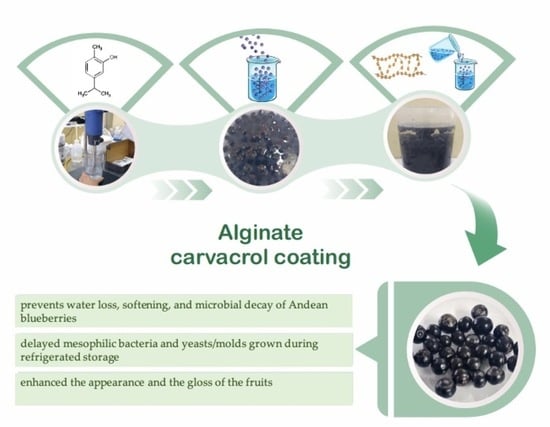
Improvement of Andean Blueberries Postharvest Preservation Using Carvacrol/Alginate-Edible Coatings
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Coating Solutions
2.3. Characterization of Edible Coatings
2.4. Application of Edible Coatings
2.5. Evaluation of Quality Attributes of Andean Blueberries along Storage
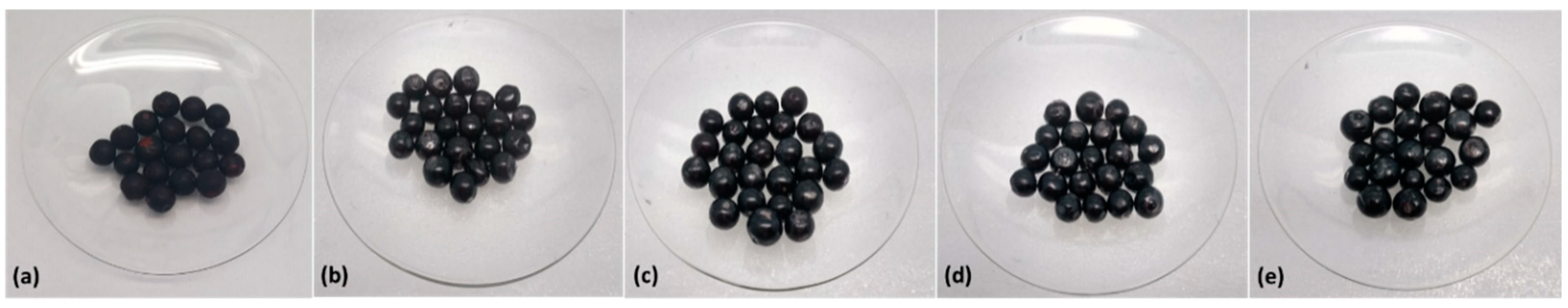
2.5.1. Color Attributes
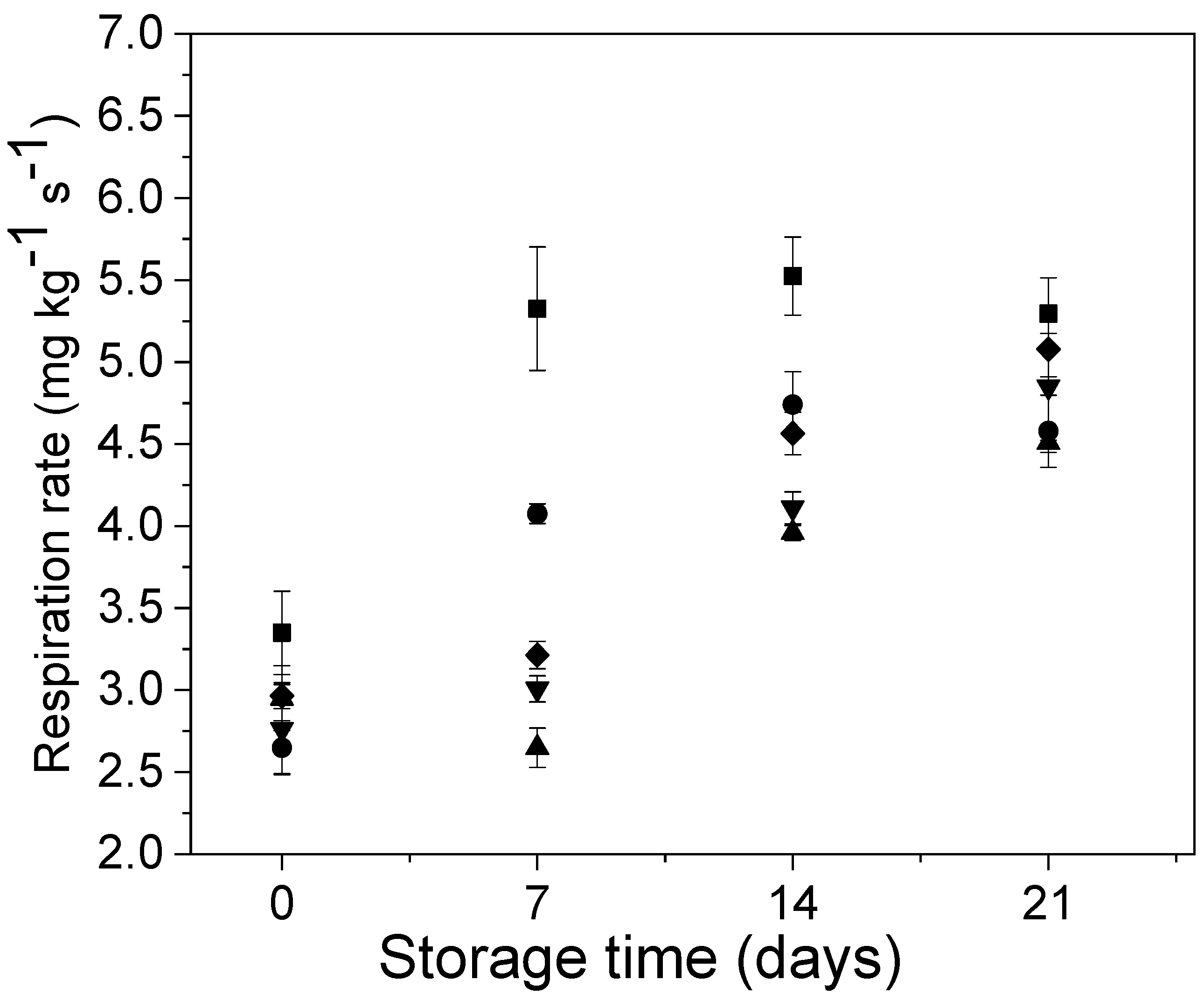
2.5.2. Respiration Rate
2.5.3. Weight Loss
2.5.4. Soluble Solids Content, pH, and Titratable Acidity (%)
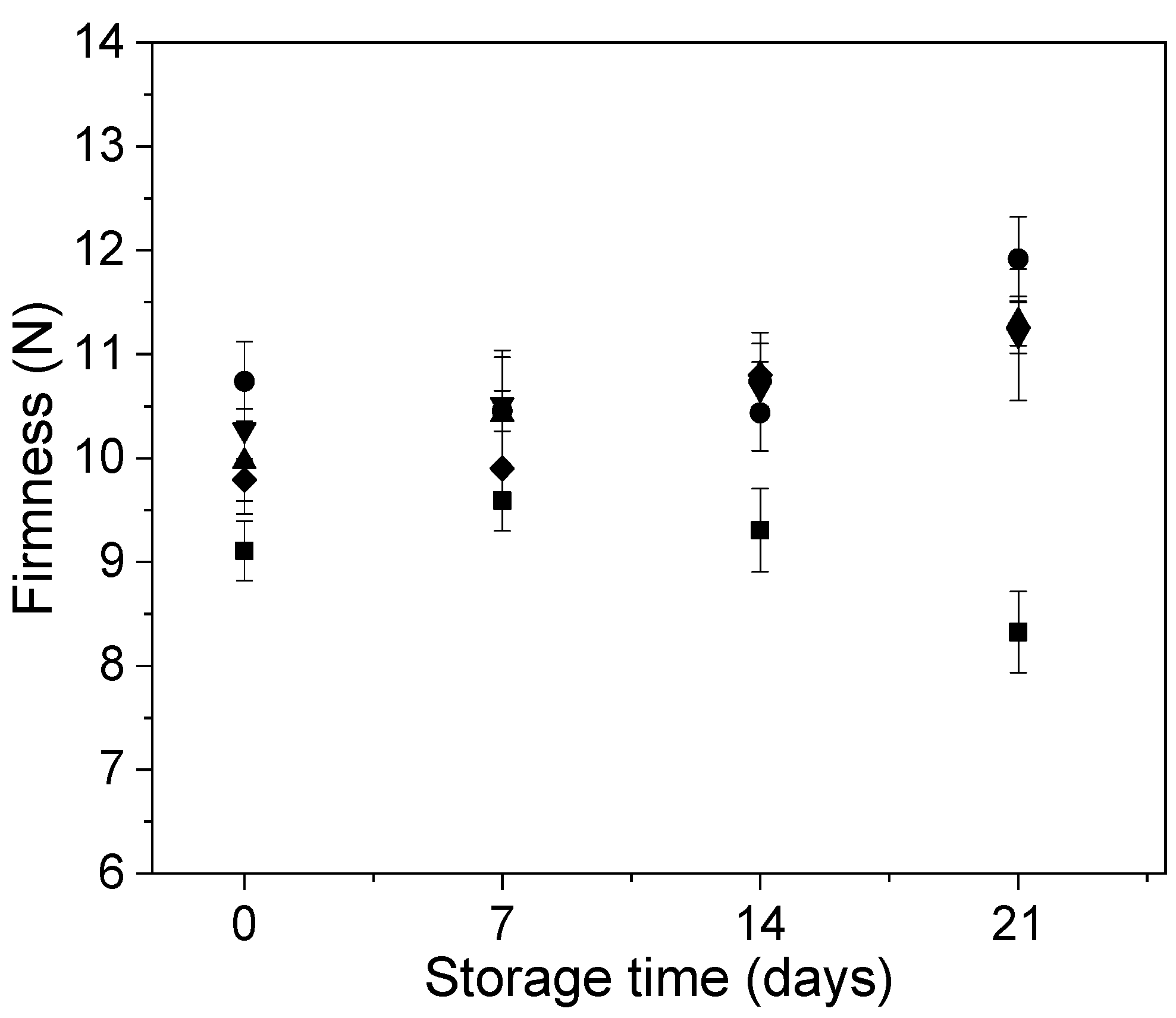
