Monitoring of Peroxide in Gamma Irradiated EVA Multilayer Film Using Methionine Probe
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample Bags
2.2. Gamma Irradiation
2.3. Ageing and Storage Times
2.4. Chemicals and Reagents
2.5. Methionine Solution Filling
2.6. HPLC System and Conditions
2.7. Results Treatment
2.8. Design of Experiments
2.8.1. Factors and Experimental Domain of Interest
2.8.2. General Protocol
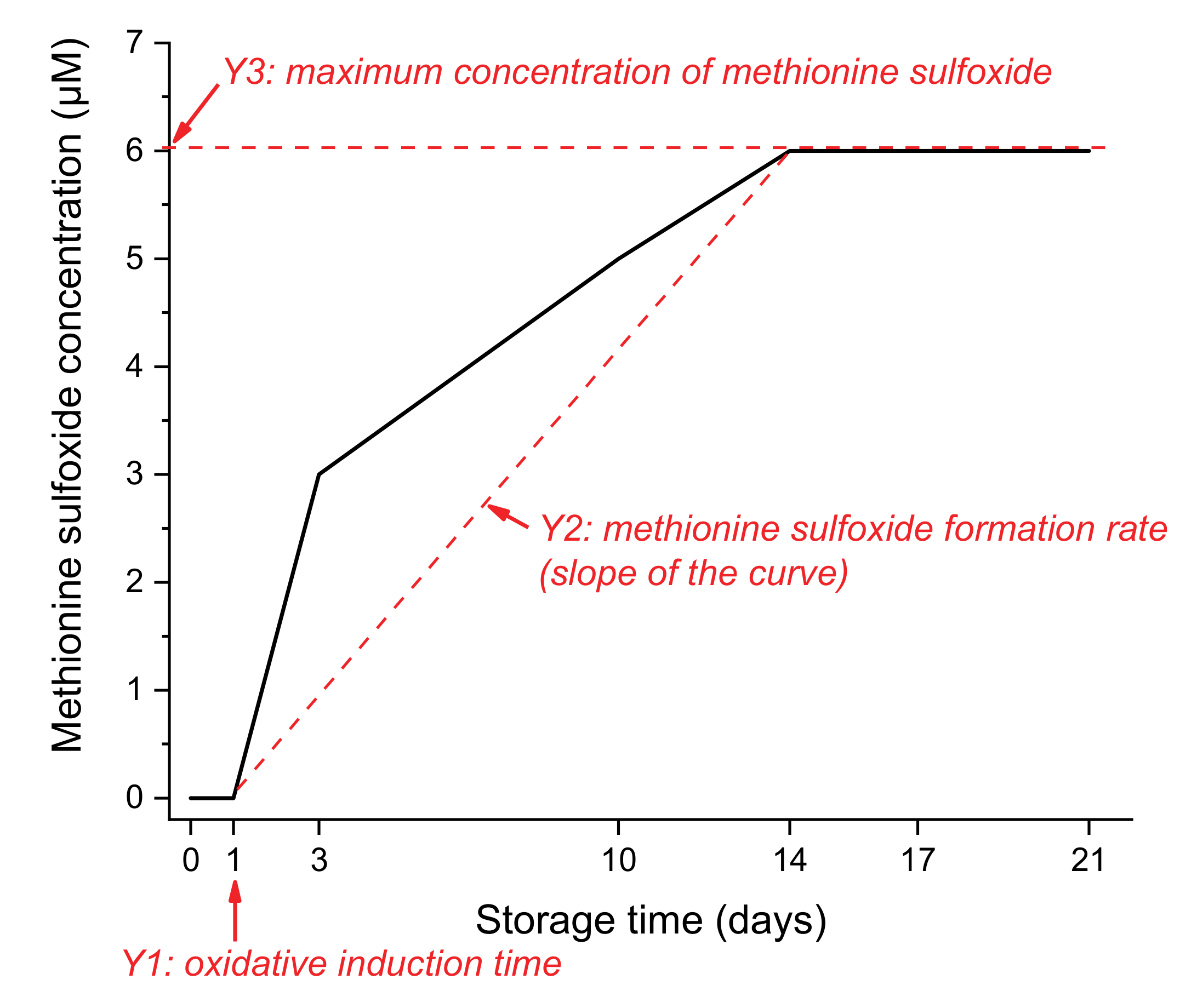
- Y1: the oxidative induction time in days. For example, in Figure 3, the oxidative induction time for the red curve is 1 day.
