Yerba Mate Extract in Microfibrillated Cellulose and Corn Starch Films as a Potential Wound Healing Bandage
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Film Formation
2.2.1. Yerba Mate Collection and Extract
2.2.2. Starch Solution
2.2.3. Microfibrillated Cellulose Gel
2.2.4. Solvent Evaporation Casting Film Formation
2.3. Fourier Transform Infrared Spectroscopy (FTIR)
2.4. Thermo and Mechanical Analysis
2.5. Yerba Mate Extract Release
2.6. Antioxidant Activities (DPPH and ABTS)
2.7. Antibacterial Evaluation
2.8. Cytotoxic Evaluation
2.8.1. Elution Assay (Cytotoxicity Testing)
2.8.2. Determination of p65-NF-κB Protein
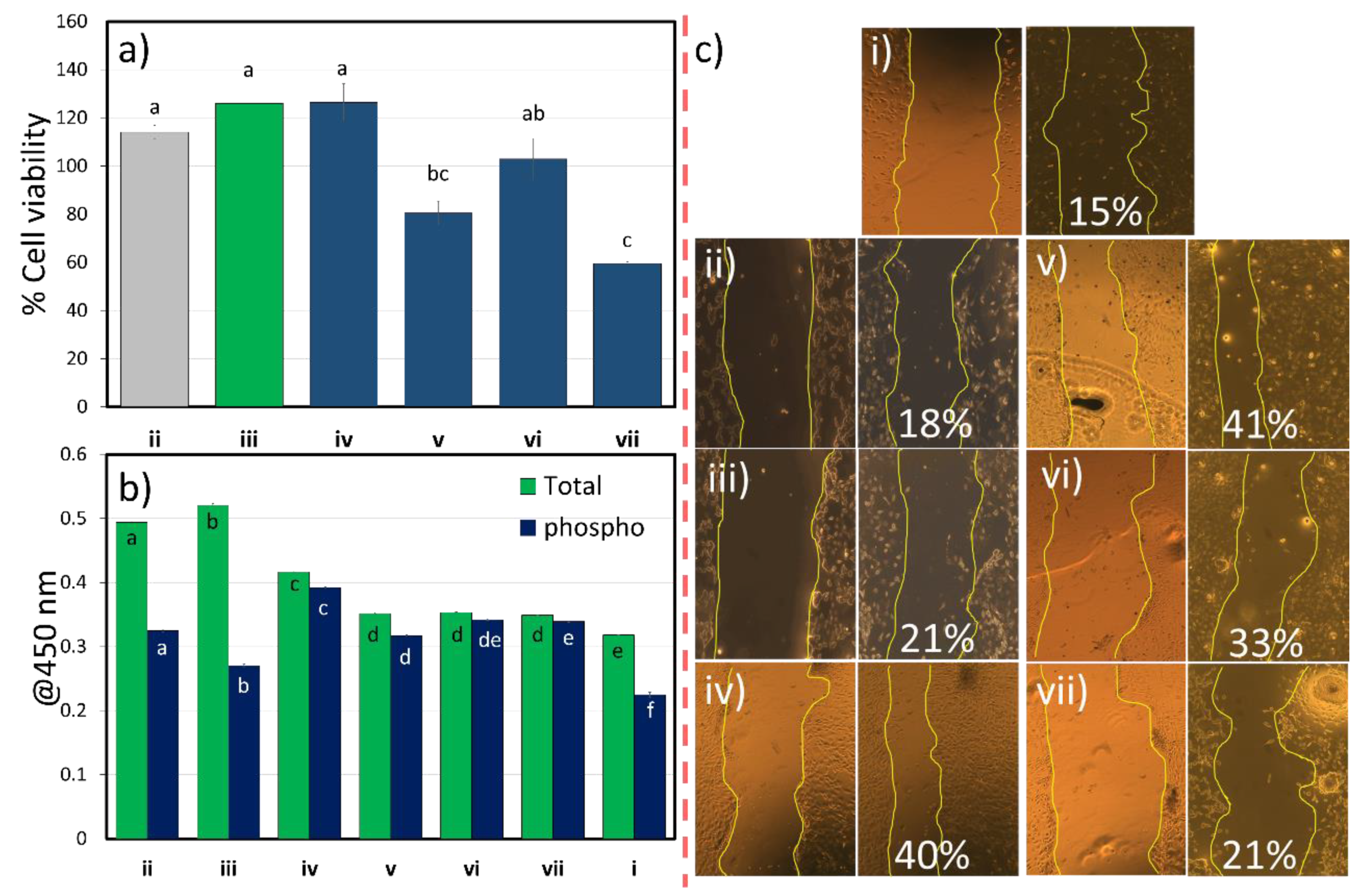
2.8.3. Wound Scratch Assay
2.8.4. Statistical Analyses
3. Results and Discussion
3.1. Characteristics of Blending the Extract with MFC at Different Stages
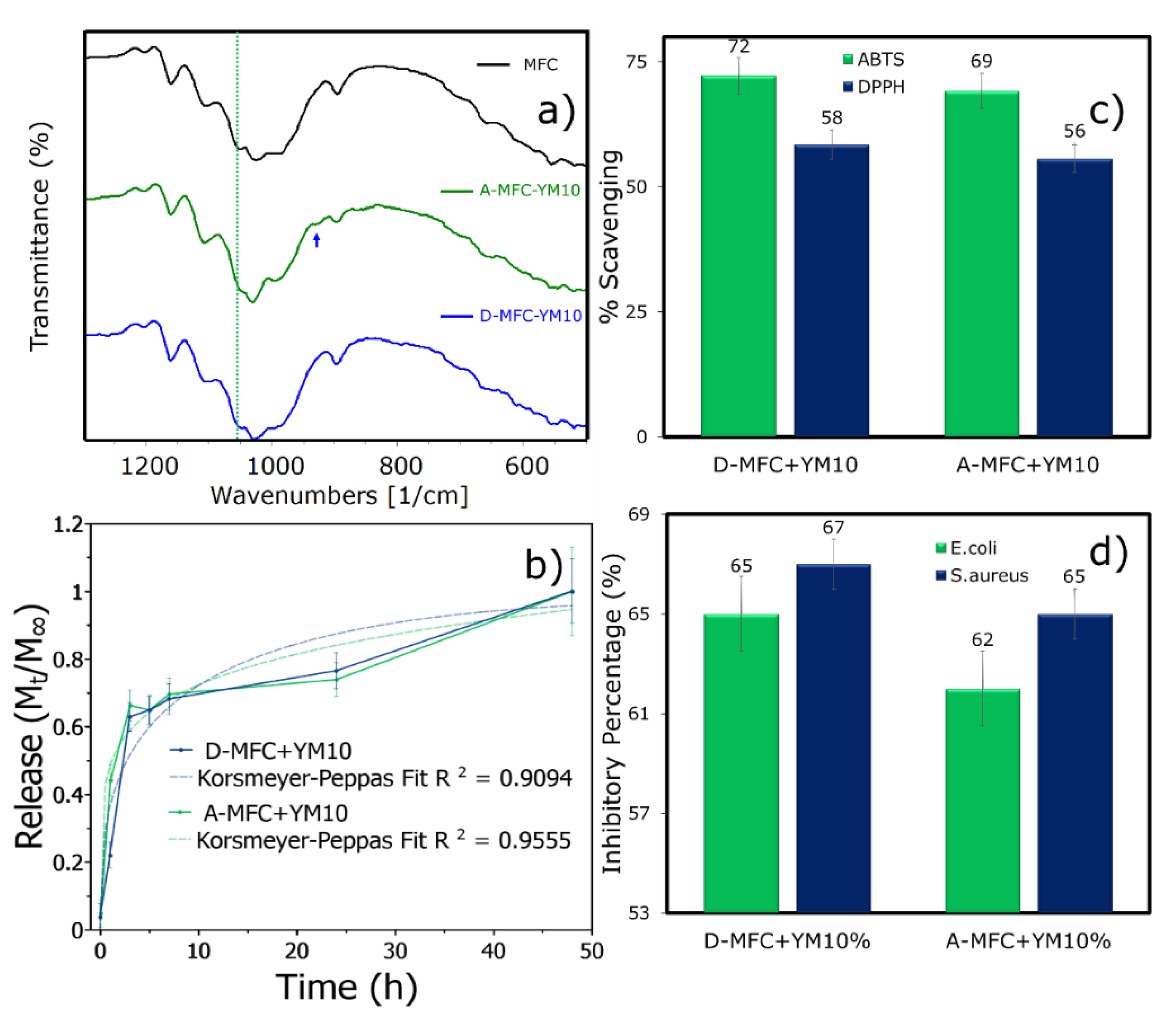
3.1.1. Microstructure
3.1.2. Yerba Mate Extract Release, Antioxidant, and Antibacterial Analysis
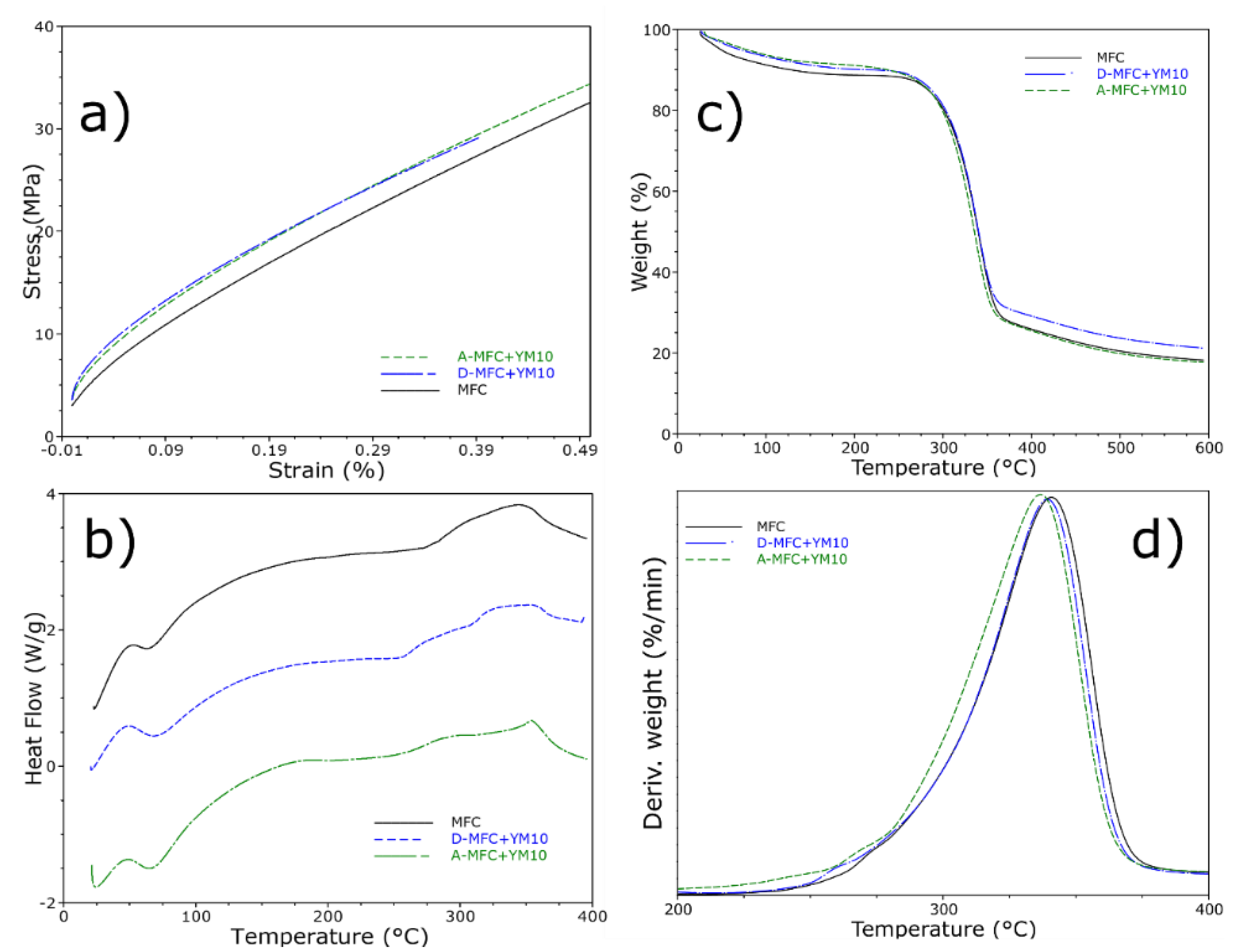
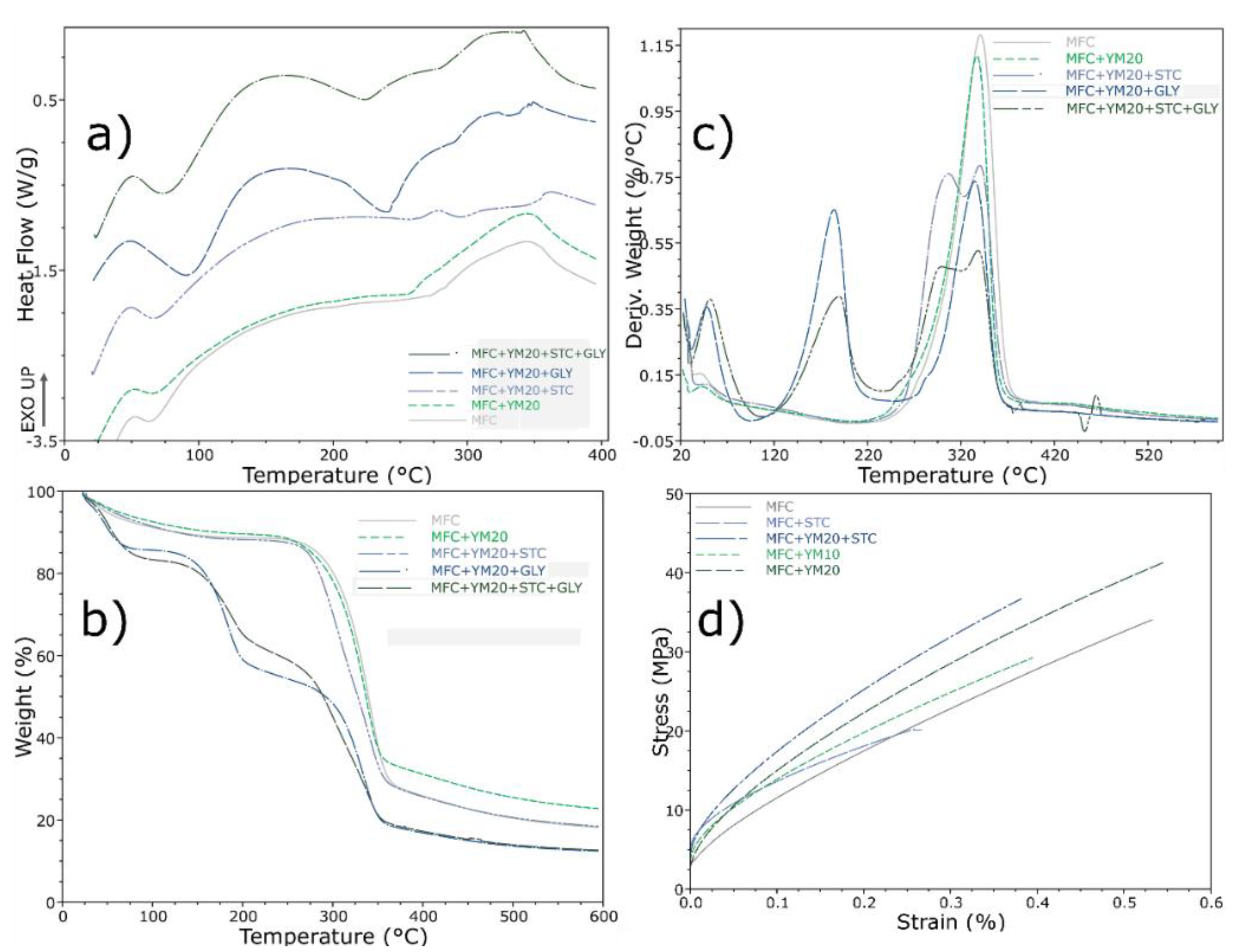
3.1.3. Mechanical and Thermal Properties
3.2. Characteristics of MFC with Extract Blended with Starch and Glycerine
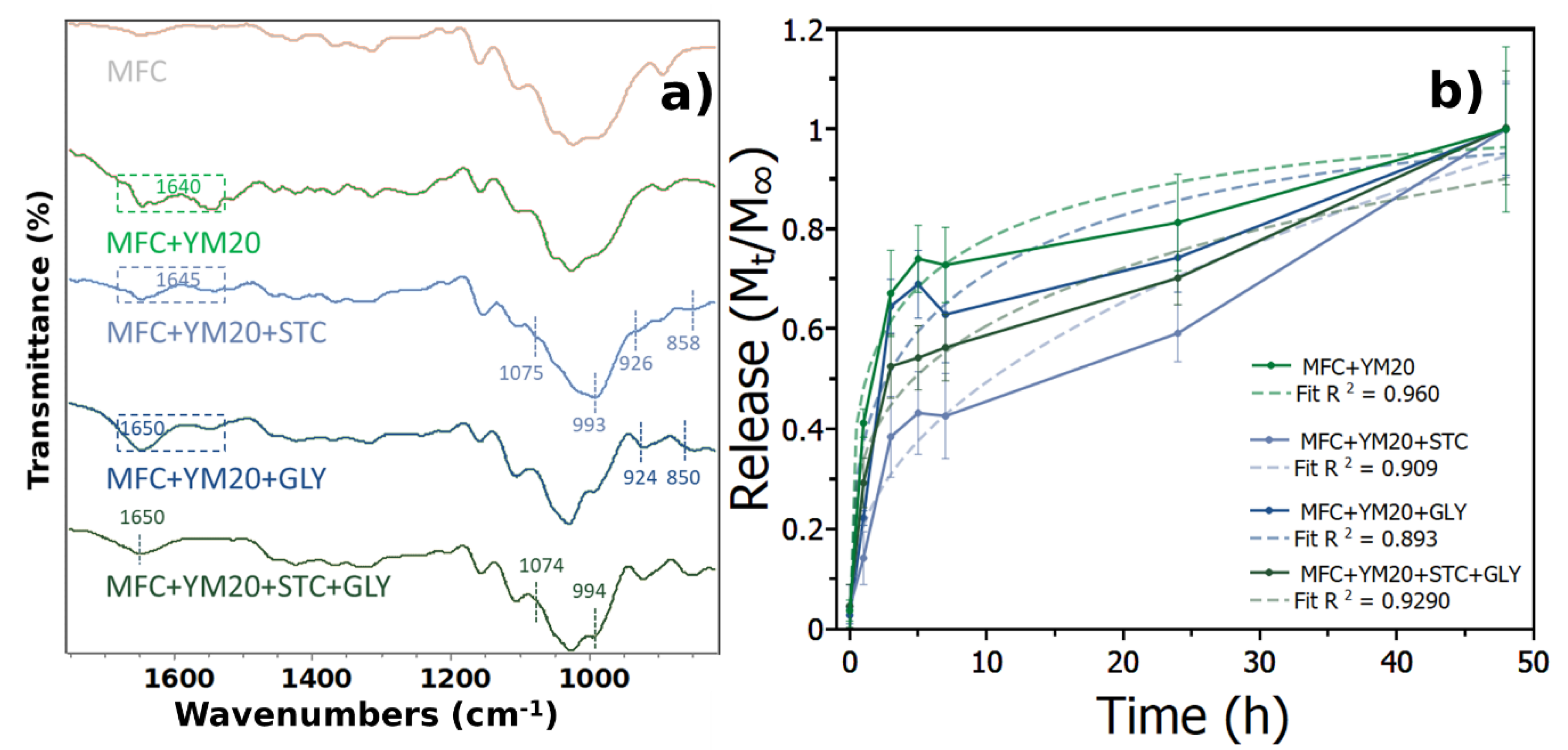
3.2.1. Microstructure of Blending Various Materials within MFC
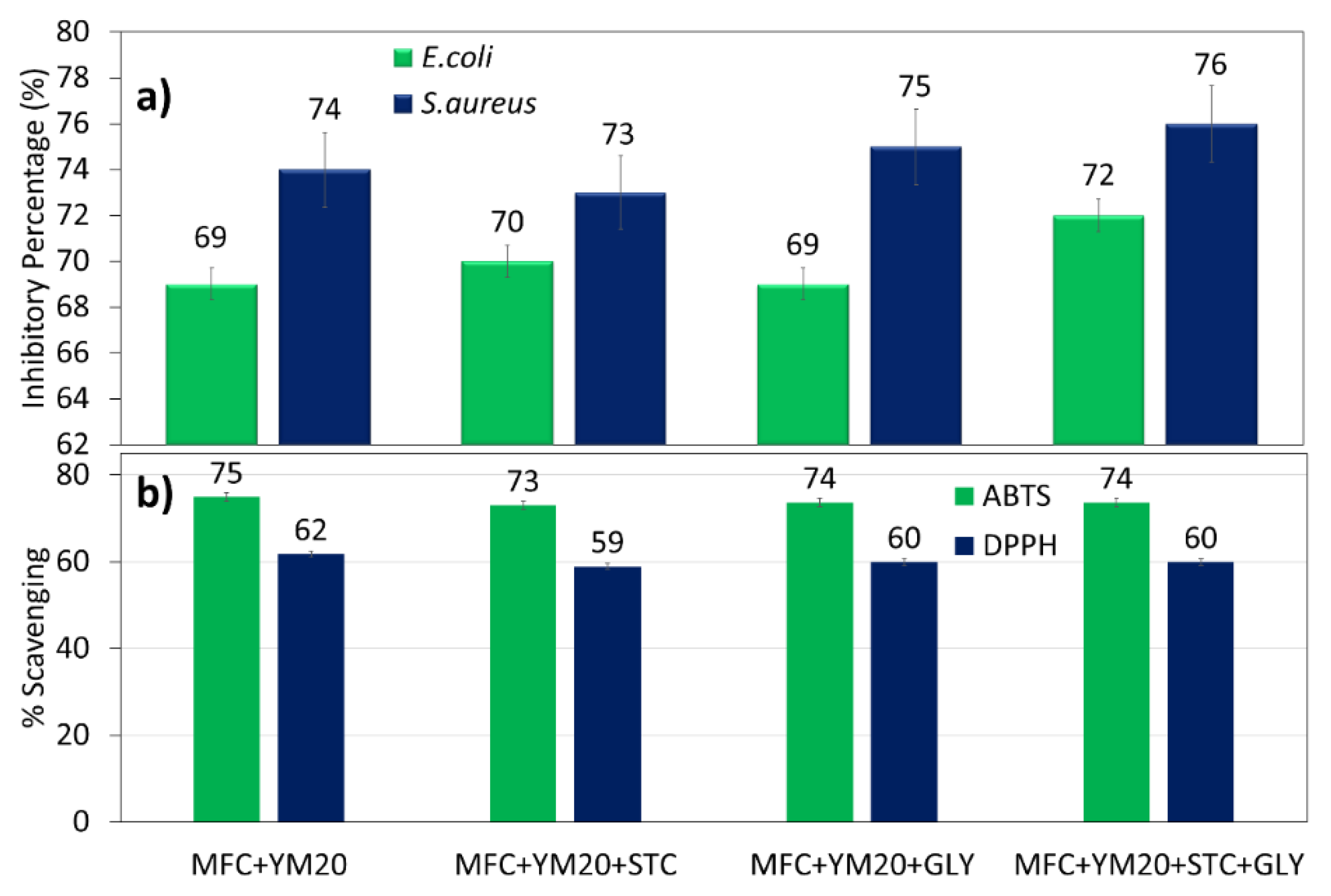
3.2.2. Extract Release, Antibacterial and Antioxidant Activities
3.2.3. Thermal and Mechanical Properties
3.2.4. Cell Activity Values
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Abdul Khalil, H.P.S.; Adnan, A.S.; Yahya, E.B.; Olaiya, N.G.; Safrida, S.; Hossain, M.S.; Balakrishnan, V.; Gopakumar, D.A.; Abdullah, C.K.; Oyekanmi, A.A.; et al. A Review on Plant Cellulose Nanofibre-Based Aerogels for Biomedical Applications. Polymers (Basel) 2020, 12, 1759. [Google Scholar] [CrossRef] [PubMed]
- Kanikireddy, V.; Varaprasad, K.; Jayaramudu, T.; Karthikeyan, C.; Sadiku, R. Carboxymethyl cellulose-based materials for infection control and wound healing: A review. Int. J. Biol. Macromol. 2020, 164, 963–975. [Google Scholar] [CrossRef]
- de Lima, G.G.; de Lima, D.W.F.; de Oliveira, M.J.A.; Lugão, A.B.; Alcântara, M.T.S.; Devine, D.M.; de Sá, M.J.C. Synthesis and in Vivo Behavior of PVP/CMC/Agar Hydrogel Membranes Impregnated with Silver Nanoparticles for Wound Healing Applications. ACS Appl. Bio Mater. 2018, 1, 1842–1852. [Google Scholar] [CrossRef]
- Boateng, J.; Catanzano, O. Advanced Therapeutic Dressings for Effective Wound Healing—A Review. J. Pharm. Sci. 2015, 104, 3653–3680. [Google Scholar] [CrossRef] [PubMed]