2.5.5. Firmness Analysis
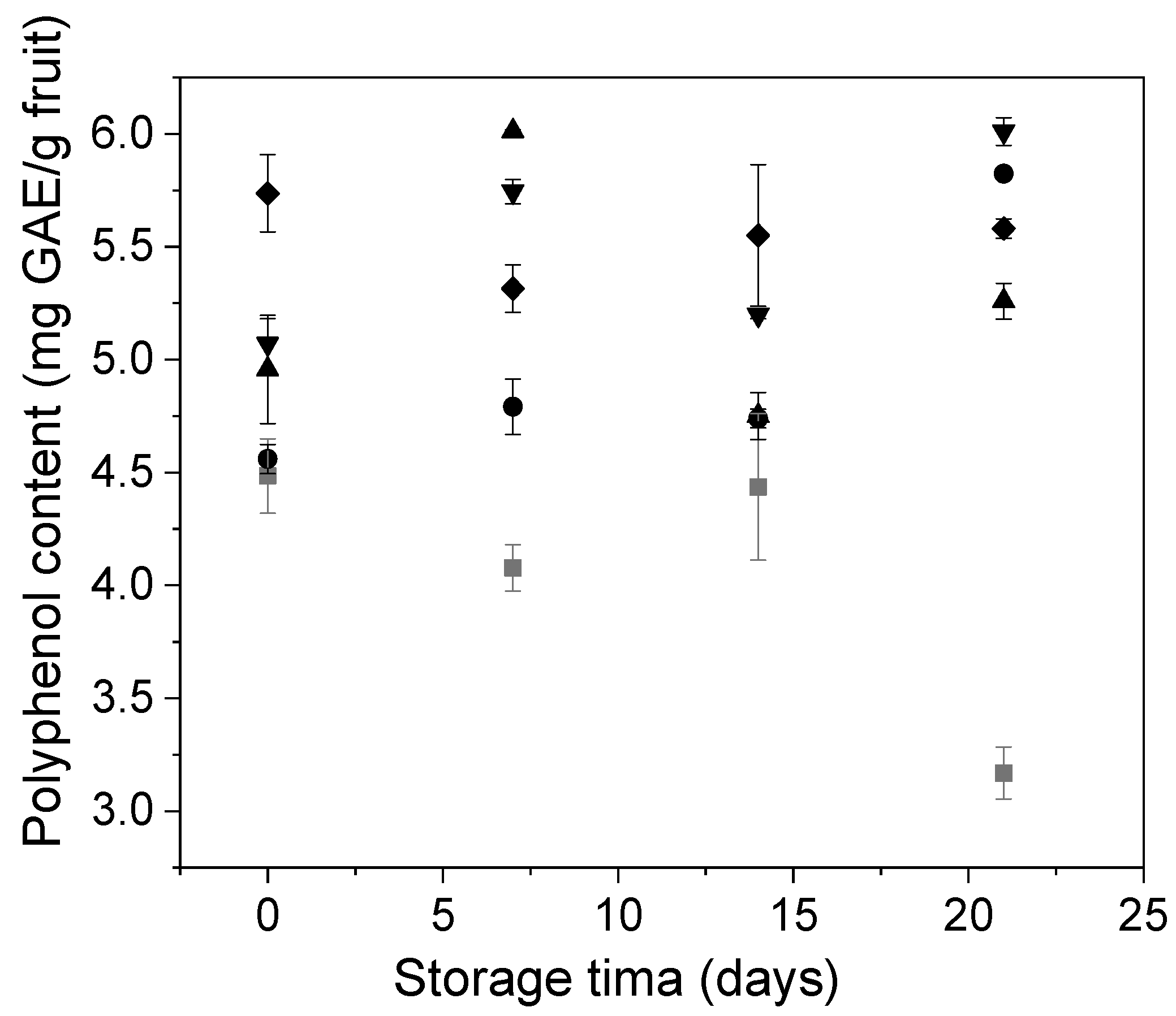
2.5.6. Total Polyphenols Content
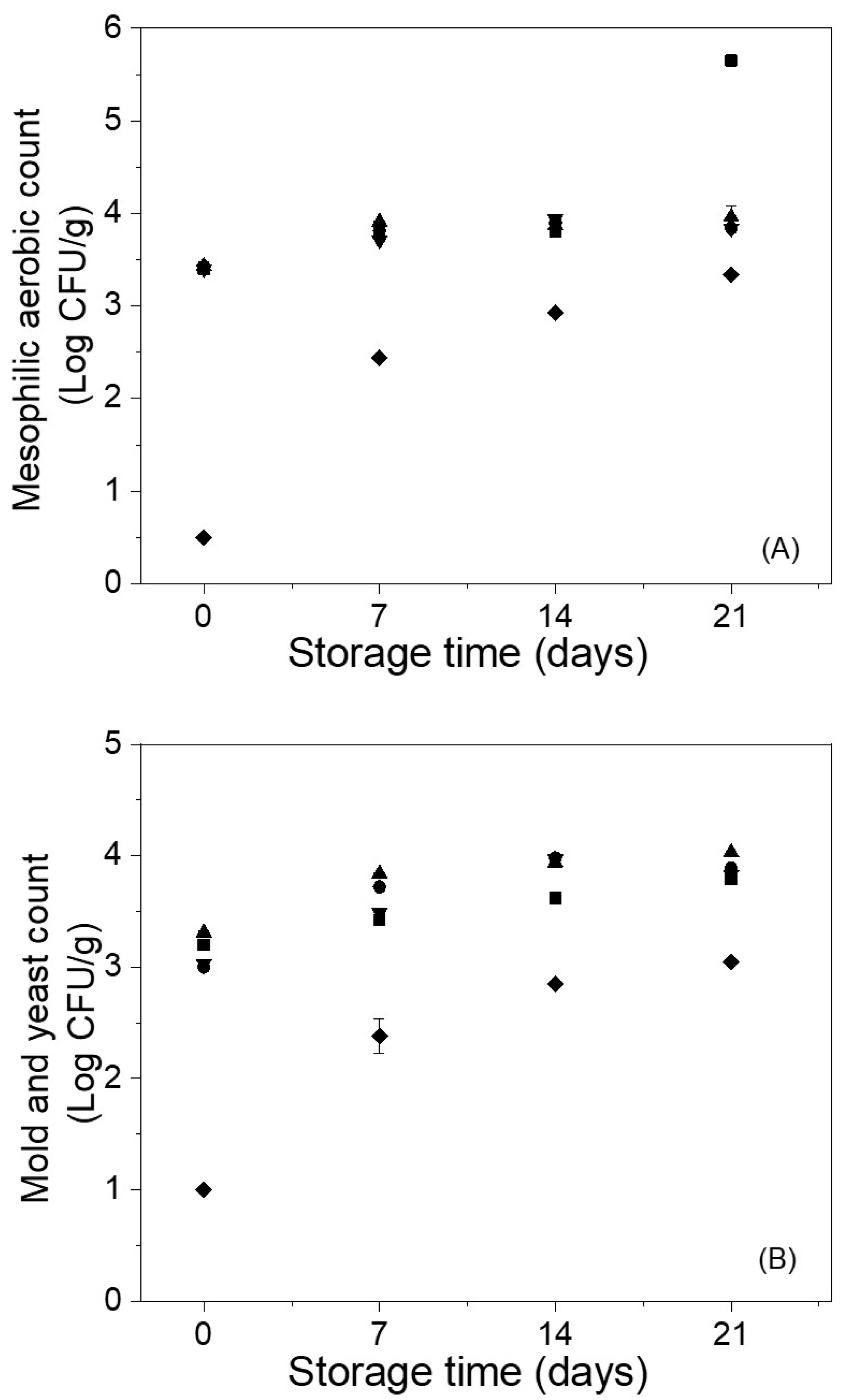
2.5.7. Microbiological Analysis
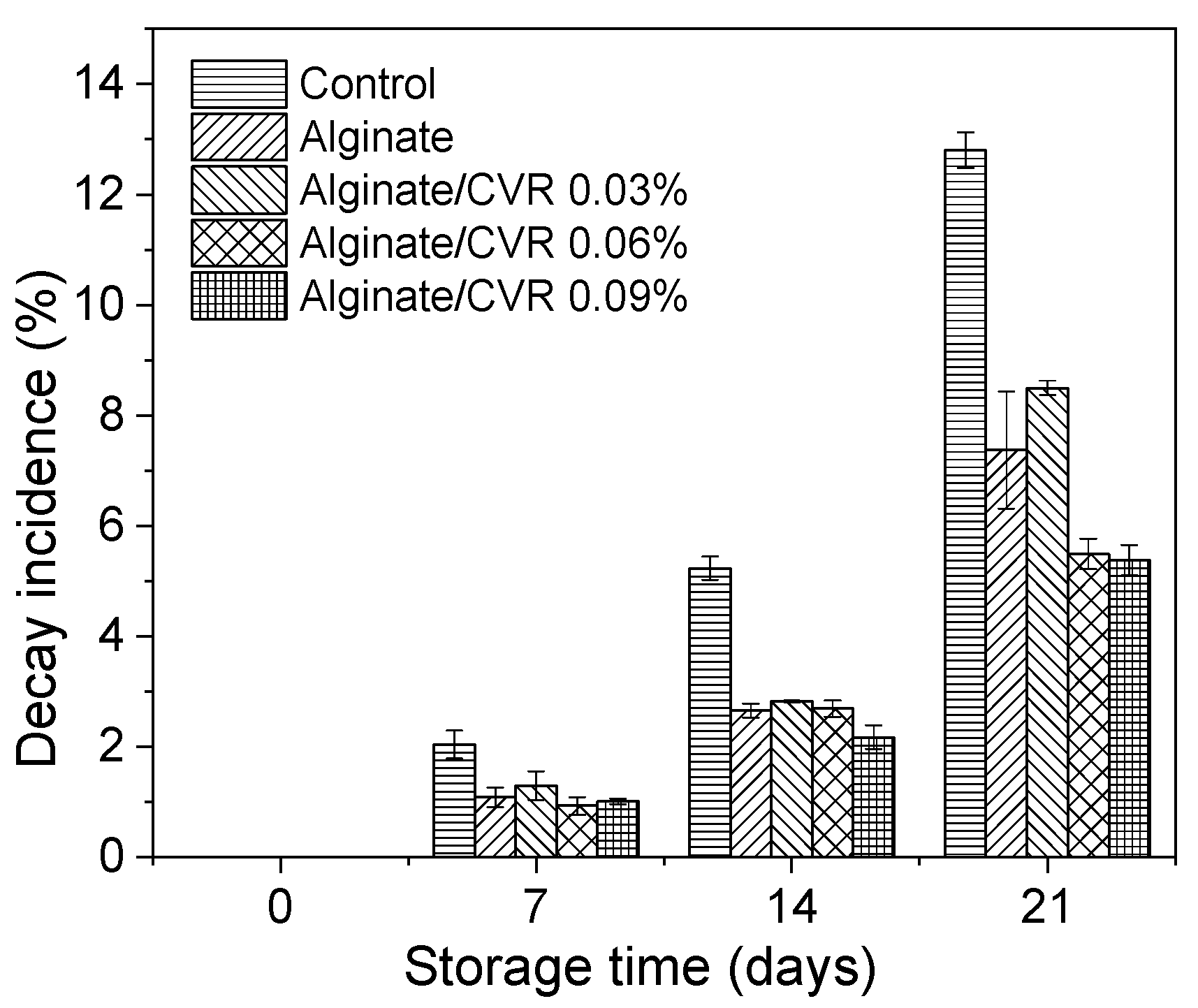
2.5.8. Fruit Decay Evaluation
2.6. Sensory Evaluation
2.7. Statistical Analysis
3. Results and Discussion
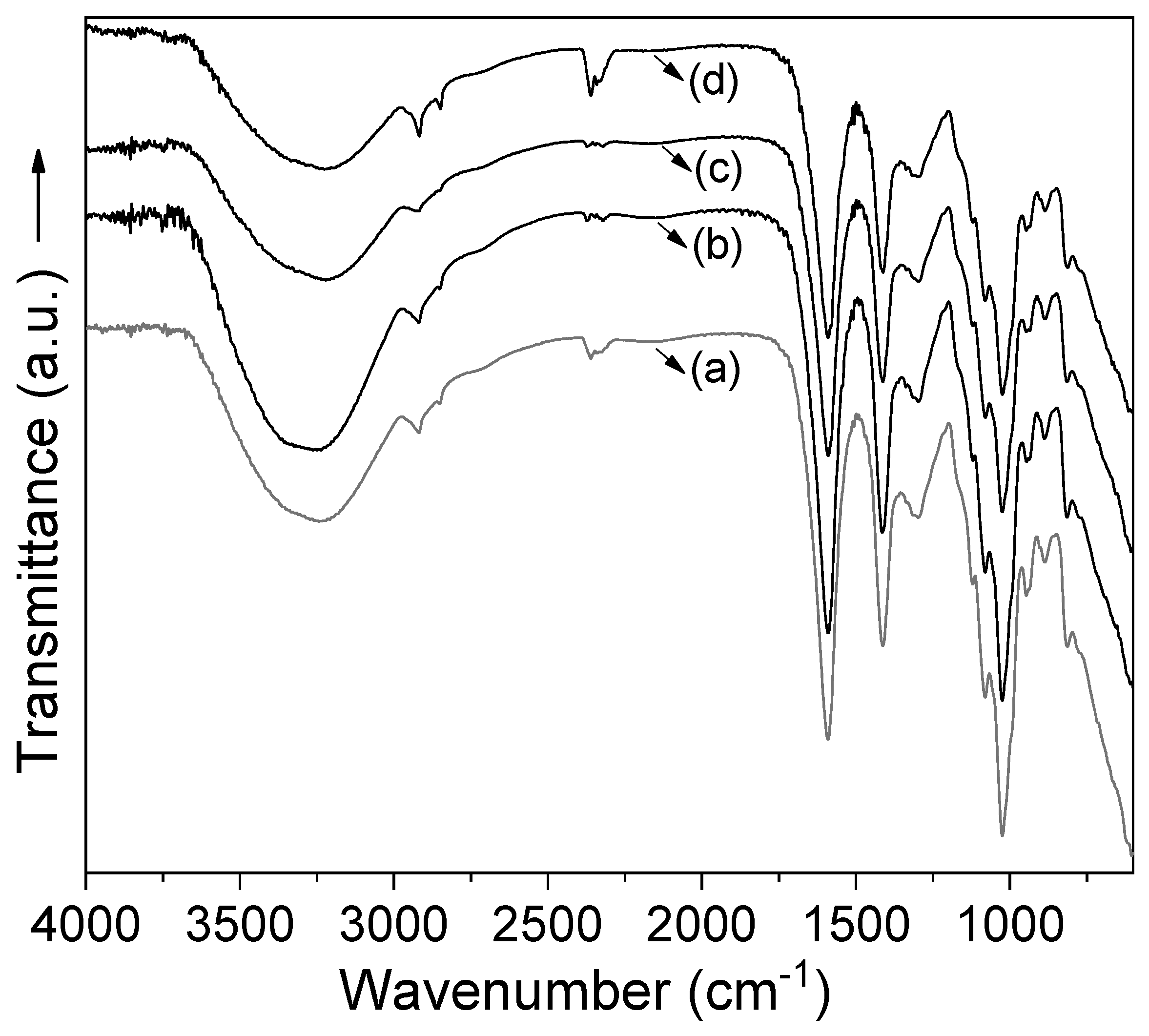
3.1. Coating Characterization
3.2. Effect of Edible Coatings on Andean Blueberries
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- FAO Global Food Losses and Food Waste. Available online: http://www.fao.org/docrep/014/mb060e/mb060e00.pdf (accessed on 19 March 2018).
