Using Thermally Crosslinkable Hole Transporting Layer to Improve Interface Characteristics for Perovskite CsPbBr3 Quantum-Dot Light-Emitting Diodes
Abstract
1. Introduction
2. Materials and Methods
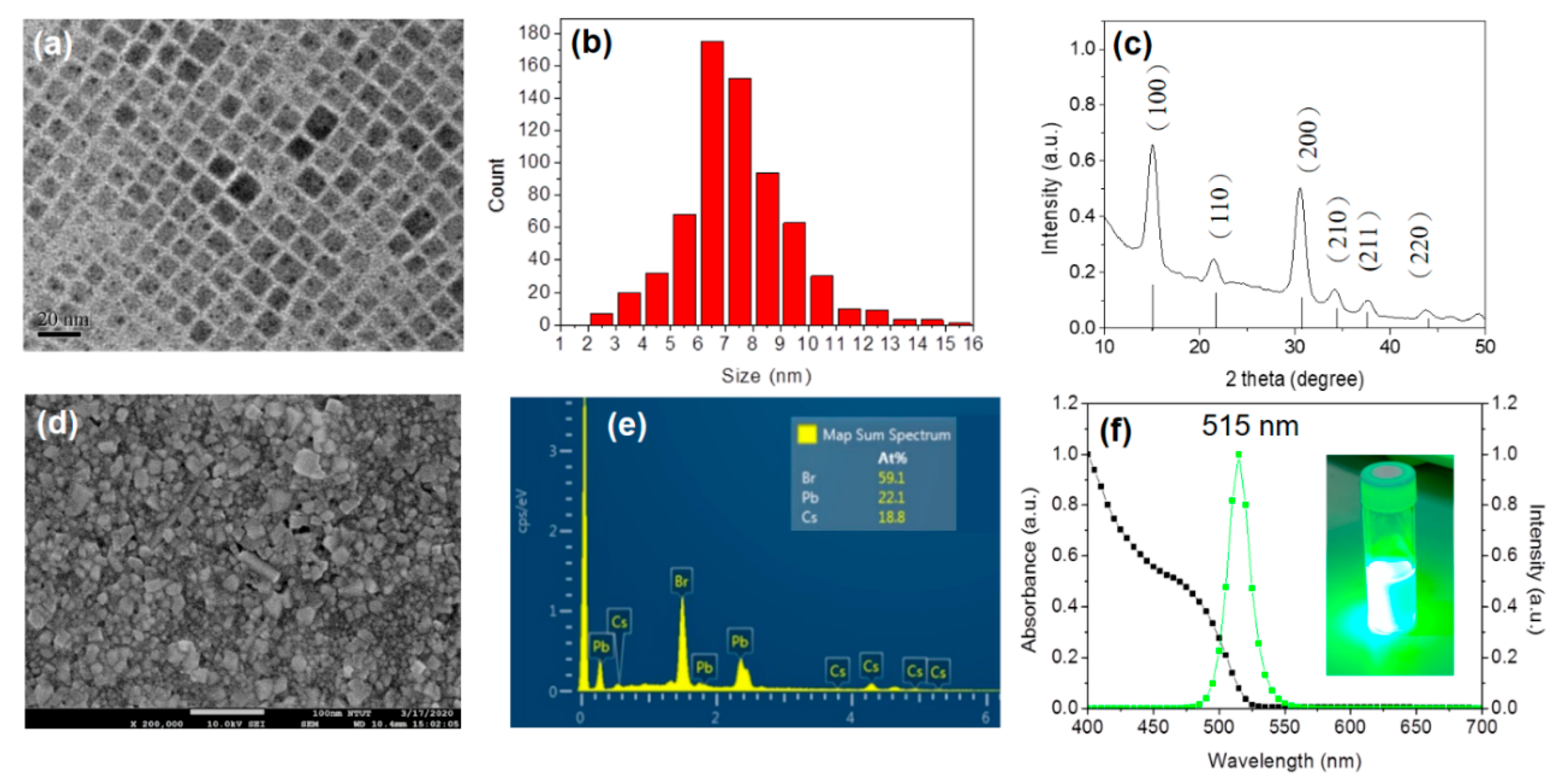
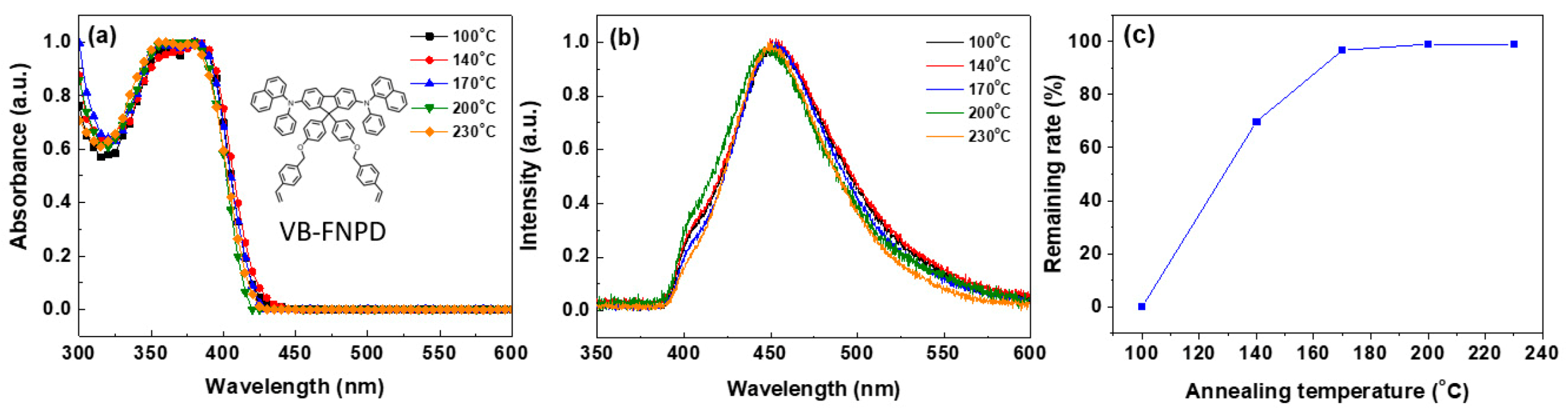
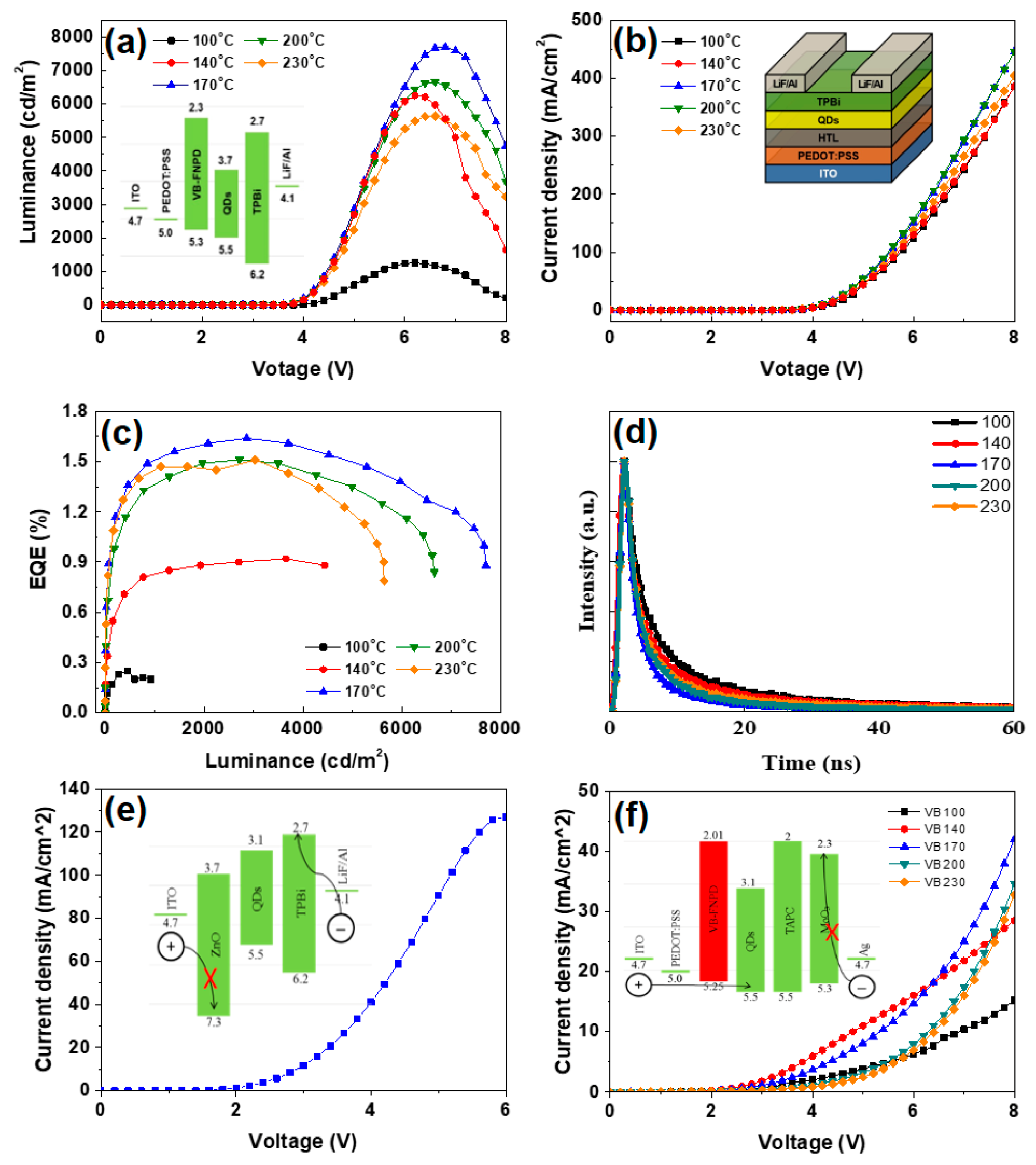
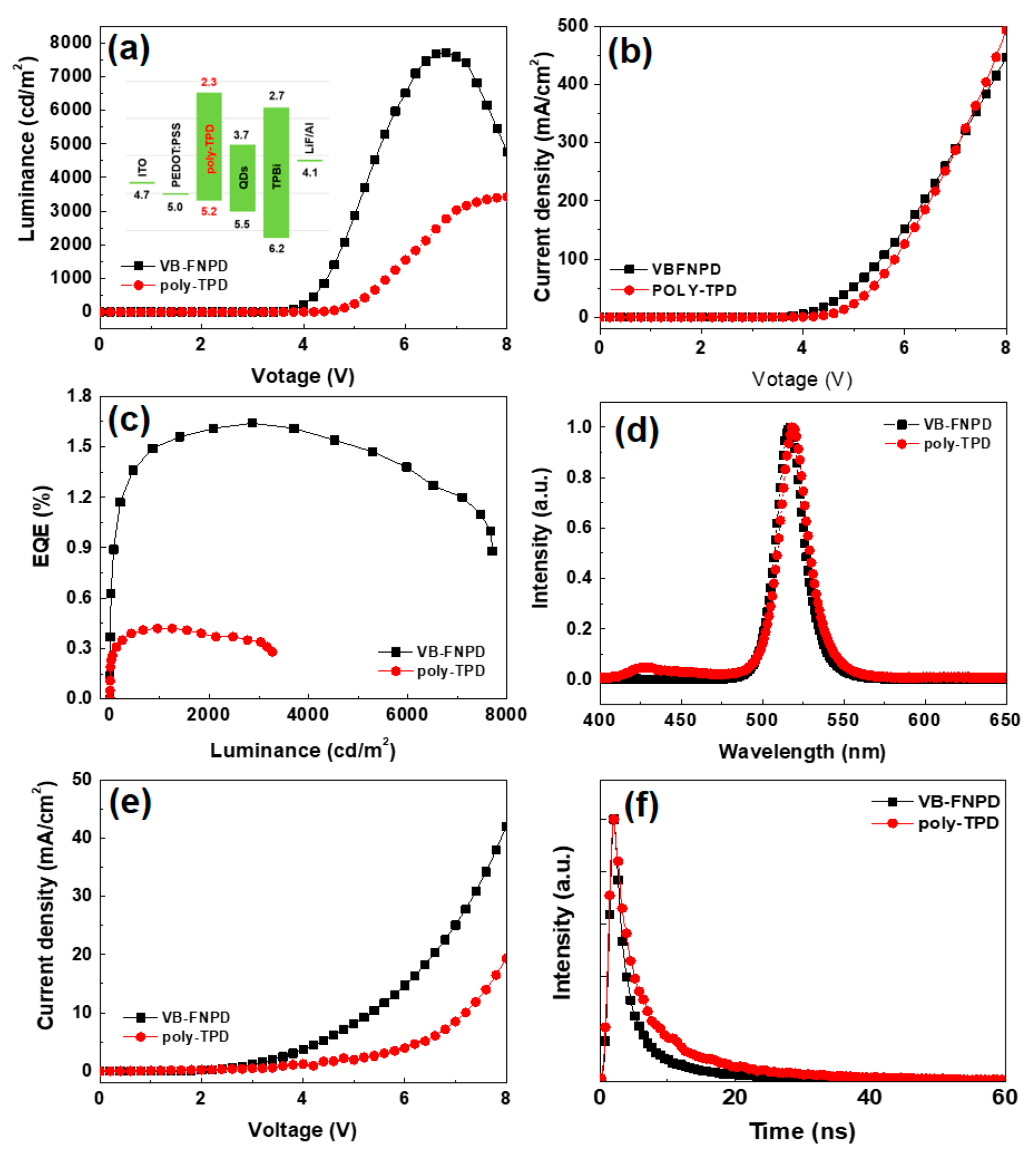
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Lee, Y.J.; Kim, S.H.; Huh, J.; Kim, G.H.; Lee, Y.H.; Cho, S.H.; Kim, Y.C.; Do, Y.R. A high-extraction-efficiency nanopatterned organic light-emitting diode. Appl. Phys. Lett. 2003, 82, 3779–3781. [Google Scholar] [CrossRef]
- Kabe, R.; Notsuka, N.; Yoshida, K.; Adachi, C. Afterglow organic light-emitting diode. Adv. Mater. 2016, 28, 655–660. [Google Scholar] [CrossRef]
- Chen, H.W.; Lee, J.H.; Lin, B.Y.; Chen, S.; Wu, S.T. Liquid crystal display and organic light-emitting diode display: Present status and future perspectives. Light Sci. Appl. 2018, 7, 17168. [Google Scholar] [CrossRef] [PubMed]
- Choi, M.; Park, Y.J.; Sharma, B.K.; Bae, S.R.; Kim, S.Y.; Ahn, J.H. Flexible active-matrix organic light-emitting diode display enabled by MoS2 thin-film transistor. Sci. Adv. 2018, 4, eaas8721. [Google Scholar] [CrossRef] [PubMed]
- Wei, Q.; Fei, N.; Islam, A.; Lei, T.; Hong, L.; Peng, R.; Fan, X.; Chen, L.; Gao, P.; Ge, Z. Small-molecule emitters with high quantum efficiency: Mechanisms, structures, and applications in OLED devices. Adv. Opt. Mater. 2018, 6, 1800512. [Google Scholar] [CrossRef]
