CuS@Corn Stalk/Chitin Composite Hydrogel for Photodegradation and Antibacterial
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of CuS@Corn Stalk/Chitin Composite Hydrogel
2.3. Characterization of CuS@corn Stalk/Chitin Composite Hydrogel
2.4. Peroxidase-Like Activity of CuS@Corn Stalk/Chitin Composite Hydrogel
2.5. Photocatalytic Activity of CuS@Corn Stalk/Chitin Composite Hydrogel
2.6. Antibacterial Experiments
3. Results and Discussion
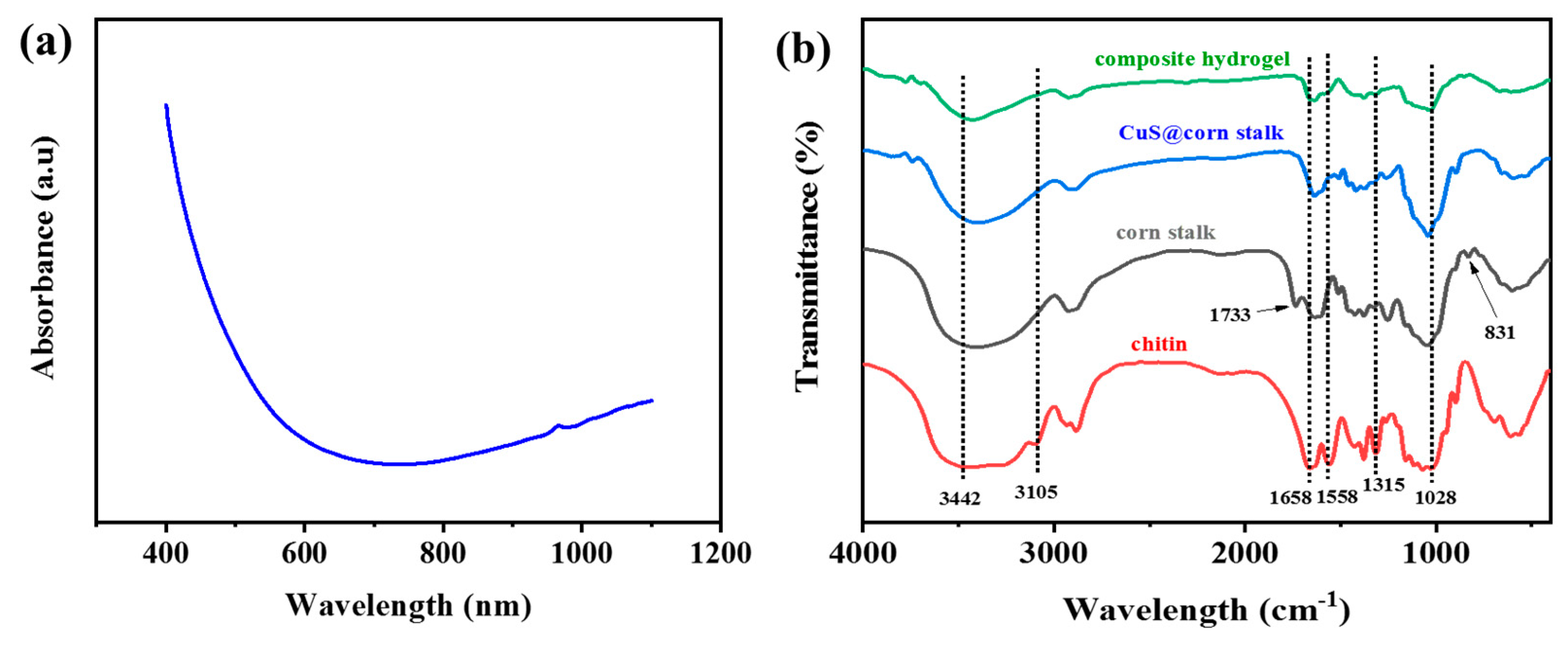
3.1. Structure and Morphology of CuS@Corn Stalk/Chitin Composite Hydrogel
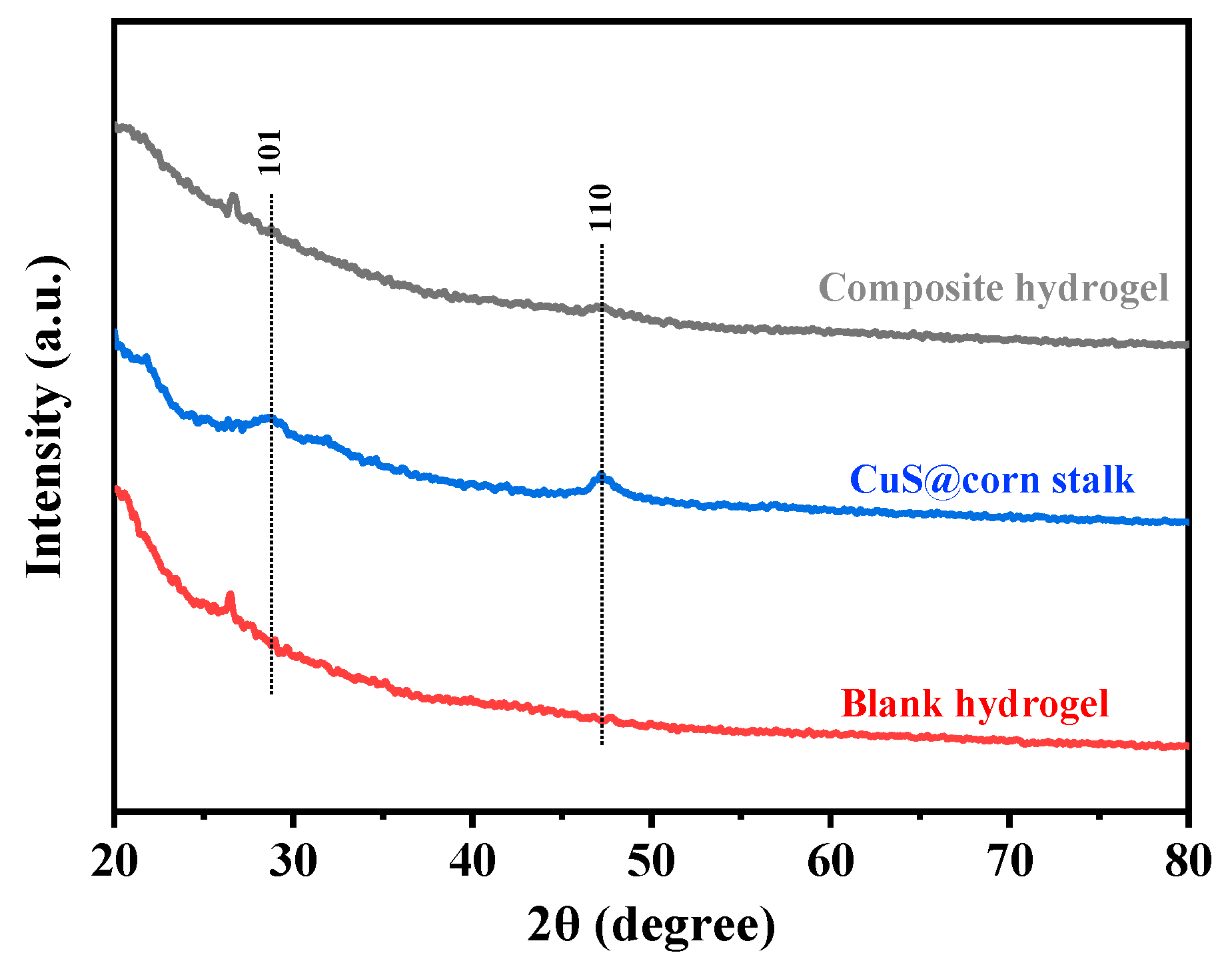
3.2. XRD Patterns of CuS@Corn Stalk/Chitin Composite Hydrogel
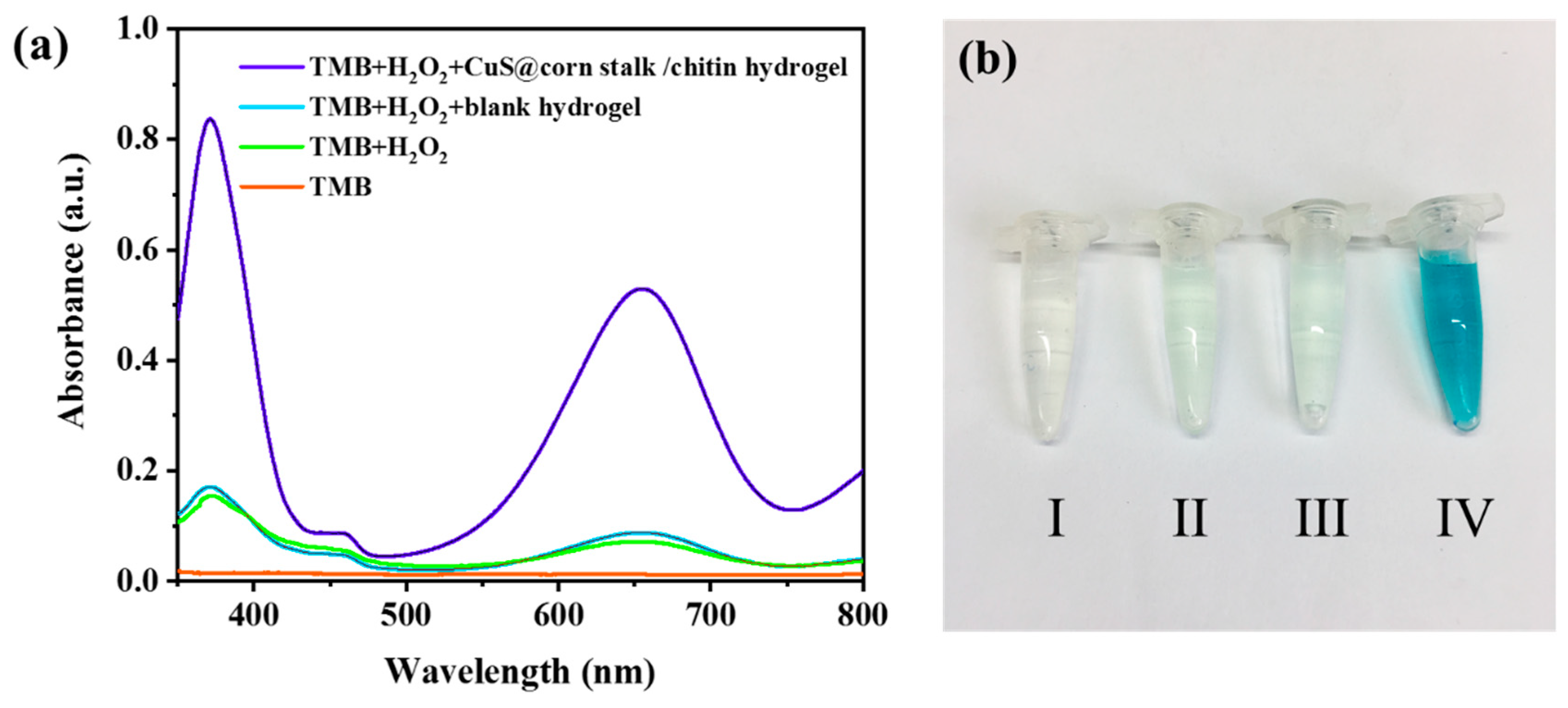
3.3. Peroxidase-Like Activity of CuS@Corn Stalk/Chitin Composite Hydrogel
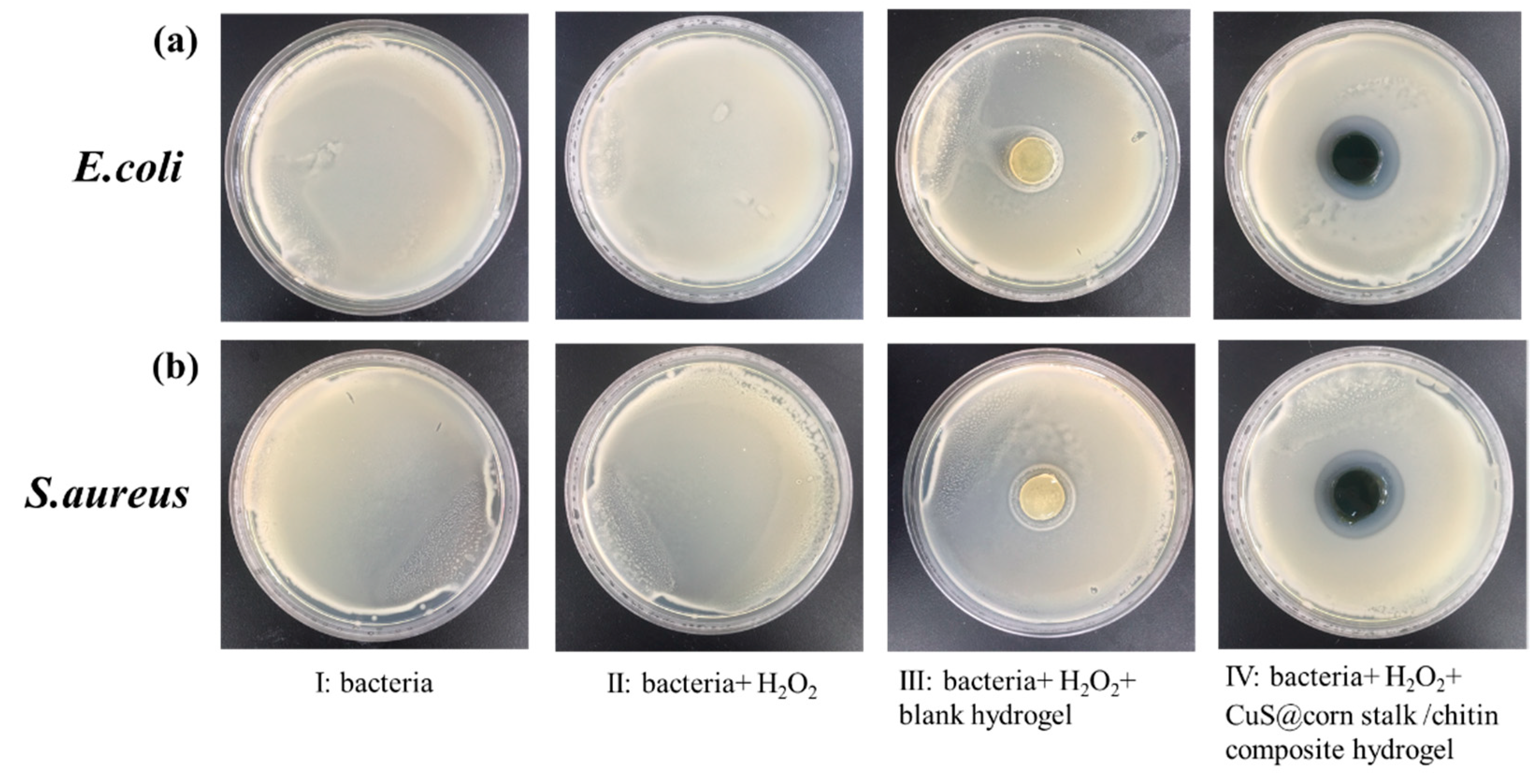
3.4. Antibacterial Study of CuS@Corn Stalk/Chitin Composite Hydrogel
3.5. Photocatalytic Activity of CuS@Corn Stalk/Chitin Composite Hydrogel
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Goel, S.; Chen, F.; Cai, W.B. Synthesis and Biomedical Applications of Copper Sulfide Nanoparticles: From Sensors to Theranostics. Small 2014, 10, 631–645. