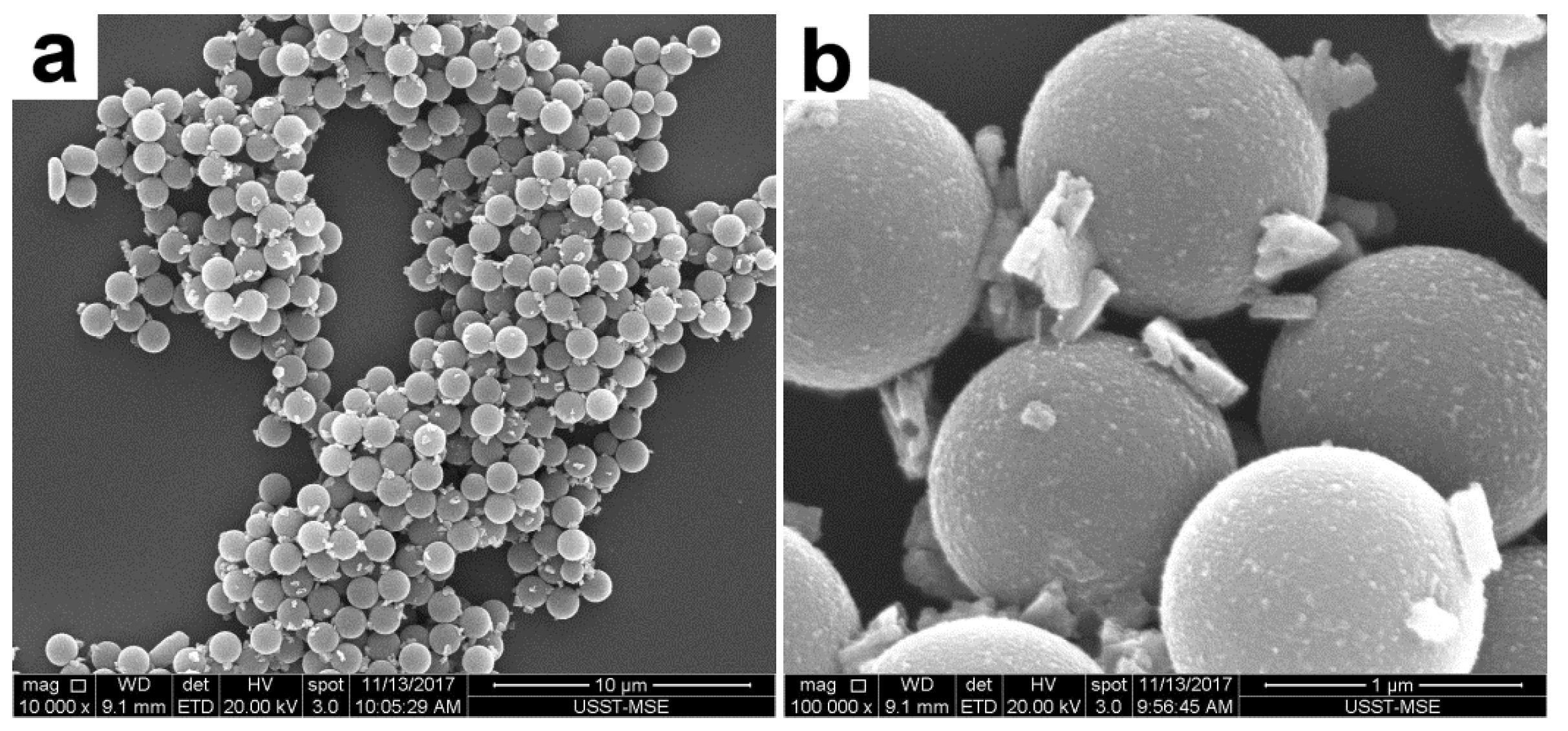
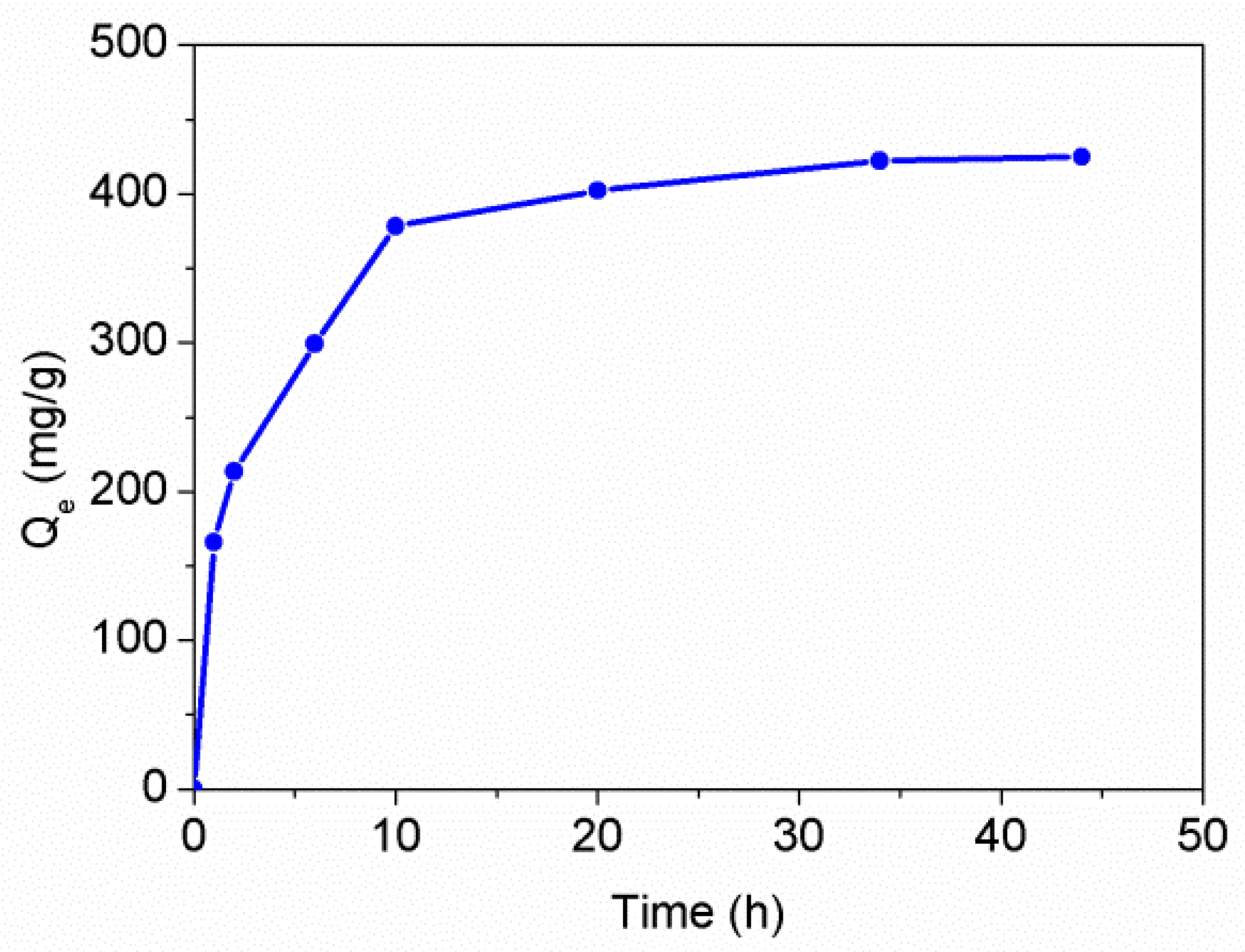
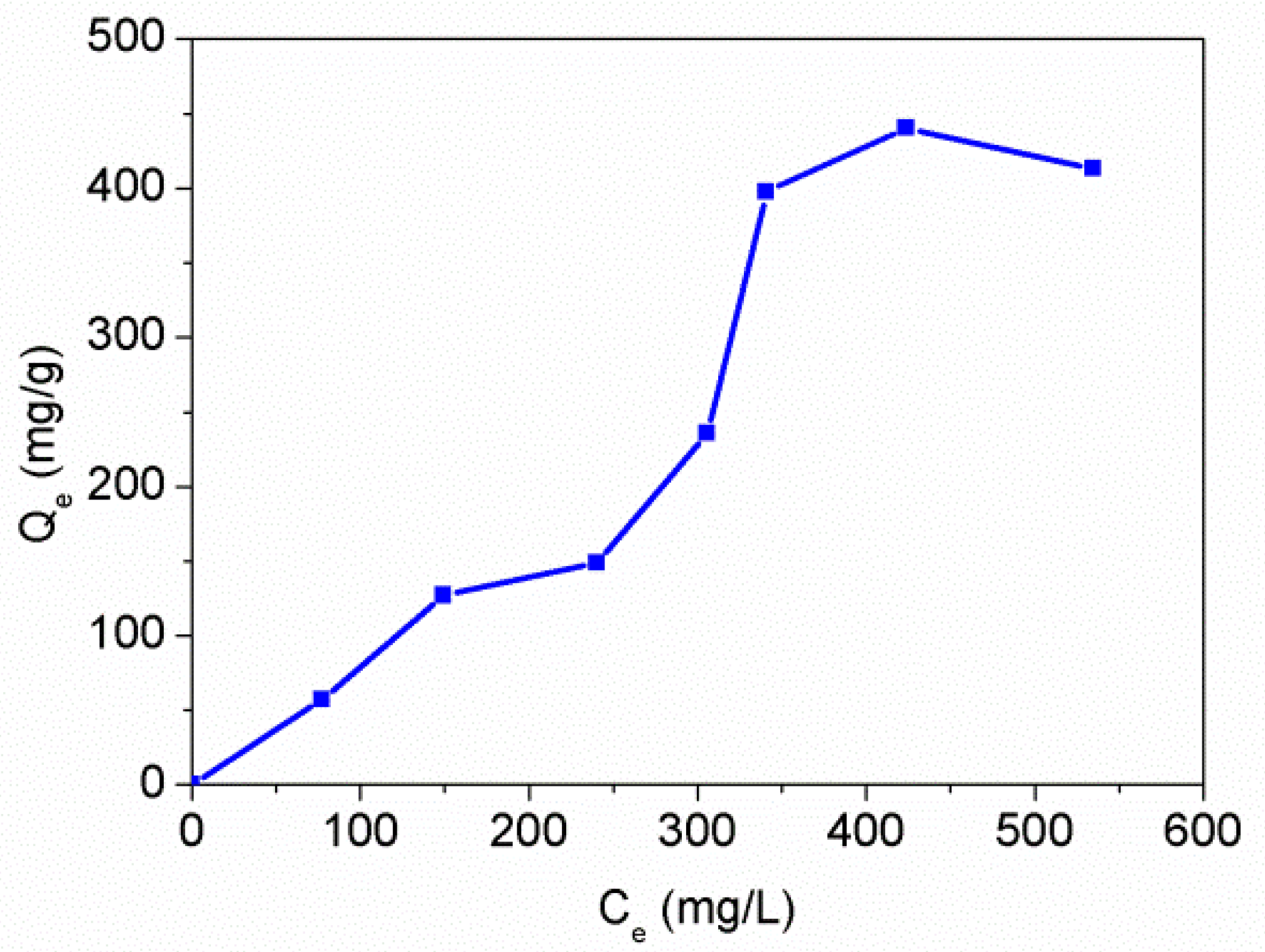
3.5. Adsorption Properties of the PAT-PS Particles
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Fielding, L.A.; Hillier, J.K.; Burchell, M.J.; Armes, S.P. Space science applications for conducting polymer particles: Synthetic mimics for cosmic dust and micrometeorites. Chem. Commun. 2015, 51, 16886–16899. [Google Scholar] [CrossRef] [PubMed]
- Morgan, M.; Fielding, L.A.; Armes, S.P. Synthesis and characterisation of sterically stabilised polypyrrole particles using a chemically reactive poly(vinyl amine)-based stabiliser. Colloid Polym. Sci. 2013, 291, 77–86. [Google Scholar] [CrossRef][Green Version]
- Zou, H.; Wu, S.S.; Shen, J. Polymer/silica nanocomposites: Preparation, characterization, properties, and applications. Chem. Rev. 2008, 108, 3893–3957. [Google Scholar] [CrossRef] [PubMed]
- Roosz, N.; Euvard, M.; Lakard, B.; Buron, C.C.; Martin, N.; Viau, L. Synthesis and characterization of polyaniline-silica composites: Raspberry vs core-shell structures. where do we stand? J. Colloid Interface Sci. 2017, 502, 184–192. [Google Scholar] [CrossRef]
- Zou, H.; Wu, D.; Sun, H.; Chen, S.W.; Wang, X. Poly(2-aminothiazole)-silica nanocomposite particles: Synthesis and morphology control. Appl. Surf. Sci. 2018, 436, 1083–1092. [Google Scholar] [CrossRef]
- Lascelles, S.F.; Armes, S.P. Synthesis and characterization of micrometer-sized polypyrrole-coated polystyrene latexes. Adv. Mater. 1995, 7, 864–866. [Google Scholar] [CrossRef]
- Fujii, S.; Matsuzawa, S.; Nakamura, Y. One-pot synthesis of conducting polymer-coated latex particles: Ammonium persulfate as free radical initiator and chemical oxidant. Chem. Commun. 2010, 46, 7217–7219. [Google Scholar] [CrossRef]