- Ma, B.; Lauterwasser, F.; Deng, L.; Zonte, C.S.; Kim, B.J.; Fréchet, J.M.J.; Borek, C.; Thompson, M.E. New Thermally cross-linkable polymer and its application as a hole-transporting layer for solution processed multilayer organic light emitting diodes. Chem. Mater. 2007, 19, 4827–4832. [Google Scholar] [CrossRef]
- Nuyken, O.; Jungermann, S.; Wiederhirn, V.; Bacher, E.; Meerholz, K. Modern trends in organic light-emitting devices (OLEDs). Mon. Für Chem. Chem. Mon. 2006, 137, 811–824. [Google Scholar] [CrossRef]
- Baek, S.; Kang, S.; Son, C.; Shin, S.J.; Kim, J.H.; Park, J.; Kim, S.W. Highly stable all-inorganic perovskite quantum dots using a znx2-trioctylphosphine-oxide: Application for high-performance full-color light-emitting diode. Adv. Opt. Mater. 2020, 8, 1901897. [Google Scholar] [CrossRef]
- Lee, J.S.; Kang, B.H.; Kim, S.H.; Lee, J.W.; Lee, S.W.; Kim, S.W.; Gopalan, S.A.; Kwon, J.B.; Bae, J.H.; Kim, E.S.; et al. All-solution-processed high-brightness hybrid white quantum-dot light-emitting devices utilizing polymer modified quantum dots. Org. Electron. 2017, 42, 393–398. [Google Scholar] [CrossRef]
- Triana, M.A.; Chen, H.; Zhang, D.; Camargoa, R.J.; Zhai, T.; Duhm, S.; Dong, Y. Bright inverted quantum-dot light-emitting diodes by all-solution processing. J. Mater. Chem. C 2018, 6, 28. [Google Scholar] [CrossRef]
