Preparation and Characterization of Potato Starch Copolymers with a High Natural Polymer Content for the Removal of Cu(II) and Fe(III) from Solutions
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
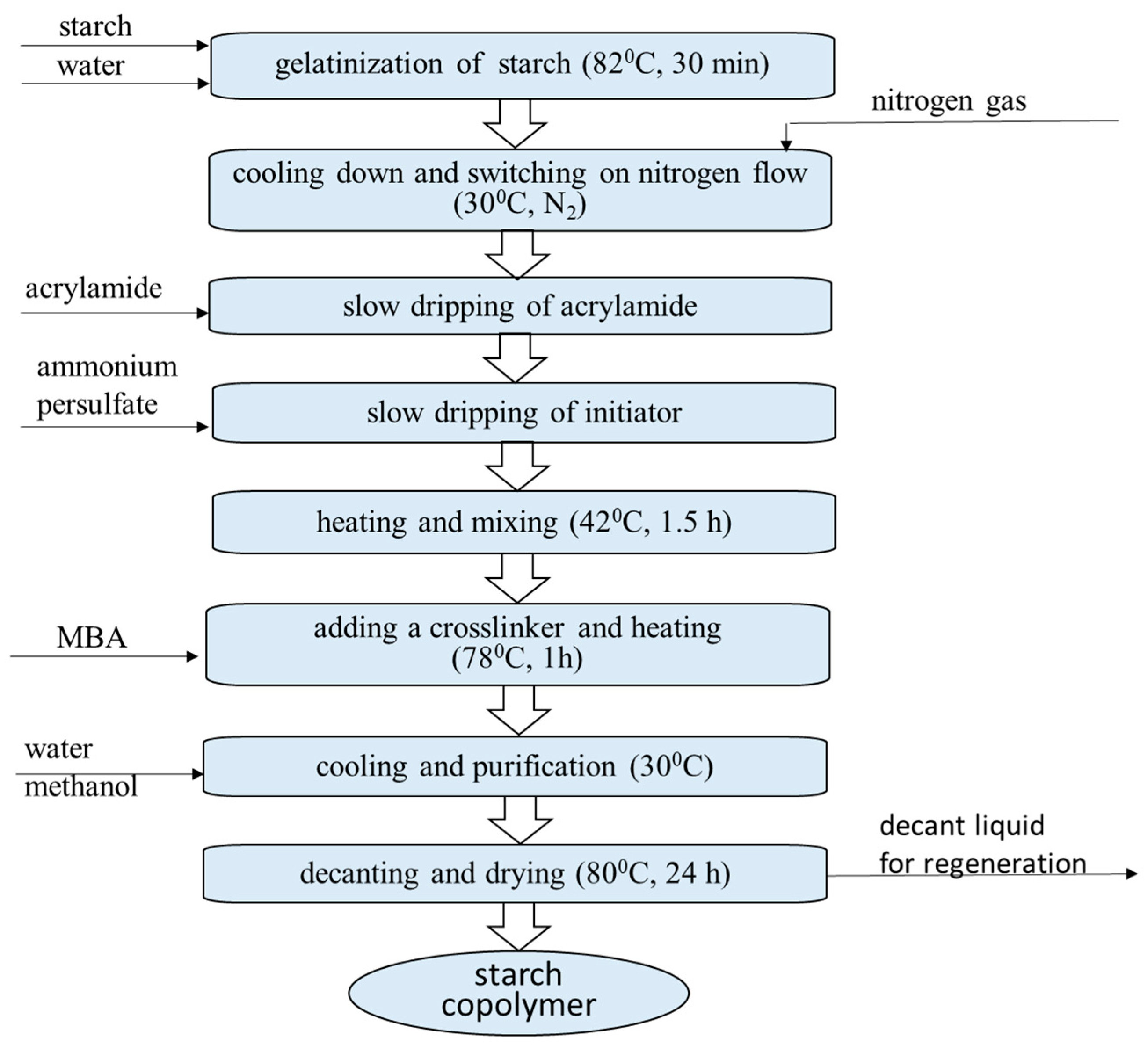
2.2. Preparation of Starch-g-Polyacrylamide SAPs with High Starch Content
2.3. Fourier Transform Infrared (FTIR) Spectroscopy
2.4. Differential Scanning Calorimetry (DSC)
2.5. Thermogravimetric Analysis (TGA)
2.6. Laser Scanning Microscopy (LSM)
2.7. X-ray Diffraction (XRD)
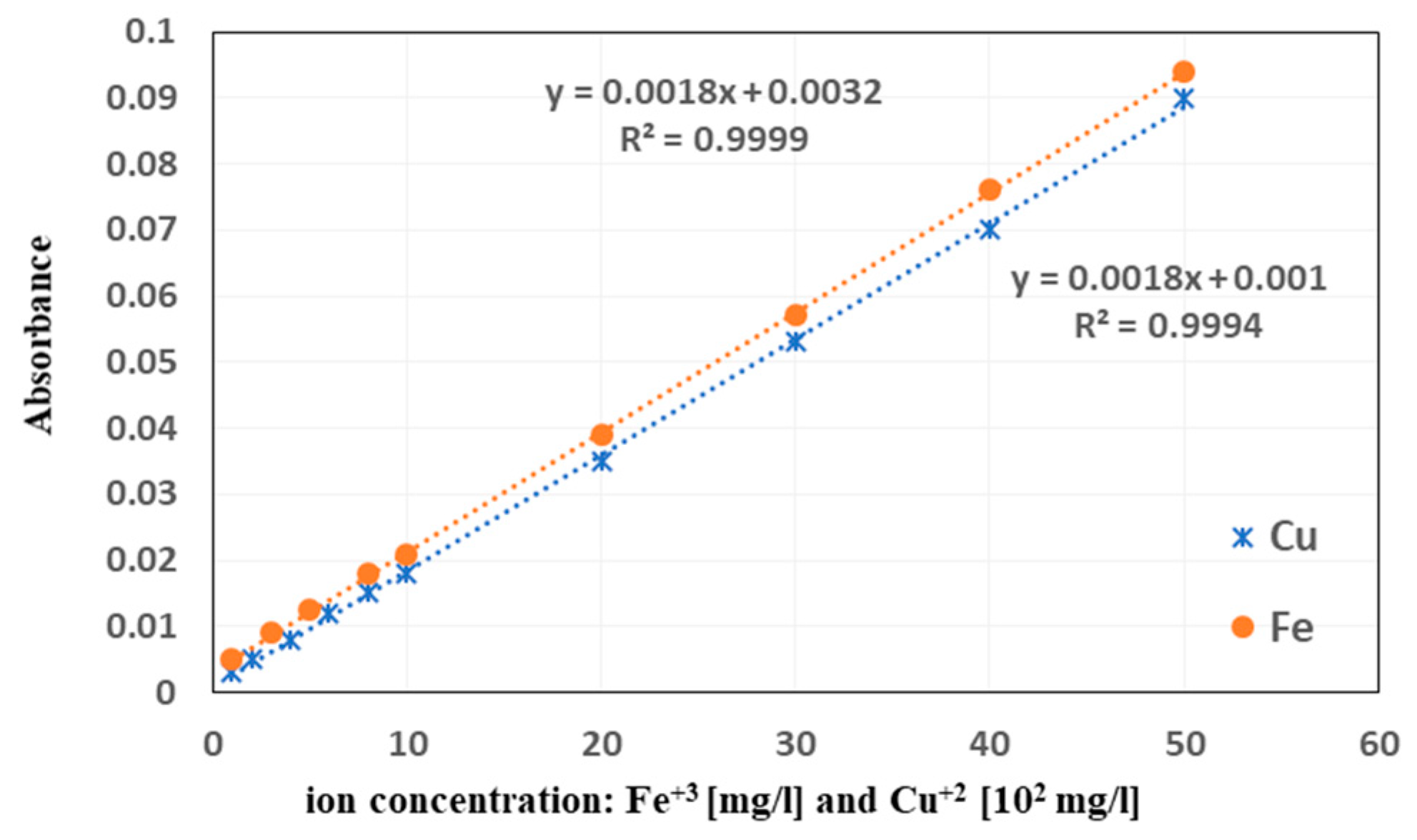
2.8. Adsorption of Metal Ions
2.9. The Swelling Properties
3. Results and Discussion
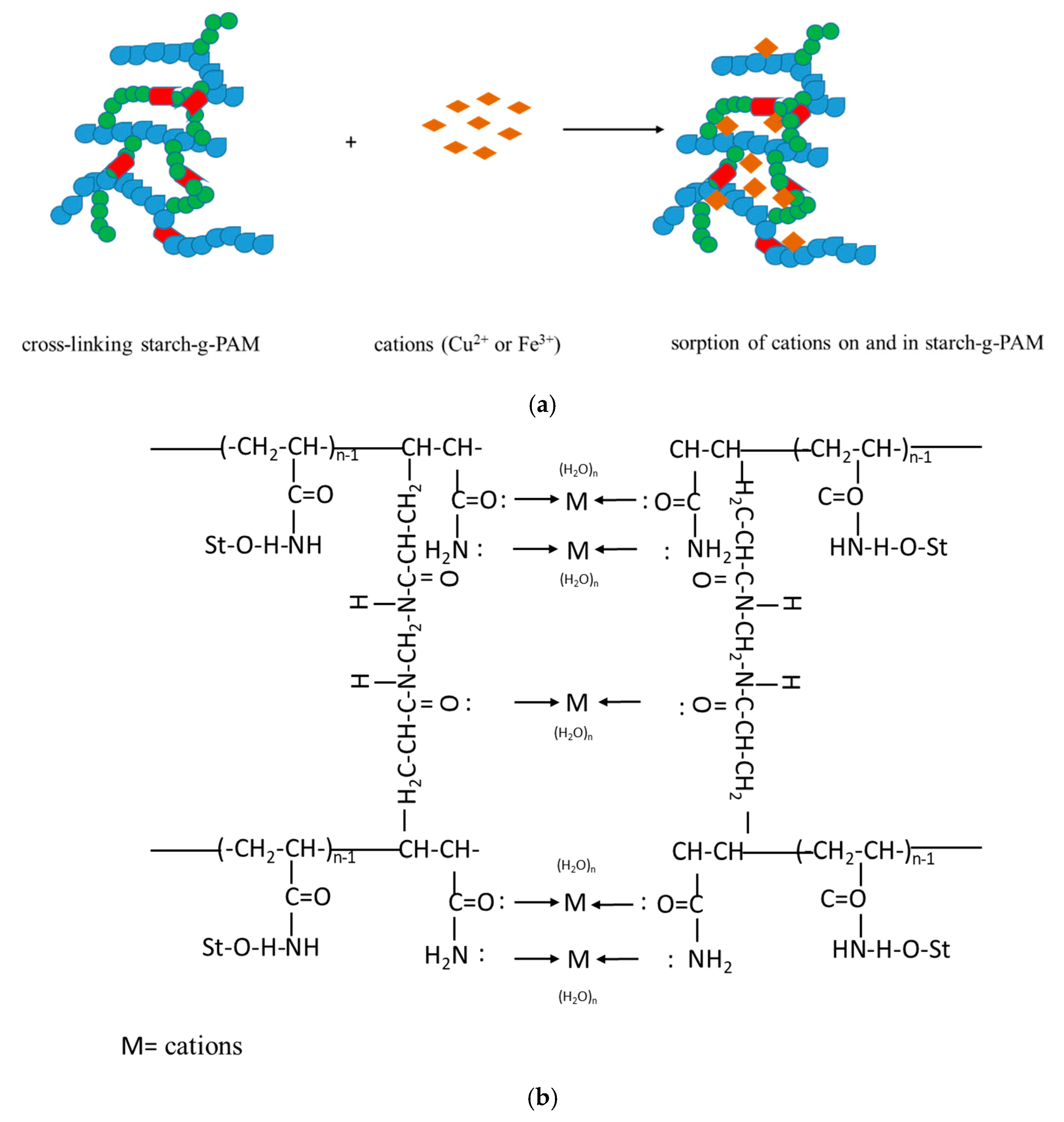
3.1. The Double Starch Modifications
3.2. Characterization of Modified Starch SAP
3.2.1. FTIR Analysis
3.2.2. The Surface Morphology
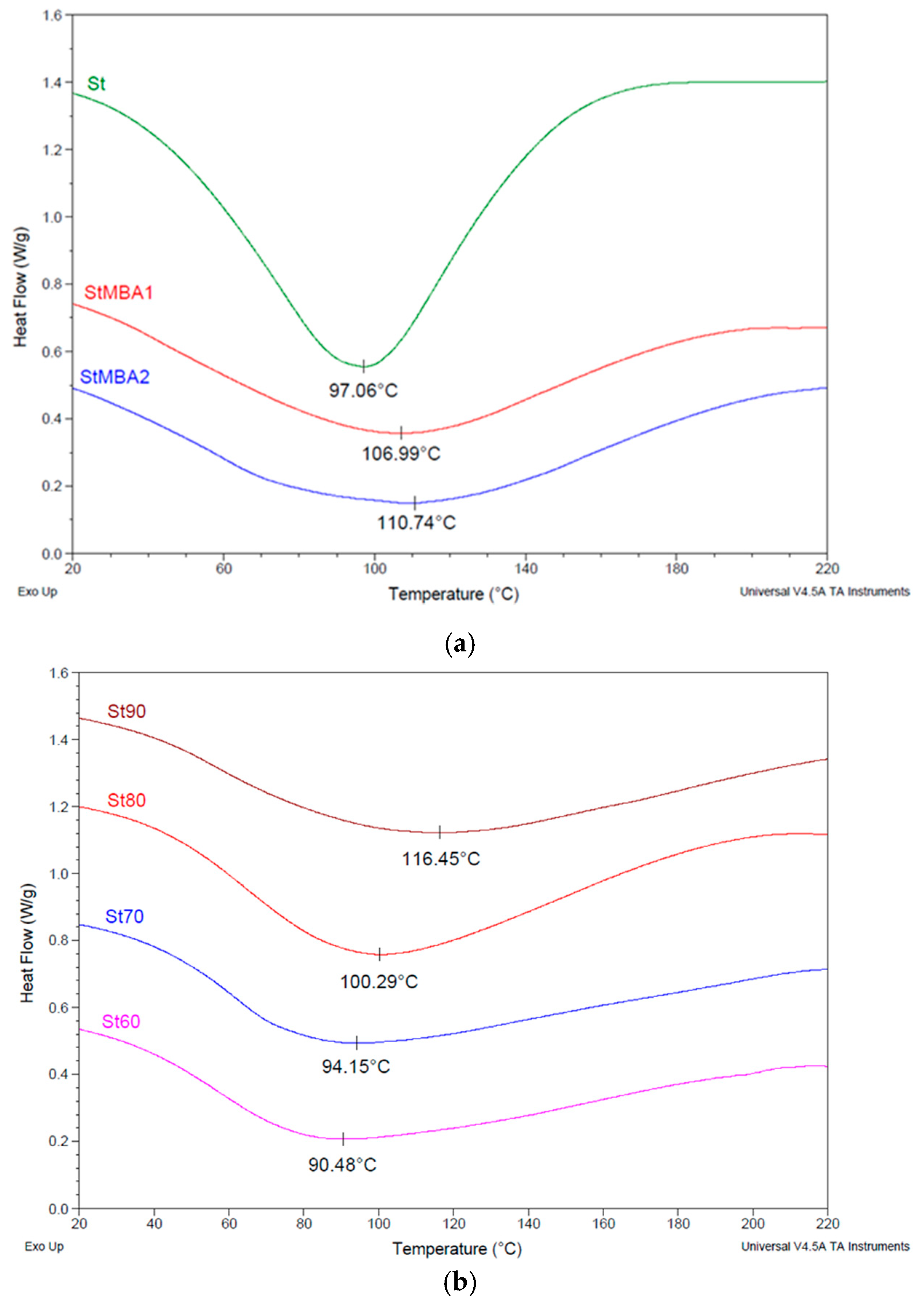
3.2.3. Differential Scanning Calorimetry
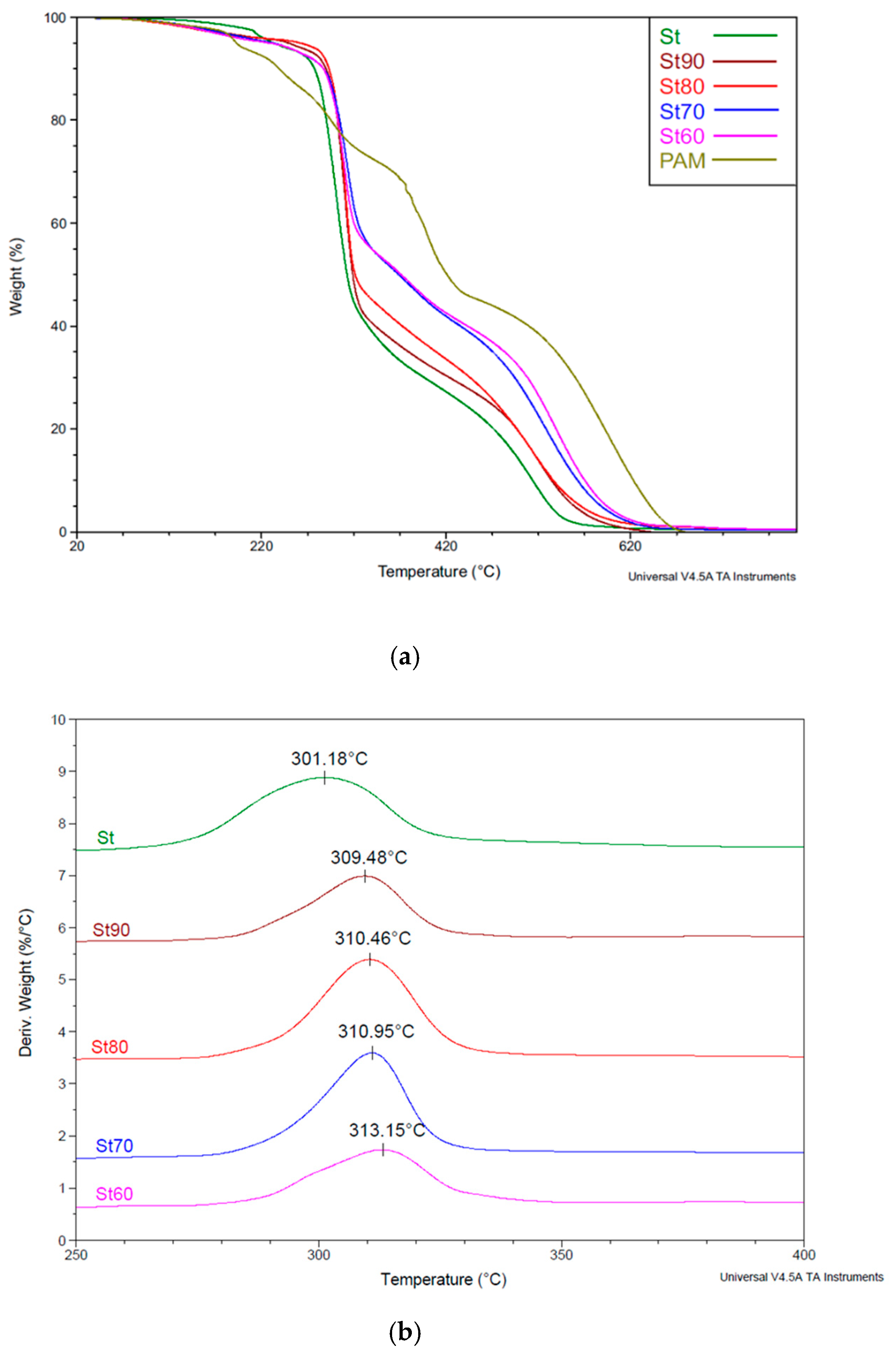
3.2.4. TGA Characterization of Starch SAPs
3.2.5. X-ray Diffraction (XRD)
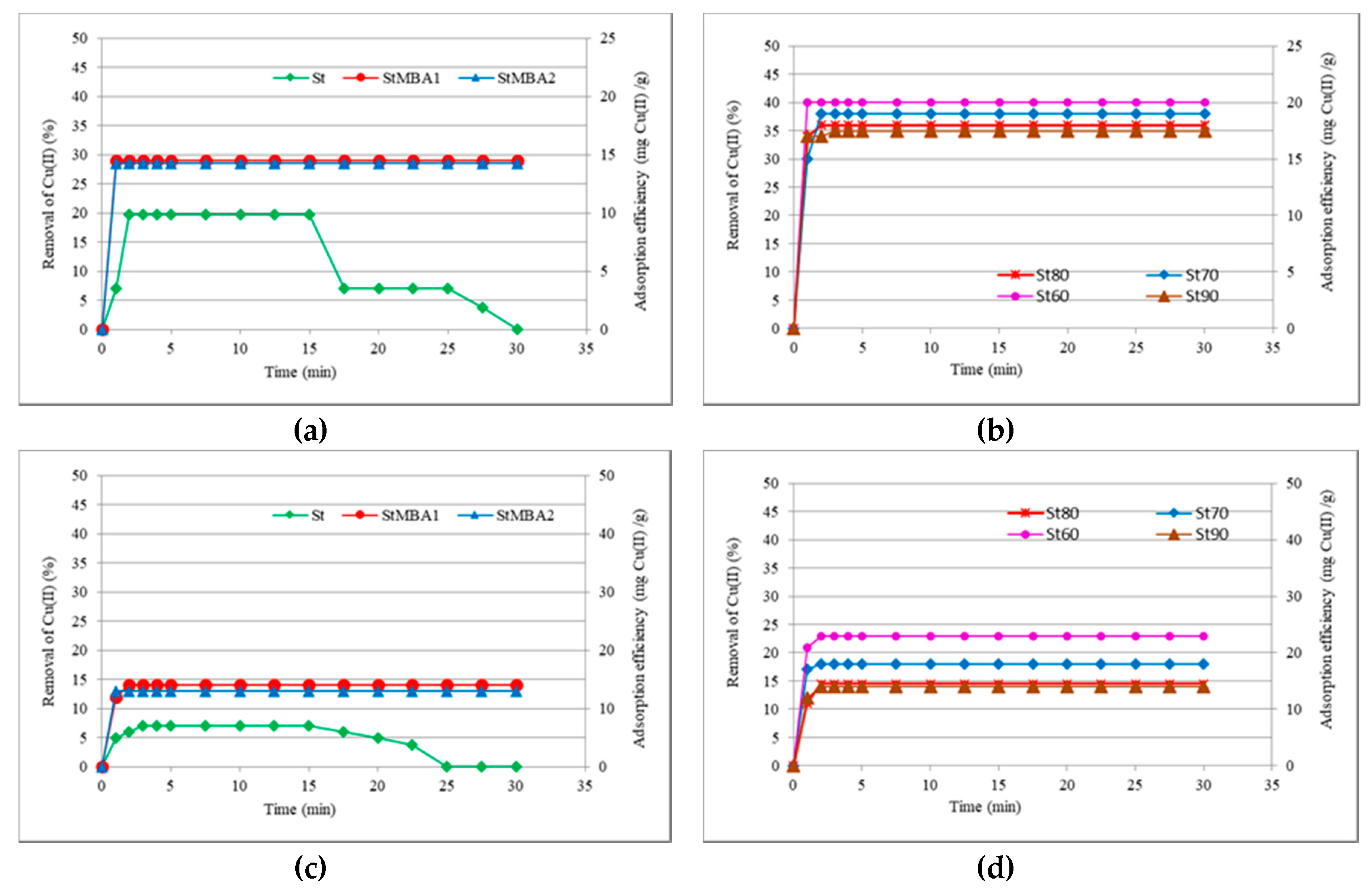
3.3. Adsorption from Cations Solution
3.3.1. Adsorption of Cu(II)
3.3.2. Adsorption of Fe(III)
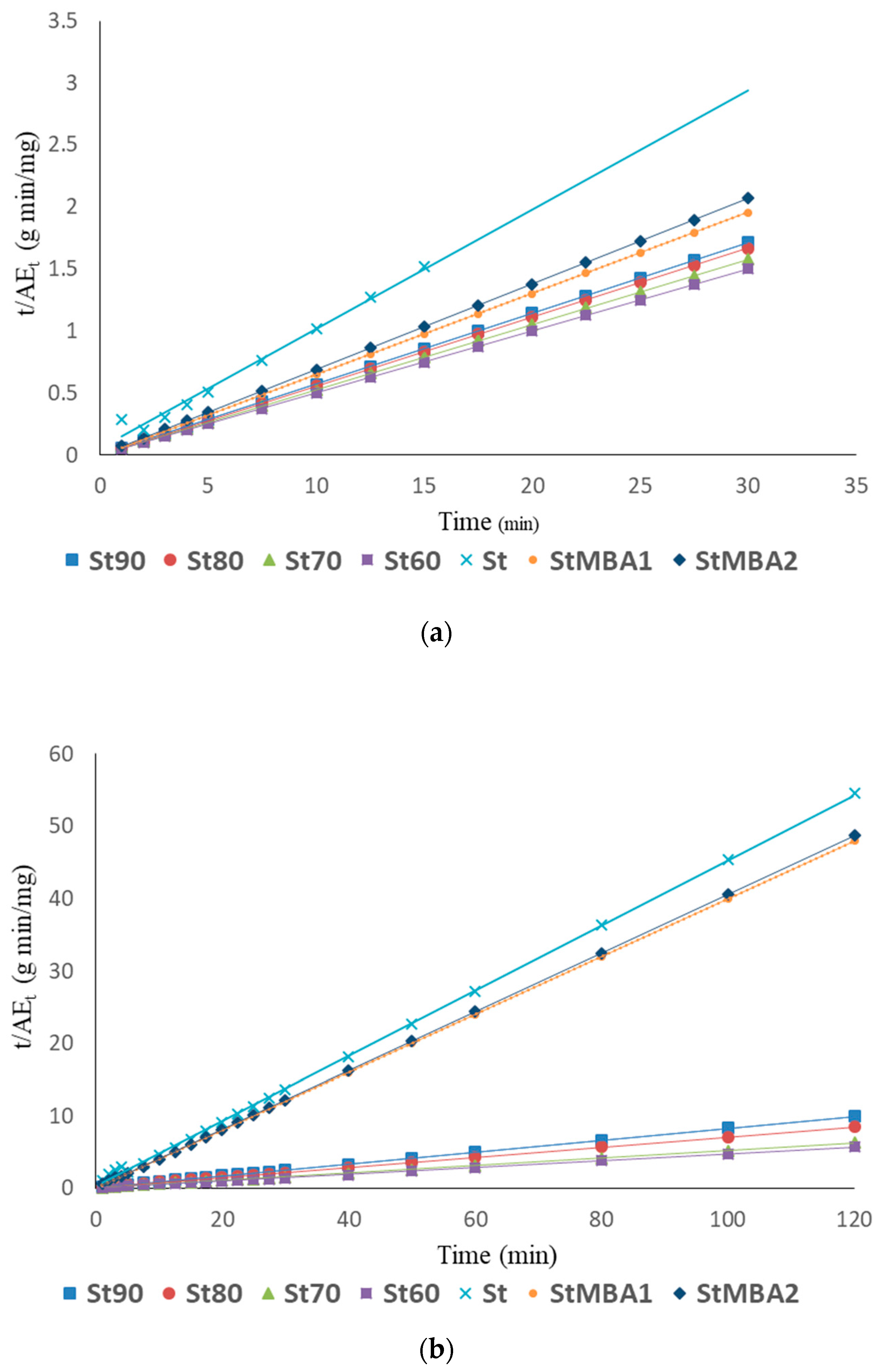
3.3.3. Pseudo-Second Order Model
3.3.4. Swelling in Cation Solution
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Santander-Ortega, M.; Stauner, T.; Loretz, B.; Ortega-Vinuesa, J.; Bastos-González, D.; Wenz, G.; Schaefer, U.; Lehr, C.-M. Nanoparticles made from novel starch derivatives for transdermal drug delivery. J. Control. Release 2010, 141, 85–92. [Google Scholar] [CrossRef] [PubMed]
- Ashogbon, A.O.; Akintayo, E.T. Recent trend in the physical and chemical modification of starches from different botanical sources: A review. Starch Stärke 2014, 66, 41–57. [Google Scholar] [CrossRef]
- Zhang, Y.; Kou, R.; Lv, S.; Zhu, L.; Tan, H.; Gu, J.; Cao, J. Effect of Mesh Number of Wood Powder and Ratio of Raw Materials on Properties of Composite Material of Starch/Wood Powder. Bioresources 2015, 10, 5356–5368. [Google Scholar] [CrossRef]
- Soltovski de Oliveira, C.S.; Andrade, M.M.P.; Colman, T.A.D.; Da Costa, F.J.O.G.; Schnitzler, E. Thermal, structural and rheological behaviour of native and modified waxy corn starch with hydrochloric acid at different temperatures. J. Therm. Anal. Calorim. 2014, 115, 13–18. [Google Scholar] [CrossRef]