- Taheri, P.; Jahanmardi, R.; Koosha, M.; Abdi, S. Physical, mechanical and wound healing properties of chitosan/gelatin blend films containing tannic acid and/or bacterial nanocellulose. Int. J. Biol. Macromol. 2020, 154, 421–432. [Google Scholar] [CrossRef]
- Chattopadhyay, S.; Raines, R.T. Collagen-based biomaterials for wound healing. Biopolymers 2014, 101, 821–833. [Google Scholar] [CrossRef] [Green Version]
- Sun, G.; Zhang, X.; Shen, Y.-I.; Sebastian, R.; Dickinson, L.E.; Fox-Talbot, K.; Reinblatt, M.; Steenbergen, C.; Harmon, J.W.; Gerecht, S. Dextran hydrogel scaffolds enhance angiogenic responses and promote complete skin regeneration during burn wound healing. Proc. Natl. Acad. Sci. USA 2011, 108, 20976–20981. [Google Scholar] [CrossRef] [Green Version]
- de Lima, G.G.; Lyons, S.; Devine, D.M.; Nugent, M.J.D. Electrospinning of Hydrogels for Biomedical Applications—Hydrogels: Recent Advances; Thakur, V.K., Thakur, M.K., Eds.; Springer: Singapore, 2018; pp. 219–258. ISBN 978-981-10-6077-9. [Google Scholar]
- de Queiroz Antonino, R.; Lia Fook, B.; de Oliveira Lima, V.; de Farias Rached, R.; Lima, E.; da Silva Lima, R.; Peniche Covas, C.; Lia Fook, M. Preparation and Characterization of Chitosan Obtained from Shells of Shrimp (Litopenaeus vannamei Boone). Mar. Drugs 2017, 15, 141. [Google Scholar] [CrossRef] [Green Version]
- Claro, F.C.; Jordão, C.; de Viveiros, B.M.; Isaka, L.J.E.; Villanova Junior, J.A.; Magalhães, W.L.E. Low cost membrane of wood nanocellulose obtained by mechanical defibrillation for potential applications as wound dressing. Cellulose 2020, 1–15. [Google Scholar] [CrossRef]
- Naomi, R.; Bt Hj Idrus, R.; Fauzi, M.B. Plant- vs. Bacterial-Derived Cellulose for Wound Healing: A Review. Int. J. Environ. Res. Public Health 2020, 17, 6803. [Google Scholar] [CrossRef]
- Claro, F.C.; Matos, M.; Jordão, C.; Avelino, F.; Lomonaco, D.; Magalhães, W.L.E. Enhanced microfibrillated cellulose-based film by controlling the hemicellulose content and MFC rheology. Carbohydr. Polym. 2019, 218, 307–314. [Google Scholar] [CrossRef] [PubMed]
- Kolakovic, R.; Peltonen, L.; Laukkanen, A.; Hirvonen, J.; Laaksonen, T. Nanofibrillar cellulose films for controlled drug delivery. Eur. J. Pharm. Biopharm. 2012, 82, 308–315. [Google Scholar] [CrossRef] [PubMed]
- de Lima, G.G.; Ferreira, B.D.; Matos, M.; Pereira, B.L.; Nugent, M.J.D.; Hansel, F.A.; Magalhães, W.L.E. Effect of cellulose size-concentration on the structure of polyvinyl alcohol hydrogels. Carbohydr. Polym. 2020, 245, 116612. [Google Scholar] [CrossRef] [PubMed]
