Coagulation of Chitin Production Wastewater from Shrimp Scraps with By-Product Chitosan and Chemical Coagulants
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample of Wastewater
2.2. Reagents and Apparatus
2.3. Analytical Methods
2.4. Experimental Design
2.4.1. Preliminary Sedimentation Experiments
2.4.2. Coagulation and Flocculation Experiments by Chitosan, PAC, and PAA
2.5. Response Surface Designs and Analysis
3. Results and Discussion
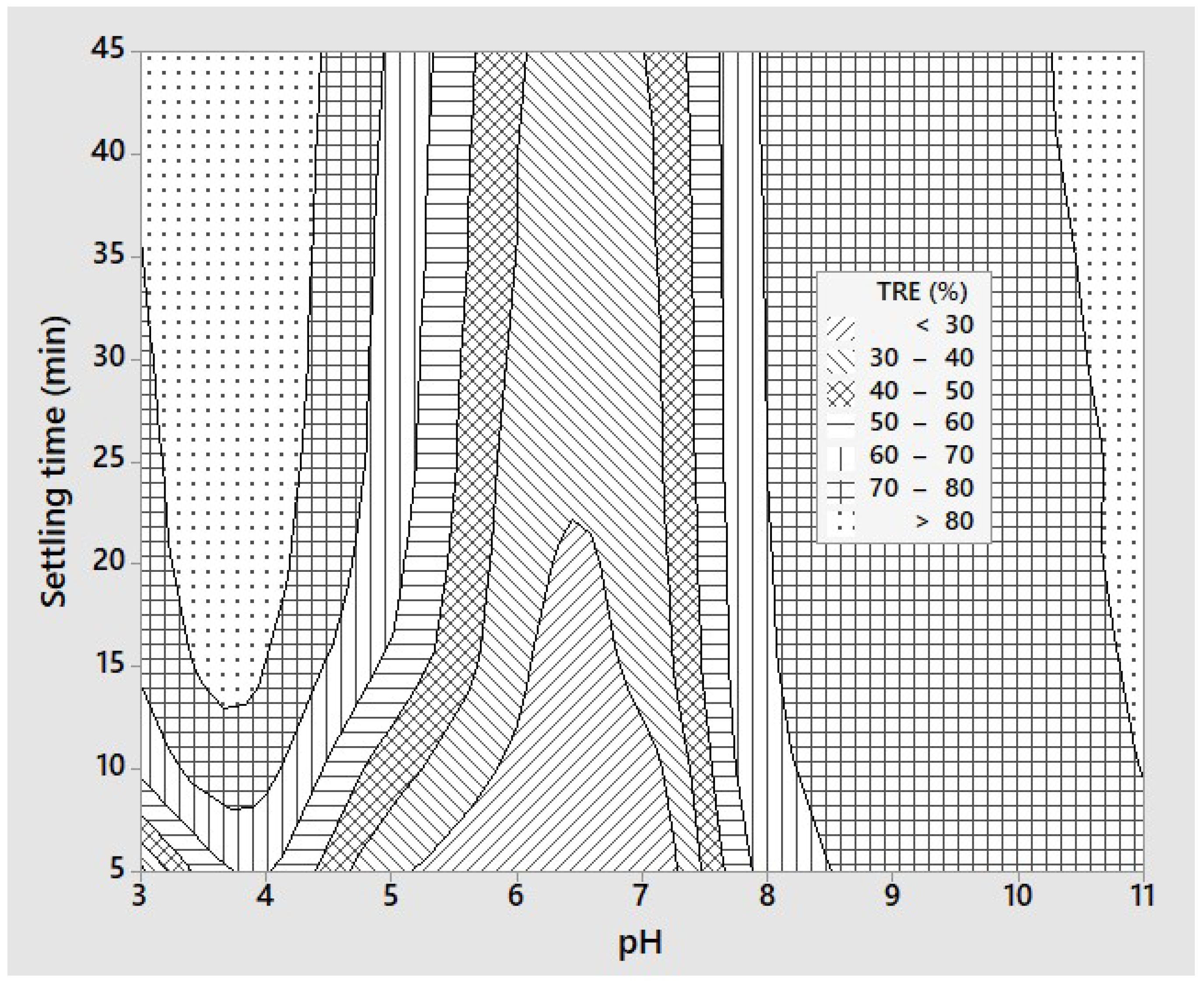
3.1. Preliminary Sedimentation Efficiency
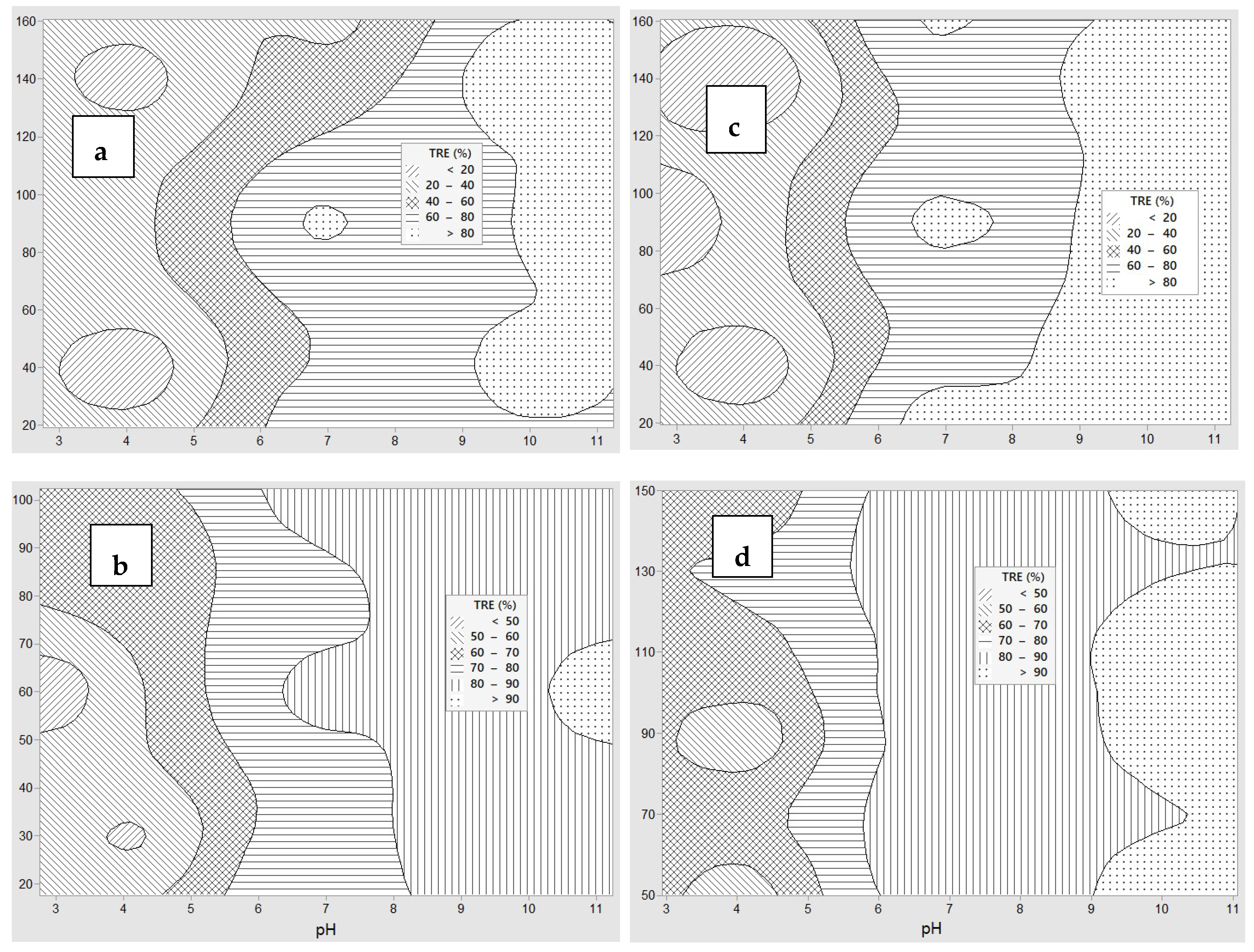
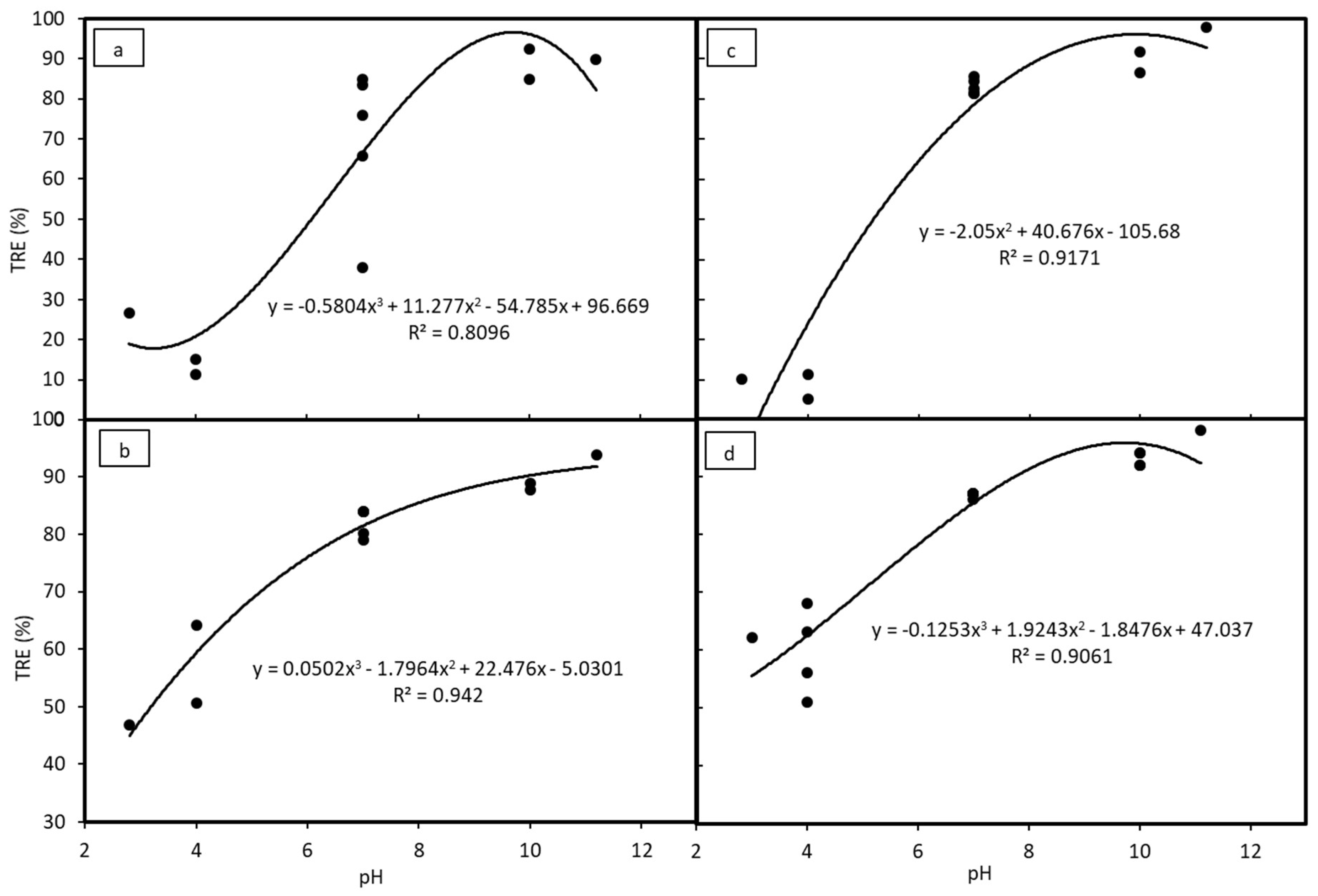
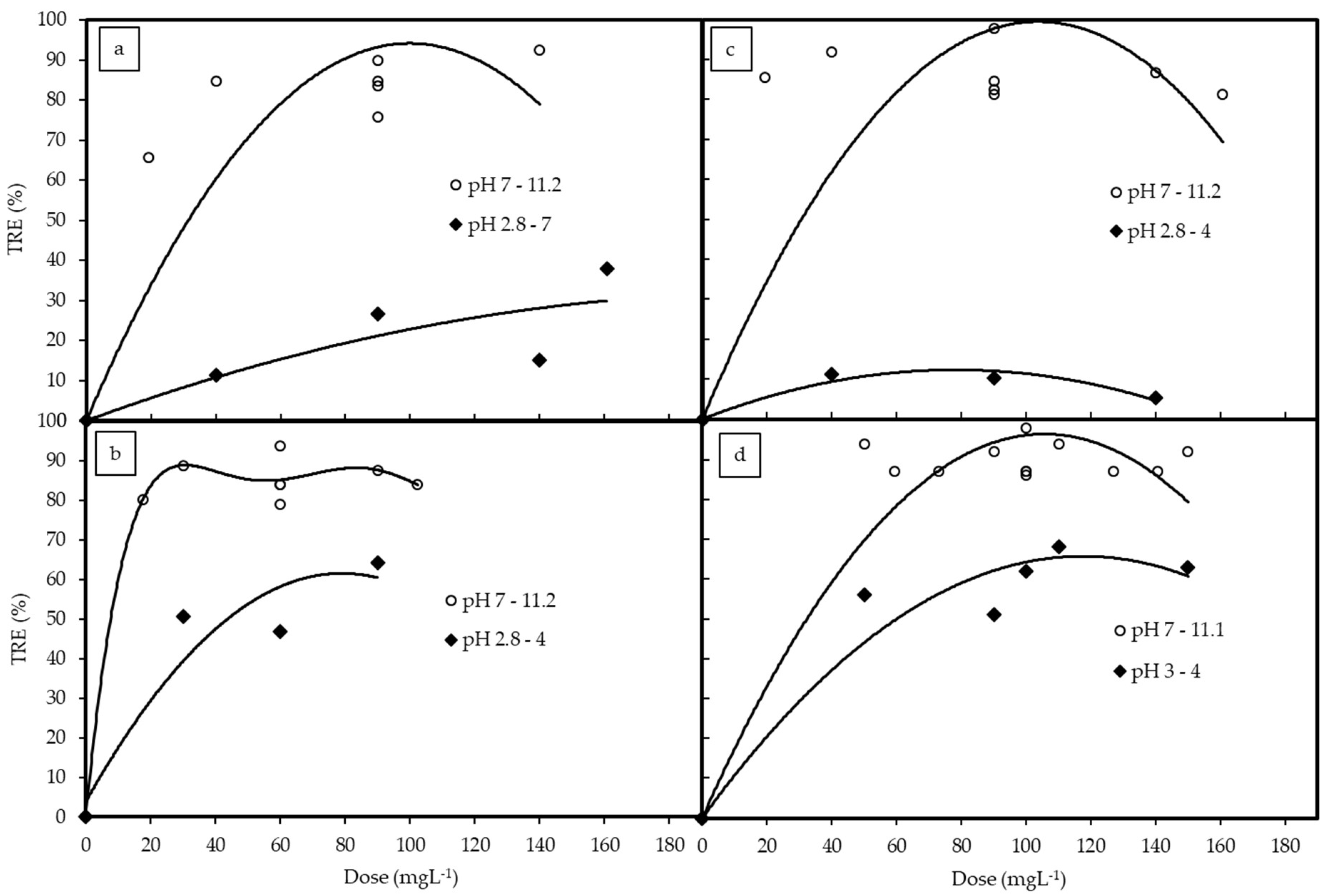
3.2. Coagulation by Chitosan (Experiment 1)
3.3. Coagulation by PAC (Experiment 2)
3.4. Coagulation by PAA (Experiment 3)
3.5. Coagulation and Flocculation by PAC and PAA (Experiment 4)
3.6. Comparison between Studied Coagulants
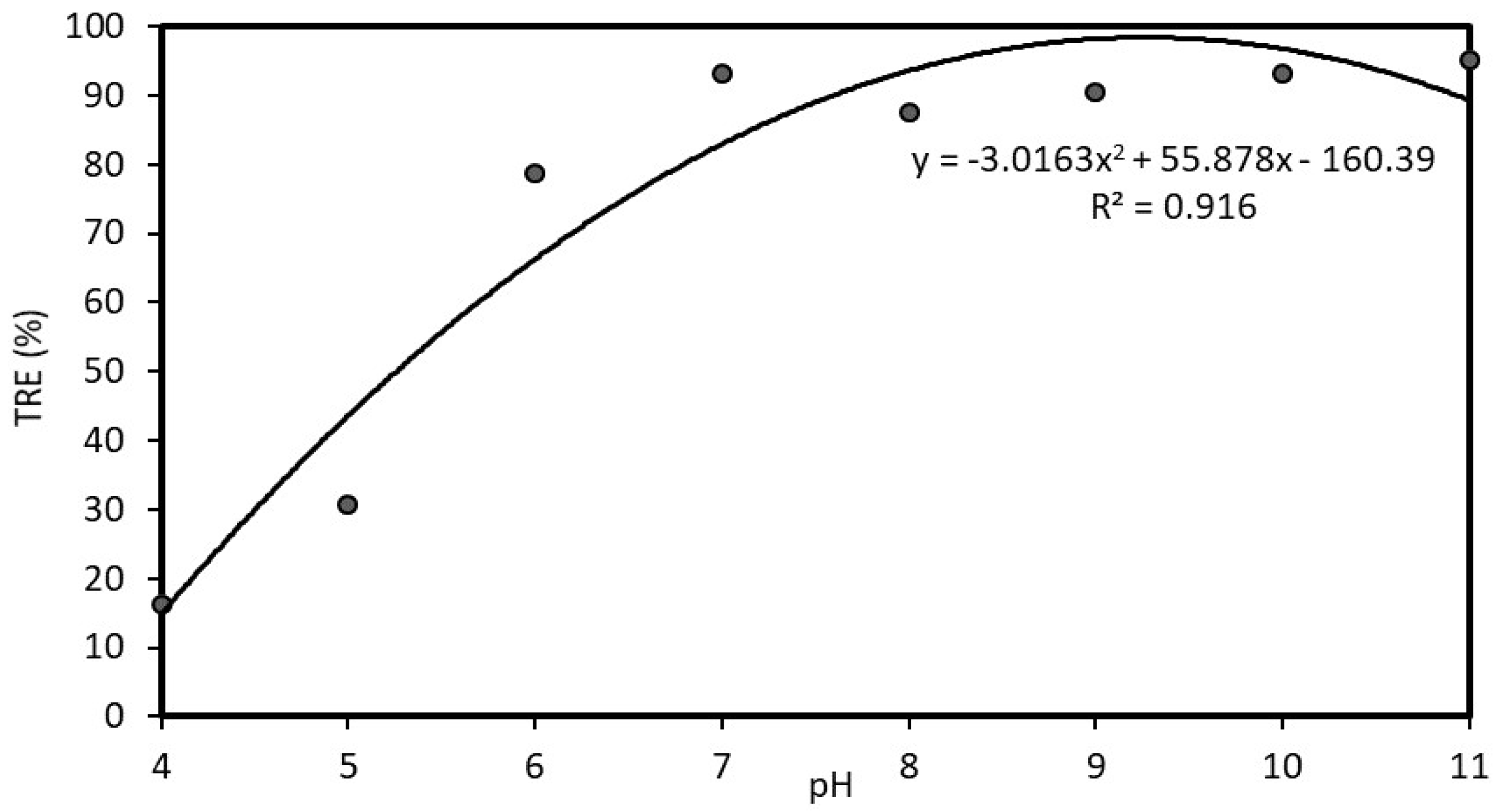
3.7. Optimal Conditions of Chitosan Coagulation
3.7.1. Traditional Experimental Design
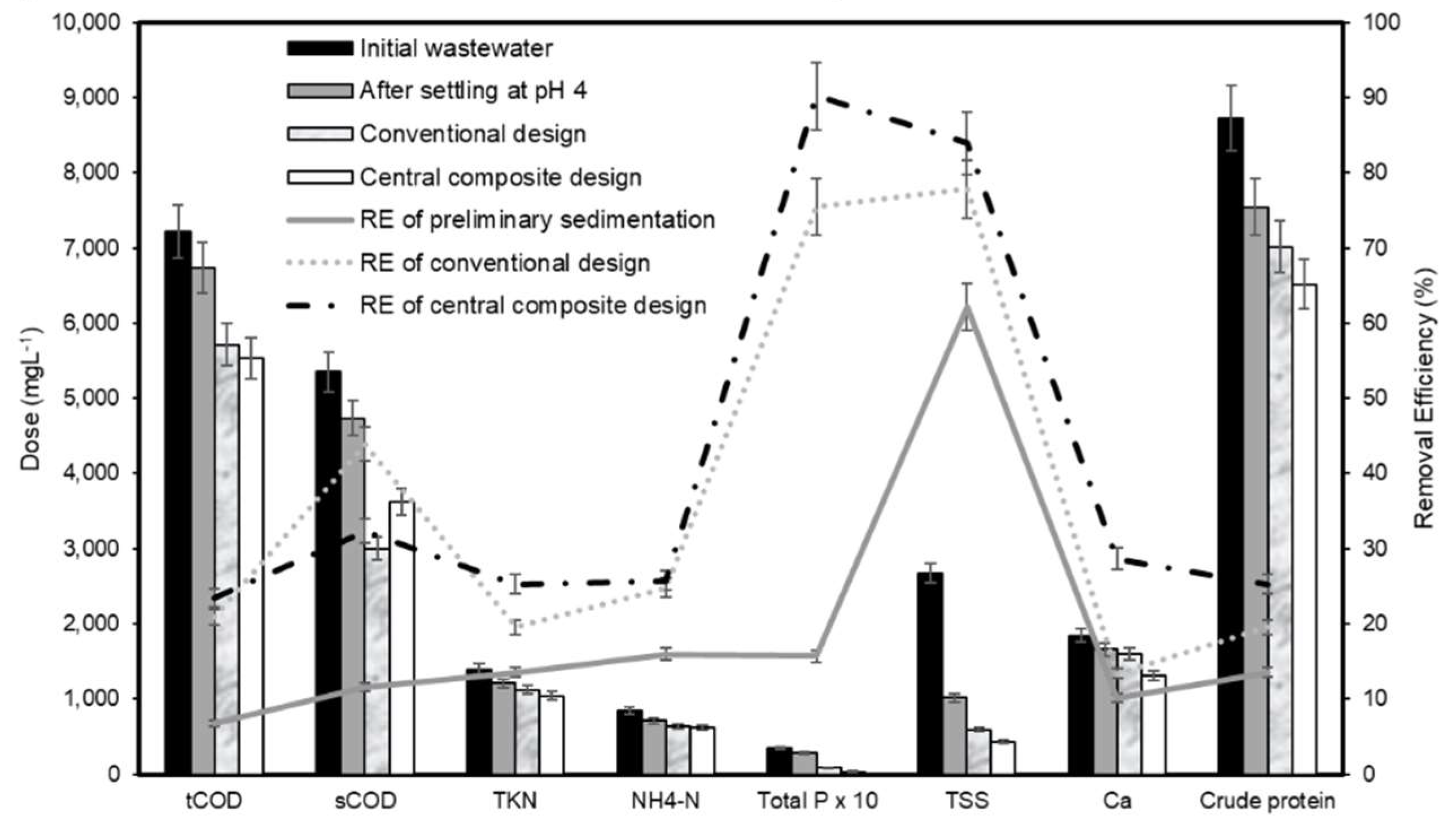
3.7.2. Box-Wilson Central Composite Design (Experiment 5)
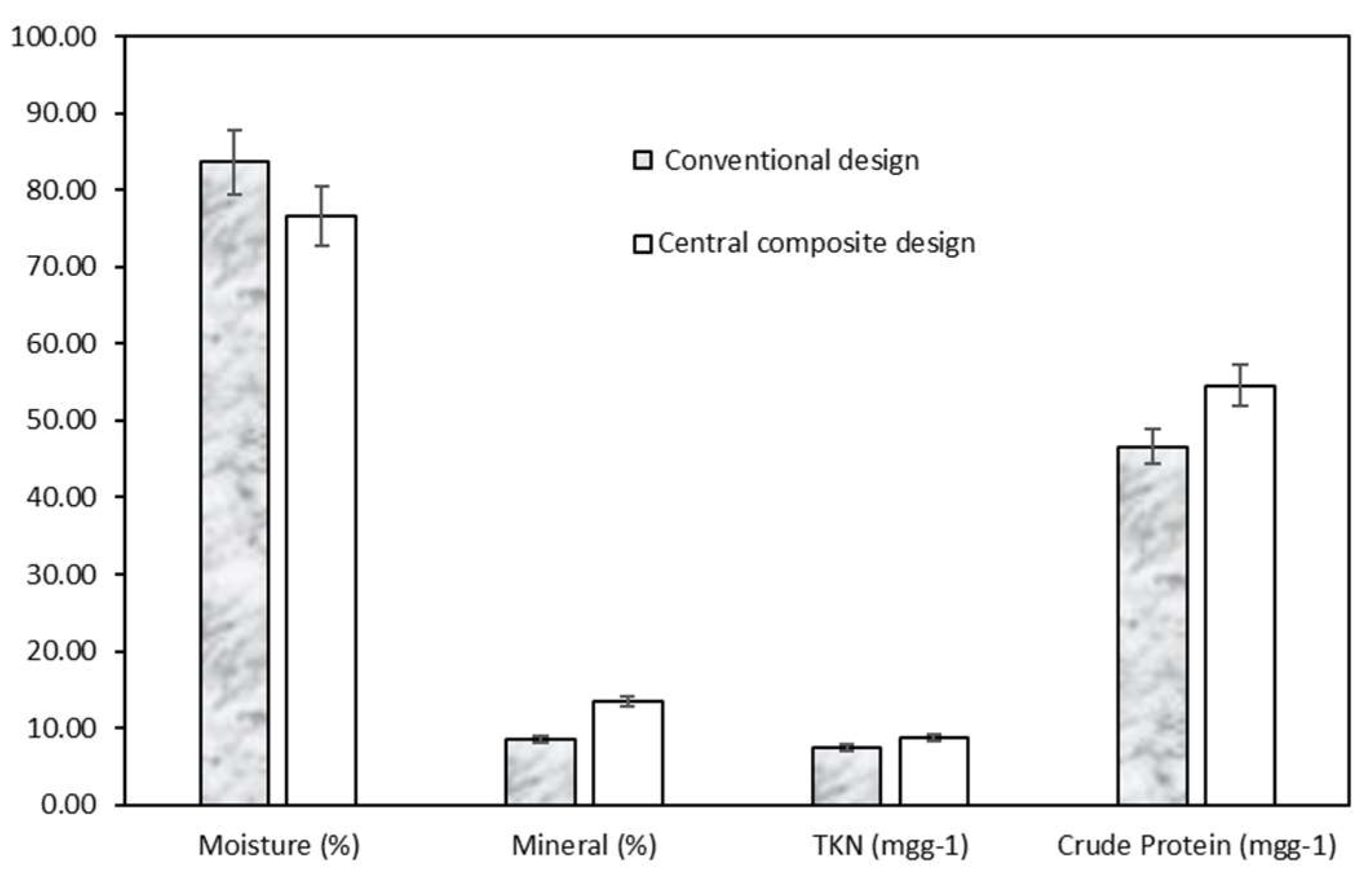
3.7.3. Comparison of Treatment Performances between Two Optimal Points from Traditional Design and Central Composite Design
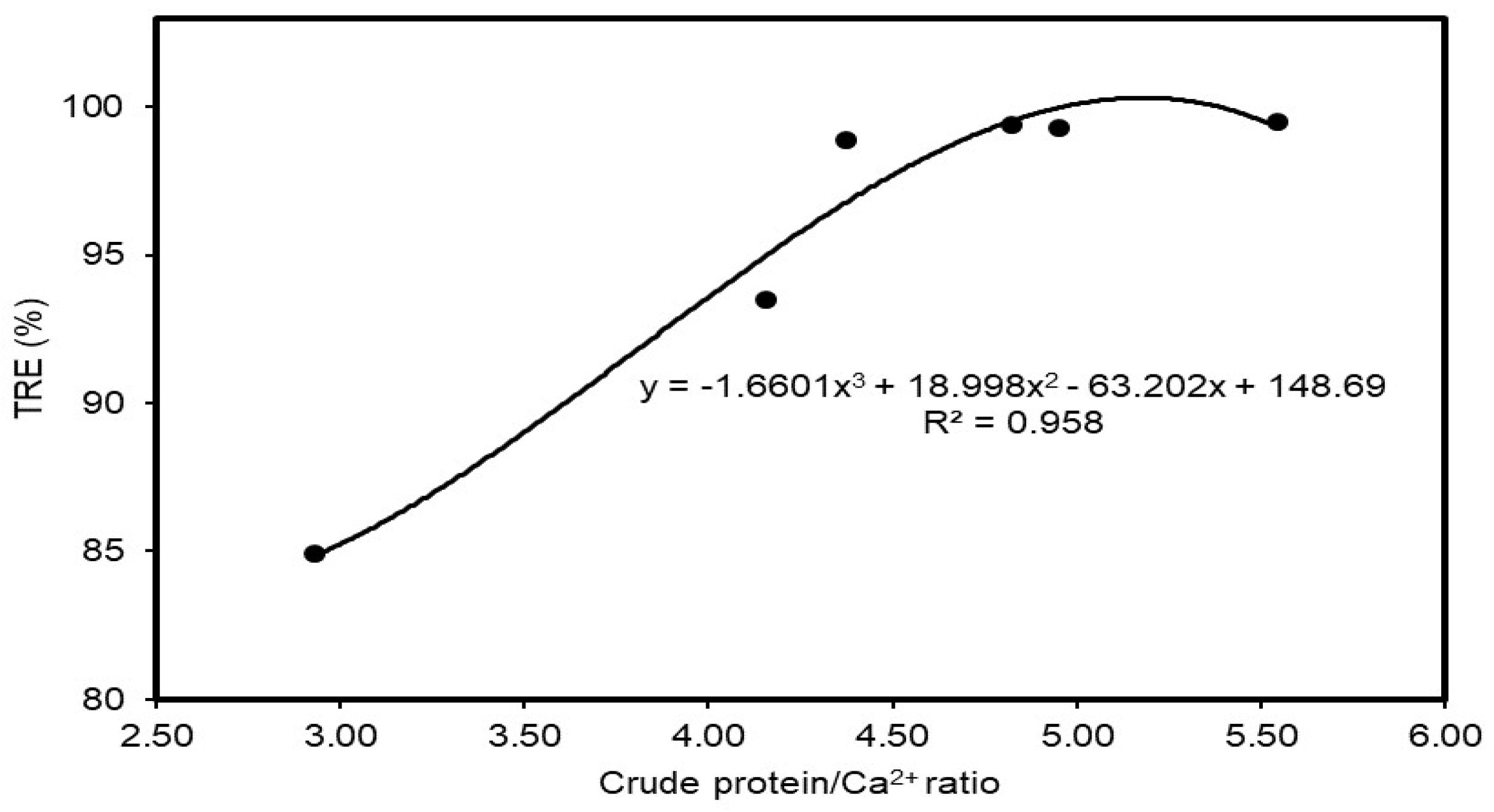
3.8. Correlation between Crude Protein and Calcium Ratio with Turbidity Removal Performances
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Trung, T.S.; Bao, H.N.D. Physicochemical properties and antioxidant activity of chitin and chitosan prepared from pacific white shrimp waste. Int. J. Carbohydr. Chem. 2015, 2015. [Google Scholar] [CrossRef]
- Mao, X.; Guo, N.; Sun, J. Cleaner production guide of chito/chitin oligosaccharides and its monomer. In Oligosaccharides of Chitin and Chitosan: Bio-Manufacture and Applications; Zhao, L., Ed.; Springer: Singapore, 2019; pp. 107–127. [Google Scholar] [CrossRef]
- Su, W.; Yu, S.; Wu, D.; Xia, M.; Wen, Z.; Yao, Z.; Tang, J.; Wu, W. A critical review of cast-off crab shell recycling from the perspective of functional ands versatile biomaterials. Environ. Sci. Pollut. Res. 2019, 31581–31591. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.-L.; Liang, T.-W. Microbial reclamation of squid pens and shrimp shells. Res. Chem. Intermed. 2017, 43, 3445–3462. [Google Scholar] [CrossRef]
