The Use of Lanthanum Ions and Chitosan for Boron Elimination from Aqueous Solutions
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Analysis and Apparatus
2.3. Preparation of the La-CTS Hydrogel Beads
2.4. Adsorption and Desorption Methodology
2.5. The Procedure of pHpzc Determination
3. Results
3.1. Calculations
3.2. La-CTS Hydrogel Beads Characteristic
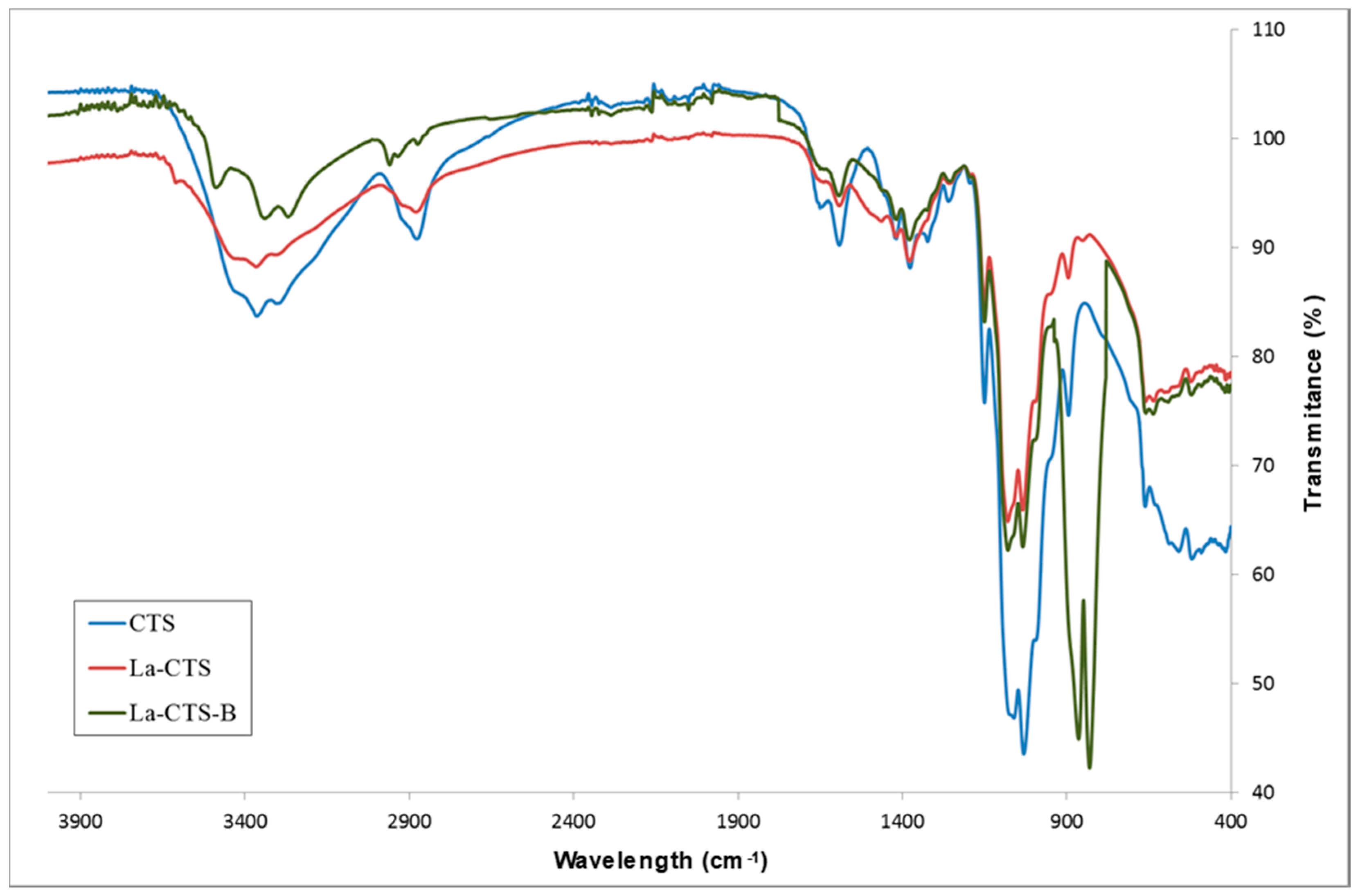
3.2.1. Fourier-Transform Infrared Spectrometry
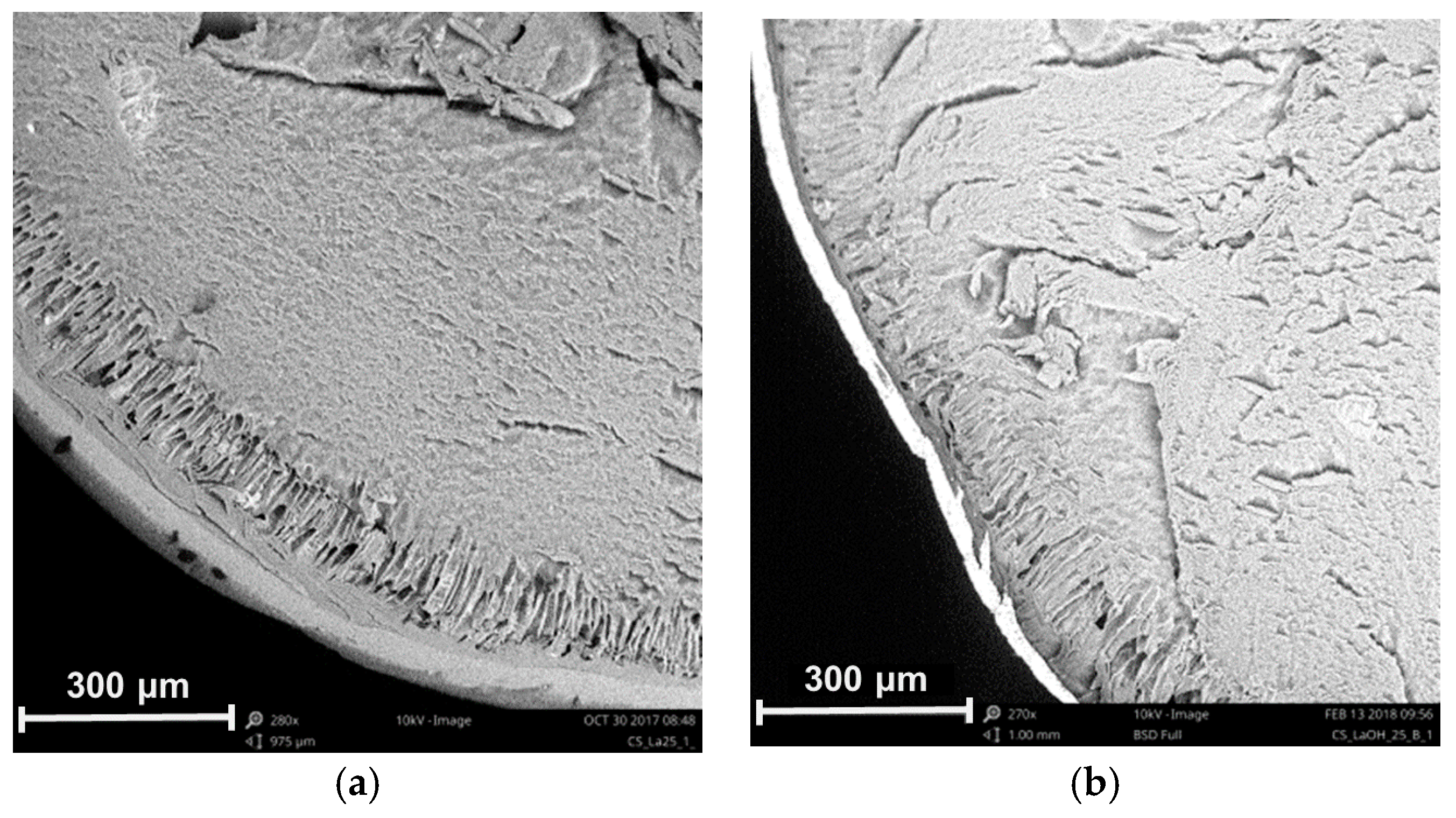
3.2.2. Scanning Electron Microscopy
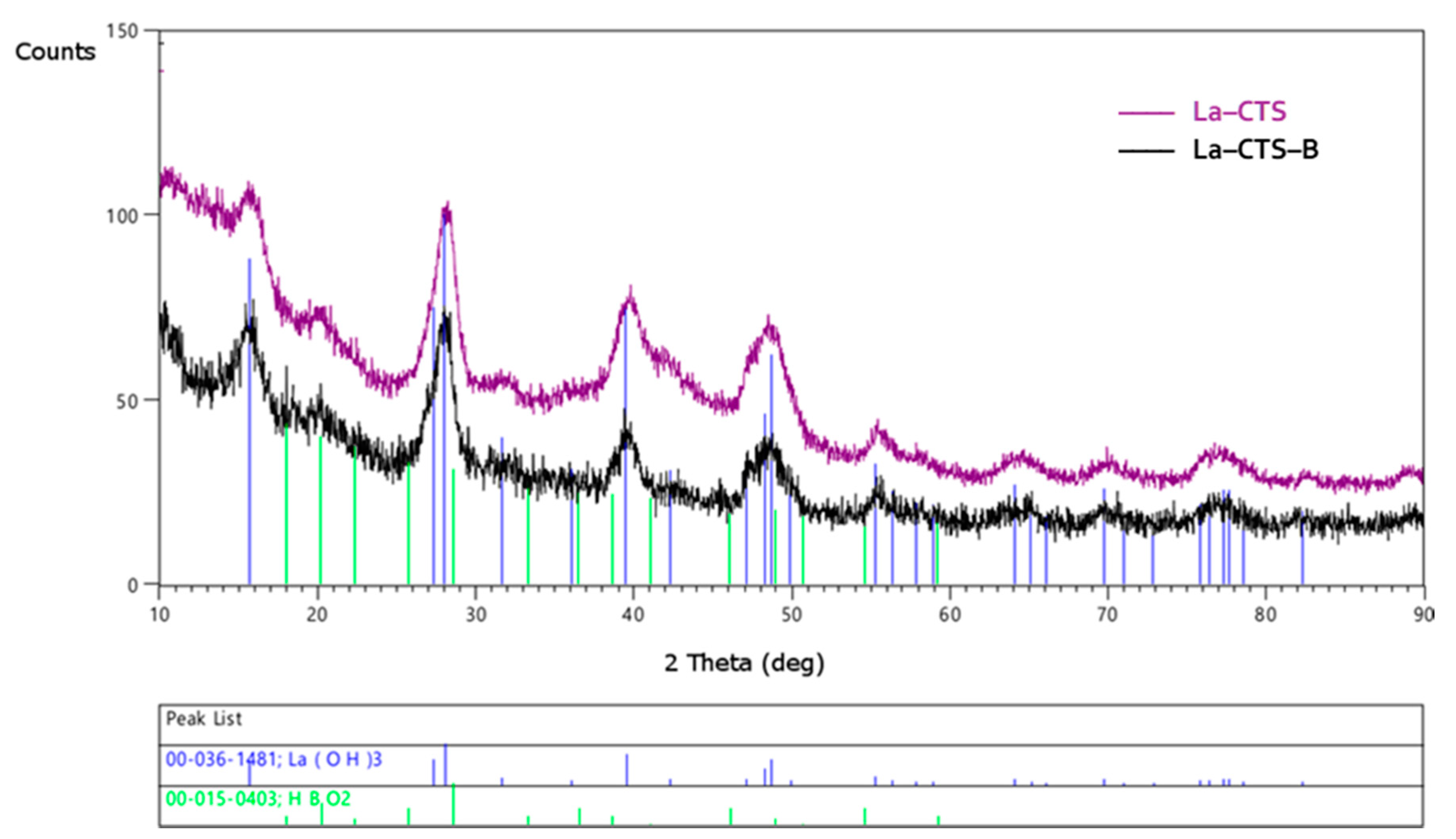
3.2.3. X-ray Diffractometry
3.2.4. Inductively Coupled Plasma Optical Emission Spectrometry
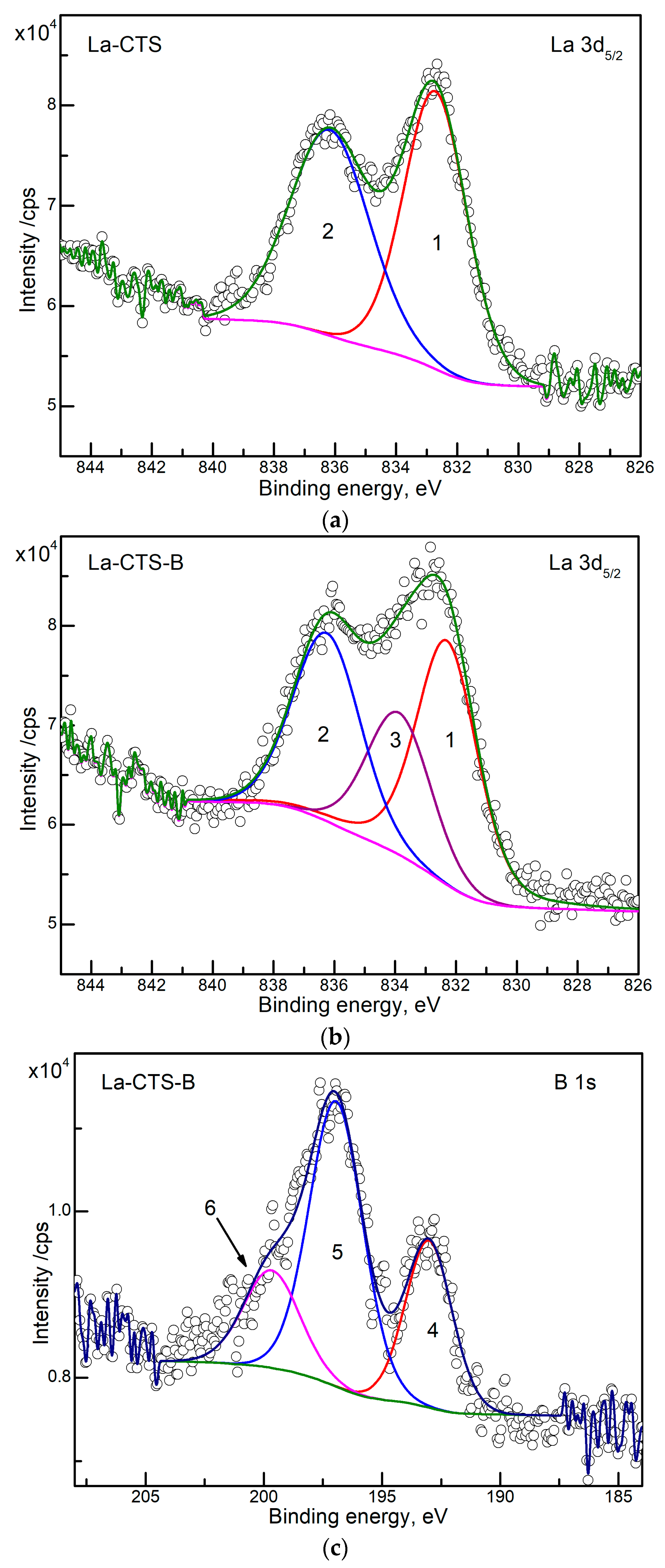
3.2.5. X-ray Photoelectron Spectroscopy
3.2.6. Point of Zero Charge Determination
3.3. The Influence of pH on Boron Adsorption
3.4. Kinetic Studies
3.5. Isotherm Models
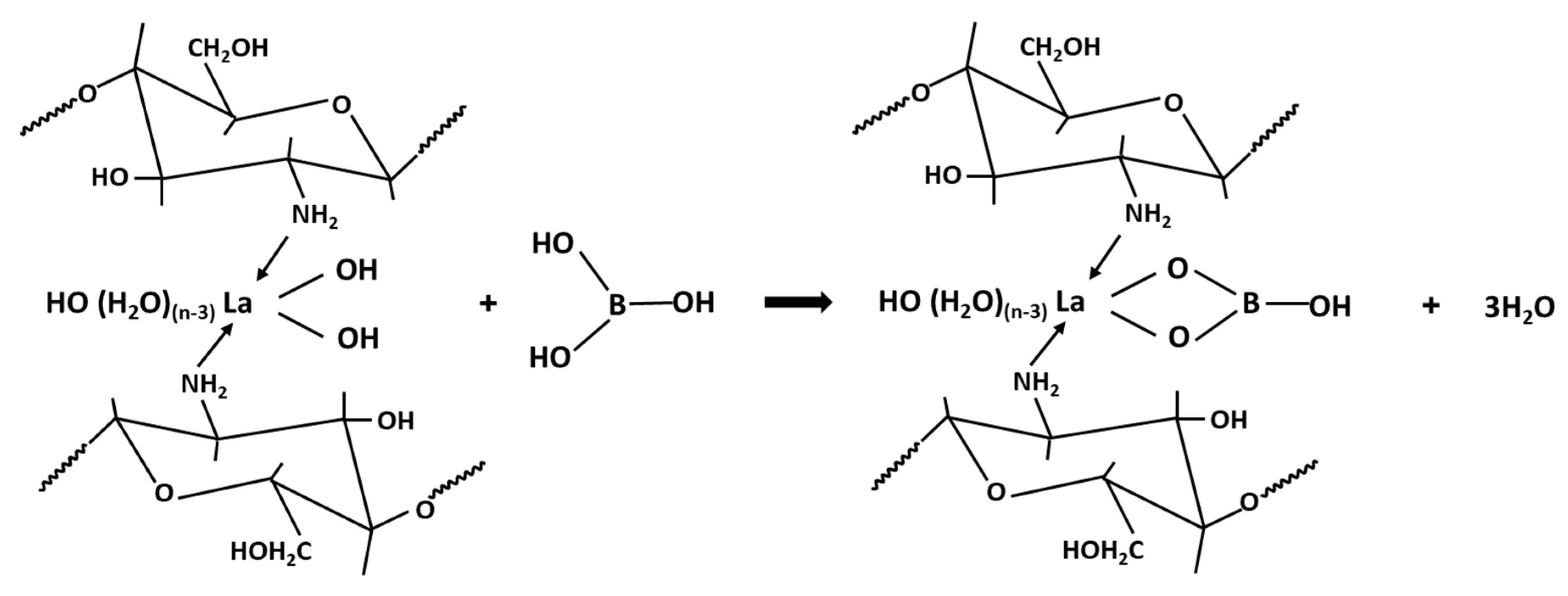
3.6. Mechanism of La-CTS Formation and Boron Adsorption
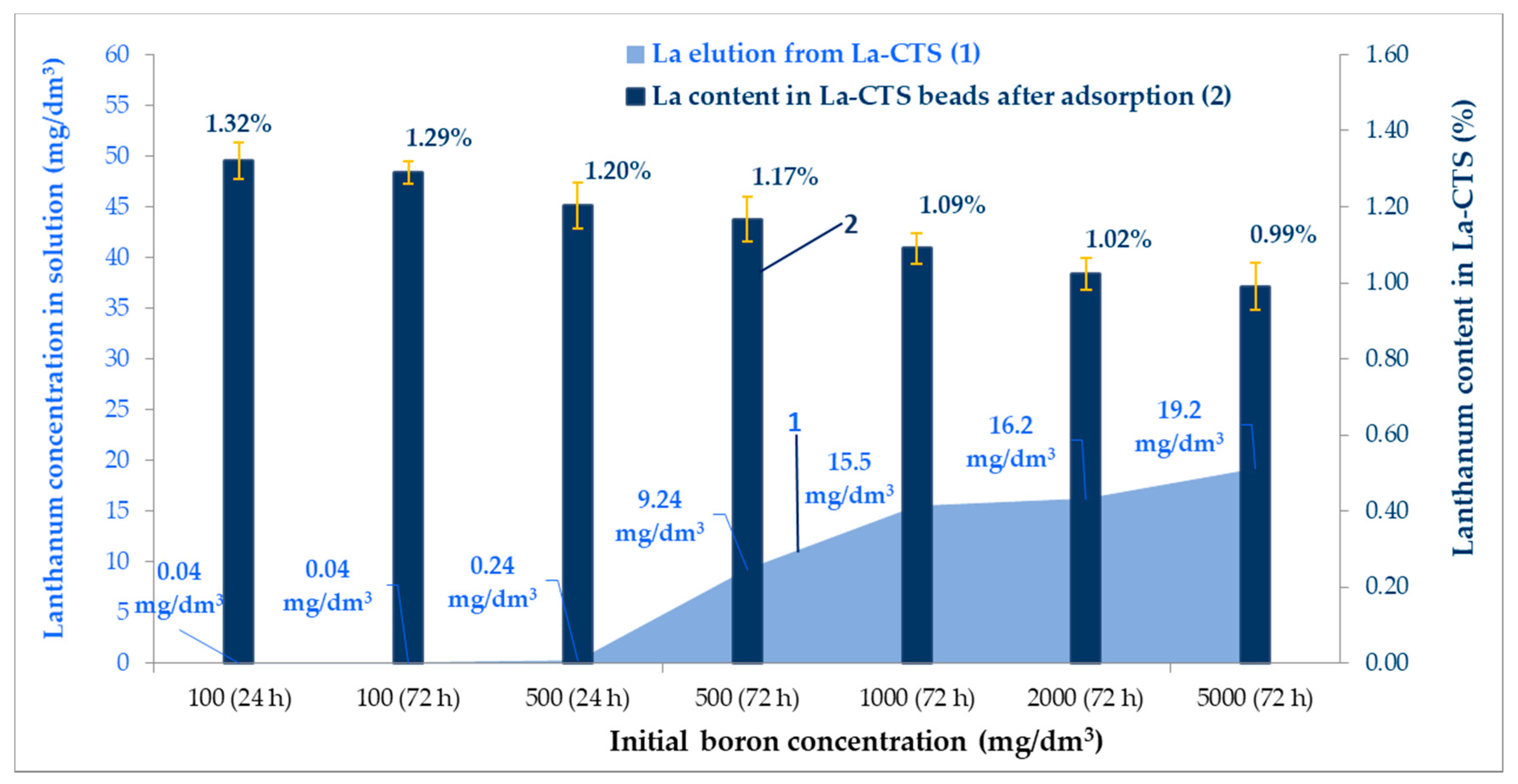
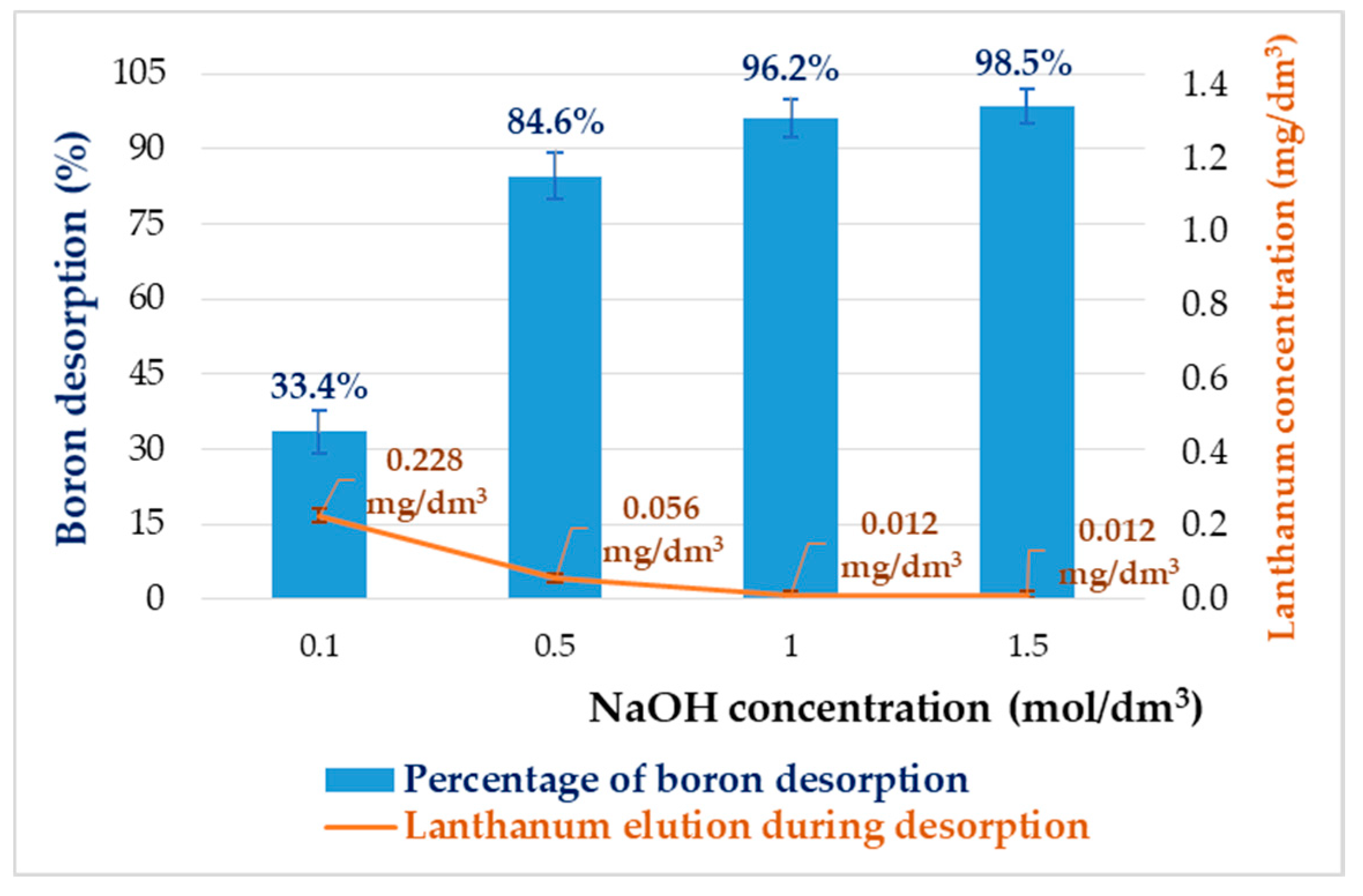
3.7. The Stability of the La-CTS and Desorption Tests
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| CTS | Chitosan |
| La-CTS | Chitosan attached with lanthanum(III) |
| La-CTS-B | Chitosan attached with lanthanum(III) after boron adsorption |
| FTIR | Fourier-transform infrared spectrometry |
| SEM | Scanning electron microscopy |
| XRD | X-ray diffractometry |
| ICP-OES | Inductively coupled plasma optical emission spectrometry |
| XPS | X-ray photoelectron spectroscopy |
| PFO model | Pseudo-first-order kinetic model |
