A Collagen-Based Scaffold for Promoting Neural Plasticity in a Rat Model of Spinal Cord Injury
Abstract
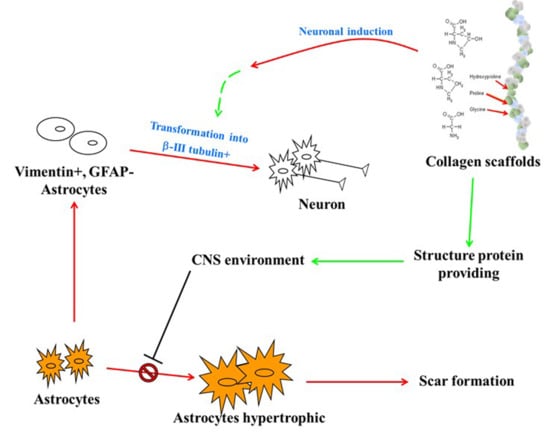
1. Introduction
2. Materials and Methods
2.1. Preparation of Collagen Scaffold
2.2. Physical Characteristics of the Collagen Scaffold
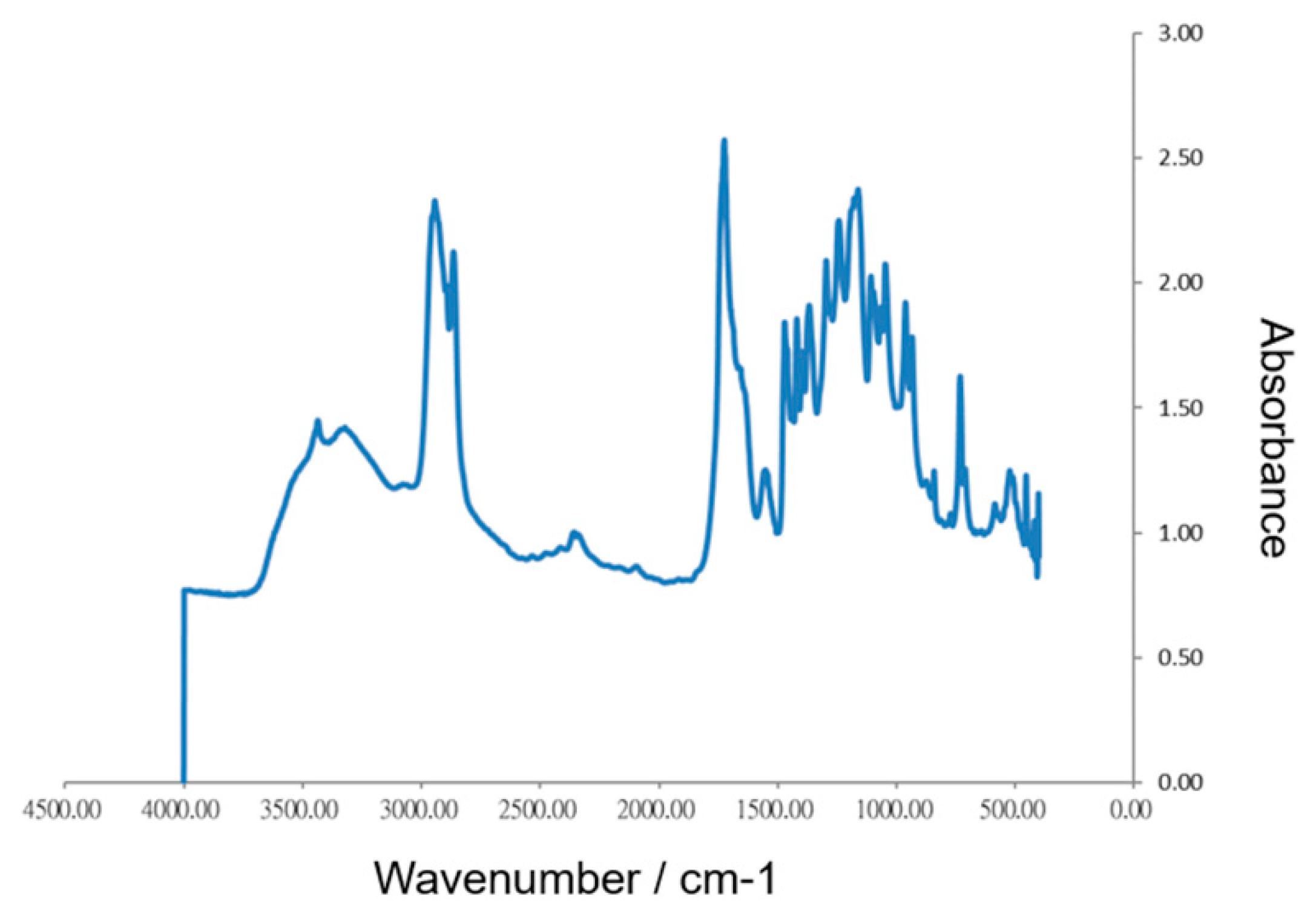
2.2.1. Fourier Transform Infra-red (FTIR) Spectroscopy
2.2.2. Scanning Electron Microscopy (SEM)
2.3. In Vitro Biocompatibility
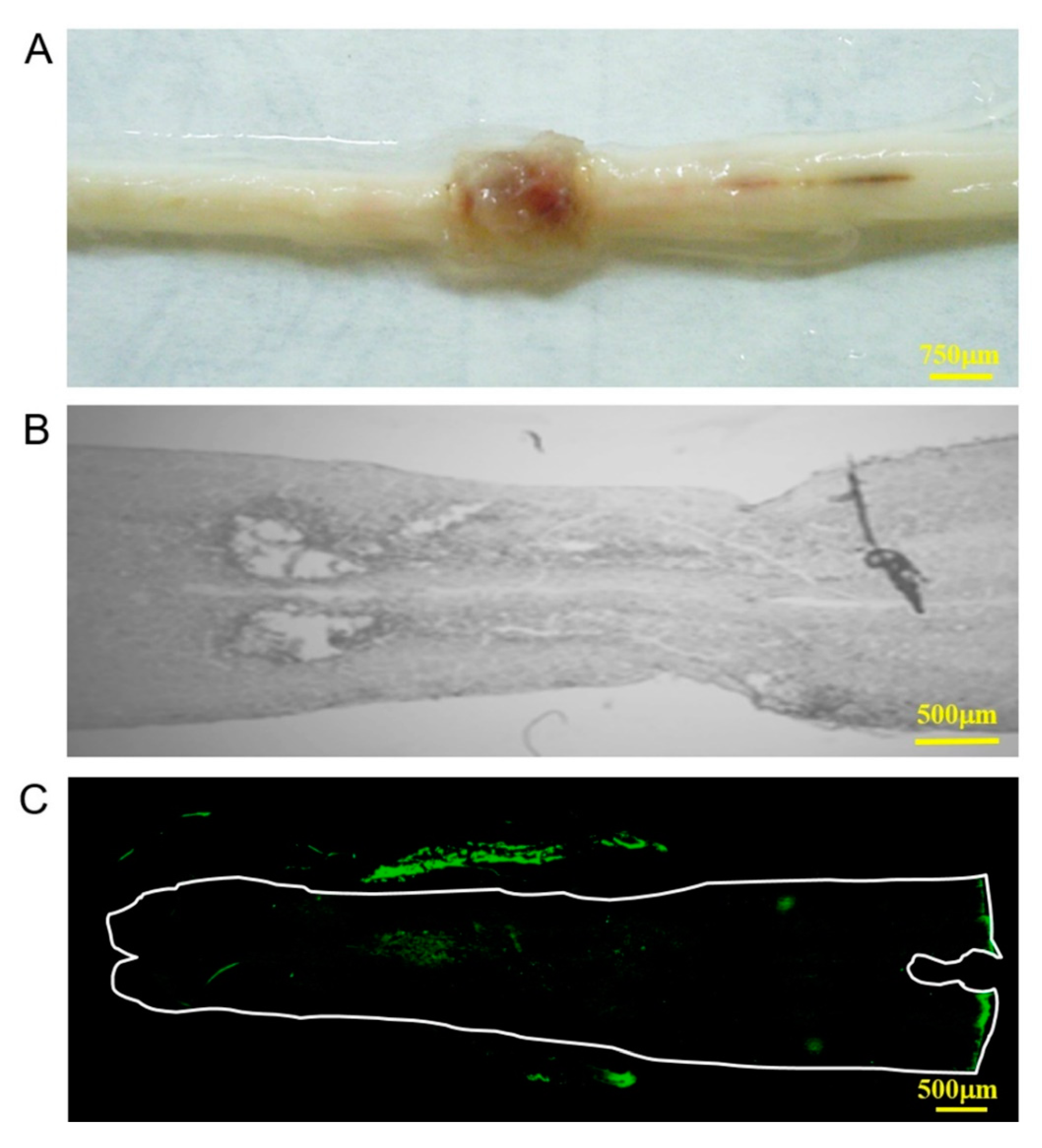
2.4. Rat Spinal Cord Injury Model
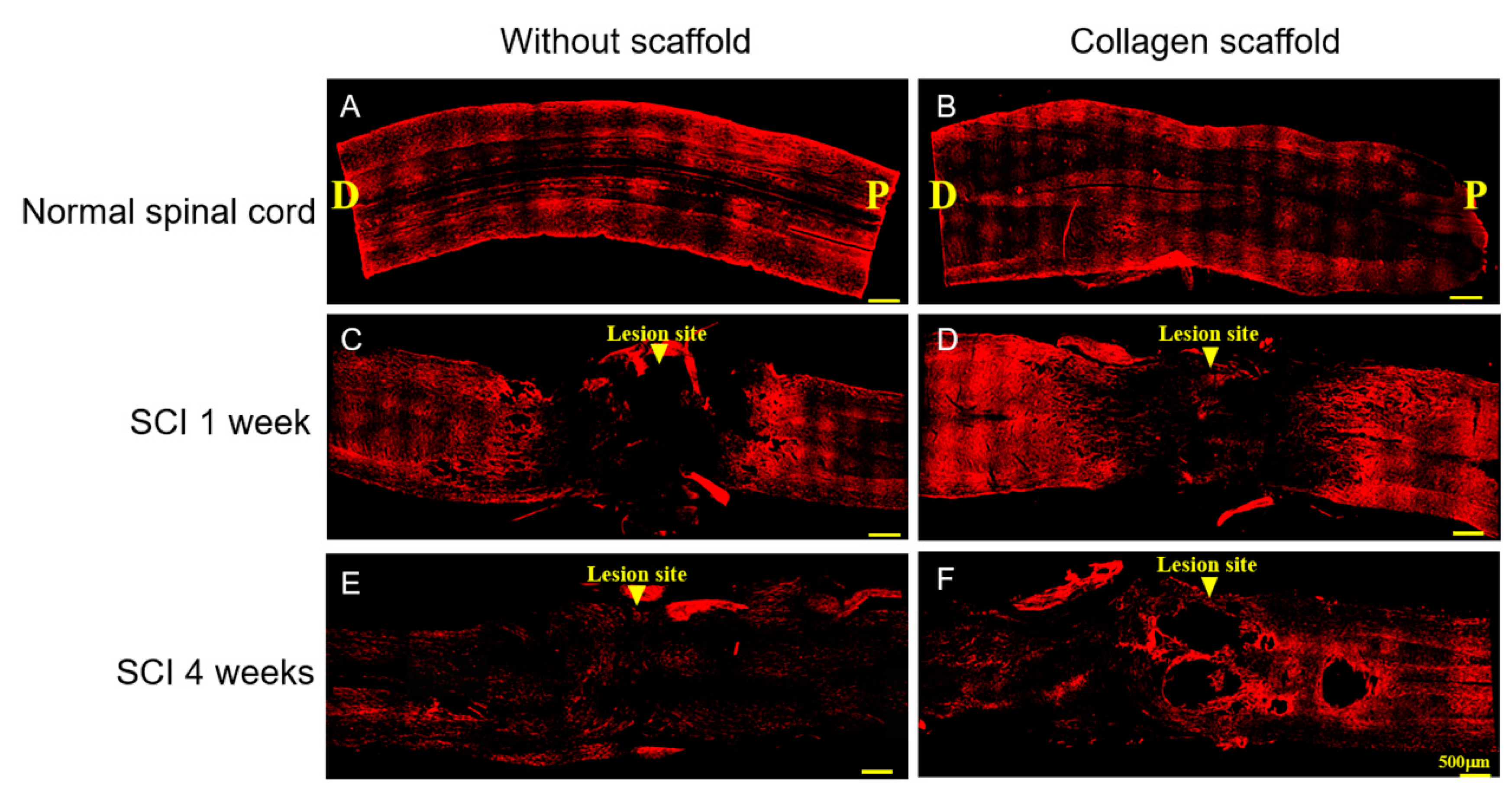
2.5. Immunofluorescence Analysis
3. Results and Discussion
3.1. Morphology and In Vitro Biocompatibility of the Collagen Scaffold
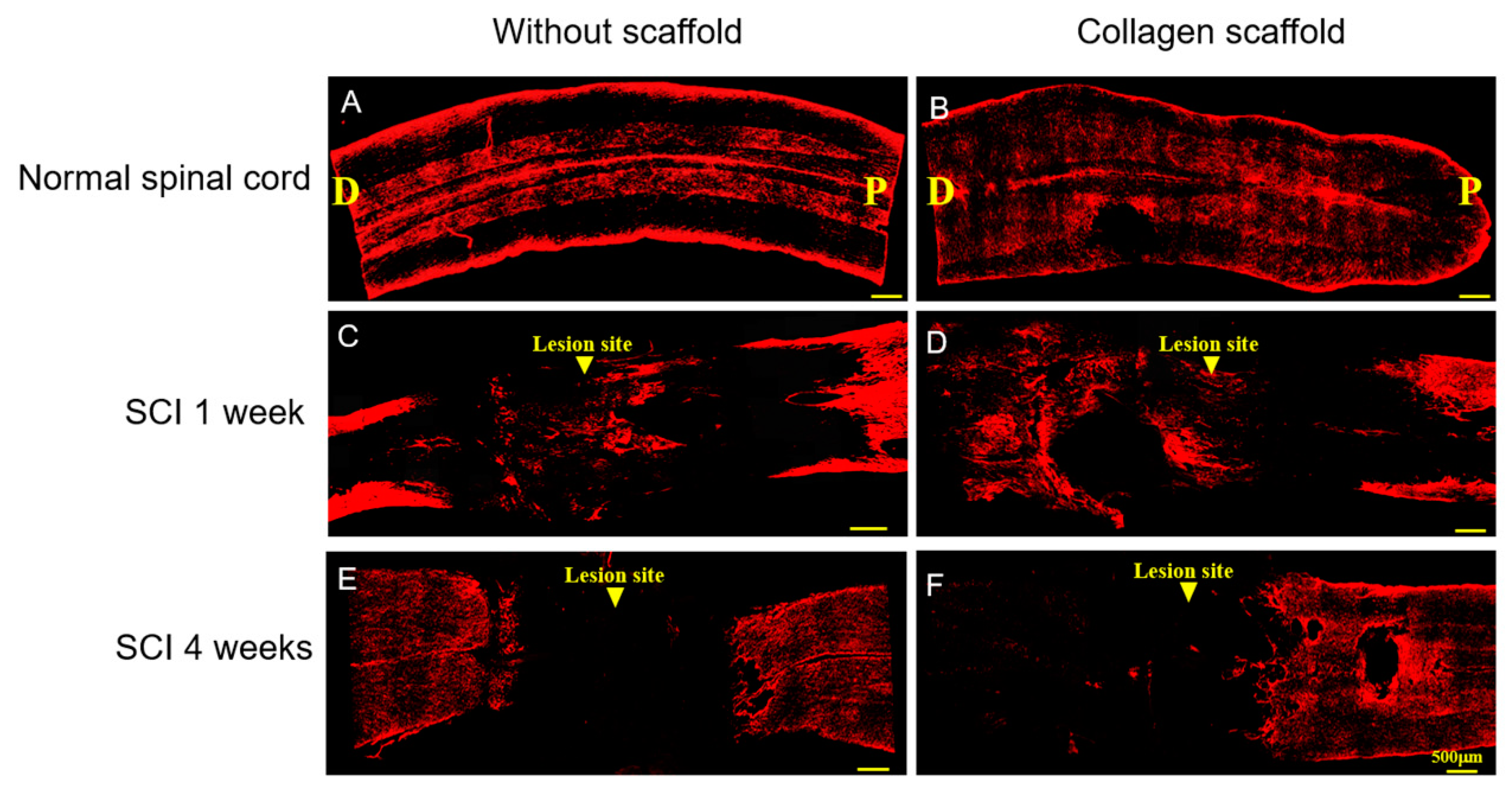
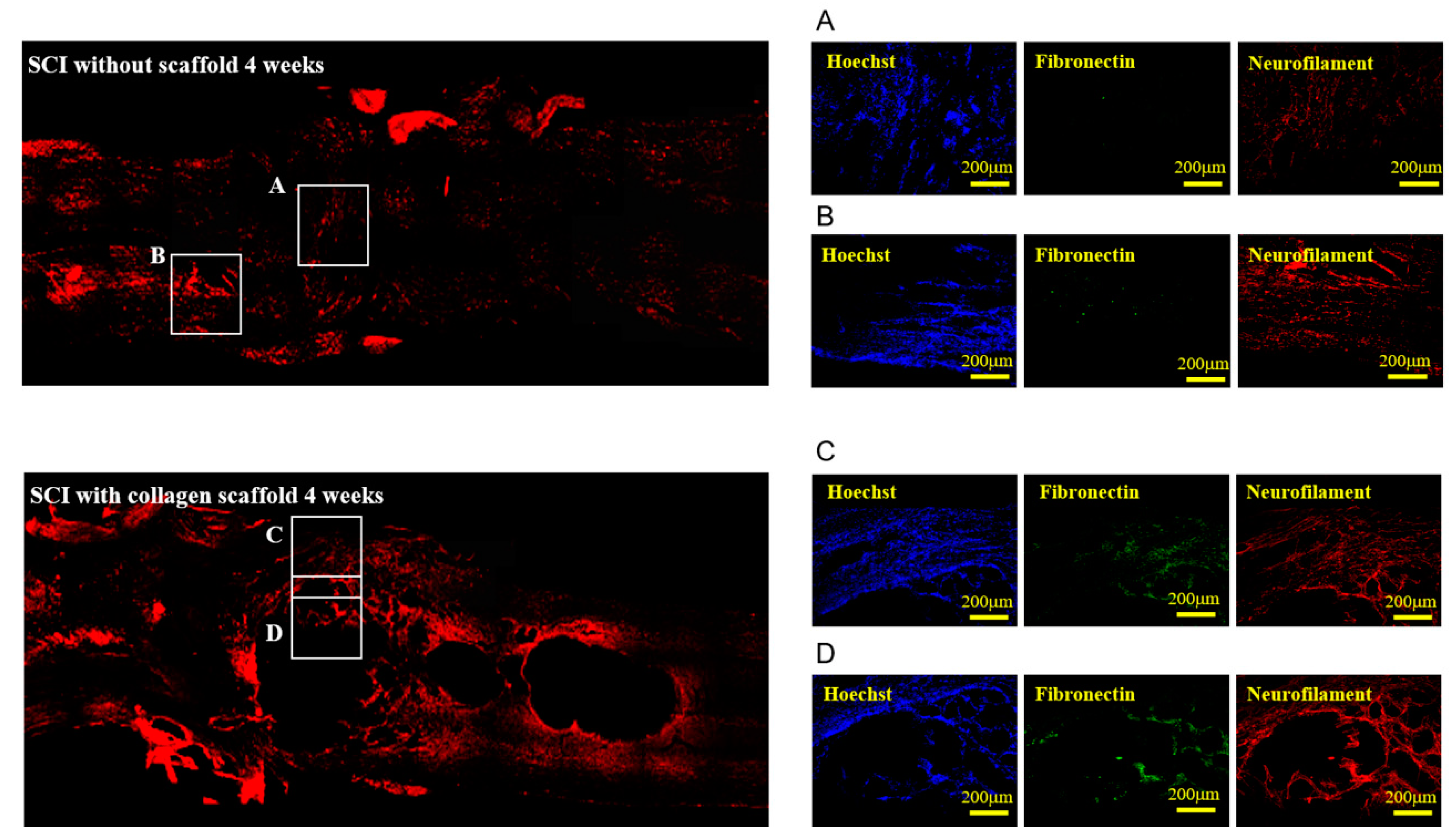
3.2. In Vivo Biocompatibility and Immunofluorescence of the Implanted Collagen Scaffold in the Rat Spinal Cord Injury Model
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Quraishe, S.; Forbes, L.H.; Andrews, M.R. The extracellular environment of the cns: Influence on plasticity, sprouting, and axonal regeneration after spinal cord injury. Neural Plast. 2018, 2018, 2952386. [Google Scholar]
- Cregg, J.M.; de Paul, M.A.; Filous, A.R.; Lang, B.T.; Tran, A.; Silver, J. Functional regeneration beyond the glial scar. Exp. Neurol. 2014, 253, 197–207. [Google Scholar]
- Silver, J.; Schwab, M.E.; Popovich, P.G. Central nervous system regenerative failure: Role of oligodendrocytes, astrocytes, and microglia. Cold Spring Harb. Perspect. Biol. 2014, 7, a020602. [Google Scholar]