- Takeoka, H.; Hamasaki, H.; Harada, Y.; Nakamura, Y.; Fujii, S. Synthesis and characterization of polypyrrole-platinum nanocomposite-coated latex particles. Colloid Polym. Sci. 2015, 293, 1483–1493. [Google Scholar] [CrossRef]
- Yildirim, M.; Kaya, I. A comparative study of aminothiazole-based polymers synthesized by chemical oxidative polymerization. Synth. Met. 2012, 162, 436–443. [Google Scholar] [CrossRef]
- Ciftci, H.; Testereci, H.N.; Öktem, Z. Ring opening polymerization of 2-aminothiazole with iron(III) chloride. Polym. Bull. 2013, 70, 1895–1909. [Google Scholar] [CrossRef]
- Biyikoglu, M.; Ciftci, H. Chemical synthesis and characterization of soluble conducting poly(2-aminothiazole). Polym. Bull. 2013, 70, 2843–2856. [Google Scholar] [CrossRef]
- Wang, X.; Wang, L.; Zou, H.; Qian, W.; Liao, Y.Z. Simple synthesis of conducting poly(2-aminothiazole) with high molecular weight. Colloid Polym. Sci. 2015, 293, 2027–2034. [Google Scholar] [CrossRef]
- Wang, X.; Lv, P.F.; Zou, H.; Li, Y.; Li, X.Y.; Liao, Y.Z. Synthesis of poly(2-aminothiazole) for selective removal of Hg(II) in aqueous solutions. Ind. Eng. Chem. Res. 2016, 55, 4911–4918. [Google Scholar] [CrossRef]
- Zou, H.; Wang, L.; Wang, X.; Lv, P.F.; Liao, Y.Z. Chemical oxidative polymerization of 2-aminothiazolein aqueous solution: Synthesis, characterization and kinetics study. Polymers 2016, 8, 407. [Google Scholar] [CrossRef] [PubMed]
- Zou, H.; Lv, P.F.; Wang, X.; Wu, D.; Yu, D.G. Electrospun poly (2-aminothiazole)/cellulose acetate fiber membrane for removing Hg(II) from water. J. Appl. Polym. Sci. 2017, 134, 44879. [Google Scholar] [CrossRef]