| PSO model | Pseudo-second-order kinetic model |
References
- Kabay, N.; Bryjak, M. Boron Separation Processes; Elsevier B.V.: Amsterdam, The Netherlands, 2015. [Google Scholar]
- Kabata-Pendias, A.; Pendias, H. Biogeochemistry of Trace Elements; PWN: Warsaw, Poland, 1999. [Google Scholar]
- Drinking-Water Quality Committee. Guidelines for Drinking-Water Quality, 4th ed.; World Health Organization: Geneve, Switzerland, 2011. [Google Scholar]
- Regulation of the Polish Minister of the Environment of 18 November 2014 on conditions to be met when introducing sewage into waters or into the ground, and on substances particularly harmful to the aquatic environment. Dziennik Ustaw. 1800. 2014.
- Kluczka, J.; Trojanowska, J.; Zolotajkin, M. Utilization of fly ash zeolite for boron removal from aqueous solution. Desalinat. Water Treat. 2015, 54, 1839–1849. [Google Scholar] [CrossRef]
- Tagliabue, M.; Reverberi, A.; Bagatin, R. Boron removal from water: Needs, challenges and perspectives. J. Clean. Product. 2014, 77, 56–64. [Google Scholar] [CrossRef]
- Wolska, J.; Bryjak, M. Methods for boron removal from aqueous solutions—A review. Desalination 2013, 310, 18–24. [Google Scholar] [CrossRef]
- Nasef, M.; Nallappan, M.; Ujang, Z. Polymer-based chelating adsorbents for the selective removal of boron from water and wastewater: A review. React. Funct. Polym. 2014, 85, 54–68. [Google Scholar] [CrossRef]
- Guan, Z.; Lv, J.; Bai, P.; Guo, X. Boron removal from aqueous solutions by adsorption—A review. Desalination 2016, 383, 29–37. [Google Scholar] [CrossRef]