- Missio, A.L.; Mattos, B.D.; de F. Ferreira, D.; Magalhães, W.L.E.; Bertuol, D.A.; Gatto, D.A.; Petutschnigg, A.; Tondi, G. Nanocellulose-tannin films: From trees to sustainable active packaging. J. Clean. Prod. 2018, 184, 143–151. [Google Scholar] [CrossRef]
- Duarte, M.M.; Tomasi, J.C.; Helm, C.V.; Amano, E.; Lazzarotto, M.; Godoy, R.C.B.; Nogueira, A.C.; Wendling, I. Caffeinated and decaffeinated mate tea: Effect of toasting on bioactive compounds and consumer acceptance. Rev. Bras. Ciências Agrárias Braz. J. Agric. Sci. 2020, 15, 1–10. [Google Scholar] [CrossRef]
- Isolabella, S.; Cogoi, L.; López, P.; Anesini, C.; Ferraro, G.; Filip, R. Study of the bioactive compounds variation during yerba mate (Ilex paraguariensis) processing. Food Chem. 2010, 122, 695–699. [Google Scholar] [CrossRef]
- Cardozo Junior, E.L.; Morand, C. Interest of mate ( Ilex paraguariensis A. St. -Hil.) as a new natural functional food to preserve human cardiovascular health—A review. J. Funct. Foods 2016, 21, 440–454. [Google Scholar] [CrossRef]
- Meinhart, A.D.; Damin, F.M.; Caldeirão, L.; da Silveira, T.F.F.; Filho, J.T.; Godoy, H.T. Chlorogenic acid isomer contents in 100 plants commercialized in Brazil. Food Res. Int. 2017, 99, 522–530. [Google Scholar] [CrossRef]
- de Mejía, E.G.; Song, Y.S.; Heck, C.I.; Ramírez-Mares, M. Yerba mate tea (Ilex paraguariensis): Phenolics, antioxidant capacity and in vitro inhibition of colon cancer cell proliferation. J. Funct. Foods 2010, 2, 23–34. [Google Scholar] [CrossRef]
- Luz, A.B.G.; da Silva, C.H.B.; Nascimento, M.V.P.S.; de Campos Facchin, B.M.; Baratto, B.; Fröde, T.S.; Reginatto, F.H.; Dalmarco, E.M. The anti-inflammatory effect of Ilex paraguariensis A. St. Hil (Mate) in a murine model of pleurisy. Int. Immunopharmacol. 2016, 36, 165–172. [Google Scholar] [CrossRef]
- Souza, A.H.P.; Corrêa, R.C.G.; Barros, L.; Calhelha, R.C.; Santos-Buelga, C.; Peralta, R.M.; Bracht, A.; Matsushita, M.; Ferreira, I.C.F.R. Phytochemicals and bioactive properties of Ilex paraguariensis: An in-vitro comparative study between the whole plant, leaves and stems. Food Res. Int. 2015, 78, 286–294. [Google Scholar] [CrossRef] [PubMed]
- Medina Jaramillo, C.; González Seligra, P.; Goyanes, S.; Bernal, C.; Famá, L. Biofilms based on cassava starch containing extract of yerba mate as antioxidant and plasticizer. Starch Stärke 2015, 67, 780–789. [Google Scholar] [CrossRef]
- Medina Jaramillo, C.; Gutiérrez, T.J.; Goyanes, S.; Bernal, C.; Famá, L. Biodegradability and plasticizing effect of yerba mate extract on cassava starch edible films. Carbohydr. Polym. 2016, 151, 150–159. [Google Scholar] [CrossRef] [PubMed]