- Doan, C.T.; Tran, T.N.; Wen, I.; Nguyen, V.B.; Nguyen, A.D.; Wang, S.-L. Conversion of shrimp head waste for production of a thermotolerant, detergent-stable, alkaline protease by paenibacillus sp. Catalysts 2019, 9, 798. [Google Scholar] [CrossRef]
- Wang, S.-L.; Su, Y.-C.; Nguyen, A.D. Reclamation of shrimp heads for the production of α-glucosidase inhibitors by Staphylococcus sp. TKU043. Res. Chem. Intermed. 2018, 44, 4929–4937. [Google Scholar] [CrossRef]
- Doan, C.T.; Tran, T.N.; Vo, T.P.K.; Nguyen, A.D.; Wang, S.-L. Chitin extraction from shrimp waste by liquid fermentation using an alkaline protease-producing strain, Brevibacillus parabrevis. Int. J. Biol. Macromol. 2019, 131, 706–715. [Google Scholar] [CrossRef]
- Wang, S.-L.; Chio, S.-H. Deproteinization of shrimp and crab shell with the protease of Pseudomonas aeruginosa K-187. Enzym. Microb. Technol. 1998, 22, 629–633. [Google Scholar] [CrossRef]
- Nguyen, V.B.; Wang, S.-L. Reclamation of marine chitinous materials for the production of α-glucosidase inhibitors via microbial conversion. Mar. Drugs 2017, 15, 350. [Google Scholar] [CrossRef]
- Hsu, C.-H.; Nguyen, A.D.; Wang, S.-L. Conversion of shrimp heads to α-glucosidase inhibitors via co-culture of Bacillus mycoides TKU040 and Rhizobium sp. TKU041. Res. Chem. Intermed. 2018, 44, 4597–4607. [Google Scholar] [CrossRef]
- Nguyen, V.B.; Wang, S.-L. Production of potent antidiabetic compounds from shrimp head powder via Paenibacillus conversion. Process Biochem. 2019, 76, 18–24. [Google Scholar] [CrossRef]
- Doan, C.T.; Tran, T.N.; Nguyen, V.B.; Nguyen, A.D.; Wang, S.-L. Production of a thermostable chitosanase from shrimp heads via Paenibacillus mucilaginosus TKU032 conversion and its application in the preparation of bioactive chitosan oligosaccharides. Mar. Drugs 2019, 17, 217. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, V.B.; Nguyen, T.H.; Doan, C.T.; Tran, T.N.; Nguyen, A.D.; Kuo, Y.-H.; Wang, S.-L. Production and bioactivity-guided isolation of antioxidants with α-glucosidase inhibitory and anti-NO properties from marine chitinous materials. Mol. J. Synth. Chem. Nat. Prod. Chem. 2018, 23, 1124. [Google Scholar] [CrossRef] [PubMed]
- Doan, C.T.; Tran, T.N.; Nguyen, V.B.; Nguyen, A.D.; Wang, S.-L. Reclamation of marine chitinous materials for chitosanase production via microbial conversion by Paenibacillus macerans. Mar. Drugs 2018, 16, 429. [Google Scholar] [CrossRef] [PubMed]