- Shah, N.; Mewada, R.K.; Mehta, T. Crosslinking of starch and its effect on viscosity behaviour. Rev. Chem. Eng. 2016, 32, 381–481. [Google Scholar] [CrossRef]
- Zdanowicz, M.; Schmidt, B.; Spychaj, T. Starch graft copolymers as superabsorbents obtained via reactive extrusion processing. Pol. J. Chem. Technol. 2010, 12, 14–17. [Google Scholar] [CrossRef]
- Xing, G.; Liu, S.; Xu, Q.; Liu, Q. Preparation and adsorption behavior for brilliant blue X-BR of the cost-effective cationic starch intercalated clay composite matrix. Carbohydr. Polym. 2012, 87, 1447–1452. [Google Scholar] [CrossRef]
- Bratskaya, S.; Schwarz, S.; Petzold, G.; Liebert, T.; Heinze, T. Cationic Starches of High Degree of Functionalization: 12. Modification of Cellulose Fibers toward High Filler Technology in Papermaking. Ind. Eng. Chem. Res. 2006, 45, 7374–7379. [Google Scholar] [CrossRef]
- Authman, M.M. Use of Fish as Bio-indicator of the Effects of Heavy Metals Pollution. J. Aquac. Res. Dev. 2015, 6, 1000321–1000328. [Google Scholar] [CrossRef]
- Jamshidi, A.; Khan Beigi, F.A.; Kabiri, K.; Zohuriaan-Mehr, M. Optimized HPLC determination of residual monomer in hygienic SAP hydrogels. Polym. Test. 2005, 24, 825–828. [Google Scholar] [CrossRef]
- Peng, N.; Wang, Y.; Ye, Q.; Liang, L.; An, Y.; Li, Q.; Chang, C. Biocompatible cellulose-based superabsorbent hydrogels with antimicrobial activity. Carbohydr. Polym. 2016, 137, 59–64. [Google Scholar] [CrossRef] [PubMed]
- Xiao, X.; Yu, L.; Xie, F.; Bao, X.; Liu, H.; Ji, Z.; Chen, L.; Xie, F. One-step method to prepare starch-based superabsorbent polymer for slow release of fertilizer. Chem. Eng. J. 2017, 309, 607–616. [Google Scholar] [CrossRef]
- Qiao, D.; Liu, H.; Yu, L.; Bao, X.; Simon, G.P.; Petinakis, E.; Chen, L. Preparation and characterization of slow-release fertilizer encapsulated by starch-based superabsorbent polymer. Carbohydr. Polym. 2016, 147, 146–154. [Google Scholar] [CrossRef] [PubMed]
- Haseeb, M.T.; Hussain, M.A.; Yuk, S.H.; Bashir, S.; Nauman, M. Polysaccharides based superabsorbent hydrogel from Linseed: Dynamic swelling, stimuli responsive on–off switching and drug release. Carbohydr. Polym. 2016, 136, 750–756. [Google Scholar] [CrossRef]
