A Review of Wood Biomass-Based Fatty Acids and Rosin Acids Use in Polymeric Materials
Abstract
1. Introduction
2. Tall Oil
2.1. Origin of Tall Oil
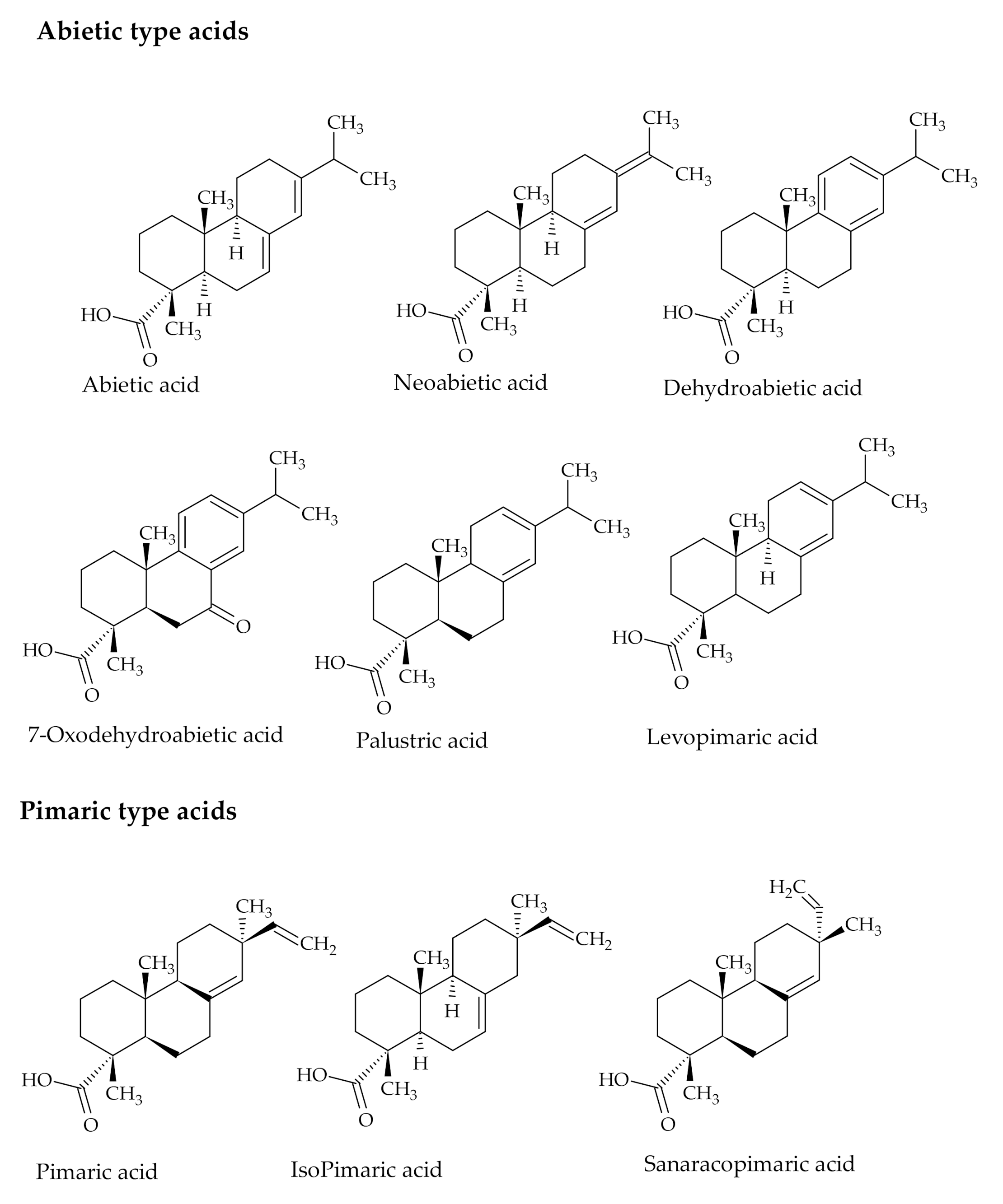
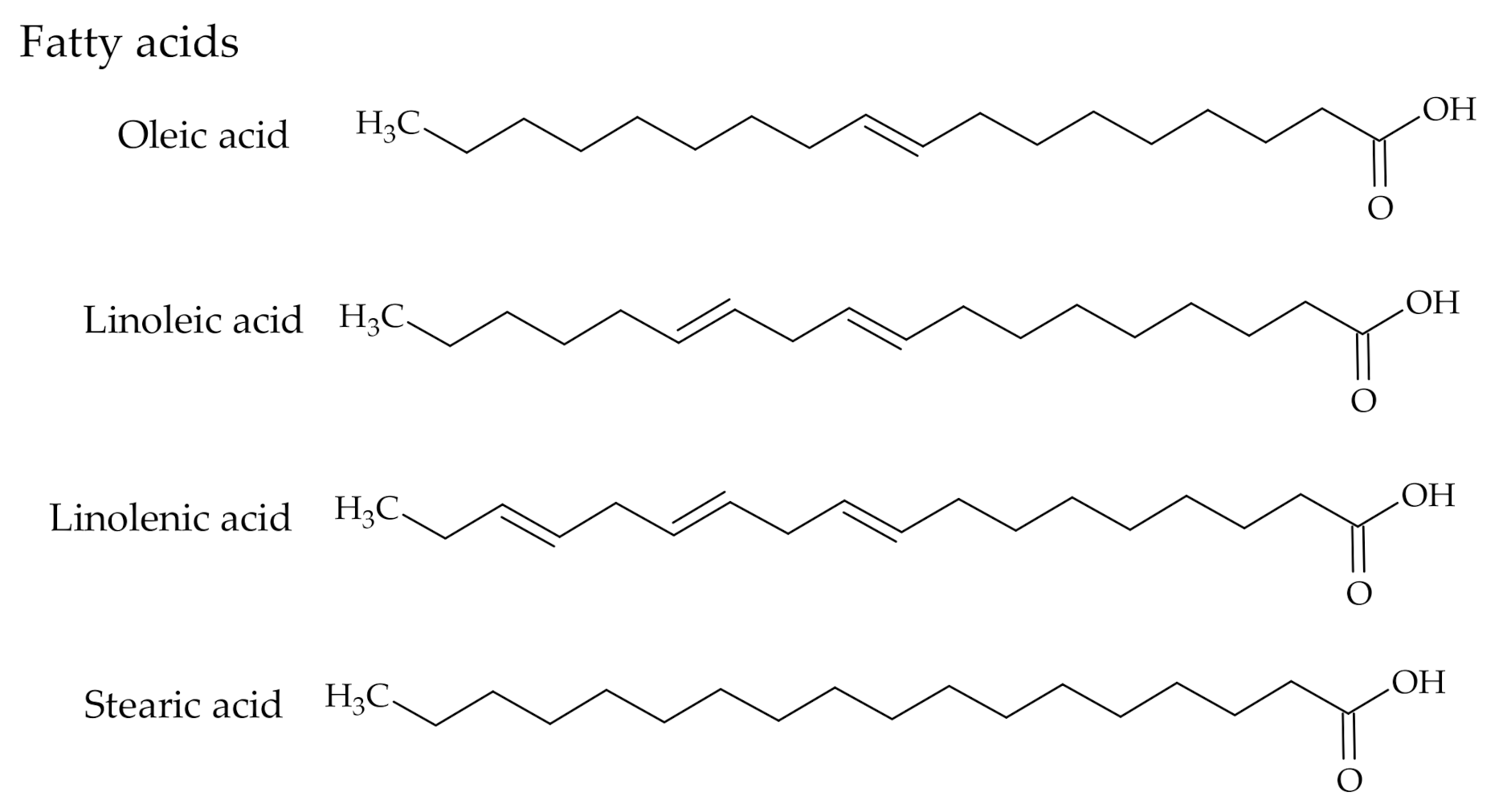
2.2. Composition
3. The Use of Tall Oil
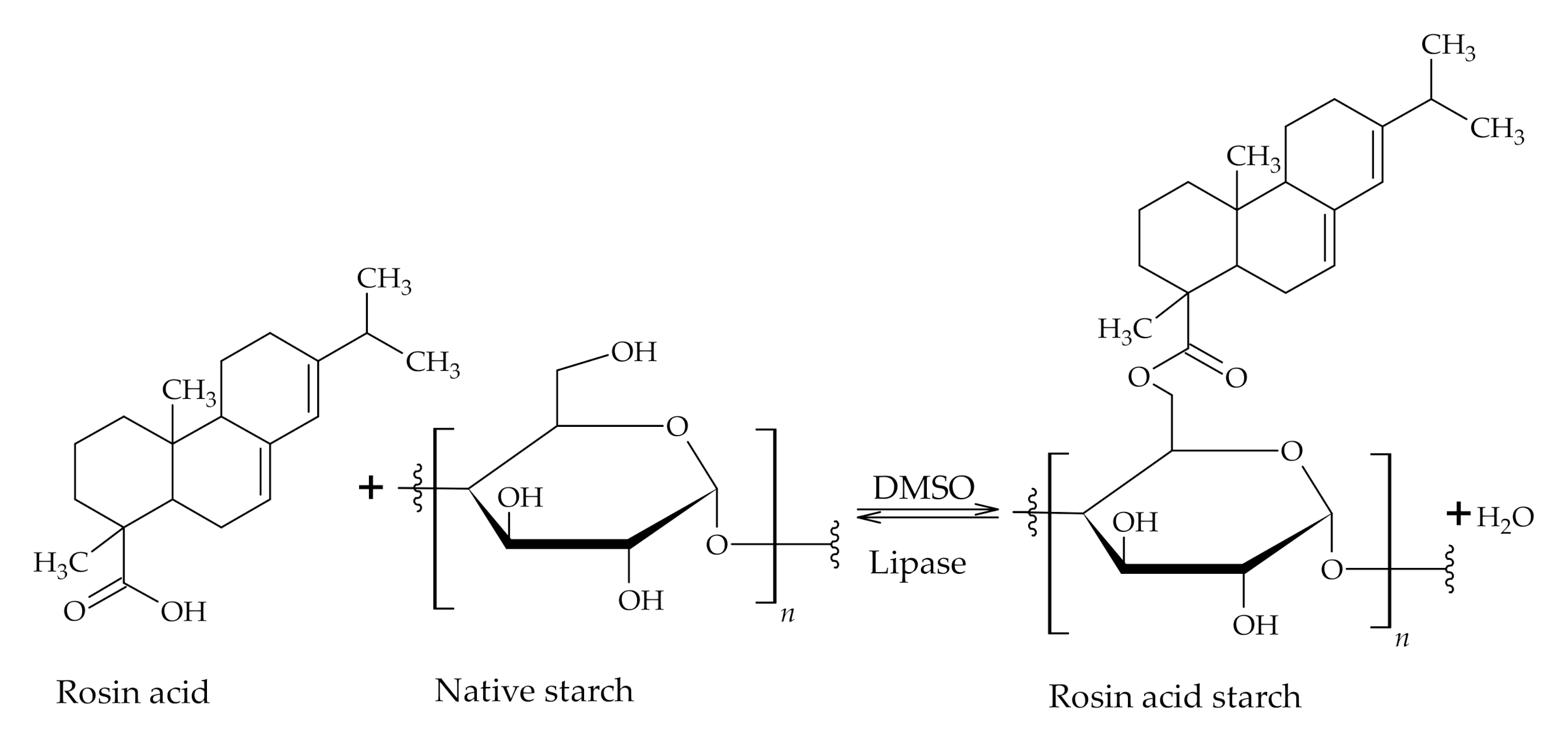
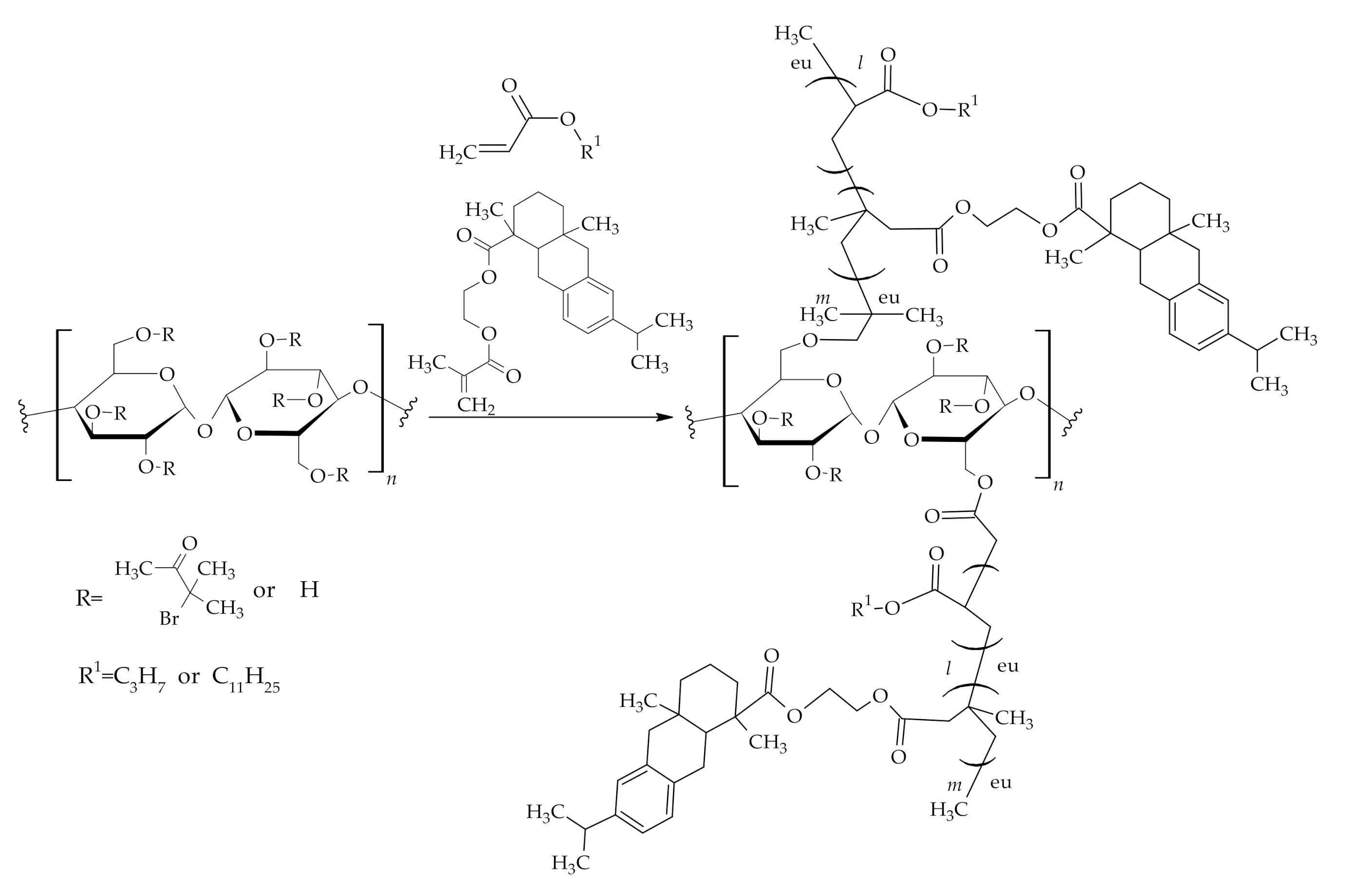
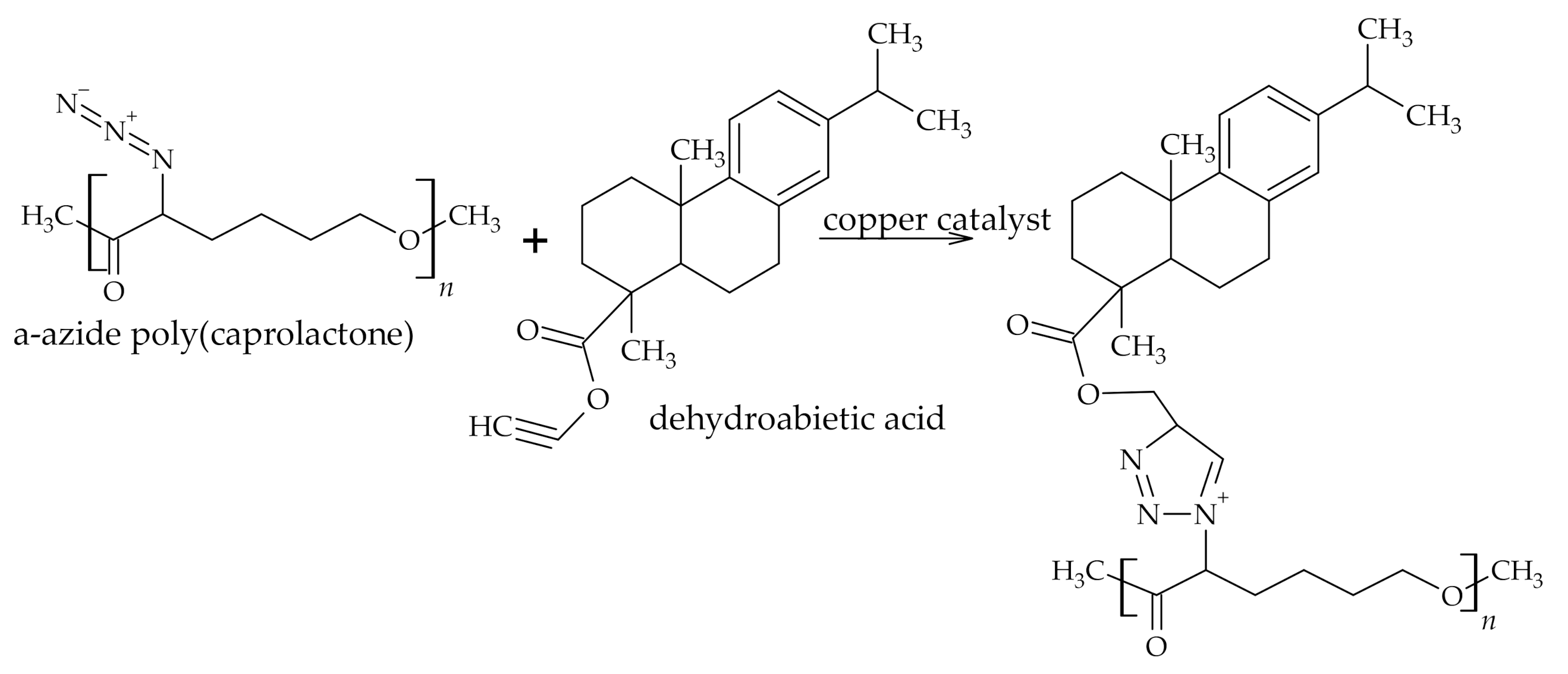
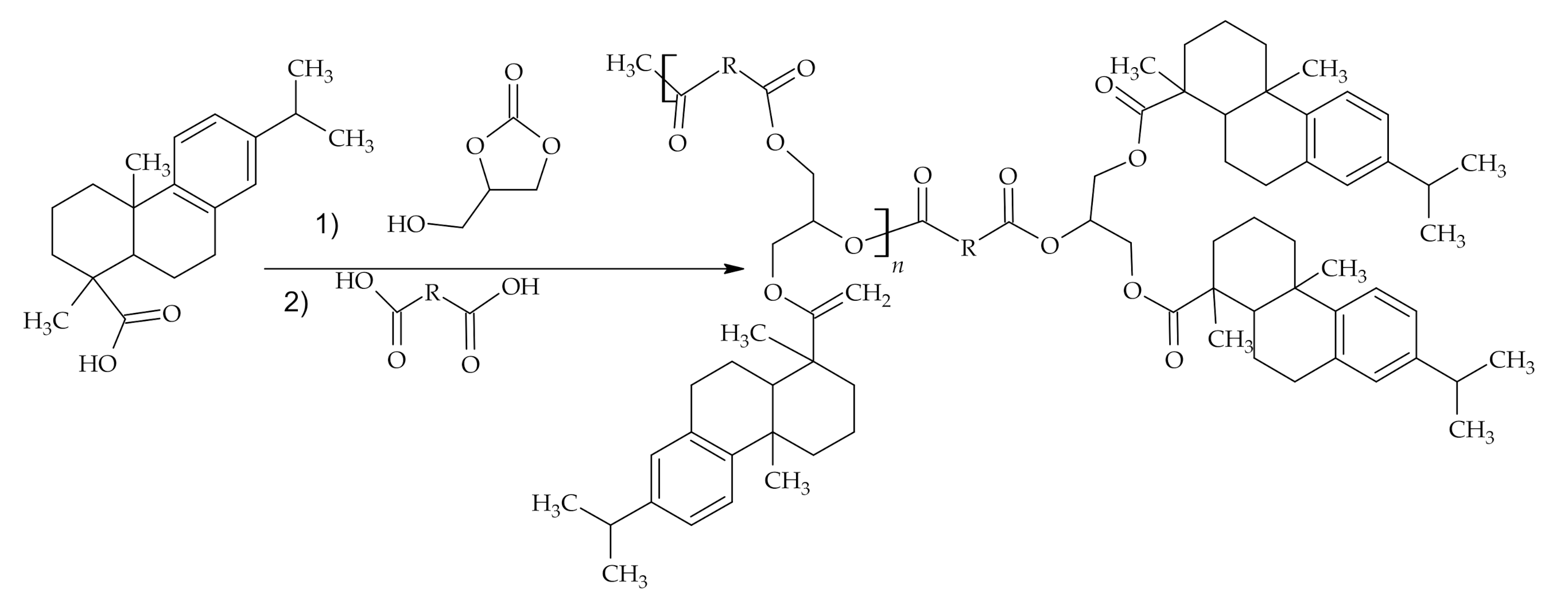
3.1. The Use of Rosin Acids in Polymer Synthesis
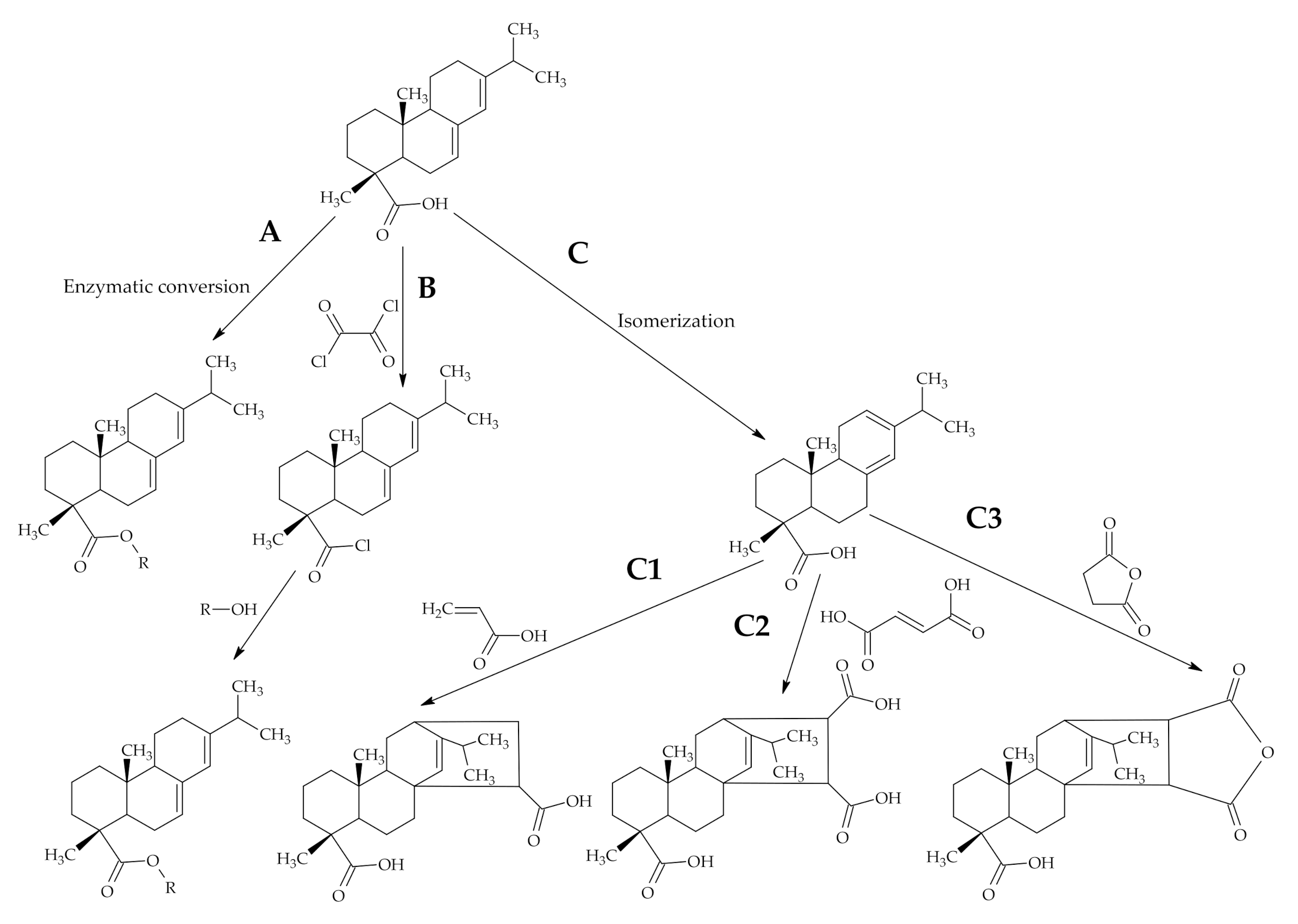
3.1.1. Reactions of Rosin Acids Carboxylic Group
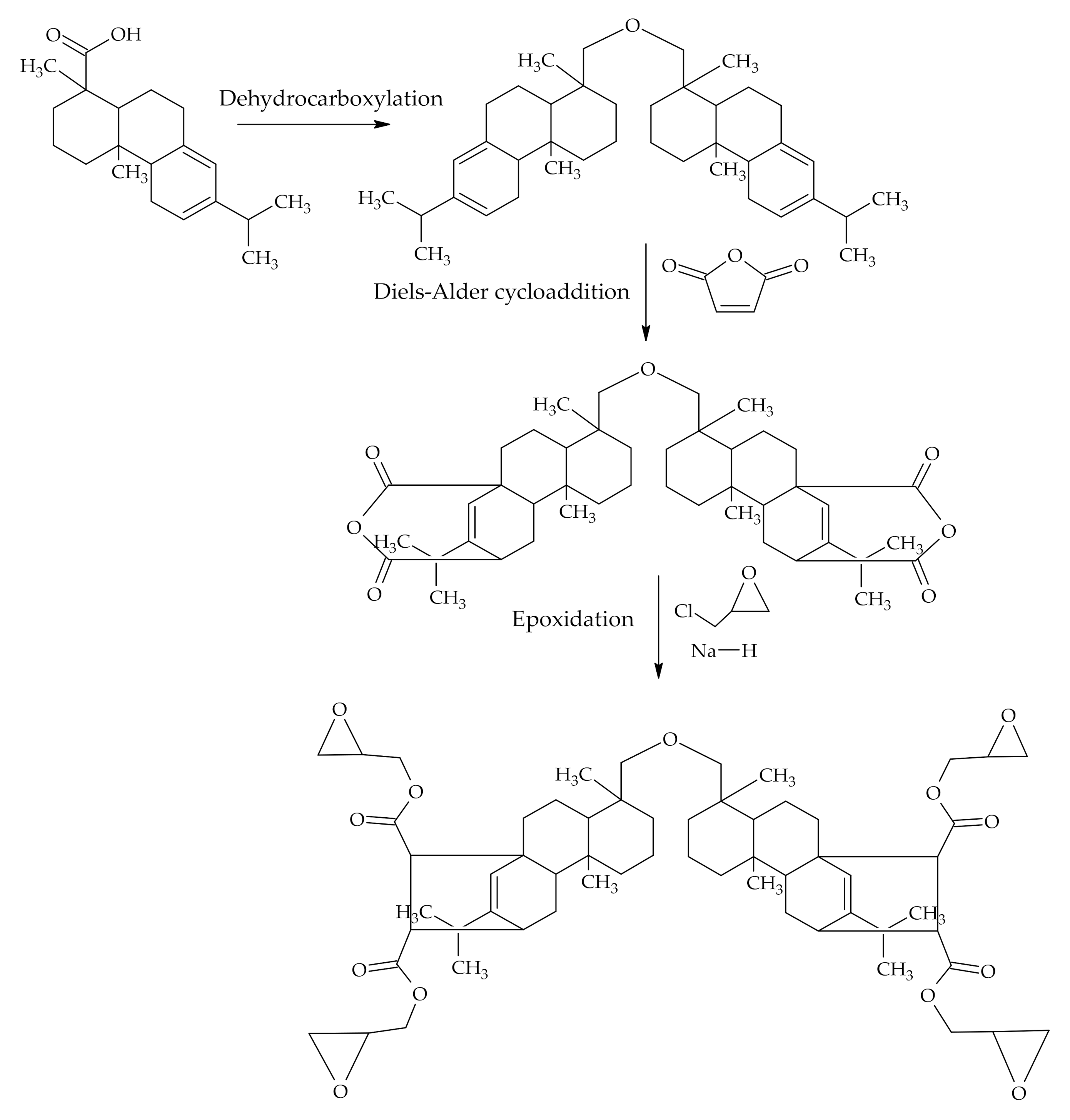
3.1.2. Modifications Starting with Diels-Alder Addition
3.2. The Use of Tall Oil Fatty Acids
3.2.1. Tall Oil-Based Polyol Development
Carboxyl Group Reaction in Tall Oil Fatty Acids
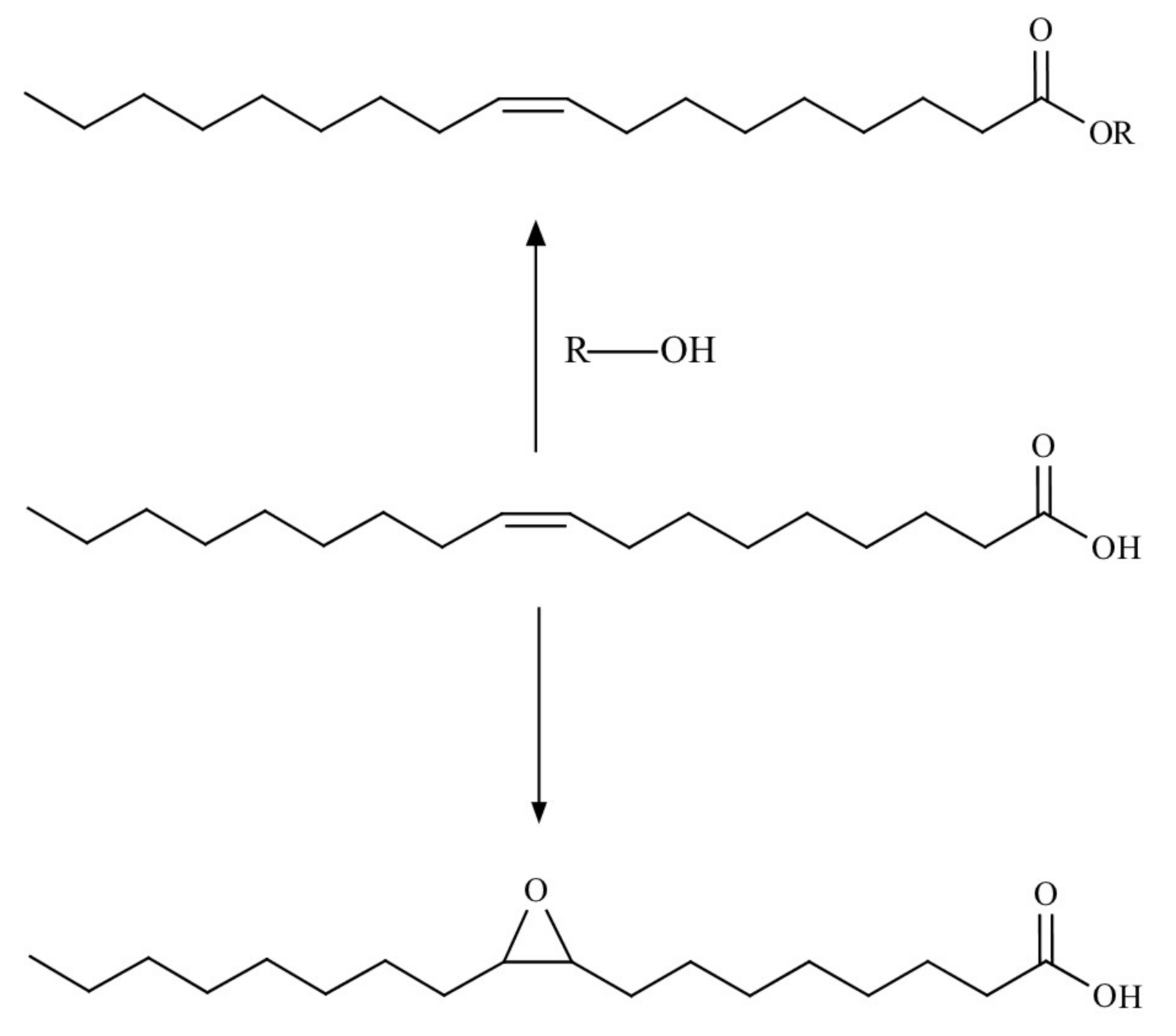
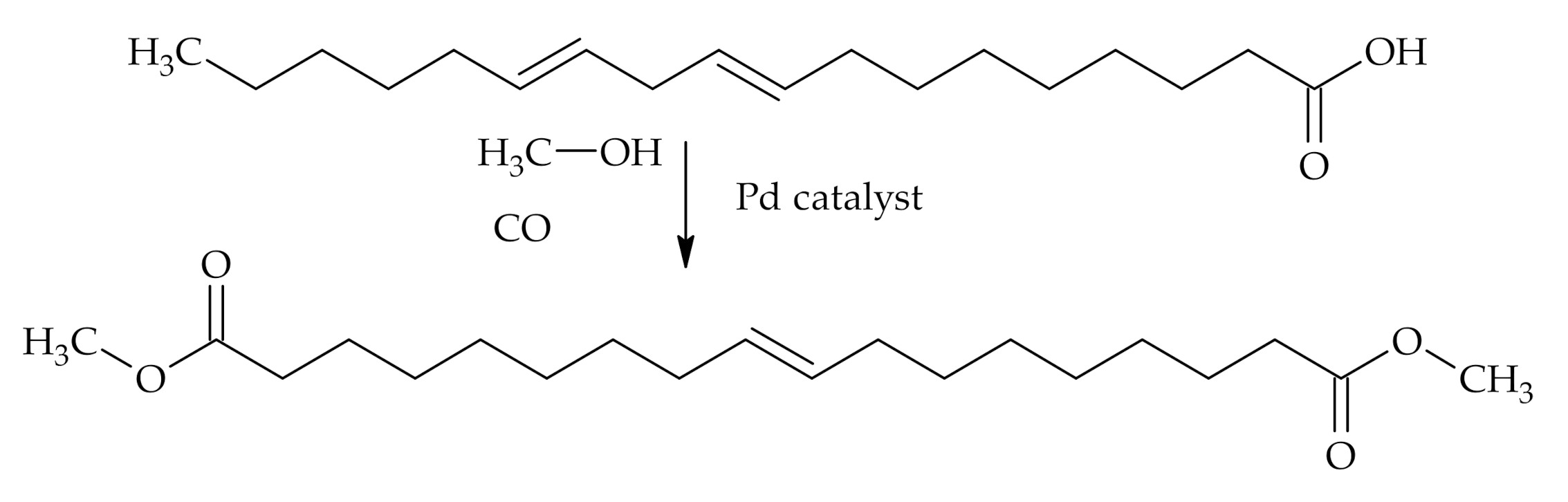
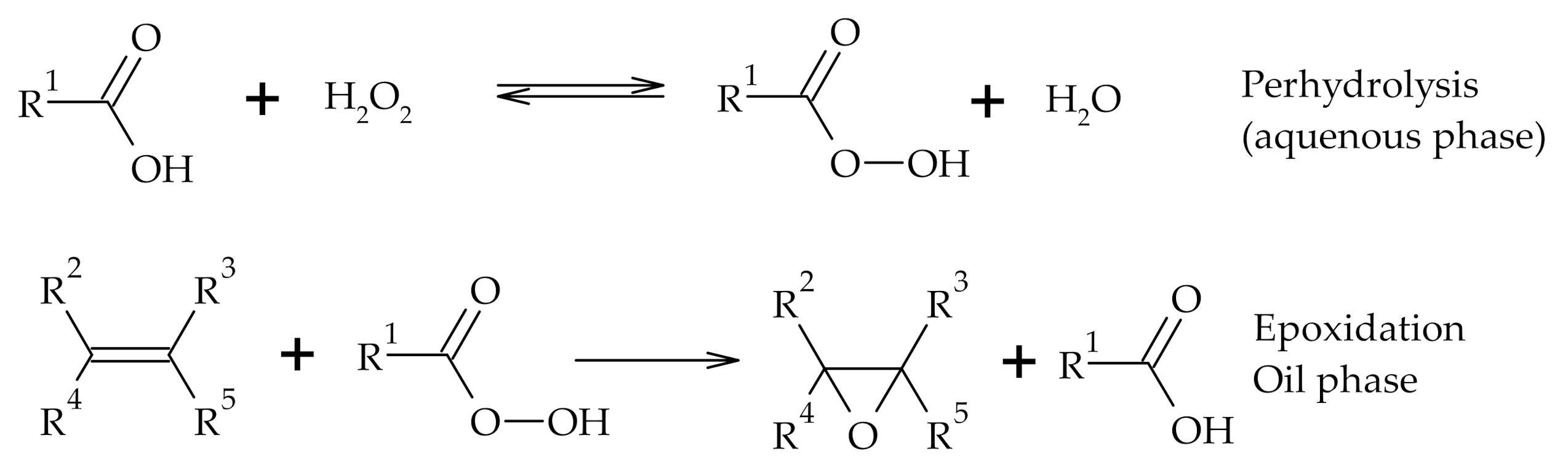
Reactions at Tall Oil Fatty Acids Double Bond
3.2.2. The Use of Tall Oil and Tall Oil Fatty Acid-Based Polyols in Polyurethane Formation
3.2.3. Tall Oil Fatty Acid Use for Alkyd Resins and Anticorrosive Smart Microcapsules Based Self-Healing Agents
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Plastics Europe. EPRO Plastics—The Facts. Available online: https://www.plasticseurope.org/application/files/9715/7129/9584/FINAL_web_version_Plastics_the_facts2019_14102019.pdf (accessed on 25 October 2020).
- Miao, S.; Wang, P.; Su, Z.; Zhang, S. Vegetable-oil-based polymers as future polymeric biomaterials. Acta Biomater. 2014, 10, 1692–1704. [Google Scholar] [CrossRef] [PubMed]
- Imre, B.; Pukánszky, B. From natural resources to functional polymeric biomaterials. Eur. Polym. J. 2015, 68, 481–487. [Google Scholar] [CrossRef]
- Peters, D.; Stojcheva, V. Crude Tall Oil Low ILUC Risk Assessment—Comparing Global Supply and Demand; ECOFYS Netherlands BV: Utrecht, The Netherlands, 2017; pp. 1–23. [Google Scholar]
- Bajpai, P. Kraft Spent Liquor Recovery Polymers for a Sustainable Environ-ment and Green Energy. In Biermann’s Hadbook of Pulp and Paper; Elsevier: Amsterdam, The Netherlands, 2018. [Google Scholar]
- Keskin, A.; Gürü, M.; Altiparmak, D. Biodiesel production from tall oil with synthesized Mn and Ni based additives: Effects of the additives on fuel consumption and emissions. Fuel 2007, 86, 1139–1143. [Google Scholar] [CrossRef]
- Adewale, P.; Vithanage, L.N.; Christopher, L. Optimization of enzyme-catalyzed biodiesel production from crude tall oil using Taguchi method. Energy Convers. Manag. 2017, 154, 81–91. [Google Scholar] [CrossRef]
- White, K.; Lorenz, N.; Potts, T.; Roy Penney, W.; Babcock, R.; Hardison, A.; Canuel, E.A.; Hestekin, J.A. Production of biodiesel fuel from tall oil fatty acids via high temperature methanol reaction. Fuel 2011, 90, 3193–3199. [Google Scholar] [CrossRef]
- Keskin, A.; Gürü, M.; Altiparmak, D. Influence of tall oil biodiesel with Mg and Mo based fuel additives on diesel engine performance and emission. Bioresour. Technol. 2008, 99, 6434–6438. [Google Scholar] [CrossRef]