- Doan, C.T.; Tran, T.N.; Nguyen, V.B.; Nguyen, A.D.; Wang, S.-L. Conversion of squid pens to chitosanases and proteases via Paenibacillus sp. TKU042. Mar. Drugs 2018, 16, 83. [Google Scholar] [CrossRef] [PubMed]
- Doan, C.T.; Tran, T.N.; Nguyen, M.T.; Nguyen, V.B.; Nguyen, A.D.; Wang, S.-L. Anti-α-glucosidase activity by a protease from Bacillus licheniformis. Molecules 2019, 24, 691. [Google Scholar] [CrossRef] [PubMed]
- Tran, T.N.; Doan, C.T.; Nguyen, A.D.; Wang, S.-L. The isolation of chitinase from Streptomyces thermocarboxydus and its application in the preparation of chitin oligomers. Res. Chem. Intermed. 2019, 45, 727–742. [Google Scholar] [CrossRef]
- Liang, T.-W.; Wang, S.-L. Recent advances in exopolysaccharides from Paenibacillus spp.: Production, isolation, structure, and bioactivities. Mar. Drugs 2015, 13, 1847–1863. [Google Scholar] [CrossRef]
- Liang, T.-W.; Wu, C.-C.; Cheng, W.-T.; Chen, Y.-C.; Wang, C.-L.; Wang, I.-L.; Wang, S.-L. Exopolysaccharides and antimicrobial biosurfactants produced by Paenibacillus macerans TKU029. Appl. Biochem. Biotechnol. 2014, 172, 933–950. [Google Scholar] [CrossRef]
- Liang, T.-W.; Tseng, S.-C.; Wang, S.-L. Production and characterization of antioxidant properties of exopolysaccharide (s) from Peanibacillus mucilaginosus TKU032. Mar. Drugs 2016, 14, 40. [Google Scholar] [CrossRef]
- Wang, S.-L.; Li, H.-T.; Zhang, L.-J.; Lin, Z.-H.; Kuo, Y.-H. Conversion of squid pen to homogentisic acid via Paenibacillus sp. TKU036 and the antioxidant and anti-inflammatory activities of homogentisic acid. Mar. Drugs 2016, 14, 183. [Google Scholar] [CrossRef]
- Nguyen, V.B.; Nguyen, A.D.; Wang, S.-L. Utilization of fishery processing by-product squid pens for α-glucosidase inhibitors production by Paenibacillus sp. Mar. Drugs 2017, 15, 274. [Google Scholar] [CrossRef] [PubMed]
- Hsu, C.-H.; Nguyen, A.D.; Chen, Y.-W.; Wang, S.-L. Tyrosinase inhibitors and insecticidal materials produced by Burkholderia cepacia using squid pen as the sole carbon and nitrogen source. Res. Chem. Intermed. 2014, 40, 2249–2258. [Google Scholar] [CrossRef]
- Liang, T.-W.; Chen, W.-T.; Lin, Z.-H.; Kuo, Y.-H.; Nguyen, A.D.; Pan, P.-S.; Wang, S.-L. An amphiprotic novel chitosanase from Bacillus mycoides and its application in the production of chitooligomers with their antioxidant and anti-inflammatory evaluation. Int. J. Mol. Sci. 2016, 17, 1302. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.-H.; Doan, C.T.; Nguyen, V.B.; Nguyen, A.D.; Wang, S.