- Hamidi, M.; Azadi, A.; Rafiei, P. Hydrogel nanoparticles in drug delivery. Adv. Drug Deliv. Rev. 2008, 60, 1638–1649. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Chen, H.; Liu, S.; Guo, J. Superabsorbent Polymer with High Swelling Ratio, and Temperature-Sensitive and Magnetic Properties Employed as an Efficient Dewatering Medium of Fine Coal. Energy Fuels 2017, 31, 1825–1831. [Google Scholar] [CrossRef]
- He, Y.; Tang, H.; Chen, Y.; Zhang, S. Facile Strategy to Construct Metal-Organic Coordination Thermoplastic Starch with High Hydrophobicity, Glass-Transition Temperature, and Improved Shape Recovery. ACS Sustain. Chem. Eng. 2020, 8, 8655–8663. [Google Scholar] [CrossRef]
- Crini, G. Recent developments in polysaccharide-based materials used as adsorbents in wastewater treatment. Prog. Polym. Sci. 2005, 30, 38–70. [Google Scholar] [CrossRef]
- Staroszczyk, H.; Ciesielski, W.; Tomasik, P. Starch-metal complexes and metal compounds. J. Sci. Food Agric. 2018, 98, 2845–2856. [Google Scholar] [CrossRef]
- Pathania, D. Graft Copolymerization of Acrylic Acid onto Gelatinized Patato Starch For Removal of Metal Ions and Organic Dyes From Aqueous System. Adv. Mater. Lett. 2012, 3, 259–264. [Google Scholar] [CrossRef]
- Spychaj, T.; Schmidt, B.; Ulfig, K.; Markowska-Szczupak, A. Starch-grafted-N-vinylformamide copolymers manufactured by reactive extrusion: Synthesis and characterization. Polimery 2012, 57, 95–100. [Google Scholar] [CrossRef]
- Cheng, R.; Xiang, B.; Li, Y. Application of nickel (II) complex of dithiocarbamate-modified starch for anionic dyes removal from aqueous solutions. J. Appl. Polym. Sci. 2011, 123, 2439–2444. [Google Scholar] [CrossRef]
- Ibrahim, B.; Fakhre, N. Crown ether modification of starch for adsorption of heavy metals from synthetic wastewater. Int. J. Biol. Macromol. 2019, 123, 70–80. [Google Scholar] [CrossRef]
- Lu, D.R.; Xiao, C.M.; Xu, S.J. Starch-based completely biodegradable polymer materials. Express Polym. Lett. 2009, 3, 366–375. [Google Scholar] [CrossRef]
- Haroon, M.; Wang, L.; Yu, H.; Abbasi, N.M.; Abdin, Z.U.; Saleem, M.; Khan, R.U.; Ullah, R.S.; Chen, Q.; Wu, J. Chemical modification of starch and its application as an adsorbent material. RSC Adv. 2016, 6, 78264–78285. [Google Scholar] [CrossRef]