- Zou, H.; Chen, S.W. Anticorrosion properties of poly(2-aminothiazole)-coated poly(n-butyl methacrylate) films for carbon steel. Prog. Org. Coat. 2020, 139, 105482. [Google Scholar]
- Zou, H.; Wu, W. Revisiting the synthesis of poly(2-aminothiazole) for removal of Hg(II) in aqueous solution. Eur. Polym. J. 2020, 125, 109514. [Google Scholar] [CrossRef]
- Naushad, M.; ALOthman, Z.A.; Awual, M.R.; Alam, M.M.; Eldesoky, G.E. Adsorption kinetics, isotherms, and thermodynamic studies for the adsorption of Pb2+ and Hg2+ metal ions from aqueous medium using Ti(IV) iodovanadate cation exchanger. Ionics 2015, 21, 2237–2245. [Google Scholar] [CrossRef]
- Naushad, M.; ALOthman, Z.A. Separation of toxic Pb2+ metal from aqueous solution using strongly acidic cation-exchange resin: Analytical applications for the removal of metal ions from pharmaceutical formulation. Desalin. Water Treat. 2015, 53, 2158–2166. [Google Scholar] [CrossRef]
- Naushad, M.; Vasudevan, S.; Sharma, G.; Kumar, A.; ALOthman, Z.A. Adsorption kinetics, isotherms, and thermodynamic studies for Hg2+ adsorption from aqueous medium using alizarin red-S-loaded amberlite IRA-400 resin. Desalin. Water Treat. 2016, 57, 18551–18559. [Google Scholar] [CrossRef]
- Mironyuk, I.; Tatarchuk, T.; Naushad, M.; Vasylyeva, H.; Mykytyn, I. Highly efficient adsorption of strontium ions by carbonated mesoporous TiO2. J. Mol. Liq. 2019, 285, 742–753. [Google Scholar] [CrossRef]
- Faisal, A.A.H.; Al-Wakel, S.F.A.; Assi, H.A.; Naji, L.A.; Naushad, M. Waterworks sludge-filter sand permeable reactive barrier for removal of toxic lead ions from contaminated groundwater. J. Water Process. Eng. 2020, 33, 101112. [Google Scholar] [CrossRef]
- Zhao, G.X.; Huang, H.B.; Tang, Z.W.; Huang, Q.F.; Niu, F.L.; Wang, X.K. Polymer-based nanocomposites for heavy metal ions removal from aqueous solution: A review. Polym. Chem. 2018, 9, 3562–3582. [Google Scholar] [CrossRef]