- Rajendran, V.K.; Breitkreuz, K.; Kraft, A.; Maga, D.; Brucart, M. Analysis of the European Crude Tall Oil Industry—Environmental Impact, Socioeconomic Value & Downstream Potential; Fraunhofer Institute for Environmental, Safety and Energy Technology UMSICHT: Oberhausen, Germany, 2016; pp. 3–77. [Google Scholar]
- Dahlen, J.; Nicholas, D.D.; Schultz, T.P. Water repellency and dimensional stability of Southern Pine decking treated with waterborne resin acids. J. Wood Chem. Technol. 2008, 28, 47–54. [Google Scholar] [CrossRef]
- Hyvönen, A.; Piltonen, P.; Niinimäki, J. Tall oil/water—Emulsions as water repellents for Scots pine sapwood. Holz als Roh und Werkst. 2006, 64, 68–73. [Google Scholar] [CrossRef]
- Hosseinpourpia, R.; Adamopoulos, S.; Parsland, C. Utilization of different tall oils for improving the water resistance of cellulosic fibers. J. Appl. Polym. Sci. 2019, 136, 47303. [Google Scholar] [CrossRef]
- Lu, C.; Yu, J.; Wang, C.; Wang, J.; Chu, F. Fabrication of UV-absorbent cellulose-rosin based thermoplastic elastomer via “graft from” ATRP. Carbohydr. Polym. 2018, 188, 128–135. [Google Scholar] [CrossRef]
- Temiz, A.; Alfredsen, G.; Eikenes, M.; Terziev, N. Decay resistance of wood treated with boric acid and tall oil derivates. Bioresour. Technol. 2008, 99, 2102–2106. [Google Scholar] [CrossRef] [PubMed]
- Wool, R.P.; Sun, X.S. Bio-Based Polymers and Composites; Academic Press: Cambridge, MA, USA, 2005; ISBN 9780127639529. [Google Scholar]
- Fengel, D.; Wegener, G. Wood—Chemistry, Ultrastructure, Reactions; Verlag Kessel: Munchen, Germany, 2003. [Google Scholar]
- Sjöström, E. Wood Chemistry: Fundamentals and Applications; Academic Press: Cambridge, MA, USA, 1993; ISBN 0126474818. [Google Scholar]
- Aro, T.; Fatehi, P. Tall oil production from black liquor: Challenges and opportunities. Sep. Purif. Technol. 2017, 175, 469–480. [Google Scholar] [CrossRef]
- Uusi-Kyyny, P.; Pakkanen, M.; Linnekoski, J.; Alopaeus, V. Hydrogen solubility measurements of analyzed tall oil fractions and a solubility model. J. Chem. Thermodyn. 2017, 105, 15–20. [Google Scholar] [CrossRef]
- Biermann, C.J.; Biermann, C.J. Kraft Spent Liquor Recovery. In Handbook of Pulping and Papermaking; Elsevier: Amsterdam, The Netherlands, 1996; ISBN 9780120973620. [Google Scholar]
- Keeling, C.I.; Bohlmann, J. Diterpene resin acids in conifers. Phytochemistry 2006, 67, 2415–2423. [Google Scholar] [CrossRef]
- Ojagh, H.; Creaser, D.; Salam, M.A.; Grennfelt, E.L.; Olsson, L. Hydroconversion of abietic acid into value-added fuel components over sulfided NiMo catalysts with varying support acidity. Fuel Process. Technol. 2019, 190, 55–66. [Google Scholar] [CrossRef]
- Zhang, C.; Wang, H.; Zhou, Q. Preparation and characterization of microcapsules based self-healing coatings containing epoxy ester as healing agent. Prog. Org. Coat. 2018, 125, 403–410. [Google Scholar] [CrossRef]