- Knapp, M.A.; dos Santos, D.F.; Pilatti-Riccio, D.; Deon, V.G.; dos Santos, G.H.F.; Pinto, V.Z. Yerba mate extract in active starch films: Mechanical and antioxidant properties. J. Food Process. Preserv. 2019, 43, e13897. [Google Scholar] [CrossRef]
- López-Rubio, A.; Lagaron, J.M.; Ankerfors, M.; Lindström, T.; Nordqvist, D.; Mattozzi, A.; Hedenqvist, M.S. Enhanced film forming and film properties of amylopectin using micro-fibrillated cellulose. Carbohydr. Polym. 2007, 68, 718–727. [Google Scholar] [CrossRef]
- Alves, V.D.; Mali, S.; Beléia, A.; Grossmann, M.V.E. Effect of glycerol and amylose enrichment on cassava starch film properties. J. Food Eng. 2007, 78, 941–946. [Google Scholar] [CrossRef]
- Syverud, K.; Chinga-Carrasco, G.; Toledo, J.; Toledo, P.G. A comparative study of Eucalyptus and Pinus radiata pulp fibres as raw materials for production of cellulose nanofibrils. Carbohydr. Polym. 2011, 84, 1033–1038. [Google Scholar] [CrossRef]
- de Lima, G.G.; de Miranda, N.B.; Timm, T.G.; Matos, M.; Angelina Moraes de Lima, T.; Luiz Esteves Magalhães, W.; Benathar Ballod Tavares, L.; Hansel, F.A.; Helm, C.V. Characterisation and in vivo evaluation of Araucaria angustifolia pinhão seed coat nanosuspension as a functional food source. Food Funct. 2020, 11, 9820–9832. [Google Scholar] [CrossRef]
- Siripatrawan, U.; Harte, B.R. Physical properties and antioxidant activity of an active film from chitosan incorporated with green tea extract. Food Hydrocoll. 2010, 24, 770–775. [Google Scholar] [CrossRef]
- Samarth, R.M.; Panwar, M.; Kumar, M.; Soni, A.; Kumar, M.; Kumar, A. Evaluation of antioxidant and radical-scavenging activities of certain radioprotective plant extracts. Food Chem. 2008, 106, 868–873. [Google Scholar] [CrossRef]
- de Lima, G.G.; Chee, B.S.; Moritz, V.F.; Cortese, Y.J.; Magalhães, W.L.E.; Devine, D.M.; Nugent, M.J.D. The production of a novel poly(vinyl alcohol) hydrogel cryogenic spheres for immediate release using a droplet system. Biomed. Phys. Eng. Express 2019, 5, 045017. [Google Scholar] [CrossRef]
- Grada, A.; Otero-Vinas, M.; Prieto-Castrillo, F.; Obagi, Z.; Falanga, V. Research Techniques Made Simple: Analysis of Collective Cell Migration Using the Wound Healing Assay. J. Investig. Dermatol. 2017, 137, e11–e16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Summerscales, J.; Gwinnett, C. Forensic identification of bast fibres. In Biocomposites for High-Performance Applications; Elsevier: Amsterdam, The Netherlands, 2017; pp. 125–164. [Google Scholar]