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.B.; Lu, W.; Huang, Q.A.; Huang, M.A.; Li, C.; Chen, W. Copper sulfide nanoparticles for photothermal ablation of tumor cells. Nanomed. UK 2010, 5, 1161–1171. [Google Scholar] [CrossRef] [PubMed]
- Jiao, S.H.; Xu, L.F.; Jiang, K.; Xu, D.S. Well-defined non-spherical copper sulfide mesocages with single-crystalline shells by shape-controlled Cu2O crystal templating. Adv. Mater. 2006, 18, 1174–1177. [Google Scholar] [CrossRef]
- Yang, Z.K.; Song, L.X.; Teng, Y.; Xia, J. Ethylenediamine-modulated synthesis of highly monodisperse copper sulfide microflowers with excellent photocatalytic performance. J. Mater. Chem. A 2014, 2, 20004–20009. [Google Scholar] [CrossRef]
- Ramadan, S.; Guo, L.R.; Li, Y.J.; Yan, B.F.; Lu, W. Hollow Copper Sulfide Nanoparticle-Mediated Transdermal Drug Delivery. Small 2012, 8, 3143–3150. [Google Scholar] [CrossRef] [PubMed]
- Ku, G.; Zhou, M.; Song, S.L.; Huang, Q.; Hazle, J.; Li, C. Copper Sulfide Nanoparticles As a New Class of Photoacoustic Contrast Agent for Deep Tissue Imaging at 1064 nm. ACS Nano 2012, 6, 7489–7496. [Google Scholar] [CrossRef] [PubMed]