- López-Córdoba, A. Antimicrobial Films and Coatings Incorporated with Food Preservatives of Microbial Origin. In Polymer for Food Applications; Gutierrez, T., Ed.; Springer: Cham, Switzerland, 2018; pp. 193–209. ISBN 9783319946252. [Google Scholar]
- Moreno, M.A.; Vallejo, A.M.; Ballester, A.-R.; Zampini, C.; Isla, M.I.; López-Rubio, A.; Fabra, M.J. Antifungal edible coatings containing Argentinian propolis extract and their application in raspberries. Food Hydrocoll. 2020, 107, 105973. [Google Scholar] [CrossRef]
- Falcó, I.; Randazzo, W.; Sánchez, G.; López-Rubio, A.; Fabra, M.J. On the use of carrageenan matrices for the development of antiviral edible coatings of interest in berries. Food Hydrocoll. 2019, 92, 74–85. [Google Scholar] [CrossRef]
- Cliff, M.A.; Li, J.B.; Stanich, K. Survey Reveals Urban Consumers’ Attitudes and Beliefs Regarding the Use of Wax on Apples. J. Food Qual. 2014, 37, 29–41. [Google Scholar] [CrossRef]
- Medina-Jaramillo, C.; Estevez-Areco, S.; Goyanes, S.; López-Córdoba, A. Characterization of Starches Isolated from Colombian Native Potatoes and Their Application as Novel Edible Coatings for Wild Andean Blueberries (Vaccinium meridionale Swartz). Polymers 2019, 11, 1937. [Google Scholar] [CrossRef] [PubMed]
- Wüstenberg, T. General Overview of Food Hydrocolloids. In Cellulose and Cellulose Derivatives in the Food industry Fundamentals and Applications; Wüstenberg, T., Ed.; Wiley-VCH: Weinheim, Germany, 2015; pp. 1–68. [Google Scholar]
- Pereira, L.; Cotas, J. Introductory Chapter: Alginates—A General Overview, Alginates—Recent Uses of This Natural Polymer. Available online: https://www.intechopen.com/books/alginates-recent-uses-of-this-natural-polymer/introductory-chapter-alginates-a-general-overview (accessed on 19 August 2020).