- Dias, D.O.; Kim, H.; Holl, D.; Solnestam, B.W.; Lundeberg, J.; Carlén, M.; Göritz, C.; Frisén, J. Reducing pericyte-derived scarring promotes recovery after spinal cord injury. Cell 2018, 173, 153–165. [Google Scholar]
- Xu, B.; Park, D.; Ohtake, Y.; Li, H.; Hayat, U.; Liu, J.; Selzer, M.E.; Longo, F.M.; Li, S. Role of CSPG receptor LAR phosphatase in restricting axon regeneration after CNS injury. Neurobiol. Dis. 2015, 73, 36–48. [Google Scholar]
- Yiu, G.; He, Z. Glial inhibition of CNS axon regeneration. Nat. Rev. Neurosci. 2006, 7, 617–627. [Google Scholar]
- Orr, M.B.; Gensel, J.C. Spinal cord injury scarring and inflammation: Therapies targeting glial and inflammatory responses. Neurotherapeutics 2018, 15, 541–553. [Google Scholar] [PubMed]
- Sofroniew, M.V.; Vinters, H.V. Astrocytes: Biology and pathology. Acta Neuropathol. 2010, 119, 7–35. [Google Scholar] [PubMed]
- Wanner, I.B.; Anderson, M.A.; Song, B.; Levine, J.; Fernandez, A.; Gray, Z.T.; Ao, Y.; Sofroniew, M.V. Glial scar borders are formed by newly proliferated, elongated astrocytes that interact to corral inflammatory and fibrotic cells via STAT3-dependent mechanisms after spinal cord injury. J. Neurosci. 2013, 33, 12870–12886. [Google Scholar] [PubMed]
- Fitch, M.T.; Silver, J. CNS injury, glial scars, and inflammation: Inhibitory extracellular matrices and regeneration failure. Exp. Neurol. 2008, 2, 294–301. [Google Scholar]