- Guo, L.; Zhang, S.-F.; Ju, B.-Z.; Yang, J.-Z. Study on adsorption of Cu(II) by water-insoluble starch phosphate carbamate. Carbohydr. Polym. 2006, 63, 487–492. [Google Scholar] [CrossRef]
- Apopei, D.F.; Dinu, M.V.; Trochimczuk, A.W.; Dragan, E.S. Sorption Isotherms of Heavy Metal Ions onto Semi-Interpenetrating Polymer Network Cryogels Based on Polyacrylamide and Anionically Modified Potato Starch. Ind. Eng. Chem. Res. 2012, 51, 10462–10471. [Google Scholar] [CrossRef]
- Zhang, J.; Li, A.; Wang, A. Study on superabsorbent composite. VI. Preparation, characterization and swelling behaviors of starch phosphate-graft-acrylamide/attapulgite superabsorbent composite. Carbohydr. Polym. 2006, 65, 150–158. [Google Scholar] [CrossRef]
- Bao, X.; Yu, L.; Shen, S.; Simon, G.P.; Liu, H.; Chen, L. How rheological behaviors of concentrated starch affect graft copolymerization of acrylamide and resultant hydrogel. Carbohydr. Polym. 2019, 219, 395–404. [Google Scholar] [CrossRef]
- Qiao, D.; Tu, W.; Wang, Z.; Yu, L.; Zhang, B.; Bao, X.; Jiang, F.; Lin, Q. Influence of crosslinker amount on the microstructure and properties of starch-based superabsorbent polymers by one-step preparation at high starch concentration. Int. J. Biol. Macromol. 2019, 129, 679–685. [Google Scholar] [CrossRef]
- Schmidt, B. Effect of crosslinking agent on potato starch grafted acrylamide copolymers and their sorption properties for water, Fe3+ and Cu2+ cations. Polimery 2018, 63, 347–352. [Google Scholar] [CrossRef]
- Dragan, E.S.; Felicia, L.D. Synthesis and swelling behavior of pH-sensitive semi-interpenetrating polymer network composite hydrogels based on native and modified potatoes starch as potential sorbent for cationic dyes. Chem. Eng. J. 2011, 178, 252–263. [Google Scholar] [CrossRef]
- Zhou, Y.; Meng, S.; Chen, D.; Zhu, X.; Yuan, H. Structure characterization and hypoglycemic effects of dual modified resistant starch from indica rice starch. Carbohydr. Polym. 2014, 103, 81–86. [Google Scholar] [CrossRef]
- Karim, A.; Sufha, E.H.; Zaidul, I.S.M. Dual Modification of Starch via Partial Enzymatic Hydrolysis in the Granular State and Subsequent Hydroxypropylation. J. Agric. Food Chem. 2008, 56, 10901–10907. [Google Scholar] [CrossRef] [PubMed]