- Tang, Y.P.; Luo, L.; Thong, Z.W.; Chung, T.S. Recent advances in membrane materials and technologies for boron removal. J. Membr. Sci. 2017, 541, 434–446. [Google Scholar] [CrossRef]
- Liu, S.; Xu, M.; Yu, T.; Han, D.; Peng, J.; Li, J.; Zhai, M. Radiation synthesis and performance of novel cellulose-based microsphere adsorbents for efficient removal of boron (III). Carbohydr. Polym. 2017, 174, 273–281. [Google Scholar] [CrossRef] [PubMed]
- Jakobik-Kolon, A.; Bok-Badura, J.; Milewski, A.; Karon, K. Long term and large-scale continuous studies on Zinc(II) sorption and desorption on hybrid pectin-guar gum biosorbent. Polymers 2019, 11, 96. [Google Scholar] [CrossRef]
- Bertagnolli, C.; Grishin, A.; Vincent, T.; Guibal, E. Boron removal by a composite sorbent: Polyethylenimine/tannic acid derivative immobilized in alginate hydrogel beads. J. Environ. Sci. Health Part A-Toxic/Hazard. Subst. Environ. Eng. 2017, 52, 359–367. [Google Scholar] [CrossRef]
- Wang, J.; Zhuang, S. Removal of various pollutants from water and wastewater by modified chitosan adsorbents. Crit. Rev. Environ. Sci. Technol. 2017, 47, 2331–2386. [Google Scholar] [CrossRef]
- Elwakeel, K. Environmental application of chitosan resins for the treatment of water and wastewater: A review. J. Dispers. Sci. Technol. 2010, 31, 273–288. [Google Scholar] [CrossRef]
- Ali, A.; Ahmed, S. A review on chitosan and its nanocomposites in drug delivery. Int. J. Biol. Macromol. 2018, 109, 273–286. [Google Scholar] [CrossRef]
- Aycan, D.; Alemdar, N. Development of pH-responsive chitosan-based hydrogel modified with bone ash for controlled release of amoxicillin. Carbohydr. Polym. 2018, 184, 401–407. [Google Scholar] [CrossRef]