- Fawcett, J.W.; Asher, R.A. The glial scar and central nervous system repair. Brain Res. Bull. 1999, 49, 377–391. [Google Scholar] [CrossRef]
- Fehlings, M.G.; Hawryluk, G.W. Scarring after spinal cord injury. J. Neurosurg. Spine 2010, 132, 165–168. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Song, G.; Chuang, H.; Chiu, C.; Abdelmaksoud, A.; Ye, Y.; Zhao, L. Portrait of glial scar in neurological diseases. Int. J. Immunopathol. Pharmacol. 2018, 31, 6. [Google Scholar] [CrossRef]
- Bradbury, E.J.; Burnside, E.R. Moving beyond the glial scar for spinal cord repair. Nat. Commun. 2019, 10, 3879. [Google Scholar] [PubMed]
- Haggerty, A.E.; Maldonado-Lasunción, I.; Oudega, M. Biomaterials for revascularization and immunomodulation after spinal cord injury. Biomed. Mater. 2018, 13, 044105. [Google Scholar] [CrossRef]
- Tsintou, M.; Dalamagkas, K.; Seifalian, A.M. Advances in regenerative therapies for spinal cord injury: A biomaterials approach. Neural Regen. Res. 2015, 10, 726–742. [Google Scholar]
- Straley, K.S.; Foo, C.W.P.; Heilshorn, S.C. Biomaterial design strategies for the treatment of spinal cord injuries. J. Neurotrauma 2010, 27, 1–19. [Google Scholar] [CrossRef]
- Schaub, N.J.; Johnson, C.D.; Cooper, B.; Gilbert, R.J. Electrospun fibers for spinal cord injury research and regeneration. J. Neurotrauma 2016, 33, 1405–1415. [Google Scholar] [CrossRef]