- Sawpan, M.A. Polyurethanes from vegetable oils and applications: A review. J. Polym. Res. 2018, 25, 184. [Google Scholar] [CrossRef]
- Mejía, M.C.; Murillo, E.A. Polyfunctional macromonomers obtained from 2,2-bis(hydroxymethyl) propanoic acid and tall oil fatty acids. J. Appl. Polym. Sci. 2015, 132. [Google Scholar] [CrossRef]
- Paberza, A.; Stiebra, L.; Cabulis, U. Photodegradation of polyurethane foam obtained from renewable resource-pulp production byproducts. J. Renew. Mater. 2015, 3, 19–27. [Google Scholar] [CrossRef]
- Yao, K.; Wang, J.; Zhang, W.; Lee, J.S.; Wang, C.; Chu, F.; He, X.; Tang, C. Degradable rosin-ester-caprolactone graft copolymers. Biomacromolecules 2011, 12, 2171–2177. [Google Scholar] [CrossRef]
- Peng, G.; Roberts, J.C. Solubility and toxicity of resin acids. Water Res. 2000, 34, 2779–2785. [Google Scholar] [CrossRef]
- El-Ghazawy, R.A.; El-Saeed, A.M.; Al-Shafey, H.I.; Abdul-Raheim, A.R.M.; El-Sockary, M.A. Rosin based epoxy coating: Synthesis, identification and characterization. Eur. Polym. J. 2015, 69, 403–415. [Google Scholar] [CrossRef]
- Wang, J.; Yao, K.; Korich, A.L.; Li, S.; Ma, S.; Ploehn, H.J.; Iovine, P.M.; Wang, C.; Chu, F.; Tang, C. Combining renewable gum rosin and lignin: Towards hydrophobic polymer composites by controlled polymerization. J. Polym. Sci. Part A Polym. Chem. 2011, 49, 3728–3738. [Google Scholar] [CrossRef]
- Lin, R.; Li, H.; Long, H.; Su, J.; Huang, W. Synthesis of rosin acid starch catalyzed by lipase. Biomed Res. Int. 2014, 2014, 647068. [Google Scholar] [CrossRef] [PubMed]
- Lin, R.; Li, H.; Long, H.; Su, J.; Huang, W. Structure and characteristics of lipase-catalyzed rosin acid starch. Food Hydrocoll. 2015, 43, 352–359. [Google Scholar] [CrossRef]
- Li, Q.; Huang, X.; Liu, H.; Shang, S.; Song, Z.; Song, J. Preparation and properties of room temperature vulcanized silicone rubber based on rosin-grafted polydimethylsiloxane. RSC Adv. 2018, 8, 14684–14693. [Google Scholar] [CrossRef]
- Avérous, L. Biodegradable multiphase systems based on plasticized starch: A review. J. Macromol. Sci. Polym. Rev. 2004, 44, 231–274. [Google Scholar] [CrossRef]
- Russo, M.A.L.; O’Sullivan, C.; Rounsefell, B.; Halley, P.J.; Truss, R.; Clarke, W.P. The anaerobic degradability of thermoplastic starch: Polyvinyl alcohol blends: Potential biodegradable food packaging materials. Bioresour. Technol. 2009, 100, 1705–1710. [Google Scholar] [CrossRef]
- Niranjana Prabhu, T.; Prashantha, K. A review on present status and future challenges of starch based polymer films and their composites in food packaging applications. Polym. Compos. 2018, 39, 2499–2522. [Google Scholar] [CrossRef]
- Winkler, H.; Vorwerg, W.; Rihm, R. Thermal and mechanical properties of fatty acid starch esters. Carbohydr. Polym. 2014, 102, 941–949. [Google Scholar] [CrossRef]