- Aguayo, M.; Fernández Pérez, A.; Reyes, G.; Oviedo, C.; Gacitúa, W.; Gonzalez, R.; Uyarte, O. Isolation and Characterization of Cellulose Nanocrystals from Rejected Fibers Originated in the Kraft Pulping Process. Polymers (Basel) 2018, 10, 1145. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rostami, J.; Mathew, A.P.; Edlund, U. Zwitterionic Acetylated Cellulose Nanofibrils. Molecules 2019, 24, 3147. [Google Scholar] [CrossRef] [Green Version]
- García, F.E.; Senn, A.M.; Meichtry, J.M.; Scott, T.B.; Pullin, H.; Leyva, A.G.; Halac, E.B.; Ramos, C.P.; Sacanell, J.; Mizrahi, M.; et al. Iron-based nanoparticles prepared from yerba mate extract. Synthesis, characterization and use on chromium removal. J. Environ. Manag. 2019, 235, 1–8. [Google Scholar] [CrossRef]
- Cheynier, V. Polyphenols in foods are more complex than often thought. Am. J. Clin. Nutr. 2005, 81, 223S–229S. [Google Scholar] [CrossRef] [Green Version]
- Travalini, A.P.; Lamsal, B.; Magalhães, W.L.E.; Demiate, I.M. Cassava starch films reinforced with lignocellulose nanofibers from cassava bagasse. Int. J. Biol. Macromol. 2019, 139, 1151–1161. [Google Scholar] [CrossRef]
- Chang-Bravo, L.; López-Córdoba, A.; Martino, M. Biopolymeric matrices made of carrageenan and corn starch for the antioxidant extracts delivery of Cuban red propolis and yerba mate. React. Funct. Polym. 2014, 85, 11–19. [Google Scholar] [CrossRef]
- Ceballos, R.L.; Ochoa-Yepes, O.; Goyanes, S.; Bernal, C.; Famá, L. Effect of yerba mate extract on the performance of starch films obtained by extrusion and compression molding as active and smart packaging. Carbohydr. Polym. 2020, 244, 116495. [Google Scholar] [CrossRef]
- Heck, C.I.; De Mejia, E.G. Yerba mate tea (Ilex paraguariensis): A comprehensive review on chemistry, health implications, and technological considerations. J. Food Sci. 2007, 72, R138–R151. [Google Scholar] [CrossRef]
- Hansel, F.A.; Domingos, D.M.; de Lima, K.M.G.; Pasquini, C. Moagem e sapeco/secagem em forno de microondas na classificação sensorial de erva-mate no infravermelho próximo. Embrapa Florestas-Comun. Técnico (INFOTECA-E) 2008. [Google Scholar]
- Tamura, A.; Sasaki, M.; Yamashita, H.; Matsui-Yuasa, I.; Saku, T.; Hikima, T.; Tabuchi, M.; Munakata, H.; Kojima-Yuasa, A. Yerba-mate (Ilex paraguariensis) extract prevents ethanol-induced liver injury in rats. J. Funct. Foods 2013, 5, 1714–1723. [Google Scholar] [CrossRef]
- Garcia-Lazaro, R.S.; Lamdan, H.; Caligiuri, L.G.; Lorenzo, N.; Berengeno, A.L.; Ortega, H.H.; Alonso, D.F.; Farina, H.G. In vitro and in vivo antitumor activity of Yerba Mate extract in colon cancer models. J. Food Sci. 2020, 85, 2186–2197. [Google Scholar] [CrossRef] [PubMed]