- Zuidema, J.M.; Gilbert, R.J.; Gottipati, M.K. Biomaterial approaches to modulate reactive astroglial response. Cells Tissues Organs 2018, 205, 372–395. [Google Scholar] [CrossRef]
- Rooney, G.E.; Vaishya, S.; Ameenuddin, S.; Currier, B.L.; Schiefer, T.K.; Knight, A.; Chen, B.; Mishra, P.K.; Spinner, R.J.; Macura, S.I. Rigid fixation of the spinal column improves scaffold alignment and prevents scoliosis in the transected rat spinal cord. Spine 2008, 33, 914–919. [Google Scholar]
- Wang, J.; Zheng, J.; Zheng, Q.; Wu, Y.; Wu, B.; Huang, S.; Fang, W.; Guo, X. FGL-functionalized self-assembling nanofiber hydrogel as a scaffold for spinal cord-derived neural stem cells. Mater. Sci. Eng. C Mater. Biol. Appl. 2015, 46, 140–147. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Xie, Y.Y.; Wang, B. Role and prospects of regenerative biomaterials in the repair of spinal cord injury. Neural Regen. Res. 2019, 14, 1352–1363. [Google Scholar] [PubMed]
- Tabesh, H.; Amoabediny, G.; Nik, N.S.; Heydari, M.; Yosefifard, M.; Siadat, S.O.; Mottaghy, K. The role of biodegradable engineered scaffolds seeded with Schwann cells for spinal cord regeneration. Neurochem. Int. 2009, 54, 73–83. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Mo, L.; Duan, H.; Li, X. Effects of chitosan/collagen substrates on the behavior of rat neural stem cells. Sci. China Life Sci. 2010, 53, 215–222. [Google Scholar] [CrossRef]
- Deumens, R.; Bozkurt, A.; Meek, M.F.; Marcus, M.A.E.; Joosten, E.A.J.; Weis, J.; Brook, G.A. Repairing injured peripheral nerves: Bridging the gap. Prog. Neurobiol. 2010, 92, 245–276. [Google Scholar] [CrossRef]