- Y2: the methionine sulfoxide formation rate represented by the slope of the methionine sulfoxide concentration-storage time curve. For example, in Figure 3, the slope of the red curve is obtained with x and y values at 1, 3, and 10 days.
- Y3: the maximum concentration of methionine sulfoxide detected in µM. This response is obtained by averaging the four concentrations obtained at 10, 14, 17, and 21 days.
3. Results
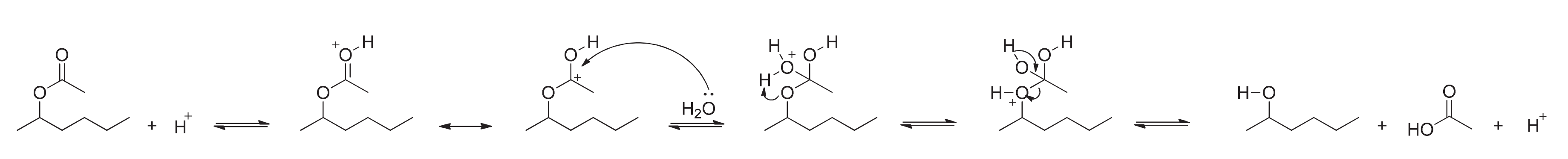
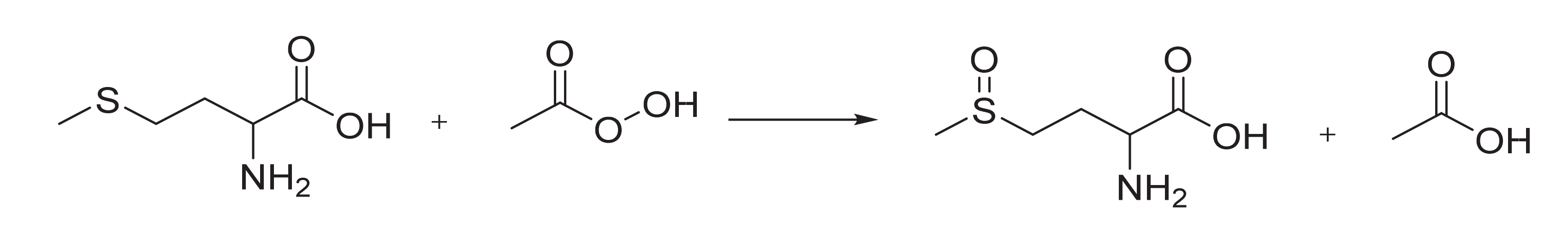
3.1. Methionine Oxidation Conditions
3.2. Methionine Oxidation Kinetics
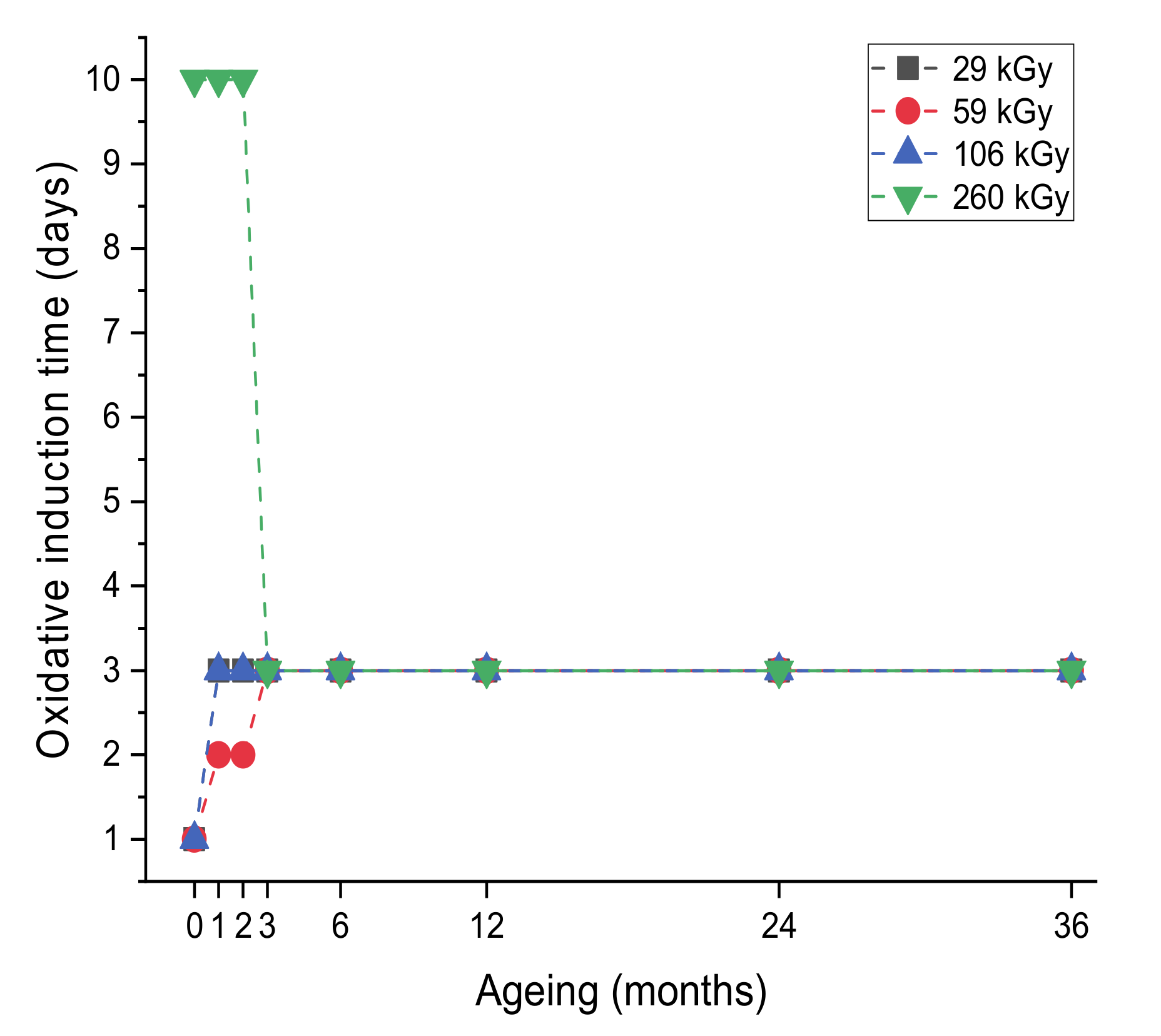
3.2.1. Evolution of the Oxidative Induction Time (Y1)
3.2.2. Evolution of Methionine Sulfoxide Formation Rate (Y2)
3.2.3. Evolution of the Maximum Methionine Sulfoxide Concentration Detected (Y3)
3.3. Modeling
3.3.1. Mathematical Modeling
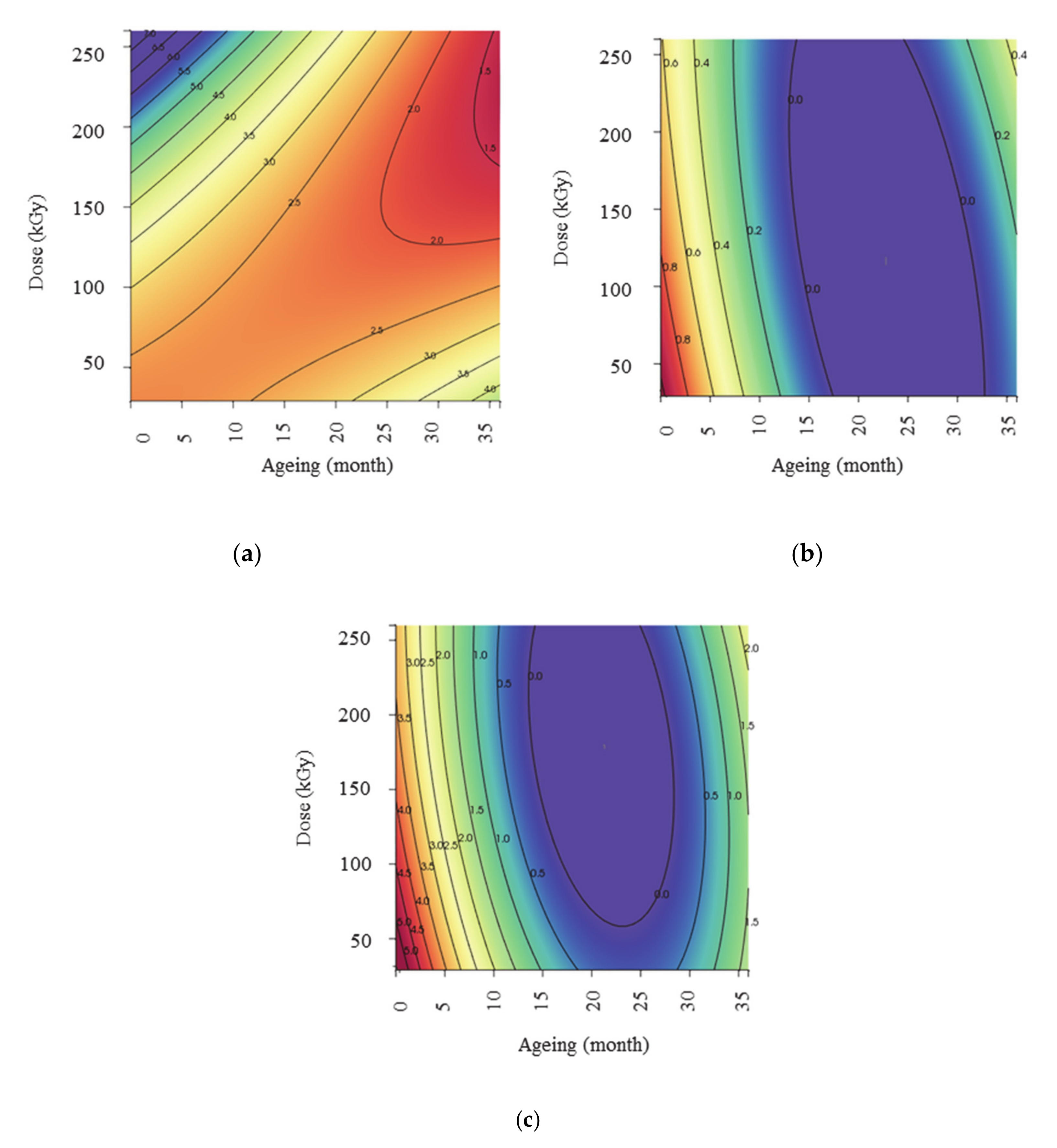
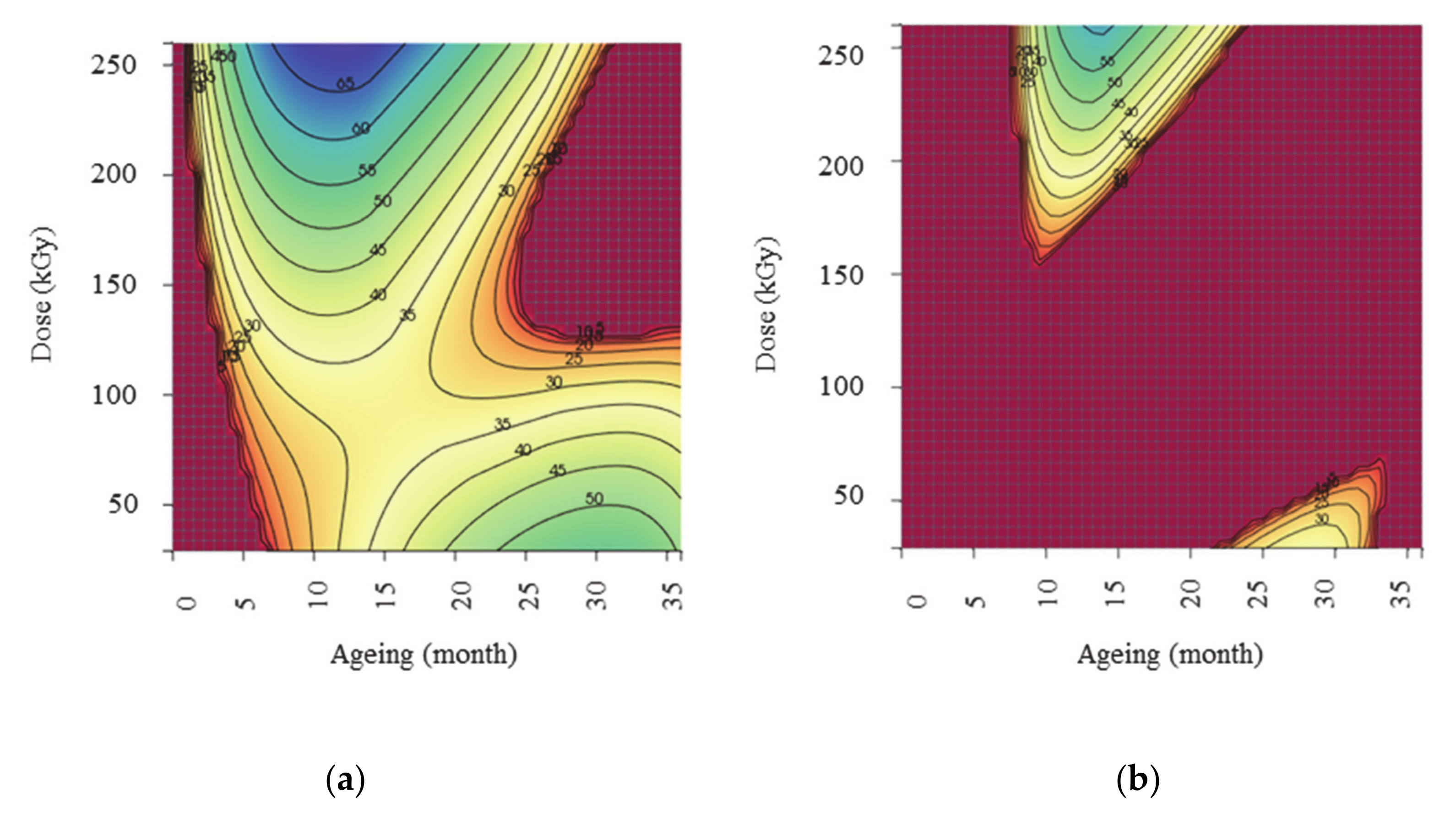
3.3.2. Interpretation of Response Surfaces
Oxidative Induction Time (Y1)
Methionine Sulfoxide Formation Rate (Y2)
Maximum Concentration of Methionine Sulfoxide Detected (Y3)