- Chen, F.; Lin, Q.; Shen, H.; Tang, A. Blue quantum dot-based electroluminescent light-emitting diodes. Mater. Chem. Front. 2020, 4, 1340. [Google Scholar] [CrossRef]
- Protesescu, L.; Yakunin, S.; Bodnarchuk, M.I.; Krieg, F.; Caputo, R.; Hendon, C.H.; Yang, R.X.; Walsh, A.; Kovalenko, M.V. Nanocrystals of cesium lead halide perovskites (CsPbX3, X = Cl, Br, and I): Novel optoelectronic materials showing bright emission with wide color gamut. Nano Lett. 2015, 15, 3692–3696. [Google Scholar] [CrossRef]
- Fauchet, P.M. Light emission from Si quantum dots. Mater. Today 2005, 8, 26–33. [Google Scholar] [CrossRef]
- Qian, L.; Zheng, Y.; Xue, J.; Holloway, P.H. Stable and efficient quantum-dot light-emitting diodes based on solution-processed multilayer structures. Nat. Photonics 2011, 5, 543–548. [Google Scholar] [CrossRef]
- Reza, K.M.; Gurung, A.; Bahrami, B.; Mabrouk, S.; Elbohy, H.; Pathak, R.; Chen, K.; Chowdhury, A.H.; Rahman, M.T.; Letourneau, S.; et al. Tailored PEDOT:PSS hole transport layer for higher performance in perovskite solar cells: Enhancement of electrical and optical properties with improved morphology. J. Energy Chem. 2020, 44, 41–50. [Google Scholar] [CrossRef]
- Saianand, G.; Sonar, P.; Wilson, G.J.; Gopalan, A.I.; Roy, V.A.L.; Unni, G.E.; Reza, K.M.; Bahrami, B.; Venkatramanan, K.; Qiao, Q. Current advancements on charge selective contact interfacial layers and electrodes in flexible hybrid perovskite photovoltaics. J. Energy Chem. 2021, 54, 151–173. [Google Scholar] [CrossRef]