- Hamedi, H.; Moradi, S.; Hudson, S.M.; Tonelli, A.E. Chitosan based hydrogels and their applications for drug delivery in wound dressings: A review. Carbohydr. Polym. 2018, 199, 445–460. [Google Scholar] [CrossRef]
- Figiela, M.; Wysokowski, M.; Galiński, M.; Jesionowski, T.; Stępniak, I. Synthesis and characterization of novel copper oxide-chitosan nanocomposites for non-enzymatic glucose sensing. Sens. Actuators B Chem. 2018, 272, 296–307. [Google Scholar] [CrossRef]
- Pakdel, P.; Peighambardoust, S. Review on recent progress in chitosan-based hydrogels for wastewater treatment application. Carbohydr. Polym. 2018, 201, 264–279. [Google Scholar] [CrossRef]
- Geissen, V.; Mol, H.; Klumpp, E.; Umlauf, G.; Nadal, M.; van der Ploeg, M.; van de Zee, S.E.A.T.M.; Ritsema, C.J. Emerging pollutants in the environment: A challenge for water resource management. Int. Soil Water Conserv. Res. 2015, 3, 57–65. [Google Scholar] [CrossRef]
- Azarova, Y.A.; Pestov, A.V.; Bratskaya, S.Y. Application of chitosan and its derivatives for solid-phase extraction of metal and metalloid ions: a mini-review. Cellulose 2016, 23, 2273–2289. [Google Scholar] [CrossRef]
- Matsumoto, M.; Matsui, T.; Kondo, K. Adsorption mechanism of boric acid on chitosan resin modified by saccharides. J. Chem. Eng. Jpn. 1999, 32, 190–196. [Google Scholar] [CrossRef]
- Harada, A.; Takagi, T.; Kataoka, S.; Yamamoto, T.; Endo, A. Boron adsorption mechanism on polyvinyl alcohol. J. Int. Adsorpt. Soc. 2011, 17, 171–178. [Google Scholar] [CrossRef]