- Raina, C.S.; Singh, S.; Bawa, A.S.; Saxena, D.C. Some characteristics of acetylated, cross-linked and dual modified Indian rice starches. Eur. Food Res. Technol. 2006, 223, 561–570. [Google Scholar] [CrossRef]
- Xiao, H.-X.; Lin, Q.-L.; Liu, G.-Q.; Yu, F.-X. A Comparative Study of the Characteristics of Cross-Linked, Oxidized and Dual-Modified Rice Starches. Molecules 2012, 17, 10946–10957. [Google Scholar] [CrossRef]
- Sun, S.; Liu, P.; Ji, N.; Hou, H.; Dong, H. Effects of various cross-linking agents on the physicochemical properties of starch/PHA composite films produced by extrusion blowing. Food Hydrocoll. 2018, 77, 964–975. [Google Scholar] [CrossRef]
- Qiao, D.; Yu, L.; Bao, X.; Zhang, B.; Jiang, F. Understanding the microstructure and absorption rate of starch-based superabsorbent polymers prepared under high starch concentration. Carbohydr. Polym. 2017, 175, 141–148. [Google Scholar] [CrossRef]
- Lapointe, M.; Barbeau, B. Substituting polyacrylamide with an activated starch polymer during ballasted flocculation. J. Water Process. Eng. 2019, 28, 129–134. [Google Scholar] [CrossRef]
- Jiraprasertkul, W.; Nuisin, R.; Jinsart, W.; Kiatkamjornwong, S. Synthesis and characterization of cassava starch graft poly(acrylic acid) and poly[(acrylic acid)-co-acrylamide] and polymer flocculants for wastewater treatment. J. Appl. Polym. Sci. 2006, 102, 2915–2928. [Google Scholar] [CrossRef]
- Gupta, S.S.; Bhattacharyya, K.G. Kinetics of adsorption of metal ions on inorganic materials: A review. Adv. Colloid Interface Sci. 2011, 162, 39–58. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Tian, T.; Xiao, Z. Preparation of cross-linked porous starch and its adsorption for chromium (VI) in tannery wastewater. Polym. Adv. Technol. 2015, 26, 1259–1266. [Google Scholar] [CrossRef]
- Ramazan, K.; Joseph, I.; Koushik, S. Characterization of Irradiated Starches by Using FT-Raman and FTIR Spectroscopy. J. Agric. Food Chem. 2002, 50, 3912–3918. [Google Scholar] [CrossRef]
- Natkański, P.; Kuśtrowski, P.; Białas, A.; Piwowarska, Z.; Michalik, M. Thermal stability of montmorillonite polyacrylamide and polyacrylate nanocomposites and adsorption of Fe(III) ions. Appl. Clay Sci. 2013, 153–157. [Google Scholar] [CrossRef]