- Schoof, H.; Apel, J.; Heschel, I.; Rau, G. Control of pore structure and size in freeze-dried collagen sponges. J. Biomed. Mater. Res. 2001, 58, 352–357. [Google Scholar] [CrossRef]
- Kourgiantaki, A.; Tzeranis, D.S.; Karali, K.; Georgelou, K.; Bampoula, E.; Psilodimitrakopoulos, S.; Yannas, I.V.; Stratakis, E.; Sidiropoulou, K.; Charalampopoulos, I.; et al. Neural stem cell delivery via porous collagen scaffolds promotes neuronal differentiation and locomotion recovery in spinal cord injury. NPJ Regen. Med. 2020, 5, 12. [Google Scholar] [CrossRef]
- Altinova, H.; Mollers, S.; Fuhrmann, T.; Deumens, R.; Bozkurt, A.; Heschel, I.; Damink, L.H.H.O.; Schügner, F.; Weis, J.; Brook, G.A. Functional improvement following implantation of a microstructured, type-I collagen scaffold into experimental injuries of the adult rat spinal cord. Brain Res. 2014, 1585, 37–50. [Google Scholar] [CrossRef]
- Breen, B.A.; Kraskiewicz, H.; Ronan, R.; Kshirsagar, A.; Patar, A.; Sargeant, T.; Pandit, A.; Mcmahon, S.S. Therapeutic effect of neurotrophin-3 treatment in an injectable collagen scaffold following rat spinal cord hemisection injury. Acs. Biomater. Sci. Eng. 2016, 3, 1287. [Google Scholar]
- Vidal, B.C.; Mello, M.L. Collagen type I amide I band infrared spectroscopy. Micron 2011, 42, 283–289. [Google Scholar] [CrossRef]