- Oladipo, A.; Gazi, M. Hydroxyl-enhanced magnetic chitosan microbeads for boron adsorption: Parameter optimization and selectivity in saline water. React. Funct. Polym. 2016, 109, 23–32. [Google Scholar] [CrossRef]
- Krzesinska, M.; Torz, A.; Zachariasz, J.; Muszynski, J.; Socha, J.; Marcinkowski, A. New chitosan/CEG (compressed expanded graphite) composites preparation and physical properties. Green Chem. 2007, 9, 842–844. [Google Scholar] [CrossRef]
- Demey, H.; Vincent, T.; Ruiz, M.; Sastre, A.; Guibal, E. Development of a new chitosan/Ni(OH)(2)-based sorbent for boron removal. Chem. Eng. J. 2014, 244, 576–586. [Google Scholar] [CrossRef]
- Kluczka, J. Boron removal from aqueous solutions using an amorphous zirconium dioxide. Int. J. Environ. Res. 2015, 9, 711–720. [Google Scholar]
- Smolik, M.; Zolotajkin, M.; Kluczka, J. Distribution of trace amounts of impurities during manganese(II) sulfate crystallization at 20-degrees-c and 2-degrees-c. Pol. J. Chem. 1995, 69, 1322–1327. [Google Scholar]
- Kluczka, J.; Torz, A.; Lacka, D.; Kazek-Kesik, A.; Adamek, J. Boron Removal by Adsorption on Cobalt(II) Doped Chitosan Bio-composite. J. Polym. Environ. 2018, 26, 2039–2048. [Google Scholar] [CrossRef]
- Demetriou, A.; Pashalidis, I. Adsorption of boron on iron-oxide in aqueous solutions. Desalinat. Water Treat. 2012, 37, 315–320. [Google Scholar] [CrossRef]
- Demetriou, A.; Pashalidis, I.; Nicolaides, A.; Kumke, M. Surface mechanism of the boron adsorption on alumina in aqueous solutions. Desalinat. Water Treat. 2013, 51, 6130–6136. [Google Scholar] [CrossRef]