-L. Reclamation of fishery processing waste: A mini-review. Molecules 2019, 24, 2234. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, V.B.; Chen, S.-P.; Nguyen, T.H.; Nguyen, M.T.; Tran, T.T.T.; Doan, C.T.; Tran, T.N.; Nguyen, A.D.; Kuo, Y.-H.; Wang, S.-L. Novel efficient bioprocessing of marine chitins into active anticancer prodigiosin. Mar. Drugs 2020, 18, 15. [Google Scholar] [CrossRef] [PubMed]
- Tran, T.N.; Doan, C.T.; Nguyen, V.B.; Nguyen, A.D.; Wang, S.-L. Anti-oxidant and anti-diabetes potential of water-soluble chitosan–glucose derivatives produced by maillard reaction. Polymers 2019, 11, 1714. [Google Scholar] [CrossRef] [PubMed]
- Tran, T.N.; Doan, C.T.; Nguyen, M.T.; Nguyen, V.B.; Vo, T.P.K.; Nguyen, A.D.; Wang, S.-L. An exochitinase with N-Acetyl-β-glucosaminidase-like activity from shrimp head conversion by streptomyces speibonae and its application in hydrolyzing β-chitin powder to produce N-Acetyl-d-glucosamine. Polymers 2019, 11, 1600. [Google Scholar] [CrossRef]
- Wang, S.-L.; Yu, H.-T.; Tsai, M.-H.; Doan, C.T.; Nguyen, A.D. Conversion of squid pens to chitosanases and dye adsorbents via Bacillus cereus. Res. Chem. Intermed. 2018, 44, 4903–4911. [Google Scholar] [CrossRef]
- Dotto, G.L.; Rosa, G.S.; Moraes, M.A.; Weska, R.F.; Pinto, L.A.A. Treatment of chitin effluents by coagulation–flocculation with chitin and aluminum sulfate. J. Environ. Chem. Eng. 2013, 1, 50–55. [Google Scholar] [CrossRef]
- Zhao, L.; Xia, W. Stainless steel membrane UF coupled with NF process for the recovery of sodium hydroxide from alkaline wastewater in chitin processing. Desalination 2009, 249, 774–780. [Google Scholar] [CrossRef]
- Chen, X.; Li, C.; Ji, X.; Zhong, Z.; Li, P. Recovery of protein from discharged wastewater during the production of chitin. Bioresour. Technol. 2008, 99, 570–574. [Google Scholar] [CrossRef] [PubMed]
- Teh, C.Y.; Budiman, P.M.; Shak, K.P.Y.; Wu, T.Y. Recent advancement of coagulation–flocculation and its application in wastewater treatment. Ind. Eng. Chem. Res. 2016, 55, 4363–4389. [Google Scholar] [CrossRef]
- Sillanpää, M.; Ncibi, M.C.; Matilainen, A.; Vepsäläinen, M. Removal of natural organic matter in drinking water treatment by coagulation: A comprehensive review. Chemosphere 2018, 190, 54–71. [Google Scholar] [CrossRef] [PubMed]
- Jarvis, P.; Sharp, E.; Pidou, M.; Molinder, R.; Parsons, S.A.; Jefferson, B. Comparison of coagulation performance and floc properties using a novel zirconium coagulant against traditional ferric and alum coagulants. Water Res. 2012, 46, 4179–4187. [Google Scholar] [CrossRef] [PubMed]