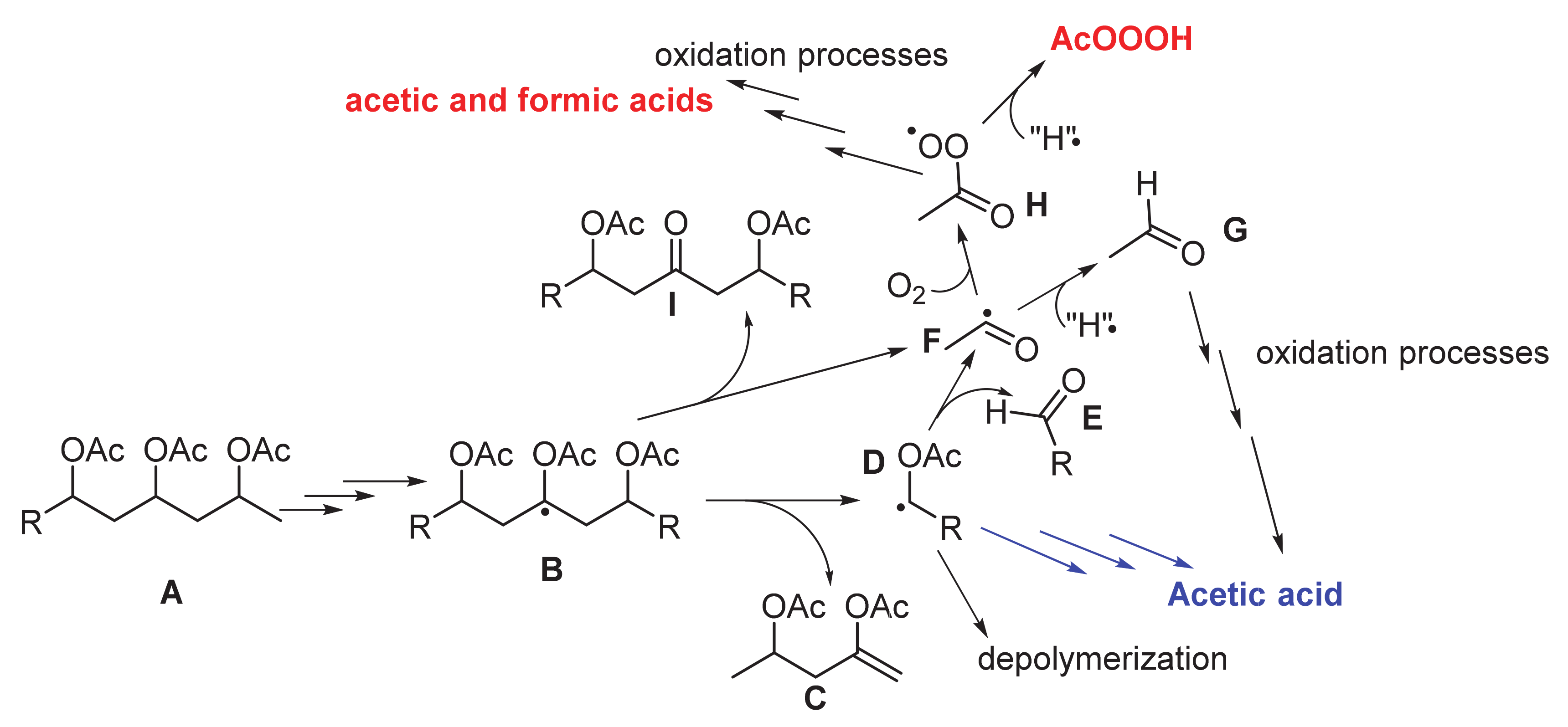
4. Discussion
- Sterilization of bags between 170 kGy and 260 kGy and storage of bags for aging between 5 and 20 months after irradiation
- Sterilization of bags from 30 kGy to 50 kGy and storage of bags for aging between 24 and 36 months after irradiation
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Gao, Y.; Allison, N. Extractables and Leachables Issues with the Application of Single Use Technology in the Biopharmaceutical Industry: Extractables and Leachables in Disposable Manufacture. J. Chem. Technol. Biot. 2016, 91, 289–295. [Google Scholar] [CrossRef]
- Shukla, A.A.; Gottschalk, U. Single-use disposable technologies for biopharmaceutical manufacturing. Trends Biotechnol. 2013, 31, 147–154. [Google Scholar] [CrossRef]
- Dorey, S.; Gaston, F.; Girard-Perier, N.; Dupuy, N.; Marque, S.R.A.; Delaunay, L. Generation of O2-Permeation Barrier during the Gamma-Irradiation of Polyethylene/Ethylene-Vinyl Alcohol/Polyethylene Multilayer Film. Ind. Eng. Chem. Res. 2019, 58, 14115–14123. [Google Scholar] [CrossRef]