- Kluczka, J.; Gnus, M.; Dudek, G.; Turczyn, R. Removal of boron from aqueous solution by composite chitosan beads. Sep. Sci. Technol. 2017, 52, 1559–1571. [Google Scholar] [CrossRef]
- Neacsu, I.A.; Stoica, A.E.; Vasile, B.S.; Andronescu, E. Luminescent hydroxyapatite doped with rare earth elements for biomedical applications. Nanomaterials 2019, 9, 239. [Google Scholar] [CrossRef] [PubMed]
- Shuai, C.; Liu, L.; Yang, Y.; Gao, C.; Zhao, M.; Yi, L.; Peng, S. Lanthanum-containing magnesium alloy with antitumor function based on increased reactive oxygen species. Appl. Sci. 2018, 8, 2109. [Google Scholar] [CrossRef]
- Spears, B.M.; Lürling, M.; Yasseri, S.; Castro-Castellon, A.T.; Gibbs, M.; Meis, S.; McDonald, C.; McIntosh, J.; Sleep, D.; Van Oosterhout, F. Lake responses following lanthanum-modified bentonite clay (Phoslock®) application: An analysis of water column lanthanum data from 16 case study lakes. Water Res. 2013, 47, 5930–5942. [Google Scholar] [CrossRef]
- Wang, C.H.; Wu, Y.; Wang, Y.Q.; Bai, L.L.; Jiang, H.L.; Yu, J.H. Lanthanum-modified drinking water treatment residue for initial rapid and long-term equilibrium phosphorus immobilization to control eutrophication. Water Res. 2018, 137, 173–183. [Google Scholar] [CrossRef]
- Kamble, S.; Jagtap, S.; Labhsetwar, N.; Thakare, D.; Godfrey, S.; Devotta, S.; Rayalu, S. Defluoridation of drinking water using chitin, chitosan and lanthanum-modified chitosan. Chem. Eng. J. 2007, 129, 173–180. [Google Scholar] [CrossRef]
- Prabhu, S.; Elanchezhiyan, S.; Lee, G.; Meenakshi, S. Defluoridation of water by Tea-bag model using La3+ modified synthetic resin@chitosan biocomposite. Int. J. Biol. Macromol. 2016, 91, 1002–1009. [Google Scholar] [CrossRef]