- Pouretedal, H.R.; Norozi, A.; Keshavarz, M.H.; Semnani, A. Nanoparticles of zinc sulfide doped with manganese, nickel and copper as nanophotocatalyst in the degradation of organic dyes. J. Hazard. Mater. 2009, 162, 674–681. [Google Scholar] [CrossRef]
- Xu, B.; Wang, H.; Wang, W.; Gao, L.; Li, S.; Pan, X.; Wang, H.; Yang, H.; Meng, X.; Wu, Q.; et al. A Single-Atom Nanozyme for Wound Disinfection Applications. Angew. Chem. Int. Ed. Engl. 2019, 58, 4911–4916. [Google Scholar] [CrossRef]
- Huang, X.J.; Li, X.Y.; Li, Y.C.; Wang, X.Y. Biopolymer as Stabilizer and Adhesive To in Situ Precipitate CuS Nanocrystals on Cellulose Nanofibers for Preparing Multifunctional Composite Papers. ACS Omega 2018, 3, 8083–8090. [Google Scholar] [CrossRef]
- Peng, H.; Yang, A.S.; Xiong, J.H. Green, microwave-assisted synthesis of silver nanoparticles using bamboo hemicelluloses and glucose in an aqueous medium. Carbohydr. Polym. 2013, 91, 348–355. [Google Scholar] [CrossRef]
