Effect of Carbon Black Nanofiller on Adhesion Properties of SBS Rubber Surfaces Treated by Low-Pressure Plasma
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Plasma Treatment
2.3. Peel Tests
2.4. X-ray Photoelectron Spectroscopy (XPS)
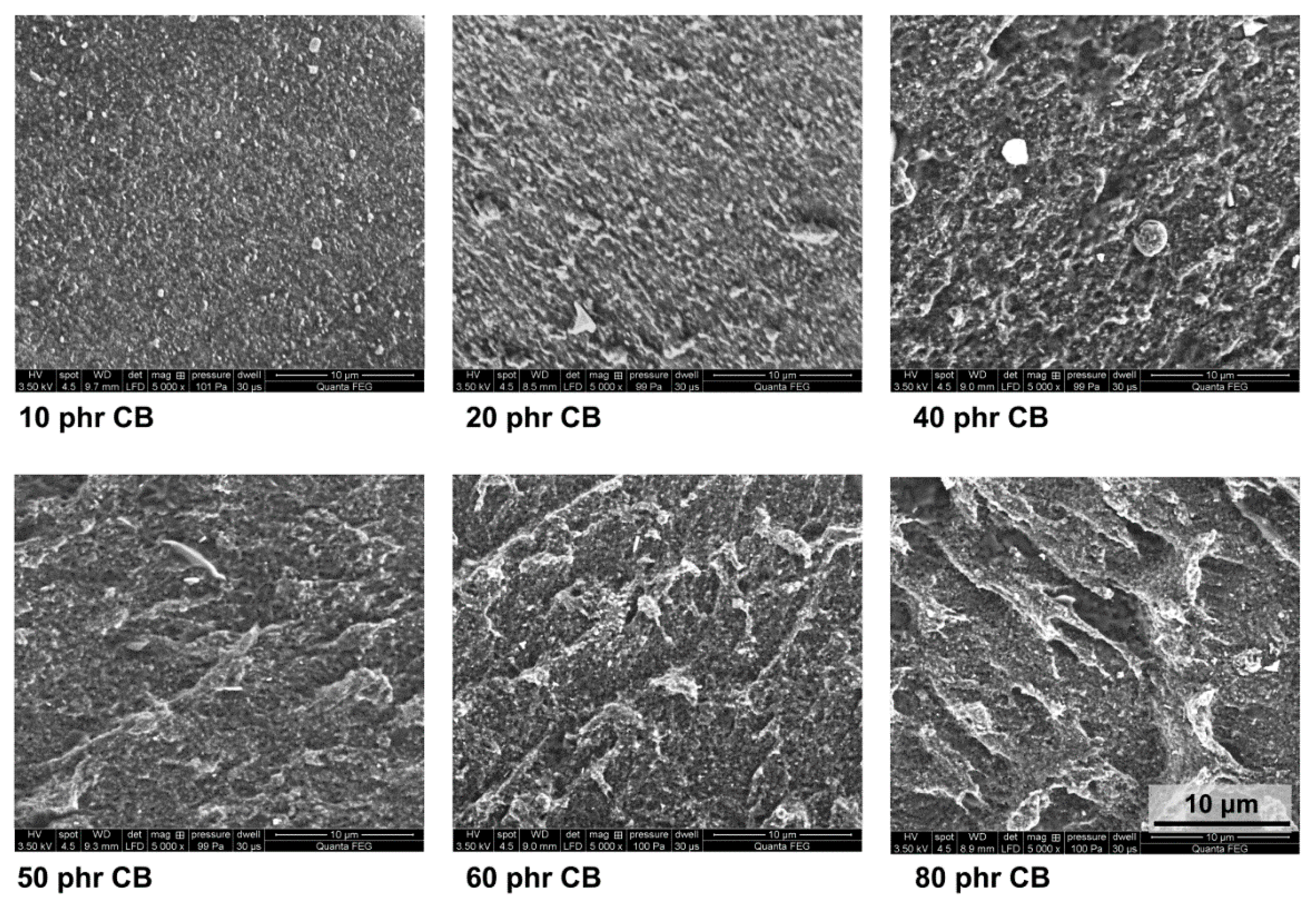
2.5. Scanning Electron Microscopy (SEM)
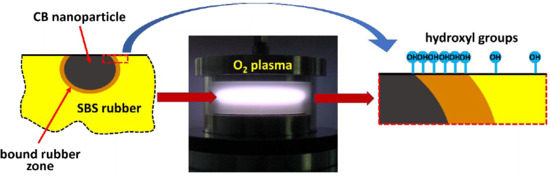
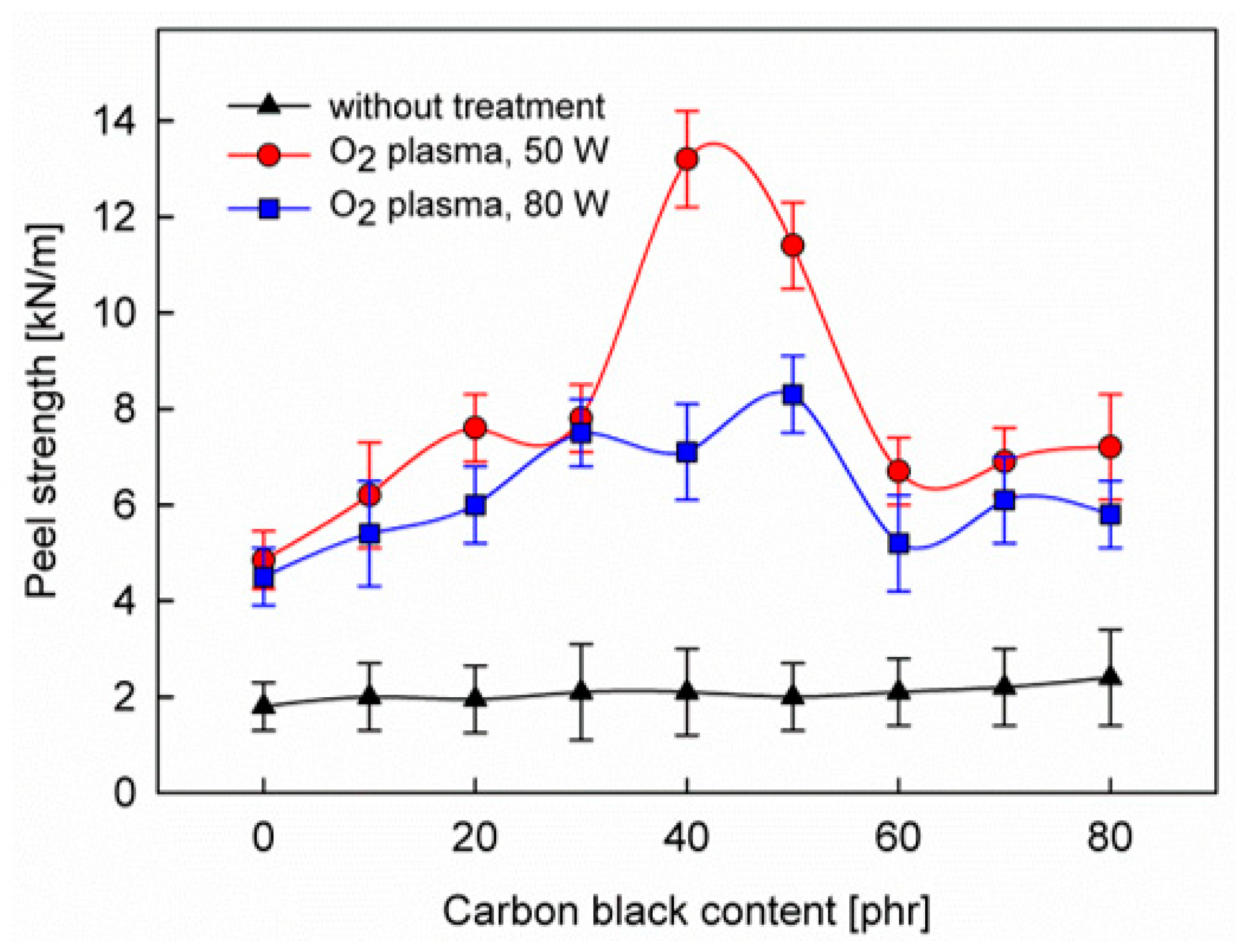
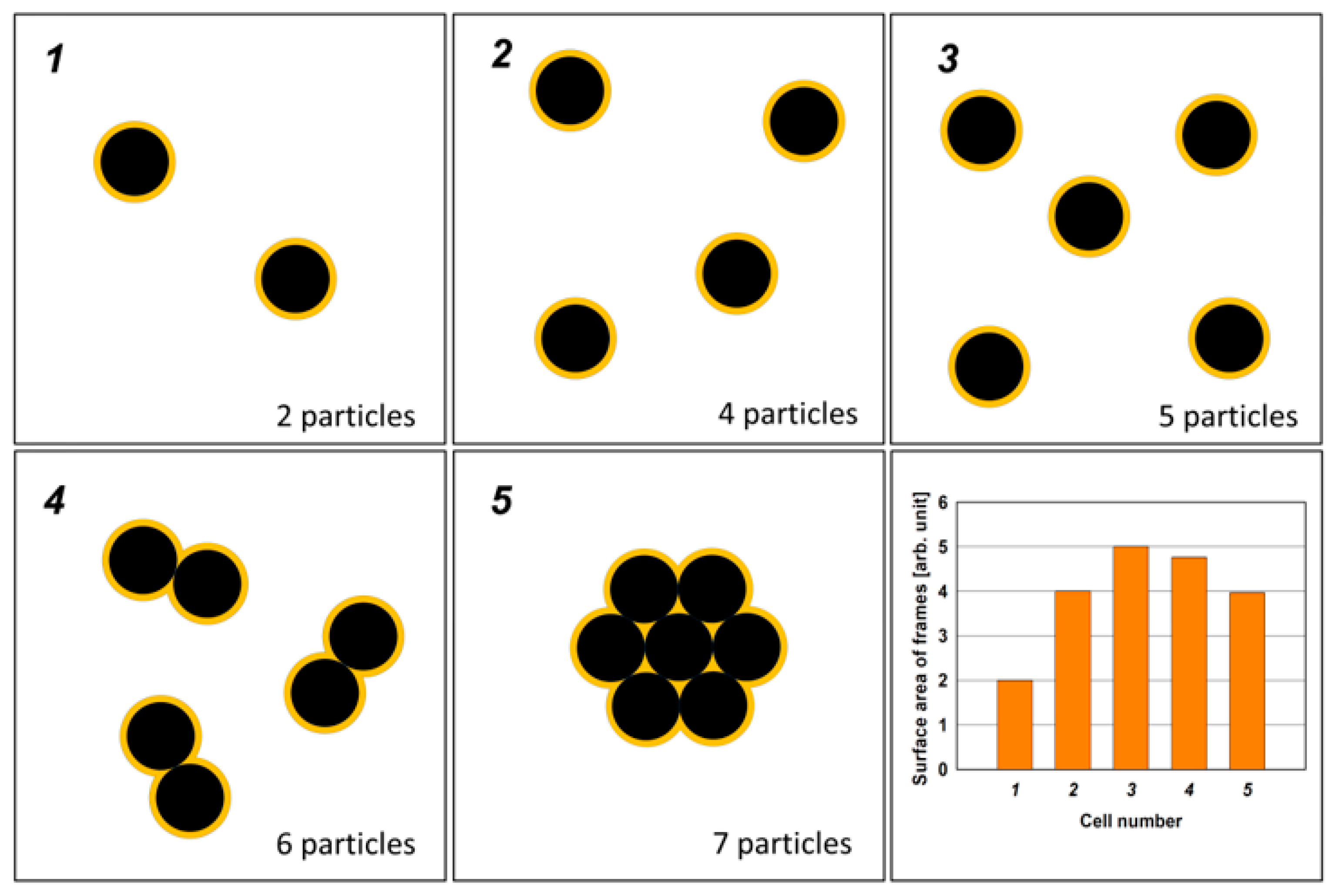
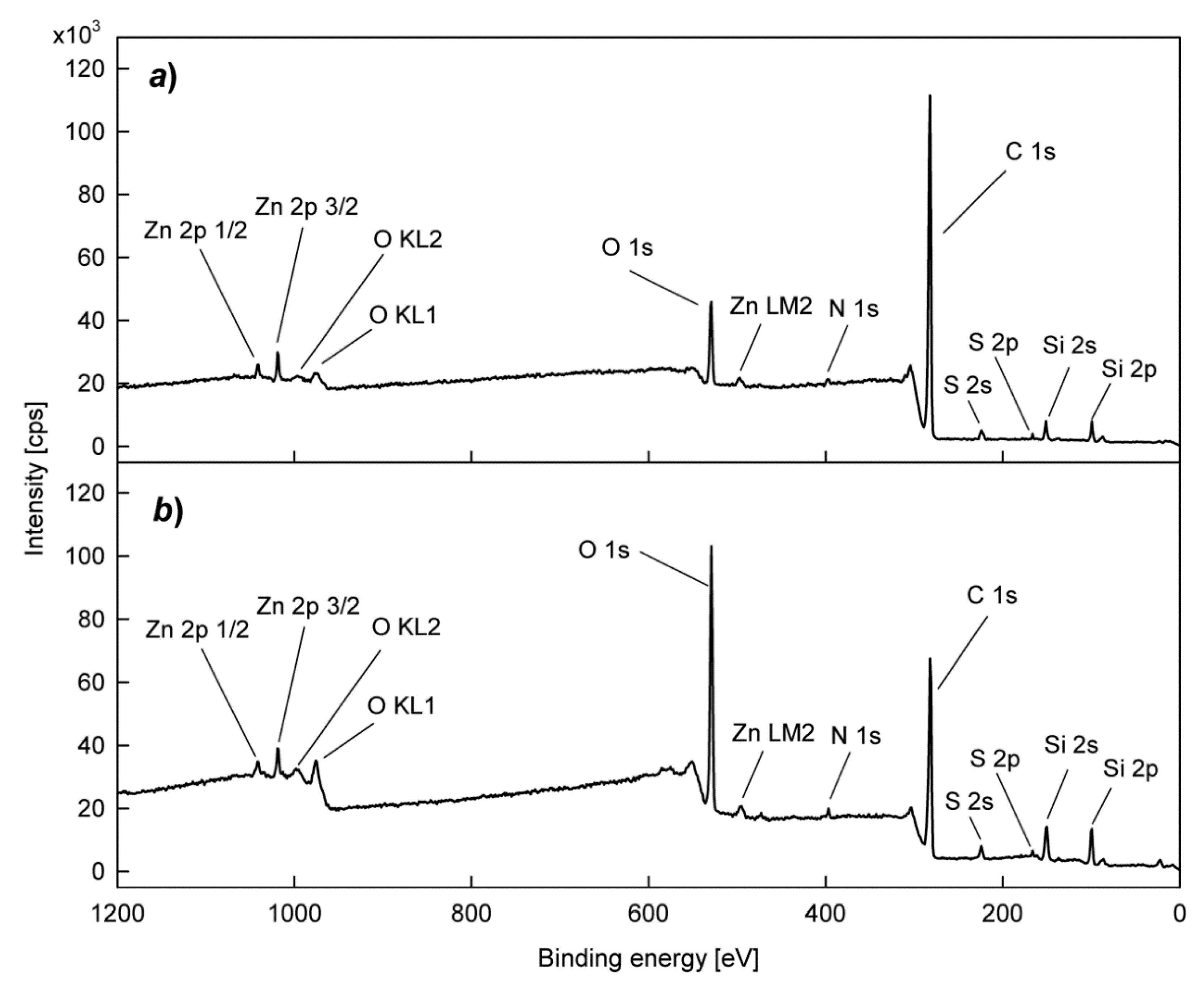
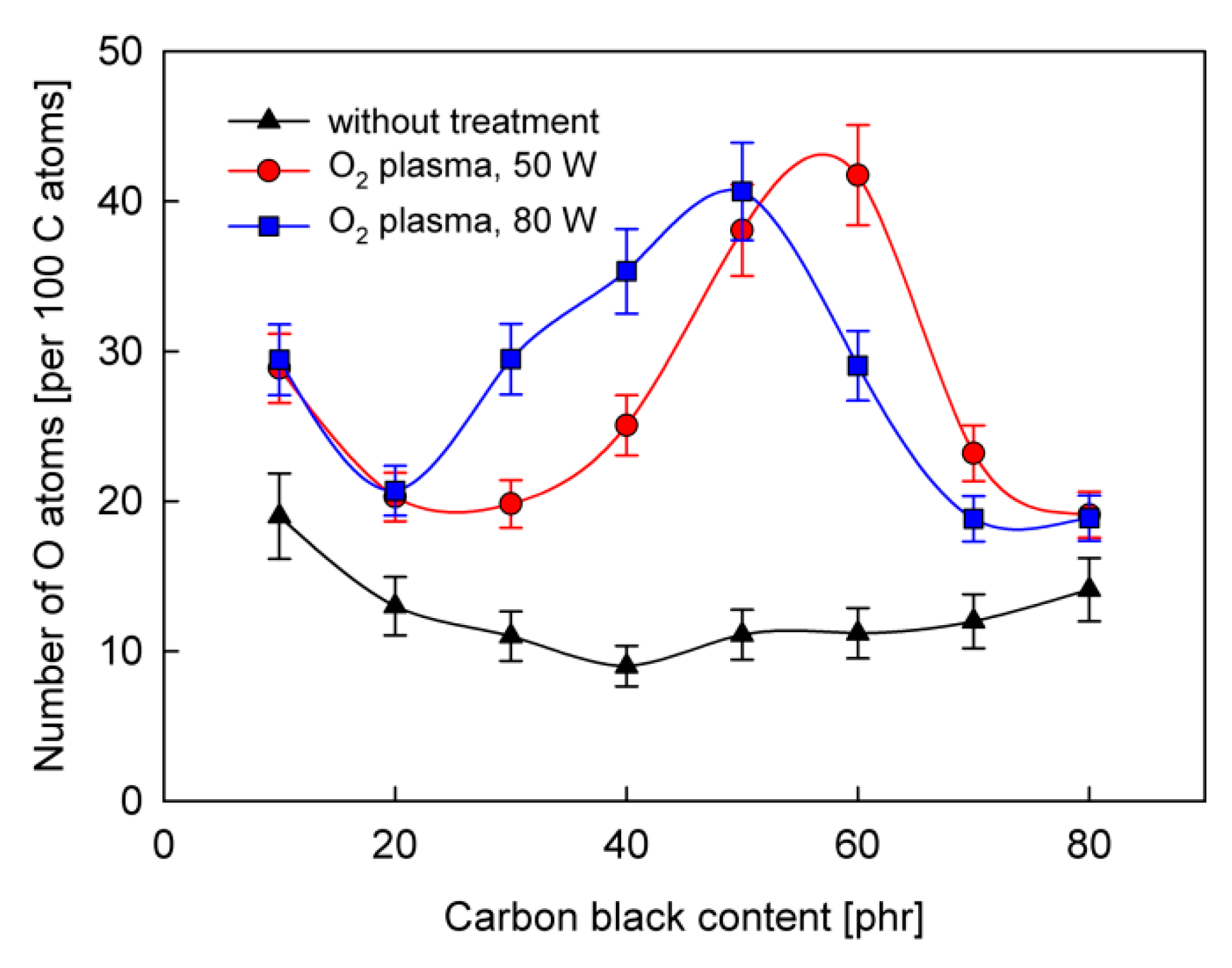
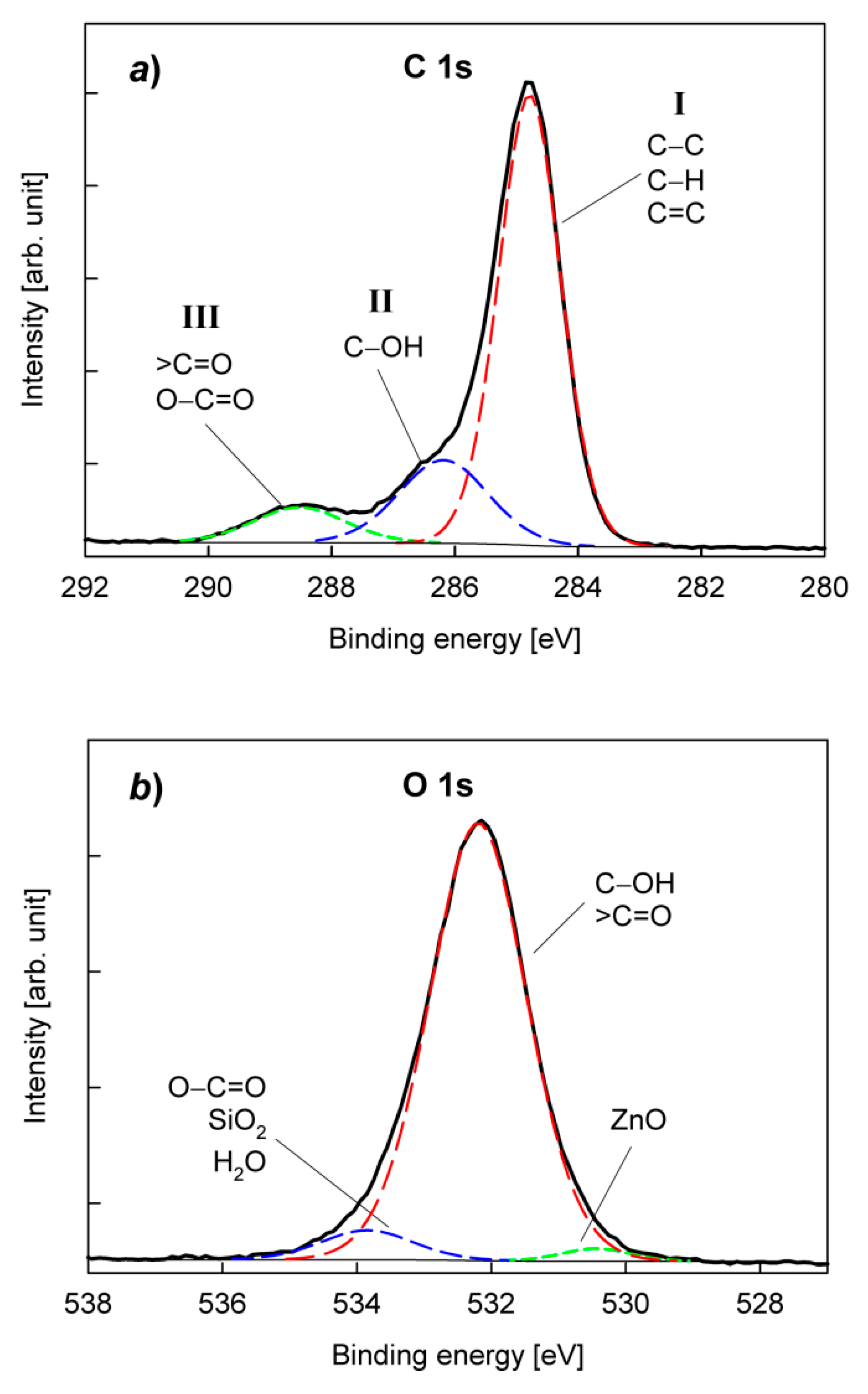
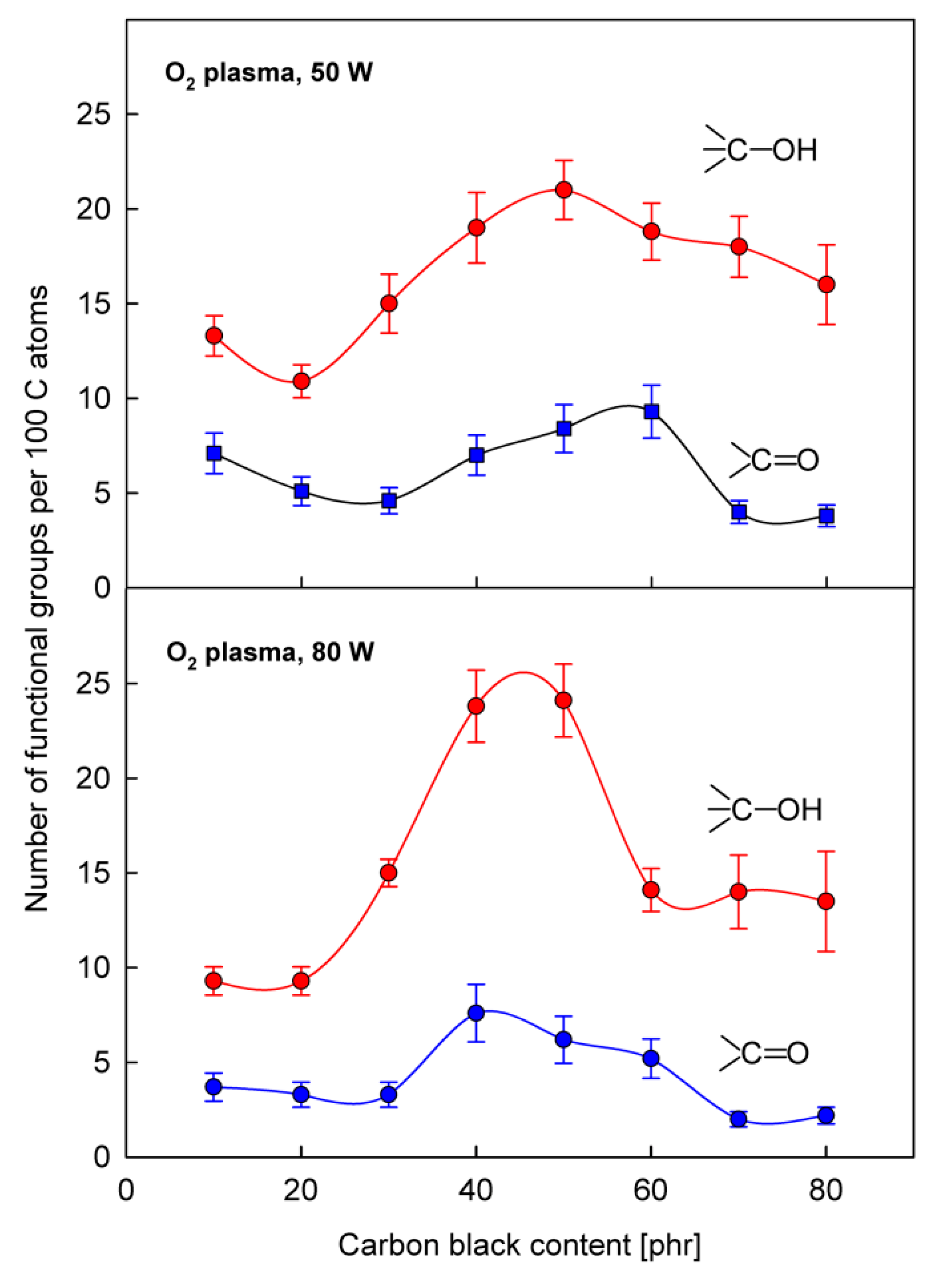
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Martin-Martinez, J.M. Improving adhesion of rubber. In Current Topics in Elastomers Research; Bhowmick, A.K., Ed.; CRC Press: Boca Raton, FL, USA, 2008; Chap. 27; pp. 765–777. [Google Scholar] [CrossRef]
- Radabutra, S.; Thanawan, S.; Amornsakchai, T. Chlorination and characterization of natural rubber and its adhesion to nitrile rubber. Eur. Polym. J. 2009, 45, 2017–2022. [Google Scholar] [CrossRef]
