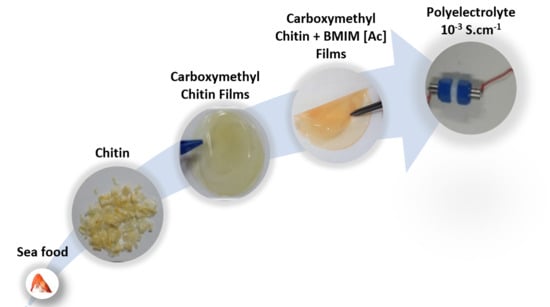
Chemical Modification and Processing of Chitin for Sustainable Production of Biobased Electrolytes
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
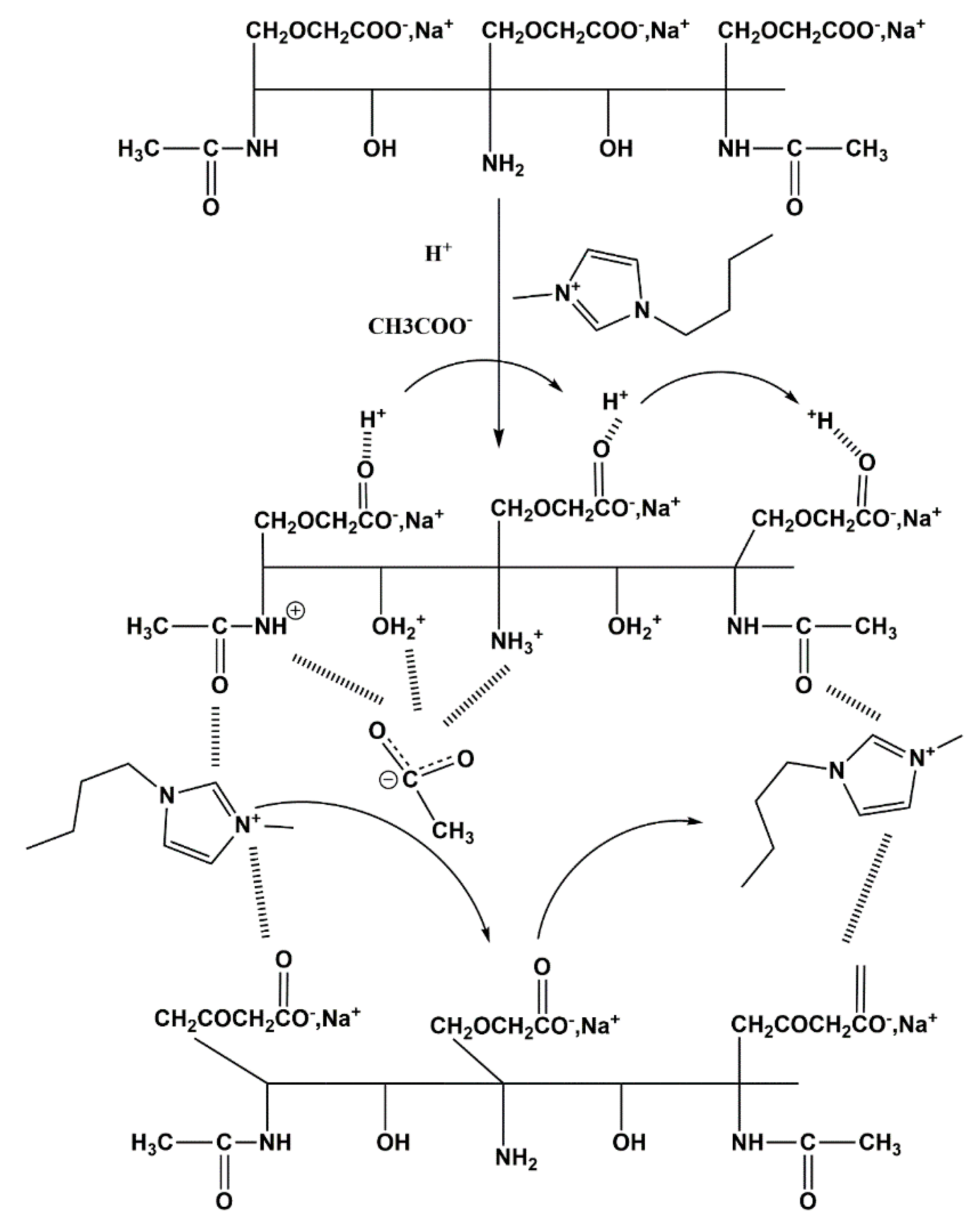
2.2. Preparation of Carboxymethylatedchitin
2.3. Films Processing
2.4. Fourier Transformed Infra-Red (FTIR)
2.5. Nuclear Magnetic Resonance Spectroscopy (NMR)
2.6. X-ray Diffraction (XRD)
2.7. Field Emission Scanning Electron Microscopy (FE-SEM)
2.8. Thermogravimetric Analyses (TGA)
2.9. Potentiometric Titration
2.10. Ionic Conductivity Measurements
3. Results and Discussion
3.1. Preparation and Characterization of Chitin Derivatives
3.2. Films Processing and Characterization
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Stephan, A.M.; Nahm, K.S. Review on composite polymer electrolytes for lithium batteries. Polymer 2006, 47, 5952–5964. [Google Scholar] [CrossRef]
- Armand, M. Polymer solid electrolytes—An overview. Solid State Ion. 1983, 9, 745–754. [Google Scholar] [CrossRef]
- Watanabe, M.; Thomas, M.L.; Zhang, S.; Ueno, K.; Yasuda, T.; Dokko, K. Application of Ionic Liquids to Energy Storage and Conversion Materials and Devices. Chem. Rev. 2017, 117, 7190–7239. [Google Scholar] [CrossRef] [PubMed]
- Angulakshmi, N.; Prem, K.T.; Sabu, T.; Manuel, S.A. Ionic conductivity and interfacial properties of nanochitin-incorporated polyethylene oxide-LiN (C2F5SO2)2 polymer electrolytes. Electrochim. Acta 2010, 55, 1401–1406. [Google Scholar] [CrossRef]
- Leones, R.; Sentanin, F.; Rodrigues, L.C.; Marrucho, I.M.; Esperança, J.M.S.S.; Pawlicka, A.; Silva, M.M. Investigation of polymer electrolytes based on agar and ionic liquids. Express Polym. Lett. 2012, 6, 1007–1016. [Google Scholar] [CrossRef]
- Liew, C.W.; Ramesh, S. Studies on ionic liquid-based corn starch biopolymer electrolytes coupling with high ionic transport number. Cellulose 2013, 20, 3227–3237. [Google Scholar] [CrossRef]
- Ou, R.; Xie, Y.; Shen, X.; Yuan, F.; Wang, H.; Wang, Q. Solid biopolymer electrolytes based on all-cellulose composites prepared by partially dissolving cellulosic fibers in the ionic liquid 1-butyl-3- methylimidazolium chloride. J. Mater. Sci. 2012, 47, 5978–5986. [Google Scholar] [CrossRef]
- Ramesh, S.; Shanti, R.; Morris, E. Employment of [Amim] Cl in the effort to upgrade the properties of cellulose acetate based polymer electrolytes. Cellulose 2013, 20, 1377–1389. [Google Scholar] [CrossRef]
- Singh, R.; Reddy, A.; Bhattacharya, B.; Rhee, H.-W.; Varlikli, C. Perspectives for solid biopolymer electrolytes in dye sensitized solar cell and battery application. Renew. Sustain. Energy Rev. 2016, 65, 1098–1117. [Google Scholar] [CrossRef]