- Huang, Q.; Pan, J.; Zhang, Y.; Chen, J.; Tao, Z.; He, C.; Zhou, K.; Tu, Y.; Lei, W. High-performance quantum dot light-emitting diodes with hybrid hole transport layer via doping engineering. Opt. Express 2016, 24, 25955–25963. [Google Scholar] [CrossRef] [PubMed]
- Olayan, H.B.; Hami, H.S.; Owen, E.D. Photochemical and thermal crosslinking of polymers. J. Macromol. Sci. Part C 1996, 36, 671–719. [Google Scholar] [CrossRef]
- Jin, X.; Li, L.; Xu, R.; Liu, Q.; Ding, L.; Pan, Y.; Wang, C.; Hung, W.; Lee, K.; Wang, T. Effects of thermal cross-linking on the structure and property of asymmetric membrane prepared from the polyacrylonitrile. Polymers 2018, 10, 539. [Google Scholar] [CrossRef] [PubMed]
- Niu, Y.H.; Liu, M.S.; Ka, J.W.; Bardeker, J.; Zin, M.T.; Schofield, R.; Chi, Y.; Jen, A.K.Y. Crosslinkable hole-transport layer on conducting polymer for high-efficiency white polymer light-emitting diodes. Adv. Mater. 2007, 19, 300–304. [Google Scholar] [CrossRef]
- Huang, F.; Cheng, Y.J.; Zhang, Y.; Liu, M.S.; Jen, A.K.Y. Crosslinkable hole-transporting materials for solution processed polymer light-emitting diodes. J. Mater. Chem. 2008, 18, 4495–4509. [Google Scholar] [CrossRef]
- Lin, C.Y.; Lin, Y.C.; Hung, W.Y.; Wong, K.T.; Kwong, R.C.; Xia, S.C.; Chen, Y.H.; Wu, C.I. A thermally cured 9,9-diarylfluorene-based triaryldiamine polymer displaying high hole mobility and remarkable ambient stability. J. Mater. Chem. 2009, 19, 3618–3623. [Google Scholar] [CrossRef]