- Zou, H.; Wang, X. Adsorption of silica nanoparticles onto poly(N-vinylpyrrolidone)-functionalized polystyrene latex. Langmuir 2017, 33, 1471–1477. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Moon, D.; Maruyama, T.; Yamamoto, T. Preparation of polymer blend colloids containing polyaniline or polypyrrole by Fe(II)-, Fe(III)-, and Cu(II)-H2O2 catalyst system. Polym. J. 1993, 25, 775–779. [Google Scholar] [CrossRef][Green Version]
- Yu, J.G.; Yue, B.Y.; Wu, X.W.; Liu, Q.; Jiao, F.P.; Jiang, X.Y.; Chen, X.Q. Removal of mercury by adsorption: A review. Environ. Sci. Pollut. Res. 2016, 23, 5056–5076. [Google Scholar] [CrossRef]
- Velempini, T.; Pillay, K. Sulphur functionalized materials for Hg(II) adsorption: A review. J. Environ. Chem. Eng. 2019, 7, 103350. [Google Scholar] [CrossRef]
- Xiong, C.H.; Jia, Q.; Chen, X.Y.; Wang, G.T.; Yao, C.P. Optimization of polyacrylonitrile-2-aminothiazole resin synthesis, characterization, and its adsorption performance and mechanism for removal of Hg(II) from aqueous solutions. Ind. Eng. Chem. Res. 2013, 52, 4978–4986. [Google Scholar] [CrossRef]
- Arsuaga, J.M.; Aguado, J.; Arencibia, A.; Lopez-Gutierrez, M.S. Aqueous mercury adsorption in a fixed bed column of thiol functionalized mesoporous silica. Adsorpt. J. Int. Adsorpt. Soc. 2014, 20, 311–319. [Google Scholar] [CrossRef]
- Naushad, M.; Ahamad, T.; Sharma, G.; Ala’a, H.; Albadarin, A.B.; Alam, M.M.; ALOthman, Z.A.; Alshehri, S.M.; Ghfar, A.A. Synthesis and characterization of a new starch/SnO2 nanocomposite for efficient adsorption of toxic Hg2+ metal ion. Chem. Eng. J. 2016, 300, 306–316. [Google Scholar] [CrossRef]





| Run | Initial PS Mass (g) | Initial AT Mass (g) | Temperature (°C) | Remarks |