- Silva, Z.S.J.; Botta, S.B.; Ana, P.A.; França, C.M.; Fernandes, K.P.S.; Mesquita, R.A.F.; Deana, A.; Bussadori, S.K. Effect of papain-based gel on type I collagen-spectroscopy applied for microstructural analysis. Sci. Rep. 2015, 5, 11448. [Google Scholar]
- Zhang, Y.; Liu, X.; Zeng, L.; Zhang, J.; Zuo, J.; Zou, J.; Ding, J.; Chen, X. Polymer fiber scaffolds for bone and cartilage tissue engineering. Adv. Funct. Mater. 2019, 29, 1903279. [Google Scholar]
- Elango, J.; Robinson, J.; Zhang, J.; Bao, B.; Ma, N.; de Val, J.E.M.S.; Wu, W. Collagen peptide upregulates osteoblastogenesis from bone marrow mesenchymal stem cells through mapk-runx2. Cells 2019, 8, 446. [Google Scholar]
- Pomeraniec, L.; Benayahu, D. Mesenchymal cell growth and differentiation on a new biocomposite material: A promising model for regeneration therapy. Biomolecules 2020, 10, 458. [Google Scholar] [CrossRef]
- Bunnell, T.M.; Burbach, B.J.; Shimizu, Y.; Ervasti, J.M. β-actin specifically controls cell growth, migration, and the G-actin pool. Mol. Biol. Cell 2011, 22, 4047–4058. [Google Scholar] [CrossRef]
- Shi, G.; Jin, Y. Role of Oct4 in maintaining and regaining stem cell pluripotency. Stem Cell Res. Ther. 2010, 1, 39. [Google Scholar]
- O’Brien, F.J. Biomaterials & scaffolds for tissue engineering. Mater. Today 2011, 14, 88–95. [Google Scholar]
- Al-Chalabi, A.; Miller, C.C.J. Neurofilaments and neurological disease. BioEssays 2003, 25, 346–355. [Google Scholar]