- Ortiz-Magán, A.B.; Pastor-Blas, M.M.; Martin-Martinez, J.M. Different performance of Ar, O2 and CO2 RF plasmas in the adhesion of thermoplastic rubber to polyurethane adhesive. In Plasma Processes and Polymers; d’Agostino, R., Favia, P., Oehr, C., Wertheimer, M.E., Eds.; Wiley-VCH: Weinheim, Germany, 2005; pp. 177–192. [Google Scholar] [CrossRef]
- Tyczkowski, J.; Krawczyk, I.; Woźniak, B. Plasma−surface modification of styrene-butadiene elastomers for improved adhesion. In Plasma Processes and Polymers; d’Agostino, R., Favia, P., Oehr, C., Wertheimer, M.E., Eds.; Wiley-VCH: Weinheim, Germany, 2005; pp. 233–252. [Google Scholar] [CrossRef]
- Romero-Sánchez, M.D.; Pastor-Blas, M.M.; Martin-Martinez, J.M. Environmental friendly surface treatments of styrene-butadiene-styrene rubber: alternatives to the solvent-based halogenation treatment. Int. J. Adhes. Adhes. 2005, 25, 19–29. [Google Scholar] [CrossRef]
- Cognard, J. Some recent progress in adhesion technology and science. Compt. Rend. Chim. 2006, 9, 3–24. [Google Scholar] [CrossRef]
- Anagreh, N.; Dorn, L.; Bilke-Krause, C. Low-pressure plasma pretreatment of polyphenylene sulfide (PPS) surfaces for adhesive bonding. Int. J. Adhes. Adhes. 2008, 28, 16–22. [Google Scholar] [CrossRef]
- Gao, S.H.; Zhou, K.S.; Lei, M.K.; Wen, L.S. Surface modification of silicone rubber by CF4 radio frequency plasma immersion. Plasma Chem. Plasma Proc. 2008, 28, 715–728. [Google Scholar] [CrossRef]