- Nair, M.S.; Tomar, M.; Punia, S.; Kukula-Koch, W.; Kumar, M. Enhancing the functionality of chitosan- and alginate-based active edible coatings/films for the preservation of fruits and vegetables: A review. Int. J. Biol. Macromol. 2020, 164, 304–320. [Google Scholar] [CrossRef]
- Zapata, P.J.; Castillo, S.; Valero, D.; Guillén, F.; Serrano, M.; Díaz-Mula, H.M. The use of alginate as edible coating alone or in combination with essential oils maintained postharvest quality of tomato. Acta Hortic. 2010, 877, 1529–1534. [Google Scholar] [CrossRef]
- Cakmak, H.; Kumcuoglu, S.; Tavman, S. Production of edible coatings with twin-nozzle electrospraying equipment and the effects on shelf-life stability of fresh-cut apple slices. J. Food Process Eng. 2017, e12627. [Google Scholar] [CrossRef]
- Valero, D.; Díaz-Mula, H.M.; Zapata, P.J.; Guillén, F.; Martínez-Romero, D.; Castillo, S.; Serrano, M. Effects of alginate edible coating on preserving fruit quality in four plum cultivars during postharvest storage. Postharvest Biol. Technol. 2013, 77, 1–6. [Google Scholar] [CrossRef]
- Mannozzi, C.; Cecchini, J.P.; Tylewicz, U.; Siroli, L.; Patrignani, F.; Lanciotti, R.; Rocculi, P.; Dalla Rosa, M.; Romani, S. Study on the efficacy of edible coatings on quality of blueberry fruits during shelf-life. LWT Food Sci. Technol. 2017, 85, 440–444. [Google Scholar] [CrossRef]
- López-Córdoba, A.; Aldana-Usme, A. Edible coatings based on sodium alginate and ascorbic acid for application on fresh-cut pineapple (Ananas comosus (L.) Merr). Agron. Colomb. 2020, 37, 233–238. [Google Scholar] [CrossRef][Green Version]
- Ebadollahi, A.; Ziaee, M.; Palla, F. Essential Oils Extracted from Different Species of the Lamiaceae Plant Family as Prospective Bioagents against Several Detrimental Pests. Molecules 2020, 25, 1556. [Google Scholar] [CrossRef] [PubMed]
- Kachur, K.; Suntres, Z. The antibacterial properties of phenolic isomers, carvacrol and thymol. Crit. Rev. Food Sci. Nutr. 2019, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Santos, A.R.; da Silva, A.F.; Amaral, V.C.S.; Ribeiro, A.B.; de Abreu Filho, B.A.; Mikcha, J.M.G. Application of edible coating with starch and carvacrol in minimally processed pumpkin. J. Food Sci. Technol. 2016, 53, 1975–1983. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Narciso, J.; Wang, Z.; Ference, C.; Bai, J.; Zhou, K. Effects of Chitosan-Essential Oil Coatings on Safety and Quality of Fresh Blueberries. J. Food Sci. 2014, 79, M955–M960. [Google Scholar] [CrossRef]
- Boyacı, D.; Iorio, G.; Sozbilen, G.S.; Alkan, D.; Trabattoni, S.; Pucillo, F.; Farris, S.; Yemenicioğlu, A. Development of flexible antimicrobial zein coatings with essential oils for the inhibition of critical pathogens on the surface of whole fruits: Test of coatings on inoculated melons. Food Packag. Shelf Life 2019, 20, 100316. [Google Scholar] [CrossRef]
- Peretto, G.; Du, W.-X.; Avena-Bustillos, R.J.; De Berrios, J.; Sambo, P.; McHugh, T.H. Electrostatic and Conventional Spraying of Alginate-Based Edible Coating with Natural Antimicrobials for Preserving Fresh Strawberry Quality. Food Bioprocess Technol. 2017, 10, 165–174. [Google Scholar] [CrossRef]
- Peretto, G.; Du, W.-X.; Avena-Bustillos, R.J.; Berrios, J.D.J.; Sambo, P.; McHugh, T.H. Optimization of antimicrobial and physical properties of alginate coatings containing carvacrol and methyl cinnamate for strawberry application. J. Agric. Food Chem. 2014, 62, 984–990. [Google Scholar] [CrossRef]
- Peretto, G.; Du, W.-X.; Avena-Bustillos, R.J.; Sarreal, S.B.L.; Hua, S.S.T.; Sambo, P.; McHugh, T.H. Increasing strawberry shelf-life with carvacrol and methyl cinnamate antimicrobial vapors released from edible films. Postharvest Biol. Technol. 2014, 89, 11–18. [Google Scholar] [CrossRef]
- Garzón, G.A.; Narváez, C.E.; Riedl, K.M.; Schwartz, S.J. Chemical composition, anthocyanins, non-anthocyanin phenolics and antioxidant activity of wild bilberry (Vaccinium meridionale Swartz) from Colombia. Food Chem. 2010, 122, 980–986. [Google Scholar] [CrossRef]
- Celis, M.E.M.; Franco Tobón, Y.N.; Agudelo, C.; Arango, S.S.; Rojano, B. Andean Berry (Vaccinium meridionale Swartz). In Fruit and Vegetable Phytochemicals: Chemestry and Human Health, 2nd ed.; Yahia, E.M., Ed.; John Wiley & Sons Ltd.: Hoboken, NJ, USA, 2017; Volume 2, pp. 869–882. [Google Scholar]