- Hulme, C.H.; Brown, S.J.; Fuller, H.R.; Riddell, J.; Osman, A.; Chowdhury, J.; Kumar, N.; Johnson, W.E.; Wright, K.T. The developing landscape of diagnostic and prognostic biomarkers for spinal cord injury in cerebrospinal fluid and blood. Spinal Cord 2017, 55, 114–125. [Google Scholar]
- Yokobori, S.; Zhang, Z.; Moghieb, A.; Mondello, S.; Gajavelli, S.; Dietrich, W.D.; Bramlett, H.; Hayes, R.L.; Wang, M.; Wang, K.K.W.; et al. Acute diagnostic biomarkers for spinal cord injury: Review of the literature and preliminary research report. World Neurosurg. 2015, 83, 867–878. [Google Scholar]
- Faulkner, J.R.; Herrmann, J.E.; Woo, M.J.; Tansey, K.E.; Doan, N.B.; Sofroniew, M.V. Reactive astrocytes protect tissue and preserve function after spinal cord injury. J. Neurosci. 2004, 24, 2143–2155. [Google Scholar] [CrossRef] [PubMed]
- Brenner, M. Role of GFAP in CNS injuries. Neurosci. Lett. 2014, 565, 7–13. [Google Scholar] [CrossRef]
- Lin, C.Y.; Lee, Y.S.; Lin, V.W.; Silver, J.S. Fibronectin inhibits chronic pain development after spinal cord injury. J. Neurotrauma 2012, 29, 589–599. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Yin, L.; Chen, Z. Neuroprotective role of fibronectin in neuron-glial extrasynaptic transmission. Neural Regen. Res. 2013, 8, 376–382. [Google Scholar] [PubMed]
- King, V.R.; Henseler, M.; Brown, R.A.; Priestley, J.V. Mats made from fibronectin support oriented growth of axons in the damaged spinal cord of the adult rat. Exp. Neurol. 2003, 182, 383–398. [Google Scholar] [CrossRef]