- Tyczkowski, J.; Makowski, P.; Krawczyk-Kłys, I.; Wójcik, J. Surface modification of SBS rubber by low-pressure inert gas plasma for enhanced adhesion to polyurethane adhesive. J. Adhes. Sci. Technol. 2012, 26, 841–856. [Google Scholar] [CrossRef]
- Henry, A.; Vallat, M.F.; Noël, C.; Belmonte, T.; Roucoules, V. Influence of plasma chamber set-up on the surface modification of non-vulcanized and pure SBR rubber treated at radio-frequencies air plasma. Plasma Proc. Polym. 2015, 12, 1139–1152. [Google Scholar] [CrossRef]
- Petrie, E.M. Handbook of Adhesives and Sealants; McGraw-Hill: New York, NY, USA, 2000; pp. 382–385. [Google Scholar]
- Segura, D.M.; Nurse, A.D.; McCourt, A.; Phelps, R.; Segura, A. Chemistry of polyurethane adhesives and sealants. In Adhesives and Sealants; Cognard, P., Ed.; Elsevier: Amsterdam, The Netherlands, 2005; pp. 101–162. [Google Scholar]
- Tyczkowski, J.; Krawczyk-Kłys, I.; Kuberski, S.; Makowski, P. Chemical nature of adhesion: Plasma modified styrene–butadiene elastomer and polyurethane adhesive joints. Eur. Polym. J. 2010, 46, 767–773. [Google Scholar] [CrossRef]