- Correa, V.G.; de Sá-Nakanishi, A.B.; de Almeida Gonçalves, G.; Barros, L.; Ferreira, I.C.F.R.; Bracht, A.; Peralta, R.M. Yerba mate aqueous extract improves the oxidative and inflammatory states of rats with adjuvant-induced arthritis. Food Funct. 2019, 10, 5682–5696. [Google Scholar] [CrossRef]
- Sharififar, F.; Dehghn-Nudeh, G.; Mirtajaldini, M. Major flavonoids with antioxidant activity from Teucrium polium L. Food Chem. 2009, 112, 885–888. [Google Scholar] [CrossRef]
- Burris, K.P.; Davidson, P.M.; Stewart, C.N., Jr.; Harte, F.M. Antimicrobial Activity of Yerba Mate (Ilex paraguariensis) Aqueous Extracts against Escherichia coli O157:H7 and Staphylococcus aureus. J. Food Sci. 2011, 76, M456–M462. [Google Scholar] [CrossRef]
- Fernandes, S.C.M.; Freire, C.S.R.; Silvestre, A.J.D.; Pascoal Neto, C.; Gandini, A.; Berglund, L.A.; Salmén, L. Transparent chitosan films reinforced with a high content of nanofibrillated cellulose. Carbohydr. Polym. 2010, 81, 394–401. [Google Scholar] [CrossRef]
- Menzel, C. Improvement of starch films for food packaging through a three-principle approach: Antioxidants, cross-linking and reinforcement. Carbohydr. Polym. 2020, 250, 116828. [Google Scholar] [CrossRef]
- Kachel-Jakubowska, M.; Matwijczuk, A.; Gagoś, M. Analysis of the physicochemical properties of post-manufacturing waste derived from production of methyl esters from rapeseed oil. Int. Agrophysics 2017, 31, 175–182. [Google Scholar] [CrossRef]
- Minakawa, A.F.K.; Faria-Tischer, P.C.S.; Mali, S. Simple ultrasound method to obtain starch micro- and nanoparticles from cassava, corn and yam starches. Food Chem. 2019, 283, 11–18. [Google Scholar] [CrossRef]
- Ma, X.; Qiao, C.; Wang, X.; Yao, J.; Xu, J. Structural characterization and properties of polyols plasticized chitosan films. Int. J. Biol. Macromol. 2019, 135, 240–245. [Google Scholar] [CrossRef] [PubMed]
- de Oliveira, J.P.; Bruni, G.P.; Fonseca, L.M.; da Silva, F.T.; da Rocha, J.C.; da Rosa Zavareze, E. Characterization of aerogels as bioactive delivery vehicles produced through the valorization of yerba-mate (Illex paraguariensis). Food Hydrocoll. 2020, 107, 105931. [Google Scholar] [CrossRef]
- Noureddine, T.; El Husseini, Z.; Nehme, A.; Abdel Massih, R. Antibacterial activity of Ilex paraguariensis (Yerba Mate) against Gram-positive and Gram-negative bacteria. J. Infect. Dev. Ctries 2018, 12, 712–719. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boateng, J.S.; Matthews, K.H.; Stevens, H.N.E.; Eccleston, G.M. Wound Healing Dressings and Drug Delivery Systems: A Review. J. Pharm. Sci. 2008, 97, 2892–2923. [Google Scholar] [CrossRef]