- Cǎpriţǎ, R.; Cǎpriţǎ, A.; Julean, C. Biochemical Aspects of Non-Starch Polysaccharides. Sci. Paper. Anim. Sci. Biotechnol. 2010, 43, 368–375. [Google Scholar]
- Azizi Samir, M.A.S.; Alloin, F.; Dufresne, A. Review of Recent Research Into Cellulose Whiskers, Their Properties and Their Application in Nanocomposite Field. Biomacromolecules 2005, 6, 612–626. [Google Scholar] [CrossRef] [PubMed]
- Azizi Samir, M.A.S.; Alloin, F.; Sanchez, J.Y.; Dufresne, A. Cross-linked nanocomposite polymer electrolytes reinforced with cellulose whiskers. Macromolecules 2004, 37, 4839–4844. [Google Scholar] [CrossRef]
- Yamazaki, S.; Takegawa, A.; Kaneko, Y.; Kadokawa, J.-I.; Yamagata, M. An acidic cellulose-chitin hybrid gel as novel electrolyte for an electric double layer capacitor. Electrochem. Commun. 2009, 11, 68–70. [Google Scholar] [CrossRef]
- Li, H.; Greene, L.H. Sequence and Structural Analysis of the Chitinase Insertion Domain Reveals Two Conserved Motifs Involved in Chitin-Binding. PLoS ONE 2010, 5, e8654. [Google Scholar] [CrossRef]
- Rinaudo, M. Chitin and chitosan: Properties and applications. Prog. Polym. Sci. 2006, 31, 603–632. [Google Scholar] [CrossRef]
- Synowiecki, J.; Al-Khateeb, N.A. Production, Properties, and Some New Applications of Chitin and Its Derivatives. Crit. Rev. Food Sci. Nutr. 2003, 43, 145–171. [Google Scholar] [CrossRef]
- Zargar, V.; Asghari, M.; Dashti, A. A Review on Chitin and Chitosan Polymers: Structure, Chemistry, Solubility, Derivatives, and Applications. ChemBioEng Rev. 2015, 2, 204–226. [Google Scholar] [CrossRef]
- Clark, J.; Clarke, H.M.; Cutler, R.; Cooper, D.; Edwards, D.E.; Rennie, R.; Ward, D.E. A Dictionary of Chemistry; Daintith, J., Ed.; Oxford University Press: Oxford, UK, 2008; p. 49. [Google Scholar]
- Krajewska, B. Application of chitin- and chitosan-based materials for enzyme immobilizations: A review. Enzym. Microb. Technol. 2004, 35, 126–139. [Google Scholar] [CrossRef]
- Feng, F.; Liu, Y.; Hu, K. Influence of alkali-freezing treatment on the solid state structure of chitin. Carbohydr. Res. 2004, 339, 2321–2324. [Google Scholar] [CrossRef]
- Jang, M.K.; Kong, B.G.; Jeong, Y.I.; Lee, C.H.; Nah, J.W. Physicochemical characterization of α-chitin, β-chitin, and γ-chitin separated from natural resources. J. Polym. Sci. Part A Polym. Chem. 2004, 42, 3423–3432. [Google Scholar] [CrossRef]
- Dutta, P.K.; Dutta, J.; Tripathi, V.S. Chitin and chitosan: Chemistry, properties and applications. J. Sci. Ind. Res. 2004, 63, 20–31. [Google Scholar]
- Jain, T.; Kumar, S.; Dutta, P.K. Carboxymethylchitin Nanocarrier (CMCNC): A Novel Therapeutic Formulation for Drug Release. Polym. Plast. Technol. Mater. 2019, 58, 55–69. [Google Scholar] [CrossRef]