- Lin, R.; Li, H.; Long, H.; Su, J.; Huang, W.; Wang, S. Optimization of lipase-catalyzed rosin acid starch synthesis by response surface methodology. J. Mol. Catal. B Enzym. 2014, 105, 104–110. [Google Scholar] [CrossRef]
- Liu, Y.; Yao, K.; Chen, X.; Wang, J.; Wang, Z.; Ploehn, H.J.; Wang, C.; Chu, F.; Tang, C. Sustainable thermoplastic elastomers derived from renewable cellulose, rosin and fatty acids. Polym. Chem. 2014, 5, 3170–3181. [Google Scholar] [CrossRef]
- Sacripante, G.G.; Zhou, K.; Farooque, M. Sustainable Polyester Resins Derived from Rosins. Macromolecules 2015, 48, 6876–6881. [Google Scholar] [CrossRef]
- De Castro, D.O.; Bras, J.; Gandini, A.; Belgacem, N. Surface grafting of cellulose nanocrystals with natural antimicrobial rosin mixture using a green process. Carbohydr. Polym. 2016, 137, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Moustafa, H.; El Kissi, N.; Abou-Kandil, A.I.; Abdel-Aziz, M.S.; Dufresne, A. PLA/PBAT bionanocomposites with antimicrobial natural rosin for green packaging. ACS Appl. Mater. Interfaces 2017, 9, 20132–20141. [Google Scholar] [CrossRef]
- Narayanan, M.; Loganathan, S.; Valapa, R.B.; Thomas, S.; Varghese, T.O. UV protective poly(lactic acid)/rosin films for sustainable packaging. Int. J. Biol. Macromol. 2017, 99, 37–45. [Google Scholar] [CrossRef]
- Niu, X.; Liu, Y.; Song, Y.; Han, J.; Pan, H. Rosin modified cellulose nanofiber as a reinforcing and co-antimicrobial agents in polylactic acid /chitosan composite film for food packaging. Carbohydr. Polym. 2018, 183, 102–109. [Google Scholar] [CrossRef]
- Yuan, L.; Hamidi, N.; Smith, S.; Clemons, F.; Hamidi, A.; Tang, C. Molecular characterization of biodegradable natural resin acid-substituted polycaprolactone. Eur. Polym. J. 2015, 62, 43–50. [Google Scholar] [CrossRef]
- Wilbon, P.A.; Zheng, Y.; Yao, K.; Tang, C. Renewable rosin acid-degradable caprolactone block copolymers by atom transfer radical polymerization and ring-opening polymerization. Macromolecules 2010, 43, 8747–8754. [Google Scholar] [CrossRef]
- Li, Q.; Huang, X.; Liu, H.; Shang, S.; Song, Z.; Song, J. Properties Enhancement of Room Temperature Vulcanized Silicone Rubber by Rosin Modified Aminopropyltriethoxysilane as a Cross-linking Agent. ACS Sustain. Chem. Eng. 2017, 5, 10002–10010. [Google Scholar] [CrossRef]
- Deng, L.; Shen, M.; Yu, J.; Wu, K.; Ha, C. Preparation, characterization, and flame retardancy of novel rosin-based siloxane epoxy resins. Ind. Eng. Chem. Res. 2012, 51, 8178–8184. [Google Scholar] [CrossRef]
- Xu, T.; Liu, H.; Song, J.; Shang, S.B.; Song, Z.; Zou, K.; Yang, C. Synthesis and characterization of maleated rosin-modified fluorosilicone resin and its fluorosilicone rubber. J. Appl. Polym. Sci. 2015, 132. [Google Scholar] [CrossRef]
- Yu, C.; Chen, C.; Gong, Q.; Zhang, F.A. Preparation of polymer microspheres with a rosin moiety from rosin ester, styrene and divinylbenzene. Polym. Int. 2012, 61, 1619–1626. [Google Scholar] [CrossRef]