|---|---|---|---|---|
| 1 | 0.4 | 0.4 | 25 | precipitation |
| 2 | 0.4 | 0.3 | 25 | flocculation |
| 3 | 0.4 | 0.2 | 25 | stable, smooth |
| 4 | 0.4 | 0.1 | 25 | stable, smooth |
| 5 | 0.2 | 0.2 | 25 | stable, smooth |
| 6 | 0.1 | 0.2 | 25 | stable, rough |
| 7 | 0.05 | 0.2 | 25 | metastable, rough |
| 8 | 0.4 | 0.2 | 50 | precipitation |
| 9 | 0.4 | 0.2 | 70 | precipitation |
| Adsorbent | Maximum Adsorption Capacity (mg/g) | Ref. |
|---|---|---|
| PAT | 325.7 mg/g at 308 K | [13] |
| PAT/cellulose acetate fiber membrane | 177 mg/g at 298 K with 6.5 wt % PAT | [15] |
| AT-functionalized polyacrylonitrile | 454.9 mg/g at 308 K | [28] |
| thiol-functionalized mesoporous silica | 47.50 mg/g at 293 K | [29] |
| starch/SnO2 nanocomposite | 192 mg/g at 298 K | [30] |
| PAT-coated PS particles | 440.25 mg/g at 298 K | this work |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zou, H.; Wang, Y. Synthesis of Poly(2-aminothiazole)-Coated Polystyrene Particles and Their Excellent Hg(II) Adsorption Properties. Polymers 2020, 12, 749. https://doi.org/10.3390/polym12040749
Zou H, Wang Y. Synthesis of Poly(2-aminothiazole)-Coated Polystyrene Particles and Their Excellent Hg(II) Adsorption Properties. Polymers. 2020; 12(4):749. https://doi.org/10.3390/polym12040749
Chicago/Turabian StyleZou, Hua, and Yiqian Wang. 2020. "Synthesis of Poly(2-aminothiazole)-Coated Polystyrene Particles and Their Excellent Hg(II) Adsorption Properties" Polymers 12, no. 4: 749. https://doi.org/10.3390/polym12040749
APA StyleZou, H., & Wang, Y. (2020). Synthesis of Poly(2-aminothiazole)-Coated Polystyrene Particles and Their Excellent Hg(II) Adsorption Properties. Polymers, 12(4), 749. https://doi.org/10.3390/polym12040749