- Pokhrel, S.; Yadav, P.N.; Adhikari, R. Applications of Chitin and Chitosan in Industry and Medical Science: A Review. Nepal J. Sci. Technol. 2015, 16, 99–104. [Google Scholar] [CrossRef]
- Sridevi, N.A.; Karuppasamy, K.; Balakumar, S.; Shajan, X.S. Mechanical and ionic conductivity studies on polymer electrolytes incorporated with chitin nanofiber for rechargeable magnesium ion batteries. Int. Journal Sci. Technol. Manag. 2015, 4, 171–180. [Google Scholar]
- Stephan, A.M.; Kumar, T.P.; Kulandainathan, M.A.; Lakshmi, N.A. Chitin-Incorporated Poly(ethylene oxide)-Based Nanocomposite Electrolytes for Lithium Batteries. J. Phys. Chem. B 2009, 113, 1963–1971. [Google Scholar] [CrossRef]
- Pushpamalar, V.; Langford, S.J.; Ahmad, M.; Lim, Y.Y. Optimization of reaction conditions for preparing carboxymethyl cellulose from sago waste. Carbohydr. Polym. 2006, 64, 312–318. [Google Scholar] [CrossRef]
- Qi, H.; Liebert, T.; Meister, F.; Heinze, T. Homogenous carboxymethylation of cellulose in the NaOH/urea aqueous solution. React. Funct. Polym. 2009, 69, 779–784. [Google Scholar] [CrossRef]
- Ramos, L.A.; Frollini, E.; Heinze, T. Carboxymethylation of cellulose in the new solvent dimethyl sulfoxide/tetrabutylammonium fluoride. Carbohydr. Polym. 2005, 60, 259–267. [Google Scholar] [CrossRef]
- Spychaj, T.; Wilpiszewska, K.; Zdanowicz, M. Medium and high substituted carboxymethyl starch: Synthesis, characterization and application. Starch-Stärke 2013, 65, 22–33. [Google Scholar] [CrossRef]
- Sun, G.-Z.; Chen, X.-G.; Li, Y.-Y.; Zheng, B.; Gong, Z.-H.; Sun, J.-J.; Chen, H.; Li, J.; Lin, W.-X. Preparation of H-oleoyl-carboxymethyl-chitosan and the function as a coagulation agent for residual oil in aqueous system. Front. Mater. Sci. China 2008, 2, 105–112. [Google Scholar] [CrossRef]
- Tokura, S.; Nishimura, S.; Nishi, N. Studies on chitin IX. specific binding of Calcium Ions by Carboxymethyl-Chitin. Polym. J. 1983, 15, 597–602. [Google Scholar] [CrossRef][Green Version]
- Ding, F.; Qian, X.; Zhang, Q.; Wu, H.; Liu, Y.; Xiao, L.; Deng, H.; Du, Y.; Shi, X. Electrochemically induced reversible formation of carboxymethyl chitin hydrogel and tunable protein release. New J. Chem. 2015, 39, 1253–1259. [Google Scholar] [CrossRef]
- Brugnerotto, J.; Lizardi, J.; Goycoolea, F.M.; Argüelles-Monal, W.; Desbrières, J.; Rinaudo, M. An infrared investigation in relation with chitin and chitosan characterization. Polymer 2001, 42, 3569–3580. [Google Scholar] [CrossRef]
- Fung, B.M.; Khitrin, A.K.; Ermolaev, K. An Improved Broadband Decoupling Sequence for Liquid Crystals and Solids. J. Magn. Reson. 2000, 142, 97–101. [Google Scholar] [CrossRef] [PubMed]