- Díaz, A.P.; Lourdin, D.; Della Valle, G.; Quintero, A.F.; Eceballos, H.; Tran, T.; Dufour, D. Thermomechanical characterization of an amylose-free starch extracted from cassava (Manihot esculenta, Crantz). Carbohydr. Polym. 2017, 157, 1777–1784. [Google Scholar] [CrossRef]
- Parvathy, P.C.; Jyothi, A.N. Rheological and thermal properties of saponified cassava starch-g-poly(acrylamide) superabsorbent polymers varying in grafting parameters and absorbency. J. Appl. Polym. Sci. 2014, 131, 40368. [Google Scholar] [CrossRef]
- Wang, S.; Li, C.; Copeland, L.; Niu, Q.; Wang, S. Starch Retrogradation: A Comprehensive Review. Compr. Rev. Food Sci. Food Saf. 2015, 14, 568–585. [Google Scholar] [CrossRef]
- Hoover, R.; Hughes, T.; Chung, H.; Liu, Q. Composition, molecular structure, properties, and modification of pulse starches: A review. Food Res. Int. 2010, 43, 399–413. [Google Scholar] [CrossRef]
- Zhang, L.-M.; Chen, D.-Q. An investigation of adsorption of lead(II) and copper(II) ions by water-insoluble starch graft copolymers. Colloids Surf. A Physicochem. Eng. Asp. 2002, 205, 231–236. [Google Scholar] [CrossRef]
- Ciesielski, W.; Lii, C.-Y.; Yen, M.-T.; Tomasik, P. Interactions of starch with salts of metals from the transition groups. Carbohydr. Polym. 2003, 51, 47–56. [Google Scholar] [CrossRef]
- Milosavljevic, N.; Debeljkovic, A.; Krušić, M.K.; Milašinović, N.; Üzüm, Ö.B.; Karadağ, E. Application of poly(acrlymide-co-sodium methacrylate) hydrogels in copper and cadmium removal from aqueous solution. Environ. Prog. Sustain. Energy 2013, 33, 824–834. [Google Scholar] [CrossRef]
- Cheng, X.; Cheng, R.; Ou, S.; Li, Y. Synthesis and adsorption performance of dithiocarbamate-modified glycidyl methacrylate starch. Carbohydr. Polym. 2013, 96, 320–325. [Google Scholar] [CrossRef] [PubMed]
- Kousalya, G.N.; Gandhi, M.R.; Sundaram, C.S.; Meenakshi, S. Synthesis of nano-hydroxyapatite chitin/chitosan hybrid biocomposites for the removal of Fe(III). Carbohydr. Polym. 2010, 82, 594–599. [Google Scholar] [CrossRef]
- Öztaş, N.; Karabakan, A.; Topal, O. Removal of Fe(III) ion from aqueous solution by adsorption on raw and treated clinoptilolite samples. Microporous Mesoporous Mater. 2008, 111, 200–205. [Google Scholar] [CrossRef]