- Chao, S.W.; Chen, W.S.; Hung, W.Y.; Chen, Y.Y.; Lin, Y.M.; Wong, K.T.; Chou, P.T. Cross-linkable hole transporting layers boost operational stability of high-performance quantum dot light-emitting device. Org. Electron. 2019, 71, 206–211. [Google Scholar] [CrossRef]
- Li, J.; Xu, L.; Wang, T.; Song, J.; Chen, J.; Xue, J.; Dong, Y.; Cai, B.; Shan, Q.; Han, B.; et al. 50-Fold EQE improvement up to 6.27% of solution-processed all-inorganic perovskite CsPbBr3 QLEDs via surface ligand density control. Adv. Mater. 2017, 29, 1603885. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Tian, G.; Xu, W.; Wang, S.; Zhang, H.; Niu, J.; Chen, X. Green light-emitting devices based on perovskite CsPbBr3 quantum dots. Front. Chem. 2018, 6, 381. [Google Scholar] [CrossRef]
- Li, C.; Zang, Z.; Chen, W.; Hu, Z.; Tang, X.; Hu, W.; Sun, K.; Liu, X.; Chen, W. Highly pure green light emission of perovskite CsPbBr3 quantum dots and their application for green light-emitting diodes. Opt. Express 2016, 24, 15071–15078. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.; Lu, H.; Rao, L.; Li, Z.; Ding, X.; Yan, C.; Yu, B. Regulating the emission spectrum of CsPbBr₃ from green to blue via controlling the temperature and velocity of microchannel reactor. Materials 2018, 11, 371. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.C.; Chou, W.C.; Fan, W.C.; Ku, J.T.; Ke, F.K.; Wang, W.J.; Yang, S.L.; Chen, W.K.; Chang, W.H.; Chia, C.H. Time-resolved photoluminescence of isoelectronic traps in ZnSe1−xTex semiconductor alloys. Appl. Phys. Lett. 2008, 93, 241909. [Google Scholar] [CrossRef]