- ISO 11137-1 International Organization for Standardization. Sterilization of Health Care Products-Radiation-Part 1: Requirements for Development, Validation and Routine Control of a Sterilization Process for Medical Devices; AFNOR: La plaine St-Denis, France, 2006. [Google Scholar]
- Colombani, J.; Herbette, G.; Rossi, C.; Joussot-Dubien, C.; Labed, V.; Gilardi, T. Leaching of plasticized PVC: Effect of irradiation. J. Appl. Polym. Sci. 2009, 112, 1372–1377. [Google Scholar] [CrossRef]
- Traboulsi, A.; Dupuy, N.; Rebufa, C.; Sergent, M.; Labed, V. Investigation of gamma radiation effect on the anion exchange resin Amberlite IRA-400 in hydroxide form by Fourier transformed infrared and 13C nuclear magnetic resonance spectroscopies. Anal. Chim. Acta 2012, 717, 110–121. [Google Scholar] [CrossRef]
- Audran, G.; Dorey, S.; Dupuy, N.; Gaston, F.; Marque, S.R.A. Degradation of γ-irradiated polyethylene-ethylene vinyl alcohol-polyethylene multilayer films: An ESR study. Polym. Degrad. Stab. 2015, 122, 169–179. [Google Scholar] [CrossRef]