- Liu, B.; Nie, J.; He, Y. From rosin to high adhesive polyurethane acrylate: Synthesis and properties. Int. J. Adhes. Adhes. 2016, 66, 99–103. [Google Scholar] [CrossRef]
- Yao, F.; Zhang, D.; Zhang, C.; Yang, W.; Deng, J. Preparation and application of abietic acid-derived optically active helical polymers and their chiral hydrogels. Bioresour. Technol. 2013, 129, 58–64. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Wilbon, P.A.; Chen, Y.P.; Zhou, J.; Nagarkatti, M.; Wang, C.; Chu, F.; Decho, A.W.; Tang, C. Amphipathic antibacterial agents using cationic methacrylic polymers with natural rosin as pendant group. RSC Adv. 2012, 2, 10275–10282. [Google Scholar] [CrossRef]
- Yu, C.L.; Wang, X.; Chen, C.; Zhang, F. Preparation of polystyrene microspheres using rosin-acrylic acid diester as a cross-linking agent. Ind. Eng. Chem. Res. 2014, 53, 2244–2250. [Google Scholar] [CrossRef]
- Mustata, F.; Bicu, I. A novel route for synthesizing esters and polyesters from the Diels-Alder adduct of levopimaric acid and acrylic acid. Eur. Polym. J. 2010, 46, 1316–1327. [Google Scholar] [CrossRef]
- Xu, X.; Shang, S.; Song, Z.; Cui, S.; Wang, H.; Wang, D. Preparation and characterization of rosin-based waterborne polyurethane from maleopimaric acid polyester polyol. BioResources 2011, 6, 2460–2470. [Google Scholar]
- Shao, J.; Yu, C.; Bian, F.; Zeng, Y.; Zhang, F. Preparation and Properties of Hydrophilic Rosin-Based Aromatic Polyurethane Microspheres. ACS Omega 2019, 4, 2493–2499. [Google Scholar] [CrossRef]
- Wang, H.; Wang, H.; Zhou, G. Synthesis of rosin-based imidoamine-type curing agents and curing behavior with epoxy resin. Polym. Int. 2011, 60, 557–563. [Google Scholar] [CrossRef]
- Maffini, M.V.; Rubin, B.S.; Sonnenschein, C.; Soto, A.M. Endocrine disruptors and reproductive health: The case of bisphenol-A. Mol. Cell. Endocrinol. 2006, 179–186. [Google Scholar] [CrossRef] [PubMed]
- Mantzaridis, C.; Brocas, A.L.; Llevot, A.; Cendejas, G.; Auvergne, R.; Caillol, S.; Carlotti, S.; Cramail, H. Rosin acid oligomers as precursors of DGEBA-free epoxy resins. Green Chem. 2013, 15, 3091–3098. [Google Scholar] [CrossRef]
- Deng, L.; Ha, C.; Sun, C.; Zhou, B.; Yu, J.; Shen, M.; Mo, J. Properties of bio-based epoxy resins from rosin with different flexible chains. Ind. Eng. Chem. Res. 2013, 52, 13233–13240. [Google Scholar] [CrossRef]
- Qin, J.; Liu, H.; Zhang, P.; Wolcott, M.; Zhang, J. Use of eugenol and rosin as feedstocks for biobased epoxy resins and study of curing and performance properties. Polym. Int. 2014, 63, 760–765. [Google Scholar] [CrossRef]
- Li, S.; Zou, T.; Liu, X.; Tao, M. Synthesis and characterization of benzoxazine monomers from rosin and their thermal polymerization. Des. Monomers Polym. 2014, 17, 40–46. [Google Scholar] [CrossRef][Green Version]
- Maisonneuve, L.; Lebarbé, T.; Grau, E.; Cramail, H. Structure-properties relationship of fatty acid-based thermoplastics as synthetic polymer mimics. Polym. Chem. 2013, 4, 5472–5517. [Google Scholar] [CrossRef]