- Taylor, S.J.; McDonald, J.W.; Sakiyama, S.E.E. Controlled release of neurotrophin-3 from fibrin gels for spinal cord injury. J. Control. Release 2004, 98, 281–294. [Google Scholar] [CrossRef]
- Silver, J.; Miller, J.H. Regeneration beyond the glial scar. Nat. Rev. Neurosci. 2004, 5, 146–156. [Google Scholar] [CrossRef]



© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yeh, J.-Z.; Wang, D.-H.; Cherng, J.-H.; Wang, Y.-W.; Fan, G.-Y.; Liou, N.-H.; Liu, J.-C.; Chou, C.-H. A Collagen-Based Scaffold for Promoting Neural Plasticity in a Rat Model of Spinal Cord Injury. Polymers 2020, 12, 2245. https://doi.org/10.3390/polym12102245
Yeh J-Z, Wang D-H, Cherng J-H, Wang Y-W, Fan G-Y, Liou N-H, Liu J-C, Chou C-H. A Collagen-Based Scaffold for Promoting Neural Plasticity in a Rat Model of Spinal Cord Injury. Polymers. 2020; 12(10):2245. https://doi.org/10.3390/polym12102245
Chicago/Turabian StyleYeh, Jue-Zong, Ding-Han Wang, Juin-Hong Cherng, Yi-Wen Wang, Gang-Yi Fan, Nien-Hsien Liou, Jiang-Chuan Liu, and Chung-Hsing Chou. 2020. "A Collagen-Based Scaffold for Promoting Neural Plasticity in a Rat Model of Spinal Cord Injury" Polymers 12, no. 10: 2245. https://doi.org/10.3390/polym12102245
APA StyleYeh, J.-Z., Wang, D.-H., Cherng, J.-H., Wang, Y.-W., Fan, G.-Y., Liou, N.-H., Liu, J.-C., & Chou, C.-H. (2020). A Collagen-Based Scaffold for Promoting Neural Plasticity in a Rat Model of Spinal Cord Injury. Polymers, 12(10), 2245. https://doi.org/10.3390/polym12102245