- Shigenobu, H.; Kikuko, H. Chemical Shift Standards in High-Resolution Solid-State NMR (1) 13C, 29Si, and 1H Nuclei. Bull. Chem. Soc. Jpn. 1991, 64, 685–687. [Google Scholar]
- Morcombe, C.R.; Zilm, K.W. Chemical shift referencing in MAS solid state NMR. J. Magn. Reson. 2003, 162, 479–486. [Google Scholar] [CrossRef]
- van Meerten, S.G.J.; Franssen, W.M.J.; Kentgens, A.P.M. ssNake: A cross-platform open-source NMR data processing and fitting application. J. Magn. Reson. 2019, 301, 56–66. [Google Scholar] [CrossRef]
- David, G.; Gontard, N.; Guerin, D.; Heux, L.; Lecomte, J.; Molina-Boisseau, S.; Angellier-Coussy, H. Exploring the potential of gas-phase esterification to hydrophobize the surface of micrometric cellulose particles. Eur. Polym. J. 2019, 115, 138–146. [Google Scholar] [CrossRef]
- Eyler, R.W.; Klug, E.D.; Diephuis, F. Determination of Degree of Substitution of Sodium Carboxymethylcellulose. Anal. Chem. 1947, 19, 24–27. [Google Scholar] [CrossRef]
- Tan, S.C.; Khor, E.; Tan, T.K.; Wong, S.M. The degree of deacetylation of chitosan: Advocating the first derivative UV-spectrophotometry method of determination. Talanta 1998, 45, 713–719. [Google Scholar] [CrossRef]
- Cárdenas, G.; Cabrera, G.; Taboada, E.; Miranda, S.P. Chitin characterization by SEM, FTIR, XRD, and13C cross polarization/mass angle spinning NMR. J. Appl. Polym. Sci. 2004, 93, 1876–1885. [Google Scholar] [CrossRef]
- Sagheer, F.A.A.; Al-Sughayer, M.A.; Muslim, S.; Elsabee, M.Z. Extraction and characterization of chitin and chitosan from marine sources in Arabian Gulf. Carbohydr. Polym. 2009, 77, 410–419. [Google Scholar] [CrossRef]
- Straus, S.K.; Bremi, T.; Ernst, R.R. Side-chain conformation and dynamics in a solid peptide: CP-MAS NMR study of valine rotamers and methyl-group relaxation in fully 13C-labelled antamanide. J.Biomol. NMR 1997, 10, 119–128. [Google Scholar] [CrossRef]
- Mobarak, N.N.; Ramli, N.; Ahmad, A.; Abdullah, M.P. Development of Carboxymethyl Chitosan Based Green Polymer Electrolyte. J. Biobased Mater. Bioenergy 2013, 7, 267–268. [Google Scholar] [CrossRef]
- Ma, G.; Yang, D.; Zhou, Y.; Xiao, M.; Kennedy, J.F.; Nie, J. Preparation and characterization of water-soluble N-alkylated chitosan. Carbohydr. Polym. 2008, 74, 121–126. [Google Scholar] [CrossRef]
- Kittur, F.S.; Prashanth, K.V.H.; Sankar, K.U.; Tharanathan, R.N. Characterization of chitin, chitosan and their carboxymethyl derivatives by differential scanning calorimetry. Carbohydr. Polym. 2002, 49, 185–193. [Google Scholar] [CrossRef]
- Zong, Z.; Kimura, Y.; Takahashi, M.; Yamane, H. Characterization of chemical and solid state structures of acylated chitosans. Polymer 2000, 41, 899–906. [Google Scholar] [CrossRef]