- Lligadas, G.; Ronda, J.C.; Galià, M.; Cádiz, V. Renewable polymeric materials from vegetable oils: A perspective. Mater. Today 2013, 16, 337–343. [Google Scholar] [CrossRef]
- Ferreira, G.R.; Braquehais, J.R.; da Silva, W.N.; Machado, F. Synthesis of soybean oil-based polymer lattices via emulsion polymerization process. Ind. Crops Prod. 2015, 65, 14–20. [Google Scholar] [CrossRef]
- Meier, M.A.R.; Metzger, J.O.; Schubert, U.S. Plant oil renewable resources as green alternatives in polymer science. Chem. Soc. Rev. 2007, 36, 1788–1802. [Google Scholar] [CrossRef]
- Islam, M.R.; Beg, M.D.H.; Jamari, S.S. Development of vegetable-oil-based polymers. J. Appl. Polym. Sci. 2014, 131. [Google Scholar] [CrossRef]
- Petrovic, Z.S. Polyurethanes from vegetable oils. Polym. Rev. 2008, 48, 109–155. [Google Scholar] [CrossRef]
- Uschanov, P.; Heiskanen, N.; Mononen, P.; Maunu, S.L.; Koskimies, S. Synthesis and characterization of tall oil fatty acids-based alkyd resins and alkyd-acrylate copolymers. Prog. Org. Coat. 2008, 63, 92–99. [Google Scholar] [CrossRef]
- Nguyen, H.D.; Löf, D.; Hvilsted, S.; Daugaard, A.E. Highly branched bio-based unsaturated polyesters by enzymatic polymerization. Polymers 2016, 8, 363. [Google Scholar] [CrossRef]
- Zeltins, V.; Yakushin, V.; Cabulis, U.; Kirpluks, M. Crude tall oil as raw material for rigid polyurethane foams with low water absorption. Solid State Phenom. 2017, 267, 17–22. [Google Scholar] [CrossRef]
- Yakushin, V.; Stirna, U.; Bikovens, O.; Misane, M.; Sevastyanova, I.; Vilsone, D. Synthesis and characterization of novel polyurethanes based on tall oil. Medziagotyra 2013, 19, 390–396. [Google Scholar] [CrossRef]
- Cabulis, U.; Kirpluks, M.; Stirna, U.; Lopez, M.J.; Del Carmen Vargas-Garcia, M.; Suárez-Estrella, F.; Moreno, J. Rigid polyurethane foams obtained from tall oil and filled with natural fibers: Application as a support for immobilization of lignin-degrading microorganisms. J. Cell. Plast. 2012, 48, 500–515. [Google Scholar] [CrossRef]
- Mizera, K.; Kirpluks, M.; Cabulis, U.; Leszczyńska, M.; Półka, M.; Ryszkowska, J. Characterisation of ureaurethane elastomers containing tall oil based polyols. Ind. Crops Prod. 2018, 113, 98–110. [Google Scholar] [CrossRef]
- Pietrzak, K.; Kirpluks, M.; Cabulis, U.; Ryszkowska, J. Effect of the addition of tall oil-based polyols on the thermal and mechanical properties of ureaurethane elastomers. Polym. Degrad. Stab. 2014, 108, 201–211. [Google Scholar] [CrossRef]
- Ivdre, A.; Soto, G.D.; Cabulis, U. Polyols Based on Poly(ethylene terephthalate) and Tall Oil: Perspectives for synthesis and production of rigid polyurethane foams. J. Renew. Mater. 2016, 4, 285–293. [Google Scholar] [CrossRef]
- Nayak, P.L. Natural oil-based polymers: Opportunities and challenges. J. Macromol. Sci. Polym. Rev. 2000, 40, 1–21. [Google Scholar] [CrossRef]