- Sheibani, A.; Shishehbor, M.R.; Hamed Alaei, H. Removal of Fe(III) ions from aqueous solution by hazelnut hull as an adsorbent. Int. J. Ind. Chem. 2012, 3, 4. [Google Scholar] [CrossRef]
- Anirudhan, T.; Suchithra, P. Heavy metals uptake from aqueous solutions and industrial wastewaters by humic acid-immobilized polymer/bentonite composite: Kinetics and equilibrium modeling. Chem. Eng. J. 2010, 156, 146–156. [Google Scholar] [CrossRef]






| Removal (%) | Absorption Efficiency (mg/g) | Removal (%) | Absorption Efficiency (mg/g) | |||||
|---|---|---|---|---|---|---|---|---|
| Concentration (mg/100 cm3) | 50 | 100 | ||||||
| Time (h) | 24 | 48 | 24 | 48 | 24 | 48 | 24 | 48 |
| St | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 |
| StMBA1 | 15.4 | 7.8 | 7.7 | 3.9 | 8.4 | 7.2 | 8.4 | 7.2 |
| StMBA2 | 14.5 | 7.6 | 7.2 | 3.8 | 7.6 | 7.1 | 7.6 | 7.1 |
| St90 | 35.0 | 30.0 | 17.5 | 15.0 | 11.0 | 10.8 | 11.0 | 10.8 |
| St80 | 35.0 | 30.2 | 17.5 | 15.1 | 11.0 | 10.8 | 11.0 | 10.8 |
| St70 | 38.0 | 30.0 | 19.0 | 15.0 | 17.2 | 15.9 | 17.2 | 15.9 |
| St60 | 39.0 | 30.0 | 19.5 | 15.0 | 21.0 | 20.0 | 21.0 | 20.0 |
| Removal of Fe(III) (%) | Absorption Efficiency of Fe(III) (mg/g) | |||
|---|---|---|---|---|
| Time (h) | 24 | 48 | 24 | 48 |
| Concentration of Fe(III) (mg/L) | 10 | |||
| St | 20 | 22 | 2.0 | 2.2 |
| StMBA1 | 34 | 40 | 3.4 | 4.0 |
| StMBA2 | 32 | 40 | 3.2 | 4.0 |
| St90 | 52 | 52 | 5.2 | 5.2 |
| St80 | 61 | 61 | 6.1 | 6.1 |
| St70 | 68 | 70 | 6.8 | 7.0 |
| St60 | 75 | 75 | 7.5 | 7.5 |
| Concentration of Fe(III) (mg/L) | 30 | |||
| St | 8.0 | 8.0 | 2.4 | 2.4 |
| StMBA1 | 15 | 16 | 4.5 | 4.8 |
| StMBA2 | 15 | 15 | 4.5 | 4.5 |
| St90 | 50 | 50 | 15.0 | 15.0 |
| St80 | 52 | 51 | 15.6 | 15.3 |
| St70 | 65 | 65 | 19.5 | 19.5 |
| St60 | 72 | 72 | 21.6 | 21.6 |
| Concentration of Fe(III) (mg/L) | 50 | |||
| St | 5.0 | 5.0 | 2.5 | 2.5 |
| StMBA1 | 10 | 11 | 5.0 | 5.5 |
| StMBA2 | 9 | 9 | 4.5 | 4.5 |
| St90 | 25 | 25 | 12.5 | 12.5 |
| St80 | 29 | 30 | 14.5 | 15.0 |
| St70 | 40 | 39 | 20 | 19.5 |
| St60 | 44 | 44 | 22 | 22 |
| Ion | AE (mg/g) | Adsorbent | Comments | Reference |
|---|---|---|---|---|
| Cu(II) | 24.05 | Poly(acrylamide-co-sodium methacrylate) | lack of starch | [51] |