- Huang, X.J.; Hu, N.; Wang, X.Y.; Zhang, Y.S.; Sun, R.C. Copper Sulfide Nanoparticle/Cellulose Composite Paper: Room-Temperature Green Fabrication for NIR Laser-Inducible Ablation of Pathogenic Microorganisms. ACS Sustain. Chem. Eng. 2017, 5, 2648–2655. [Google Scholar] [CrossRef]
- Raveendran, P.; Fu, J.; Wallen, S.L. Completely “green” synthesis and stabilization of metal nanoparticles. J. Am. Chem. Soc. 2003, 125, 13940–13941. [Google Scholar] [CrossRef]
- Huang, X.J.; Xu, C.L.; Li, Y.C.; Cheng, H.B.; Wang, X.Y.; Sun, R.C. Quaternized chitosan-stabilized copper sulfide nanoparticles for cancer therapy. Mater. Sci. Eng. C Mater. 2019, 96, 129–137. [Google Scholar] [CrossRef]
- Behera, S.; Arora, R.; Nandhagopal, N.; Kumar, S. Importance of chemical pretreatment for bioconversion of lignocellulosic biomass. Renew. Sustain. Energy Rev. 2014, 36, 91–106. [Google Scholar] [CrossRef]
- Johnson, A.R.; McQueen, T.M.; Rodolfa, K.T. Species distribution diagrams in the copper-ammonia system: An updated and expanded demonstration illustrating complex equilibria. J. Chem. Educ. 2005, 82, 408–414. [Google Scholar] [CrossRef]
- Zhang, P.; Gao, L. Copper sulfide flakes and nanodisks. J. Mater. Chem. 2003, 13, 2007–2010. [Google Scholar] [CrossRef]