- Krawczyk-Kłys, I.; Makowski, P.; Wójcik, J.; Tyczkowski, J. Plasma surface modification of commercial SBS rubbers for enhanced adhesive bonding. Mater. Sci-Medzg. 2012, 18, 132–137. [Google Scholar] [CrossRef]
- Cantos-Delegido, B.; Martin-Martinez, J.M. Treatment with Ar−O2 low-pressure plasma of vulcanized rubber sole containing noticeable amount of processing oils for improving adhesion to upper in shoe industry. J. Adhes. Sci. Technol. 2015, 29, 1301–1314. [Google Scholar] [CrossRef]
- Tyczkowski, J.; Krawczyk, I.; Woźniak, B. Modification of styrene−butadiene rubber surfaces by plasma chlorination. Surf. Coat. Technol. 2003, 174–175, 849–853. [Google Scholar] [CrossRef]
- Ayala, J.A.; Hess, W.M.; Kistler, F.D.; Joyce, G.A. Carbon-black−elastomer interaction. Rubber Chem. Technol. 1991, 64, 19–39. [Google Scholar] [CrossRef]
- Demirhan, E.; Kandemirli, F.; Kandemirli, M. The effects of furnace carbon blacks on the mechanical and the rheological properties of SBR1502 styrene butadiene rubber. Mater. Des. 2007, 28, 1326–1329. [Google Scholar] [CrossRef]
- Ao, G.; Hu, Q.; Kim, M.S. Properties of activated carbon blacks filled SBR rubber composites. Carbon Lett. 2008, 9, 115–120. [Google Scholar] [CrossRef]