| - | Starch-g-polyacrylamide | [25] | ||
| 20.33 | Dithiocarbamate-modifed glycidyl methacrylate starch | lack of PAM | [52] | |
| 40.72 | polyacrylamide with anionically modified potato starch | theoretical sorption, 12.28 wt.% of starch | [27] | |
| 23.0 | Starch-g-polyacrylamide | 60–90 wt.% of starch | This work | |
| Fe(III) | 12.9 | nano-hydroxyapatite (n-HAp) with chitin and chitosan | lack of starch and PAM | [53] |
| 20.9 | clinoptilolite | lack of starch and PAM | [54] | |
| 13.6 | hazelnut hull | lack of starch and PAM | [55] | |
| 21.2 | Starch-g-polyacrylamide | This work |
| Ion | Cu(II) | Fe(III) | |||
|---|---|---|---|---|---|
| Initial Concentration1 | 50 | 100 | 10 | 30 | 50 |
| St | 0.5890 | 0.2186 | 1.6971 | 0.9706 | 0.7902 |
| StMBA1 | 0.0046 | 0.0005 | 0.5446 | 0.2109 | 0.1114 |
| StMBA2 | 0.0039 | 0.0028 | 0.5668 | 0.2360 | 0.1947 |
| St90 | 0.0158 | 0.0323 | 1.3330 | 2.2934 | 1.5104 |
| St80 | 0.0108 | 0.0610 | 1.3050 | 1.5110 | 1.7124 |
| St70 | 0.0512 | 0.0108 | 0.7060 | 2.0338 | 1.8482 |
| St60 | 0.0004 | 0.0184 | 1.0290 | 1.6576 | 2.0086 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schmidt, B.; Rokicka, J.; Janik, J.; Wilpiszewska, K. Preparation and Characterization of Potato Starch Copolymers with a High Natural Polymer Content for the Removal of Cu(II) and Fe(III) from Solutions. Polymers 2020, 12, 2562. https://doi.org/10.3390/polym12112562
Schmidt B, Rokicka J, Janik J, Wilpiszewska K. Preparation and Characterization of Potato Starch Copolymers with a High Natural Polymer Content for the Removal of Cu(II) and Fe(III) from Solutions. Polymers. 2020; 12(11):2562. https://doi.org/10.3390/polym12112562
Chicago/Turabian StyleSchmidt, Beata, Joanna Rokicka, Jolanta Janik, and Katarzyna Wilpiszewska. 2020. "Preparation and Characterization of Potato Starch Copolymers with a High Natural Polymer Content for the Removal of Cu(II) and Fe(III) from Solutions" Polymers 12, no. 11: 2562. https://doi.org/10.3390/polym12112562
APA StyleSchmidt, B., Rokicka, J., Janik, J., & Wilpiszewska, K. (2020). Preparation and Characterization of Potato Starch Copolymers with a High Natural Polymer Content for the Removal of Cu(II) and Fe(III) from Solutions. Polymers, 12(11), 2562. https://doi.org/10.3390/polym12112562