- Murillo, E.A.; Vallejo, P.P.; López, B.L. Synthesis and characterization of hyperbranched alkyd resins based on tall oil fatty acids. Prog. Org. Coat. 2010, 69, 235–240. [Google Scholar] [CrossRef]
- Mejía, M.C.; Murillo, E.A. Styrene–hydroxyethyl acrylate copolymer based alkyd resins with a comb-type structural morphology obtained with a high solid content. J. Appl. Polym. Sci. 2016, 133. [Google Scholar] [CrossRef]
- Yakushin, V.; Misane, M.; Bikovens, O.; Vilsone, D.; Sevastyanova, I. Synthesis of trimethylolpropane esters of tall oil fatty acids and properties of polyurethane coatings on their basis. J. Coat. Technol. Res. 2016, 13, 317–324. [Google Scholar] [CrossRef]
- Furst, M.R.L.; Seidensticker, T.; Cole-Hamilton, D.J. Polymerisable di- and triesters from Tall Oil Fatty Acids and related compounds. Green Chem. 2013, 15, 1218–1225. [Google Scholar] [CrossRef]
- Yadav, G.D.; Manjula Devi, K. A kinetic model for the enzyme-catalyzed self-epoxidation of oleic acid. JAOCS J. Am. Oil Chem. Soc. 2001, 78, 347–351. [Google Scholar] [CrossRef]
- Törnvall, U.; Orellana-Coca, C.; Hatti-Kaul, R.; Adlercreutz, D. Stability of immobilized Candida antarctica lipase B during chemo-enzymatic epoxidation of fatty acids. Enzyme Microb. Technol. 2007, 40, 447–451. [Google Scholar] [CrossRef]
- Findley, T.W.; Swern, D.; Scanlan, J.T. Epoxidation of Unsaturated Fatty Materials with Peracetic Acid in Glacial Acetic Acid Solution. J. Am. Chem. Soc. 1945, 67, 412–414. [Google Scholar] [CrossRef]
- Aguilera, A.F.; Tolvanen, P.; Eränen, K.; Wärnå, J.; Leveneur, S.; Marchant, T.; Salmi, T. Kinetic modelling of Prileschajew epoxidation of oleic acid under conventional heating and microwave irradiation. Chem. Eng. Sci. 2019, 199, 426–438. [Google Scholar] [CrossRef]
- de Quadros, J.V.; Giudici, R. Epoxidation of soybean oil at maximum heat removal and single addition of all reactants. Chem. Eng. Process. Process Intensif. 2016, 100, 87–93. [Google Scholar] [CrossRef]
- Chua, S.C.; Xu, X.; Guo, Z. Emerging sustainable technology for epoxidation directed toward plant oil-based plasticizers. Process Biochem. 2012, 47, 1439–1451. [Google Scholar] [CrossRef]
- Vanags, E.; Kirpluks, M.; Cabulis, U.; Walterova, Z. Highly Functional Polyol Synthesis from Epoxidized Tall Oil Fatty Acids. J. Renew. Mater. 2018. [Google Scholar] [CrossRef]
- Kirpluks, M.; Vanags, E.; Abolins, A.; Fridrihsone, A.; Cabulis, U. Chemo-enzymatic oxidation of tall oil fatty acids as a precursor for further polyol production. J. Clean. Prod. 2019, 215, 390–398. [Google Scholar] [CrossRef]
- Orellana-Coca, C.; Camocho, S.; Adlercreutz, D.; Mattiasson, B.; Hatti-Kaul, R. Chemo-enzymatic epoxidation of linoleic acid: Parameters influencing the reaction. Eur. J. Lipid Sci. Technol. 2005, 107, 864–870. [Google Scholar] [CrossRef]
- Yakushin, V.; Sevastyanova, I.; Vilsone, D.; Kirpluks, M. Effect of intumescent flame retardants on the properties of polyurethanes based on tall oil fatty acids esters. Medziagotyra 2015, 21, 225–226. [Google Scholar] [CrossRef][Green Version]