- Lübtow, M.M.; Lorson, T.; Finger, T.; Gröber-Becker, F.; Luxenhofer, R. Combining Ultra-High Drug-Loaded Micelles and Injectable Hydrogel Drug Depots for Prolonged Drug Release. Macromol. Chem. Phys. 2020, 221, 1900341. [Google Scholar] [CrossRef] [Green Version]
- Semalty, M.; Semalty, A.; Kumar, G. Formulation and characterization of mucoadhesive buccal films of glipizide. Indian J. Pharm. Sci. 2008, 70, 43. [Google Scholar] [CrossRef] [Green Version]
- Saraiva, B.R.; Vital, A.C.P.; Anjo, F.A.; Ribas, J.C.R.; Matumoto Pintro, P.T. Effect of yerba mate (Ilex paraguariensis A. St.-Hil.) addition on the functional and technological characteristics of fresh cheese. J. Food Sci. Technol. 2019, 56, 1256–1265. [Google Scholar] [CrossRef]
- Deladino, L.; Teixeira, A.S.; Navarro, A.S.; Alvarez, I.; Molina-García, A.D.; Martino, M. Corn starch systems as carriers for yerba mate (Ilex paraguariensis) antioxidants. Food Bioprod. Process. 2015, 94, 463–472. [Google Scholar] [CrossRef]
- dos Santos, L.F.; Vargas, B.K.; Bertol, C.D.; Biduski, B.; Bertolin, T.E.; dos Santos, L.R.; Brião, V.B. Clarification and concentration of yerba mate extract by membrane technology to increase shelf life. Food Bioprod. Process. 2020, 122, 22–30. [Google Scholar] [CrossRef]
- Kweon, M.; Slade, L.; Levine, H. Role of glassy and crystalline transitions in the responses of corn starches to heat and high pressure treatments: Prediction of solute-induced barostabilty from solute-induced thermostability. Carbohydr. Polym. 2008, 72, 293–299. [Google Scholar] [CrossRef]
- Arık Kibar, E.A.; Us, F. Thermal, mechanical and water adsorption properties of corn starch–carboxymethylcellulose/methylcellulose biodegradable films. J. Food Eng. 2013, 114, 123–131. [Google Scholar] [CrossRef]
- Machado, B.A.S.; Nunes, I.L.; Pereira, F.V.; Druzian, J.I. Desenvolvimento e avaliação da eficácia de filmes biodegradáveis de amido de mandioca com nanocelulose como reforço e com extrato de erva-mate como aditivo antioxidante. Ciência Rural 2012, 42, 2085–2091. [Google Scholar] [CrossRef] [Green Version]
- Salehi, H.; Mehrasa, M.; Nasri-Nasrabadi, B.; Doostmohammadi, M.; Seyedebrahimi, R.; Davari, N.; Rafienia, M.; Hosseinabadi, M.; Agheb, M.; Siavash, M. Effects of nanozeolite/starch thermoplastic hydrogels on wound healing. J. Res. Med. Sci. 2017, 22, 110. [Google Scholar] [CrossRef] [PubMed]
- Dinsdale, C.; Mirza, F.; Wiebe, J. Glycerol alters cytoskeleton and cell adhesion while inhibiting cell proliferation. Cell Biol. Int. Rep. 1992, 16, 591–602. [Google Scholar] [CrossRef]