- Wan, Y.; Creber, K.A.M.; Peppley, B.; Bui, V.T. Ionic conductivity of chitosan membranes. Polymer 2003, 44, 1057–1065. [Google Scholar] [CrossRef]
- Shamsudin, I.J.; Ahmad, A.; Hassan, N.H.; Kaddami, H. Bifunctional ionic liquid in conductive biopolymer based on chitosan for electrochemical devices application. Solid State Ion. 2015, 278, 11–19. [Google Scholar] [CrossRef]
- Shamsudin, I.J. Investigation on the Effects of Imidazolium Ionic Liquids on the Derivatives of Chitosan and Kappa Carrageenan Based Biopolymer Electrolytes for Application in Electrochemical Devices. In Faculty of Science and Technology; Universiti Kebangsaan Malaysia: Bangi, Malaysia, 2016; pp. 1–170. [Google Scholar]









| Chemical Shift (ppm) | ||||||||
|---|---|---|---|---|---|---|---|---|
| Samples | C1 | C2 | C3/C5 | C4 | C6 | CH3 | C = O * | C = O ** |
| Chitin | 105.2 | 57.8 | 75.46 | 83.5 | 59.9 | 24.0 | 164.8 | 174.8 |
| CMChit_15 | 108.7 | 61.5 | 78.7 | 87.4 | 64.8 | 27.4 | 168.3 | 180.2 |
| CMChit_30 | 108.6 | 61.5 | 78.7 | 87.4 | 64.8 | 27.2 | 168.3 | 180.7 |
| CMChit_45 | 108.6 | 61.3 | 78.6 | 87.4 | 64.8 | 27.4 | 168.4 | 180.6 |
| Potentiometric Titration | FTIR Analyses | NMR | XRD | TGA | EIS | ||||
|---|---|---|---|---|---|---|---|---|---|
| Samples | DS | DA | DA | DS | DA | CI (Crystalline Index) | Td (Onset) °C | T (Peak) °C | Ionic Conductivity S/cm |
| Chitin | - | 69 ± 1 | 68 | - | 82 | 63% | 215 | 273 | 1.55 × 10−9 * |
| CMChit_15 | 0.93 ± 0.05 | 64 ± 3 | 63 | 1.73 | 75 | 27% | 215 | 273 | 9.21 × 10−6 |
| CMChit_30 | 0.95 ± 0.05 | 60 ± 2 | 61 | 1.90 | 70 | 24% | 215 | 270 | 4.17 × 10−6 |
| CMChit_45 | 0.92 ± 0.10 | 51 ± 4 | 56 | 2.85 | 65 | 24% | 210 | 272 | 3.08 × 10−6 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Latifi, M.; Ahmad, A.; Kaddami, H.; Hasyareeda Hassan, N.; Dieden, R.; Habibi, Y. Chemical Modification and Processing of Chitin for Sustainable Production of Biobased Electrolytes. Polymers 2020, 12, 207. https://doi.org/10.3390/polym12010207
Latifi M, Ahmad A, Kaddami H, Hasyareeda Hassan N, Dieden R, Habibi Y. Chemical Modification and Processing of Chitin for Sustainable Production of Biobased Electrolytes. Polymers. 2020; 12(1):207. https://doi.org/10.3390/polym12010207
Chicago/Turabian StyleLatifi, Meriem, Azizan Ahmad, Hamid Kaddami, Nur Hasyareeda Hassan, Reiner Dieden, and Youssef Habibi. 2020. "Chemical Modification and Processing of Chitin for Sustainable Production of Biobased Electrolytes" Polymers 12, no. 1: 207. https://doi.org/10.3390/polym12010207
APA StyleLatifi, M., Ahmad, A., Kaddami, H., Hasyareeda Hassan, N., Dieden, R., & Habibi, Y. (2020). Chemical Modification and Processing of Chitin for Sustainable Production of Biobased Electrolytes. Polymers, 12(1), 207. https://doi.org/10.3390/polym12010207