- Ma, J.H.; Zhang, L.Q.; Wu, Y.P. Characterization of filler-rubber interaction, filler network structure, and their effects on viscoelasticity for styrene-butadiene rubber filled with different fillers. J. Macromol. Sci. B: Phys. 2013, 52, 1128–1141. [Google Scholar] [CrossRef]
- Yang, H.; Gong, J.; Wen, X.; Xue, J.; Chen, Q.; Jiang, Z.; Tian, N.; Tang, T. Effect of carbon black on improving thermal stability, flame retardancy and electrical conductivity of polypropylene/carbon fiber composites. Comp. Sci. Technol. 2015, 113, 31–37. [Google Scholar] [CrossRef]
- Zhang, Q.; Wang, J.; Yu, J.; Guo, Z.X. Improved electrical conductivity of TPU/carbon black composites by addition of COPA and selective localization of carbon black at the interface of sea-island structured polymer blends. Soft Matter 2017, 13, 3431–3439. [Google Scholar] [CrossRef]
- Qu, M.; Deng, F.; Kalkhoran, S.M.; Gouldstone, A.; Robisson, A.; Van Vliet, K.J. Nanoscale visualization and multiscale mechanical implications of bound rubber interphases in rubber−carbon black nanocomposites. Soft Matter 2011, 7, 1066–1077. [Google Scholar] [CrossRef]