- Liu, T.; Zhang, L.; Joo, D.; Sun, S.-C. NF-κB signaling in inflammation. Signal Transduct. Target. Ther. 2017, 2, 17023. [Google Scholar] [CrossRef] [Green Version]
- Gupta, S.C.; Sundaram, C.; Reuter, S.; Aggarwal, B.B. Inhibiting NF-κB activation by small molecules as a therapeutic strategy. Biochim. Biophys. Acta Gene Regul. Mech. 2010, 1799, 775–787. [Google Scholar] [CrossRef] [Green Version]
- Park, Y.R.; Sultan, M.T.; Park, H.J.; Lee, J.M.; Ju, H.W.; Lee, O.J.; Lee, D.J.; Kaplan, D.L.; Park, C.H. NF-κB signaling is key in the wound healing processes of silk fibroin. Acta Biomater. 2018, 67, 183–195. [Google Scholar] [CrossRef]
- de Lima, G.G.; Elter, J.K.; Chee, B.S.; Magalhães, W.L.E.; Devine, D.M.; Nugent, M.J.D.; de Sá, M.J.C. A tough and novel dual-response PAA/P(NiPAAM-co-PEGDMA) IPN hydrogels with ceramics by photopolymerization for consolidation of bone fragments following fracture. Biomed. Mater. 2019, 14, 054101. [Google Scholar] [CrossRef]
- Ambrozova, N.; Ulrichova, J.; Galandakova, A. Models for the study of skin wound healing. The role of Nrf2 and NF-κB. Biomed. Pap. 2017, 161, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Yang, F.; Jin, S.; Tang, Y. Marine Collagen Peptides Promote Cell Proliferation of NIH-3T3 Fibroblasts via NF-κB Signaling Pathway. Molecules 2019, 24, 4201. [Google Scholar] [CrossRef] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aliabadi, M.; Chee, B.S.; Matos, M.; Cortese, Y.J.; Nugent, M.J.D.; de Lima, T.A.M.; Magalhães, W.L.E.; de Lima, G.G. Yerba Mate Extract in Microfibrillated Cellulose and Corn Starch Films as a Potential Wound Healing Bandage. Polymers 2020, 12, 2807. https://doi.org/10.3390/polym12122807
Aliabadi M, Chee BS, Matos M, Cortese YJ, Nugent MJD, de Lima TAM, Magalhães WLE, de Lima GG. Yerba Mate Extract in Microfibrillated Cellulose and Corn Starch Films as a Potential Wound Healing Bandage. Polymers. 2020; 12(12):2807. https://doi.org/10.3390/polym12122807
Chicago/Turabian StyleAliabadi, Meysam, Bor Shin Chee, Mailson Matos, Yvonne J. Cortese, Michael J. D. Nugent, Tielidy A. M. de Lima, Washington L. E. Magalhães, and Gabriel Goetten de Lima. 2020. "Yerba Mate Extract in Microfibrillated Cellulose and Corn Starch Films as a Potential Wound Healing Bandage" Polymers 12, no. 12: 2807. https://doi.org/10.3390/polym12122807
APA StyleAliabadi, M., Chee, B. S., Matos, M., Cortese, Y. J., Nugent, M. J. D., de Lima, T. A. M., Magalhães, W. L. E., & de Lima, G. G. (2020). Yerba Mate Extract in Microfibrillated Cellulose and Corn Starch Films as a Potential Wound Healing Bandage. Polymers, 12(12), 2807. https://doi.org/10.3390/polym12122807