- Suarez, J.C.M.; Mano, E.B. Characterization of degradation on gamma-irradiated recycled polyethylene blends by scanning electron microscopy. Polym. Degrad. Stab. 2001, 72, 217–221. [Google Scholar] [CrossRef]
- Dorey, S.; Gaston, F.; Dupuy, N.; Barbaroux, M.; Marque, S.R.A. Reconciliation of PH, Conductivity, Total Organic Carbon with Carboxylic Acids Detected by Ion Chromatography in Solution after Contact with Multilayer Films after γ-Irradiation. Eur. J. Pharm. Sci. 2018, 117, 216–226. [Google Scholar] [CrossRef]
- Gaston, F.; Dupuy, N.; Marque, S.R.A.; Barbaroux, M.; Dorey, S. FTIR study of ageing of γ-irradiated biopharmaceutical EVA based film. Polym. Degrad. Stab. 2016, 129, 19–25. [Google Scholar] [CrossRef]
- Dorey, S.; Gaston, F.; Marque, S.R.A.; Bortolotti, B.; Dupuy, N. XPS analysis of PE and EVA samples irradiated at different γ-doses. Appl. Surf. Sci. 2018, 427, 966–972. [Google Scholar] [CrossRef]
- Gaber, M.H. Effect of γ-irradiation on the molecular properties of bovine serum albumin. J. Biosci. Bioeng. 2005, 100, 203–206. [Google Scholar] [CrossRef]