- Wolff, S.; Wang, M.J.; Tan, E.H. Filler−elastomer interaction. Part VII. Study on bound rubber. Rubber Chem. Technol. 1993, 66, 163–177. [Google Scholar] [CrossRef]
- Leblanc, J.L. A molecular explanation for the origin of bound rubber in carbon black filler rubber compounds. J. Appl. Polym. Sci. 1997, 66, 2257–2268. [Google Scholar] [CrossRef]
- Choi, S.S. Characterization of bound rubber of filled styrene-butadiene rubber compounds using pyrolysis-gas chromatography. J. Analyt. Appl. Pyrol. 2000, 55, 161–170. [Google Scholar] [CrossRef]
- Choi, S.S.; Kim, I.S. Filler–polymer interactions in filled polybutadiene compounds. Eur. Polym. J. 2002, 38, 1265–1269. [Google Scholar] [CrossRef]
- Kablov, V.F.; Patryuk, I.P. The influence of the carbon black morphology on the interphase layer content in filled elastomers. Inter. Polym. Sci. Technol. 2016, 43, T17–T19. [Google Scholar] [CrossRef]
- Choi, S.S.; Ko, E. Novel test method to estimate bound rubber formation of silica-filled solution styrene-butadiene rubber compounds. Polym. Test. 2014, 40, 170–177. [Google Scholar] [CrossRef]
- Tyczkowski, J.; Krawczyk, I.; Woźniak, B.; Martin-Martinez, J.M. Low-pressure plasma chlorination of styrene–butadiene block copolymer for improved adhesion to polyurethane adhesives. Eur. Polym. J. 2009, 45, 1826–1835. [Google Scholar] [CrossRef]