- Cardenas, G.; Cabrera, G.; Taboada, E.; Miranda, S.P. Chitin characterization by SEM, FTIR, XRD, and C-13 cross polarization/mass angle spinning NMR. J. Appl. Polym. Sci. 2004, 93, 1876–1885. [Google Scholar] [CrossRef]
- Zhao, X.Y.; Chen, J.; Chen, F.L.; Wang, X.C.; Zhu, Q.J.; Ao, Q. Surface characterization of corn stalk superfine powder studied by FTIR and XRD. Colloid Surf. B 2013, 104, 207–212. [Google Scholar] [CrossRef]
- Chang, C.Y.; Lue, A.; Zhang, L. Effects of crosslinking methods on structure and properties of cellulose/PVA hydrogels. Macromol. Chem. Phys. 2008, 209, 1266–1273. [Google Scholar] [CrossRef]
- Dutta, A.; Dolui, S.K. Preparation of colloidal dispersion of CuS nanoparticles stabilized by SDS. Mater. Chem. Phys. 2008, 112, 448–452. [Google Scholar] [CrossRef]
- Ji, H.W.; Dong, K.; Yan, Z.Q.; Ding, C.; Chen, Z.W.; Ren, J.S.; Qu, X.G. Bacterial Hyaluronidase Self-Triggered Prodrug Release for Chemo-Photothermal Synergistic Treatment of Bacterial Infection. Small 2016, 12, 6200–6206. [Google Scholar] [CrossRef]