- Medina-Jaramillo, C.; Quintero-Pimiento, C.; Gómez-Hoyos, C.; Zuluaga-Gallego, R.; López-Córdoba, A. Alginate-Edible Coatings for Application on Wild Andean Blueberries (Vaccinium meridionale Swartz): Effect of the Addition of Nanofibrils Isolated from Cocoa By-Products. Polymers 2020, 12, 824. [Google Scholar] [CrossRef]
- López-Córdoba, A.; Estevez-Areco, S.; Goyanes, S. Potato starch-based biocomposites with enhanced thermal, mechanical and barrier properties comprising water-resistant electrospun poly (vinyl alcohol) fibers and yerba mate extract. Carbohydr. Polym. 2019, 215, 377–387. [Google Scholar] [CrossRef] [PubMed]
- Mercado, J.A.; Matas, A.J.; Posé, S. Fruit and Vegetable Texture: Role of Their Cell Walls; Melton, L., Shahidi, F., Varelis, P., Eds.; Academic Press: Oxford, UK, 2019; pp. 1–7. ISBN 978-0-12-814045-1. [Google Scholar]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventos, R.M. Analysis of total phenols and other oxidation substrates and antioxidants by means of Folin-Ciocalteu reagent. In Methods in Enzymology (Oxidants and Antioxidants, Part A); Lester Packer, L., Ed.; Academic Press: San Diego, CA, USA, 1999; Volume 299, pp. 152–178. [Google Scholar]
- Estevez-Areco, S.; Guz, L.; Famá, L.; Candal, R.; Goyanes, S. Bioactive starch nanocomposite films with antioxidant activity and enhanced mechanical properties obtained by extrusion followed by thermo-compression. Food Hydrocoll. 2019, 96, 518–528. [Google Scholar] [CrossRef]
- ISO 4833-1:2013. Microbiology of the Food Chain—Horizontal Method for the Enumeration of Microorganisms—Part 1: Colony Count at 30 °C by the Pour Plate Technique; International Standard Organization: Geneva, Switzerland, 2013. [Google Scholar]
- ISO 21527-2. Microbiology of Food and Animal Feeding Stuffs—Horizontal Method for the Enumeration of Yeasts and Moulds—Part 2: Colony Count Technique in Products with Water Activity Less Than or Equal to 0.95; International Standard Organization: Geneva, Switzerland, 2008. [Google Scholar]
- Chiumarelli, M.; Hubinger, M.D. Evaluation of edible films and coatings formulated with cassava starch, glycerol, carnauba wax and stearic acid. Food Hydrocoll. 2014, 38, 20–27. [Google Scholar] [CrossRef]
- Rojas-Graü, M.A.; Avena-Bustillos, R.J.; Olsen, C.; Friedman, M.; Henika, P.R.; Martín-Belloso, O.; Pan, Z.; McHugh, T.H. Effects of plant essential oils and oil compounds on mechanical, barrier and antimicrobial properties of alginate–apple puree edible films. J. Food Eng. 2007, 81, 634–641. [Google Scholar] [CrossRef]
- Tapia, M.S.; Rojas-Graü, M.A.; Rodríguez, F.J.; Ramírez, J.; Carmona, A.; Martin-Belloso, O. Alginate- and Gellan-Based Edible Films for Probiotic Coatings on Fresh-Cut Fruits. J. Food Sci. 2007, 72, E190–E196. [Google Scholar] [CrossRef] [PubMed]
- Costa, M.J.; Marques, A.M.; Pastrana, L.M.; Teixeira, J.A.; Sillankorva, S.M.; Cerqueira, M.A. Physicochemical properties of alginate-based films: Effect of ionic crosslinking and mannuronic and guluronic acid ratio. Food Hydrocoll. 2018, 81, 442–448. [Google Scholar] [CrossRef]
- Vargas, M.; Albors, A.; Chiralt, A.; González-Martínez, C. Quality of cold-stored strawberries as affected by chitosan–oleic acid edible coatings. Postharvest Biol. Technol. 2006, 41, 164–171. [Google Scholar] [CrossRef]
- Zapata, P.J.; Díaz-Mula, H.M.; Guillén, F.; Martínez-Romero, D.; Castillo, S.; Valero, D. The combination of alginate coating and essential oils delayed postharvest ripening and increased the antioxidant potential of two sweet cherries. Acta Hortic. 2017, 1161, 633–638. [Google Scholar] [CrossRef]
- Garzón, G.A.; Soto, C.Y.; López-R, M.; Riedl, K.M.; Browmiller, C.R.; Howard, L. Phenolic profile, in vitro antimicrobial activity and antioxidant capacity of Vaccinium meridionale Swartz pomace. Heliyon 2020, 6, e03845. [Google Scholar] [CrossRef]
- Reque, P.M.; Steffens, R.S.; Jablonski, A.; Flôres, S.H.; Rios, A.d.O.; de Jong, E.V. Cold storage of blueberry (Vaccinium spp.) fruits and juice: Anthocyanin stability and antioxidant activity. J. Food Compos. Anal. 2014, 33, 111–116. [Google Scholar] [CrossRef]
- Mannozzi, C.; Tylewicz, U.; Chinnici, F.; Siroli, L.; Rocculi, P.; Dalla Rosa, M.; Romani, S. Effects of chitosan based coatings enriched with procyanidin by-product on quality of fresh blueberries during storage. Food Chem. 2018, 251, 18–24. [Google Scholar] [CrossRef] [PubMed]