- Jiang, J.-Q.; Lloyd, B. Progress in the development and use of ferrate(VI) salt as an oxidant and coagulant for water and wastewater treatment. Water Res. 2002, 36, 1397–1408. [Google Scholar] [CrossRef]
- Zhang, M.; Xiao, F.; Xu, X.Z.; Wang, D.S. Novel ferromagnetic nanoparticle composited PACls and their coagulation characteristics. Water Res. 2012, 46, 127–135. [Google Scholar] [CrossRef] [PubMed]
- Ahmadi, M.; Jorfi, S.; Kujlu, R.; Ghafari, S.; Soltani, R.D.C.; Haghighifard, N.J. A novel salt-tolerant bacterial consortium for biodegradation of saline and recalcitrant petrochemical wastewater. J. Environ. Manage. 2017, 191, 198–208. [Google Scholar] [CrossRef]
- Rao, L.N. Coagulation and flocculation of industrial wastewater by chitosan. IJEAS 2015, 2, 50–52. [Google Scholar]
- Das, R. Polymeric Materials for Clean Water; Springer: Berlin, Germany, 2019. [Google Scholar]
- Choy, S.Y.; Prasad, K.M.N.; Wu, T.Y.; Raghunandan, M.E.; Ramanan, R.N. Utilization of plant-based natural coagulants as future alternatives towards sustainable water clarification. J. Environ. Sci. 2014, 26, 2178–2189. [Google Scholar] [CrossRef]
- Choy, S.; Prasad, K.; Wu, T.; Ramanan, R. A review on common vegetables and legumes as promising plant-based natural coagulants in water clarification. Int. J. Environ. Sci. Technol. 2015, 12, 367. [Google Scholar] [CrossRef]
- Zeng, D.; Wu, J.; Kennedy, J.F. Application of a chitosan flocculant to water treatment. Carbohydr. Polym. 2008, 71, 135–139. [Google Scholar] [CrossRef]
- Renault, F.; Sancey, B.; Badot, P.M.; Crini, G. Chitosan for coagulation/flocculation processes—An eco-friendly approach. Eur. Polym. J. 2009, 45, 1337–1348. [Google Scholar] [CrossRef]
- Sahoo, D.; Sahoo, S.; Mohanty, P.; Sasmal, S.; Nayak, P.L. Chitosan: A new versatile bio-polymer for various applications. Des. Monomers Polym. 2012, 12, 377–404. [Google Scholar] [CrossRef]
- Muzzarelli, R.A. Chitin; Elsevier: Amsterdam, The Netherlands, 2013. [Google Scholar]
- Sakkayawong, N.; Thiravetyan, P.; Nakbanpote, W. Adsorption mechanism of synthetic reactive dye wastewater by chitosan. J. Colloid Interf. 2005, 286, 36–42. [Google Scholar] [CrossRef] [PubMed]
- Wan Ngah, W.S.; Teong, L.C.; Hanafiah, M.A.K.M. Adsorption of dyes and heavy metal ions by chitosan composites: A review. Carbohydr. Polym. 2011, 83, 1446–1456. [Google Scholar] [CrossRef]