- Darabdhara, G.; Sharma, B.; Das, M.R.; Boukherroub, R.; Szunerits, S. Cu-Ag bimetallic nanoparticles on reduced graphene oxide nanosheets as peroxidase mimic for glucose and ascorbic acid detection. Sens. Actuators B Chem. 2017, 238, 842–851. [Google Scholar] [CrossRef]



© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xiong, Y.; Luo, B.; Chen, G.; Cai, J.; Jiang, Q.; Gu, B.; Wang, X. CuS@Corn Stalk/Chitin Composite Hydrogel for Photodegradation and Antibacterial. Polymers 2019, 11, 1393. https://doi.org/10.3390/polym11091393
Xiong Y, Luo B, Chen G, Cai J, Jiang Q, Gu B, Wang X. CuS@Corn Stalk/Chitin Composite Hydrogel for Photodegradation and Antibacterial. Polymers. 2019; 11(9):1393. https://doi.org/10.3390/polym11091393
Chicago/Turabian StyleXiong, Yutong, Bichong Luo, Guixin Chen, Jihai Cai, Qimeng Jiang, Bin Gu, and Xiaoying Wang. 2019. "CuS@Corn Stalk/Chitin Composite Hydrogel for Photodegradation and Antibacterial" Polymers 11, no. 9: 1393. https://doi.org/10.3390/polym11091393
APA StyleXiong, Y., Luo, B., Chen, G., Cai, J., Jiang, Q., Gu, B., & Wang, X. (2019). CuS@Corn Stalk/Chitin Composite Hydrogel for Photodegradation and Antibacterial. Polymers, 11(9), 1393. https://doi.org/10.3390/polym11091393