- Chiba, T.; Hoshi, K.; Pu, Y.-J.; Takeda, Y.; Hayashi, Y.; Ohisa, S.; Kawata, S.; Kido, J. High-efficiency perovskite quantum-dot light-emitting devices by effective washing process and interfacial energy level alignment. ACS Appl. Mater. Interfaces 2017, 9, 18054–18060. [Google Scholar] [CrossRef]
- Gerhard, M.; Louis, B.; Camacho, R.; Merdasa, A.; Li, J.; Kiligaridis, A.; Dobrovolsky, A.; Hofkens, J.; Scheblykin, I.G. Microscopic insight into non-radiative decay in perovskite semiconductors from temperature-dependent luminescence blinking. Nat. Commun. 2019, 10, 1698. [Google Scholar] [CrossRef]
- Lin, W.C.; Lin, H.W.; Mondal, E.; Wong, K.T. Efficient solution-processed green and white phosphorescence organic light-emitting diodes based on bipolar host materials. Org. Electron. 2015, 17, 1–8. [Google Scholar] [CrossRef]
- Wang, H.; Zhang, X.; Wu, Q.; Cao, F.; Yang, D.; Shang, Y.; Ning, Z.; Zhang, W.; Zheng, W.; Yan, Y.; et al. Trifluoroacetate induced small-grained CsPbBr3 perovskite films result in efficient and stable light-emitting devices. Nat. Commun. 2019, 10, 665. [Google Scholar] [CrossRef] [PubMed]
| 100 °C | 140 °C | 170 °C | 200 °C | 230 °C | poly-TPD | |
|---|---|---|---|---|---|---|