- American Public Health Association. Standard methods for the examination of water and wastewater. In 5220 Chemical Oxygen Demand (COD), 20th ed.; American Public Health Association: Washington, DC, USA, 1999. [Google Scholar]
- American Public Health Association. Standard Methods for the Examination of Water and Wastewater, 22nd ed.; American Water Works Association: Denver, CO, USA; Water Environment Federation: Virginia, VA, USA, 2012. [Google Scholar]
- FAO. Food energy-methods of analysis and conversion factors. Report of a technical workshop. In FAO Food and Nutrition Paper No. 77; Food and Agricultural Organazation of the United Nations: Rome, Italy, 2003. [Google Scholar]
- Box, G.E.; Wilson, K.G. On the experimental attainment of optimum conditions. J. Royal Stat. Soc. 1951, 13, 1–45. [Google Scholar] [CrossRef]
- Saïed, N.; Aider, M. Zeta potential and turbidimetry analyzes for the evaluation of chitosan/phytic acid complex formation. J. Food Res. 2014, 3, 71–81. [Google Scholar] [CrossRef]
- Rioyo, J.; Aravinthan, V.; Bundschuh, J.; Lynch, M. Research on ‘high-pH precipitation treatment’ for RO concentrate minimization and salt recovery in a municipal groundwater desalination facility. Desalination 2018, 439, 168–178. [Google Scholar] [CrossRef]
- Cui, X.; Zhou, D.; Fan, W.; Huo, M.; Crittenden, J.C.; Yu, Z.; Ju, P.; Wang, Y. The effectiveness of coagulation for water reclamation from a wastewater treatment plant that has a long hydraulic and sludge retention times: A case study. Chemosphere 2016, 157, 224–231. [Google Scholar] [CrossRef]
- Subramonian, W.; Wu, T.Y.; Chai, S.-P. An application of response surface methodology for optimizing coagulation process of raw industrial effluent using Cassia obtusifolia seed gum together with alum. Ind. Crop. Prod. 2015, 70, 107–115. [Google Scholar] [CrossRef]

| Parameters | Values |
|---|---|
| PH | 3.55–7.41 |
| COD (mg·L−1) | 4245–23,600 |
| TKN (mg·L−1) | 639–1395 |
| NH4+–N (mg·L−1) | 145–842 |
| TP (mg·L−1) | 53–366 |
| TSS (mg·L−1) | 1880–10,400 |
| Ca2+ (mg·L−1) | 1200–3163 |
| Crude protein (mg·L−1) | 3994–8719 |
| Turbidity (NTU) | 363–1254 |