- Štajner, D.; Milošević, M.; Popović, B. Irradiation Effects on Phenolic Content, Lipid and Protein Oxidation and Scavenger Ability of Soybean Seeds. Int. J. Mol. Sci. 2007, 8, 618–627. [Google Scholar] [CrossRef] [Green Version]
- Dalle-Donne, I.; Rossi, R.; Giustarini, D.; Gagliano, N.; Di Simplicio, P.; Colombo, R.; Milzani, A.D.G. Methionine oxidation as a major cause of the functional impairment of oxidized actin. Free. Radic. Biol. Med. 2002, 32, 927–937. [Google Scholar] [CrossRef]
- Folzer, E.; Diepold, K.; Bomans, K.; Finkler, C.; Schmidt, R.; Bulau, P.; Huwyler, J.; Mahler, H.-C.; Koulov, A.V. Selective Oxidation of Methionine and Tryptophan Residues in a Therapeutic IgG1 Molecule. J. Pharm. Sci. 2015, 104, 2824–2831. [Google Scholar] [CrossRef]
- Li, S.; Schöneich, C.; Borchardt, R.T. Chemical instability of protein pharmaceuticals: Mechanisms of oxidation and strategies for stabilization. Biotechnol. Bioeng. 1995, 48, 490–500. [Google Scholar] [CrossRef]
- Masato, A.; Kiichi, F.; Uchiyama, S. Suppression of Methionine Oxidation of a Pharmaceutical Antibody Stored in a Polymer-Based Syringe. J. Pharm. Sci. 2016, 105, 623–629. [Google Scholar] [CrossRef] [Green Version]