- Prabhu, S.; Subaramanian, M.; Meenakshi, S. A simple one-pot in-situ method for the synthesis of aluminum and lanthanum binary oxyhydroxides in chitosan template towards defluoridation of water. Chem. Eng. J. 2016, 283, 1081–1089. [Google Scholar] [CrossRef]
- Preethi, J.; Meenakshi, S. Fabrication of La3+ impregnated chitosan/beta-cyclodextrin biopolymeric materials for effective utilization of chromate and fluoride adsorption in single systems. J. Chem. Eng. Data 2018, 63, 723–731. [Google Scholar] [CrossRef]
- Cheng, R.; Shen, L.; Zhang, Y.; Dai, D.; Zheng, X.; Liao, L.; Wang, L.; Shi, L. Enhanced phosphate removal from water by honeycomb-like microporous lanthanum-chitosan magnetic spheres. Water 2018, 10, 1659. [Google Scholar] [CrossRef]
- Elanchezhiyan, S.; Sivasurian, N.; Meenakshi, S. Efficacy of La3+ entrapped chitosan bio-polymeric matrix for the recovery of oil from oil-in-water emulsion. J. Appl. Polym. Sci. 2016, 133. [Google Scholar] [CrossRef]
- Beamson, G.; Briggs, D. High Resolution XPS of Organic Polymers; John Wiley & Sons: Chichester, UK, 1992. [Google Scholar]
- Balistrieri, L.S.; Murray, J.W. The surface chemistry of goethite-FeOOH in major ion seawater. Am. J. Sci. 1981, 281, 788–806. [Google Scholar] [CrossRef]
- Bansiwal, A.; Thakre, D.; Labhshetwar, N.; Meshram, S.; Rayalu, S. Fluoride removal using lanthanum incorporated chitosan beads. Colloids Surf. B Biointerfaces 2009, 74, 216–224. [Google Scholar] [CrossRef]