| No | Experiment 1 (Chitosan) | Experiment 2 (PAC) | Experiment 3 (PAA) | Experiment 4 (PAC and PAA) | Experiment 5 (optimization) | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Initial turbidity: 523 NTU Turbidity after settling: 79 NTU | Initial turbidity: 493 NTU Turbidity after settling: 81 NTU | Initial turbidity: 511 NTU Turbidity after settling: 97 NTU | Initial turbidity: 511 NTU Turbidity after settling: 100 NTU | Initial turbidity: 963 NTU Turbidity after settling: 184 NTU | |||||||||||||||||
| pH/Chitosan (mg·L−1)/Turbidity (NTU) | TRE * (%) | pH/PAC (mg·L−1)/Turbidity (NTU) | TRE * (%) | pH/PAA (mg·L−1)/Turbidity (NTU) | TRE * (%) | pH/PAC (mg·L−1)/PAA (mg·L−1)/Turbidity (NTU) | TRE * (%) | pH/Chitosan (mg·L−1)/Turbidity (NTU) | TRE * (%) | ||||||||||||
| 1 | 10.0 | 140.0 | 6 | 92.4 | 10.0 | 90.0 | 10 | 87.7 | 10.0 | 140.0 | 13 | 86.6 | 10.0 | 90.0 | 60.0 | 8 | 92.0 | 5.0 | 50.0 | 148 | 19.6 |
| 2 | 4.0 | 140.0 | 67 | 15.2 | 4.0 | 90.0 | 29 | 64.2 | 4.0 | 140.0 | 92 | 5.2 | 4.0 | 90.0 | 60.0 | 37 | 63.0 | 9.0 | 50.0 | 23 | 87.5 |
| 3 | 10.0 | 40.0 | 12 | 84.8 | 10.0 | 30.0 | 9 | 88.9 | 10.0 | 40.0 | 8 | 91.8 | 10.0 | 30.0 | 60.0 | 8 | 92.0 | 5.0 | 100.0 | 150 | 18.5 |
| 4 | 4.0 | 40.0 | 70 | 11.4 | 4.0 | 30.0 | 40 | 50.6 | 4.0 | 40.0 | 86 | 11.3 | 4.0 | 30.0 | 60.0 | 49 | 51.0 | 9.0 | 100.0 | 20 | 89.1 |
| 5 | 11.2 | 90.0 | 8 | 89.9 | 11.2 | 60.0 | 5 | 93.8 | 11.2 | 90.0 | 2 | 97.9 | 10.0 | 90.0 | 20.0 | 6 | 94.0 | 9.8 | 75.0 | 19 | 89.7 |
| 6 | 2.8 | 90.0 | 58 | 26.6 | 2.8 | 60.0 | 43 | 46.9 | 2.8 | 90.0 | 87 | 10.3 | 4.0 | 90.0 | 20.0 | 32 | 68.0 | 4.2 | 75.0 | 153 | 16.8 |
| 7 | 7.0 | 160.7 | 49 | 38.0 | 7.0 | 102.4 | 13 | 84.0 | 7.0 | 160.7 | 18 | 81.4 | 10.0 | 30.0 | 20.0 | 6 | 94.0 | 7.0 | 110.4 | 12 | 93.48 |
| 8 | 7.0 | 19.3 | 27 | 65.8 | 7.0 | 17.6 | 16 | 80.2 | 7.0 | 19.3 | 14 | 85.6 | 4.0 | 30.0 | 20.0 | 44 | 56.0 | 7.0 | 39.7 | 13 | 92.93 |
| 9 | 7.0 | 90.0 | 12 | 84.8 | 7.0 | 60.0 | 13 | 84.0 | 7.0 | 90.0 | 15 | 84.5 | 11.1 | 60.0 | 40.0 | 2 | 98.0 | 7.0 | 75.0 | 21 | 88.6 |
| 10 | 7.0 | 90.0 | 13 | 83.5 | 7.0 | 60.0 | 13 | 84.0 | 7.0 | 90.0 | 18 | 81.4 | 3.0 | 60.0 | 40.0 | 38 | 62.0 | 7.0 | 75.0 | 19 | 89.7 |
| 11 | 7.0 | 90.0 | 19 | 75.9 | 7.0 | 60.0 | 17 | 79.0 | 7.0 | 90.0 | 17 | 82.5 | 7.0 | 100.6 | 40.0 | 13 | 87.0 | 7.0 | 75.0 | 18 | 90.2 |
| 12 | 7.0 | 19.4 | 40.0 | 13 | 87.0 | ||||||||||||||||
| 13 | 7.0 | 60.0 | 67.1 | 13 | 87.0 | ||||||||||||||||
| 14 | 7.0 | 60.0 | 12.9 | 13 | 87.0 | ||||||||||||||||
| 15 | 7.0 | 60.0 | 40.0 | 14 | 86.0 | ||||||||||||||||
| 16 | 7.0 | 60.0 | 40.0 | 13 | 87.0 | ||||||||||||||||
| 17 | 7.0 | 60.0 | 40.0 | 13 | 87.0 | ||||||||||||||||