- Goldstein, J.I.; Newbury, D.E.; Michael, J.R.; Ritchie, N.W.M.; Scott, J.H.J.; Joy, D.C. Scanning Electron Microscopy and X-ray Microanalysis, 4th ed.; Springer: New York, NY, USA, 2018; pp. 173–185. [Google Scholar] [CrossRef]
- Tyczkowski, J.; Kierzkowska-Pawlak, H.; Sielski, J.; Krawczyk-Kłys, I. Low-temperature plasma modification of styrene–butadiene block copolymer surfaces for improved adhesion – a kinetic approach. Polymers 2020. in preparation for the Special Issue “Surface Chemistry of Polymers”. [Google Scholar]
- Roy, S.; Yue, C.Y. Surface modification of COC microfluidic devices: A comparative study of nitrogen plasma treatment and its advantages over argon and oxygen plasma treatments. Plasma Proc. Polym. 2011, 8, 432–443. [Google Scholar] [CrossRef]
- The GIMP Development Team. GIMP, 2020. Available online: https://www.gimp.org (accessed on 10 January 2020).
| Ingredient | Content [phr] a |
|---|---|
| Styrene-butadiene (SBS) copolymer (KER 1502) | 100 |
| Nitrile-butadiene copolymer (KER N-29) | 42.9 |
| Carbon black (N330) | 10 to 80 |
| Silica (Arsil) | 11.5 |
| Zinc oxide | 5.7 |
| Phthalates | 5.7 |