- Kleijnen, J.P.C. Response Surface Methodology. In Handbook of Simulation Optimization; International Series in Operations Research & Management Science; Springer: Berlin/Heidelberg, Germany, 2014; pp. 81–104. [Google Scholar] [CrossRef] [Green Version]
- Box, G.E.P.; Hunter, J.S. The study and exploitation of response regions. J. Am. Stat. Assoc. 1954, 49, 328–352. [Google Scholar]
- Box, G.E.P.; Hunter, J.S. Multi-Factor Experimental Designs for Exploring Response Surfaces. Ann. Math. Stat. 1957, 28, 195–241. [Google Scholar] [CrossRef]
- Droesbeke, J.J.; Fine, J.; Saporta, G. Plans d’expériences-Applications à L’entreprise; Technip Edition: Paris, France, 1997. [Google Scholar]
- Goupy, J. Les plans d’expériences. Modulad 2006, 34. [Google Scholar]
- ASTM F1980-16. Guide for Accelerated Aging of Sterile Barrier Systems for Medical Devices; ASTM International: West Conshohocken, PA, USA, 2016.
- Azurad (2019): Intuitive and Powerful Software for the Construction and Processing of DOE, Marseille, France. Available online: http://www.azurad.fr/logiciel-plans-experiences.php (accessed on 2 June 2020).