| PLQY (%) | 18 | 18.5 | 19 | 18.5 | 18.3 | 10 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, C.-C.; Yeh, S.-Y.; Huang, W.-L.; Xu, Y.-X.; Huang, Y.-S.; Yeh, T.-H.; Tien, C.-H.; Chen, L.-C.; Tseng, Z.-L. Using Thermally Crosslinkable Hole Transporting Layer to Improve Interface Characteristics for Perovskite CsPbBr3 Quantum-Dot Light-Emitting Diodes. Polymers 2020, 12, 2243. https://doi.org/10.3390/polym12102243
Lin C-C, Yeh S-Y, Huang W-L, Xu Y-X, Huang Y-S, Yeh T-H, Tien C-H, Chen L-C, Tseng Z-L. Using Thermally Crosslinkable Hole Transporting Layer to Improve Interface Characteristics for Perovskite CsPbBr3 Quantum-Dot Light-Emitting Diodes. Polymers. 2020; 12(10):2243. https://doi.org/10.3390/polym12102243
Chicago/Turabian StyleLin, Chun-Cheng, Shao-Yang Yeh, Wei-Lun Huang, You-Xun Xu, Yan-Siang Huang, Tzu-Hung Yeh, Ching-Ho Tien, Lung-Chien Chen, and Zong-Liang Tseng. 2020. "Using Thermally Crosslinkable Hole Transporting Layer to Improve Interface Characteristics for Perovskite CsPbBr3 Quantum-Dot Light-Emitting Diodes" Polymers 12, no. 10: 2243. https://doi.org/10.3390/polym12102243
APA StyleLin, C.-C., Yeh, S.-Y., Huang, W.-L., Xu, Y.-X., Huang, Y.-S., Yeh, T.-H., Tien, C.-H., Chen, L.-C., & Tseng, Z.-L. (2020). Using Thermally Crosslinkable Hole Transporting Layer to Improve Interface Characteristics for Perovskite CsPbBr3 Quantum-Dot Light-Emitting Diodes. Polymers, 12(10), 2243. https://doi.org/10.3390/polym12102243