| 18 | 10.6 | 86.4 | - | 99.4 | 11.2 | 17.6 | - | 93.5 | 9.8 | 79.3 | - | 99.5 | 11.0 | 48.1 | 39.2 | - | 98.9 | 8.3 | 77.5 | - | 99.3 |
| Coagulants | Samples | pH | tCOD (mg·L−1) | sCOD (mg·L−1) | TKN (mg·L−1) | NH4+-N (mg·L−1) | TP (mg·L−1) | TSS (mg·L−1) | Ca2+ (mg·L−1) | Crude Protein (mg·L−1) |
|---|---|---|---|---|---|---|---|---|---|---|
| Preliminary sedimentation | Inlet wastewater | 5.6 | 4245 | 1621 | 639 | 145 | 53 | 1880 | 1200 | 3996 |
| After settling | 4.0 | 2612 | 1584 | 579 | 133 | 40 | 139 | 1233 | 3617 | |
| * RE (%) | 38 | 2 | 9 | 8 | 25 | 93 | −3 | 9 | ||
| Chitosan 86.4 mg·L−1 | After coagulation | 10.6 | 1741 | 1463 | 453 | 103 | 5.2 | 39 | 587 | 2829 |
| ** REC (%) | 33 | 8 | 22 | 23 | 87 | 72 | 52 | 22 | ||
| *** ToRE (%) | 59 | 10 | 29 | 29 | 90 | 98 | 51 | 29 | ||
| PAC 17.6 mg·L−1 | After coagulation | 11.2 | 2116 | 1403 | 448 | 95 | 4.9 | 51 | 673 | 2800 |
| ** REC (%) | 19 | 11 | 23 | 29 | 88 | 63 | 45 | 23 | ||
| *** ToRE (%) | 50 | 13 | 30 | 34 | 91 | 97 | 44 | 30 | ||
| PAA 79.3 mg·L−1 | After coagulation | 9.8 | 2298 | 1246 | 520 | 146 | 4.2 | 38 | 587 | 3252 |
| ** REC (%) | 12 | 21 | 10 | −10 | 90 | 73 | 52 | 10 | ||
| *** ToRE (%) | 46 | 23 | 19 | −1 | 92 | 98 | 51 | 19 | ||
| Mixture of PAC and PAA 87.3 mg·L−1 | After coagulation | 11.0 | 1875 | 1645 | 462 | 98 | 4.3 | 44 | 660 | 2888 |
| ** REC (%) | 28 | −4 | 20 | 26 | 89 | 68 | 46 | 20 | ||
| *** ToRE (%) | 56 | −1 | 28 | 32 | 92 | 98 | 45 | 28 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tran, N.V.N.; Yu, Q.J.; Nguyen, T.P.; Wang, S.-L. Coagulation of Chitin Production Wastewater from Shrimp Scraps with By-Product Chitosan and Chemical Coagulants. Polymers 2020, 12, 607. https://doi.org/10.3390/polym12030607
Tran NVN, Yu QJ, Nguyen TP, Wang S-L. Coagulation of Chitin Production Wastewater from Shrimp Scraps with By-Product Chitosan and Chemical Coagulants. Polymers. 2020; 12(3):607. https://doi.org/10.3390/polym12030607
Chicago/Turabian StyleTran, Nguyen Van Nhi, Qiming Jimmy Yu, Tan Phong Nguyen, and San-Lang Wang. 2020. "Coagulation of Chitin Production Wastewater from Shrimp Scraps with By-Product Chitosan and Chemical Coagulants" Polymers 12, no. 3: 607. https://doi.org/10.3390/polym12030607
APA StyleTran, N. V. N., Yu, Q. J., Nguyen, T. P., & Wang, S.-L. (2020). Coagulation of Chitin Production Wastewater from Shrimp Scraps with By-Product Chitosan and Chemical Coagulants. Polymers, 12(3), 607. https://doi.org/10.3390/polym12030607