- Thakur, R.; Pristijono, P.; Golding, J.B.; Stathopoulos, C.E.; Scarlett, C.J.; Bowyer, M.; Singh, S.P.; Vuong, Q. V Development and application of rice starch based edible coating to improve the postharvest storage potential and quality of plum fruit (Prunus salicina). Sci. Hortic. (Amst.) 2018, 237, 59–66. [Google Scholar] [CrossRef]
- Bonilla, J.; Atarés, L.; Vargas, M.; Chiralt, A. Edible films and coatings to prevent the detrimental effect of oxygen on food quality: Possibilities and limitations. J. Food Eng. 2012, 110, 208–213. [Google Scholar] [CrossRef]
- Alharaty, G.; Ramaswamy, H.S. The Effect of Sodium Alginate-Calcium Chloride Coating on the Quality Parameters and Shelf Life of Strawberry Cut Fruits. J. Compos. Sci. 2020, 4, 123. [Google Scholar] [CrossRef]
- de Souza, V.R.; Pereira, P.A.P.; da Silva, T.L.T.; de Oliveira Lima, L.C.; Pio, R.; Queiroz, F. Determination of the bioactive compounds, antioxidant activity and chemical composition of Brazilian blackberry, red raspberry, strawberry, blueberry and sweet cherry fruits. Food Chem. 2014, 156, 362–368. [Google Scholar] [CrossRef]
- Chiabrando, V.; Giacalone, G. Anthocyanins, phenolics and antioxidant capacity after fresh storage of blueberry treated with edible coatings. Int. J. Food Sci. Nutr. 2015, 66, 248–253. [Google Scholar] [CrossRef]
- Rojas-Graü, M.A.; Raybaudi-Massilia, R.M.; Soliva-Fortuny, R.C.; Avena-Bustillos, R.J.; McHugh, T.H.; Martín-Belloso, O. Apple puree-alginate edible coating as carrier of antimicrobial agents to prolong shelf-life of fresh-cut apples. Postharvest Biol. Technol. 2007, 45, 254–264. [Google Scholar] [CrossRef]
- Alvarez, M.V.; Ponce, A.G.; Moreira, M.R. Influence of polysaccharide-based edible coatings as carriers of prebiotic fibers on quality attributes of ready-to-eat fresh blueberries. J. Sci. Food Agric. 2017, 98, 2587–2597. [Google Scholar] [CrossRef]
- IFT. Development and use of microbiological criteria for foods. Inst. Food Sci. Technol. 1997, 11, 137–177. [Google Scholar]
- Yuan, G.; Lv, H.; Yang, B.; Chen, X.; Sun, H. Physical Properties, Antioxidant and Antimicrobial Activity of Chitosan Films Containing Carvacrol and Pomegranate Peel Extract. Molecules 2015, 20, 11034–11045. [Google Scholar] [CrossRef]
- López-Córdoba, A.; Deladino, L.; Agudelo-Mesa, L.; Martino, M. Yerba mate antioxidant powders obtained by co-crystallization: Stability during storage. J. Food Eng. 2014, 124, 158–165. [Google Scholar] [CrossRef]
- Sipahi, R.E.; Castell-Perez, M.E.; Moreira, R.G.; Gomes, C.; Castillo, A. Improved multilayered antimicrobial alginate-based edible coating extends the shelf life of fresh-cut watermelon (Citrullus lanatus). LWT Food Sci. Technol. 2013, 51, 9–15. [Google Scholar] [CrossRef]


| Weight Loss (%) | |||||
|---|---|---|---|---|---|
| Storage Time (Days) | Control | Alginate | Alg/CVR 0.03% | Alg/CVR 0.06% | Alg/CVR 0.09% |
| 7 | 4.4 ± 0.2 a | 2.7 ± 0.5 b | 3.0 ± 0.9 b | 2.8 ± 0.6 b | 3.3 ± 0.5 b |
| 14 | 8.1 ± 1.6 a | 4.9 ± 0.8 b | 6.3 ± 1.1 b | 6.3 ± 0.7 b | 6.2 ± 0.4 b |
| 21 | 15.2 ± 1.0 a | 9.7 ± 2.0 b | 7.4 ± 1.2 b | 7.3 ± 0.6 b | 7.3 ± 0.4 b |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Medina-Jaramillo, C.; Quintero-Pimiento, C.; Díaz-Díaz, D.; Goyanes, S.; López-Córdoba, A. Improvement of Andean Blueberries Postharvest Preservation Using Carvacrol/Alginate-Edible Coatings. Polymers 2020, 12, 2352. https://doi.org/10.3390/polym12102352
Medina-Jaramillo C, Quintero-Pimiento C, Díaz-Díaz D, Goyanes S, López-Córdoba A. Improvement of Andean Blueberries Postharvest Preservation Using Carvacrol/Alginate-Edible Coatings. Polymers. 2020; 12(10):2352. https://doi.org/10.3390/polym12102352
Chicago/Turabian StyleMedina-Jaramillo, Carolina, Carmen Quintero-Pimiento, Darío Díaz-Díaz, Silvia Goyanes, and Alex López-Córdoba. 2020. "Improvement of Andean Blueberries Postharvest Preservation Using Carvacrol/Alginate-Edible Coatings" Polymers 12, no. 10: 2352. https://doi.org/10.3390/polym12102352
APA StyleMedina-Jaramillo, C., Quintero-Pimiento, C., Díaz-Díaz, D., Goyanes, S., & López-Córdoba, A. (2020). Improvement of Andean Blueberries Postharvest Preservation Using Carvacrol/Alginate-Edible Coatings. Polymers, 12(10), 2352. https://doi.org/10.3390/polym12102352