| Tetramethyl thiuram disulfide | 2.3 |
| Oiled sulfur | 1.7 |
| N-cyclohexyl-2-benzothiazole sulfenamide | 1.7 |
| Antioxidant | 1.4 |
| Protector G35 | 1.1 |
| Stearin | 1.0 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tyczkowski, J.; Balcerzak, J.; Sielski, J.; Krawczyk-Kłys, I. Effect of Carbon Black Nanofiller on Adhesion Properties of SBS Rubber Surfaces Treated by Low-Pressure Plasma. Polymers 2020, 12, 616. https://doi.org/10.3390/polym12030616
Tyczkowski J, Balcerzak J, Sielski J, Krawczyk-Kłys I. Effect of Carbon Black Nanofiller on Adhesion Properties of SBS Rubber Surfaces Treated by Low-Pressure Plasma. Polymers. 2020; 12(3):616. https://doi.org/10.3390/polym12030616
Chicago/Turabian StyleTyczkowski, Jacek, Jacek Balcerzak, Jan Sielski, and Iwona Krawczyk-Kłys. 2020. "Effect of Carbon Black Nanofiller on Adhesion Properties of SBS Rubber Surfaces Treated by Low-Pressure Plasma" Polymers 12, no. 3: 616. https://doi.org/10.3390/polym12030616
APA StyleTyczkowski, J., Balcerzak, J., Sielski, J., & Krawczyk-Kłys, I. (2020). Effect of Carbon Black Nanofiller on Adhesion Properties of SBS Rubber Surfaces Treated by Low-Pressure Plasma. Polymers, 12(3), 616. https://doi.org/10.3390/polym12030616