- Schöneich, C. Methionine oxidation by reactive oxygen species: Reaction mechanisms and relevance to Alzheimer’s disease. Biochim. Biophys. Acta BBA Proteins Proteom. 2005, 1703, 111–119. [Google Scholar] [CrossRef]
- Boonvisut, S.; Aksnes, A.; Njaa, L.R. Oxidation of methionine. Effects of hydrogen peroxide alone and in combination with iodide and selenite. Food Chem. 1982, 9, 183–194. [Google Scholar] [CrossRef]
- Du, P.; Liude, W.; Cao, H.; Zhao, H.; Huang, C.-H. Oxidation of amino acids by peracetic acid: Reaction kinetics, pathways and theoretical calculations. Water Res. X 2018, 1, 100002. [Google Scholar] [CrossRef]
- Dorey, S.; Gaston, F.; Girard-Perier, N.; Dupuy, N.; Marque, S.R.A.; Delaunay, L. Effect of gamma irradiation on the oxygen barrier properties in ethyl-vinyl acetate/ethylene-vinyl alcohol/ethyl-vinyl acetate multilayer film. J. Appl. Polym. Sci. 2020, 137. [Google Scholar] [CrossRef]
- Derringer, G.; Suich, R. Simultaneous Optimization of Several Response Variables. J. Qual. Technol. 1980, 12, 214–219. [Google Scholar] [CrossRef]





| Variable | Factor | Unit | Quantitative Factors |
|---|---|---|---|
| X1 | Gamma irradiation dose | kGy | 29-59-106-260 |
| X2 | Aging time | months | 0-1-2-3-6-12-24-36 |
| Sulfoxide Methionine Concentration (µM) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Time | Experiment 1 | Experiment 2 | Experiment 3 | Experiment 4 | Experiment 5 | Experiment 6 | Experiment 7 | Time | Experiment 8 | |
| Conditions | (1) Met a 50 µM | (1) H2O2 50 µM | (1) AA b 50 µM | (1) H2O2 30 µM | (1) H2O2 25 µM | (1) Met a 100 µM | (1) H2O2 50 µM | (1) AA b 256 mM | ||
| (2) H2O2 50 µM | (2) AA b 50 µM | (2) Met a 50 µM | (2) Met a 100 µM | (2) AA b 25 µM | (2) AA b pH 4,9 | (2) Met a 50 µM | (2) H2O2 76 mM | |||
| (3) Met a 50 µM | (3) Met a 100 µM | (3) H2O2 25 µM | (3) Met a 50 µM | |||||||
| 0 days | 0.4 | 0.0 | 0.0 | 0.6 | 0.5 | 0.4 | 0.6 | 20 min | 0.0 | |
| 3 days | 7.1 | 4.3 | 0.0 | 7.2 | 6.5 | 3.5 | 7.5 | 60 min | 3.7 | |
| 14 days | 20.0 | 12.0 | 0.4 | 20.7 | 18.6 | 10.2 | 21.5 | 80 min | 4.3 | |
| 21 days | 18.3 | 16.4 | 0.4 | 23.0 | 20.6 | 11.6 | 26.4 | 100 min | 4.2 | |
| 120 min | 5.0 | |||||||||
| 720 min | 6.6 | |||||||||
| Coefficient | Y1 Oxidative Induction Time | Y2 Methionine Sulfoxide Formation Rate (µM/day) | Y3 Maximum Concentration of Methionine Sulfoxide Detected |
|---|---|---|---|
| b0 | 2.30 | −0.10 | −0.32 |
| b1 | 0.58 | −0.02 | −0.40 |
| b2 | −1.02 | −0.27 | −1.23 |
| b11 | 1.06 | 0.07 | 0.47 |
| b22 | 0.53 | 0.59 | 3.07 |
| b12 | −2.01 | 0.19 | 0.70 |
| Case 1 | Case 2 | |
|---|---|---|
| Y1 (days) Oxidative induction time | Min: 2 Target: 10 | Min: 3 Target: 10 |
| Y2 (µM/day) Methionine sulfoxide formation rate | Target: 0 Max: 3 | Target: 0 Max: 2 |
| Y3 (µM) Methionine sulfoxide concentration | Target: 0 Max: 3 | Target: 0 Max: 1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Girard-Perier, N.; Claeys-Bruno, M.; Marque, S.R.A.; Dupuy, N.; Gaston, F.; Dorey, S. Monitoring of Peroxide in Gamma Irradiated EVA Multilayer Film Using Methionine Probe. Polymers 2020, 12, 3024. https://doi.org/10.3390/polym12123024
Girard-Perier N, Claeys-Bruno M, Marque SRA, Dupuy N, Gaston F, Dorey S. Monitoring of Peroxide in Gamma Irradiated EVA Multilayer Film Using Methionine Probe. Polymers. 2020; 12(12):3024. https://doi.org/10.3390/polym12123024
Chicago/Turabian StyleGirard-Perier, Nina, Magalie Claeys-Bruno, Sylvain R. A. Marque, Nathalie Dupuy, Fanny Gaston, and Samuel Dorey. 2020. "Monitoring of Peroxide in Gamma Irradiated EVA Multilayer Film Using Methionine Probe" Polymers 12, no. 12: 3024. https://doi.org/10.3390/polym12123024
APA StyleGirard-Perier, N., Claeys-Bruno, M., Marque, S. R. A., Dupuy, N., Gaston, F., & Dorey, S. (2020). Monitoring of Peroxide in Gamma Irradiated EVA Multilayer Film Using Methionine Probe. Polymers, 12(12), 3024. https://doi.org/10.3390/polym12123024