- Kasprzyk, P.; Datta, J. Novel bio-based thermoplastic poly(ether-urethane)s. Correlations between the structure, processing and properties. Polymer 2019, 160, 1–10. [Google Scholar] [CrossRef]
- Kairytė, A.; Kirpluks, M.; Ivdre, A.; Cabulis, U.; Vaitkus, S.; Pundienė, I. Cleaner production of polyurethane foam: Replacement of conventional raw materials, assessment of fire resistance and environmental impact. J. Clean. Prod. 2018, 183, 760–771. [Google Scholar] [CrossRef]
- Meshram, P.D.; Puri, R.G.; Patil, A.L.; Gite, V.V. High performance moisture cured poly(ether-urethane) amide coatings based on renewable resource (cottonseed oil). J. Coat. Technol. Res. 2013, 10, 331–338. [Google Scholar] [CrossRef]
- Saravari, O.; Phapant, P.; Pimpan, V. Synthesis of water-reducible acrylic-alkyd resins based on modified palm oil. J. Appl. Polym. Sci. 2005, 96, 1170–1175. [Google Scholar] [CrossRef]
- Godfrey, O.O.; Ifijen, I.H.; Mohammed, F.U.; Aigbodion, A.I.; Ikhuoria, E.U. Alkyd resin from rubber seed oil/linseed oil blend: A comparative study of the physiochemical properties. Heliyon 2019, 5, e01621. [Google Scholar] [CrossRef]
- Kirk, O. Encyclopedia of Chemical Technology, 2nd ed.; Interscience Publishers: New York, NY, USA, 1966; Volume 1. [Google Scholar]
- Chiplunkar, P.P.; Pratap, A.P. Utilization of sunflower acid oil for synthesis of alkyd resin. Prog. Org. Coat. 2016, 93, 61–67. [Google Scholar] [CrossRef]
- Rämänen, P.; Pitkänen, P.; Jämsä, S.; Maunu, S.L. Natural Oil-Based Alkyd-Acrylic Copolymers: New Candidates for Barrier Materials. J. Polym. Environ. 2012, 20, 950–958. [Google Scholar] [CrossRef]
- Ikladious, N.; Mansour, S.; Asaad, J.N.; Emira, H.S.; Hilt, M. Synthesis and evaluation of new hyperbranched alkyds for coatings. Prog. Org. Coat. 2015, 89, 252–259. [Google Scholar] [CrossRef]
- Rämänen, P.; Maunu, S.L. Structure of tall oil fatty acid-based alkyd resins and alkyd-acrylic copolymers studied by NMR spectroscopy. Prog. Org. Coat. 2014, 77, 361–368. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vevere, L.; Fridrihsone, A.; Kirpluks, M.; Cabulis, U. A Review of Wood Biomass-Based Fatty Acids and Rosin Acids Use in Polymeric Materials. Polymers 2020, 12, 2706. https://doi.org/10.3390/polym12112706
Vevere L, Fridrihsone A, Kirpluks M, Cabulis U. A Review of Wood Biomass-Based Fatty Acids and Rosin Acids Use in Polymeric Materials. Polymers. 2020; 12(11):2706. https://doi.org/10.3390/polym12112706
Chicago/Turabian StyleVevere, Laima, Anda Fridrihsone, Mikelis Kirpluks, and Ugis Cabulis. 2020. "A Review of Wood Biomass-Based Fatty Acids and Rosin Acids Use in Polymeric Materials" Polymers 12, no. 11: 2706. https://doi.org/10.3390/polym12112706
APA StyleVevere, L., Fridrihsone, A., Kirpluks, M., & Cabulis, U. (2020). A Review of Wood Biomass-Based Fatty Acids and Rosin Acids Use in Polymeric Materials. Polymers, 12(11), 2706. https://doi.org/10.3390/polym12112706