- Lee, S.; Jang, S.; Kang, J.; Sohn, Y. Luminescent Eu(III) and Tb(III) activator ions in La(OH)3 and La2O3 nanowire matrices. Mater. Sci. Eng. B Adv. Funct. Solid State Mater. 2015, 201, 35–44. [Google Scholar] [CrossRef]
- Jagtap, S.; Yenkie, M.; Das, S.; Rayalu, S. Synthesis and characterization of lanthanum impregnated chitosan flakes for fluoride removal in water. Desalination 2011, 273, 267–275. [Google Scholar] [CrossRef]
- Ranjan, M.; Srivastav, A.; Shaktibala, M. Effects of addition of cationic ligands in hydrous bismuth oxide on removal of fluoride from aqueous solutions. Curr. Sci. 2015, 108, 1673–1682. [Google Scholar]
- Available online: https://xpssimplified.com/elements/lanthanum.php/ (accessed on 5 April 2019).
- Wagner, C.D.; Naumkin, A.V.; Kraut-Vass, A.; Allison, J.W.; Powell, C.J.; Rumble, J.R., Jr. NIST Standard Reference Database 20, Version 3.4 (Web Version). Available online: http:/srdata.nist.gov/xps/ (accessed on 5 April 2019).
- Bodzek, M.; Konieczny, K. Usuwanie Zanieczyszczeń Nieorganicznych ze Środowiska Wodnego Metodami Membranowymi; Wydawnictwo Seidel-Przywecki Sp. z o.o.: Warszawa, Poland, 2011. [Google Scholar]
- Cotton, F.A.; Wilkinson, G.; Gaus, P.L. Basic Inorganic Chemistry; John Wiley and Sons, Inc.: Chichester, UK, 1987. [Google Scholar]
- Brzyska, W. Lantanowce i Aktynowce; WNT: Warszawa, Poland, 1987. [Google Scholar]
- Skwarczyńska, A.; Warchoł, J. Sorpcja Cu(II) na złożu Bet-Dagan. Zeszyty Naukowe Politechniki Rzeszowskiej Budownictwo i Inżynieria Środowiska 2008, 254, 81–90. [Google Scholar]
- Lin, J.; Wang, L. Comparison between linear and non-linear forms of pseudo-first-order and pseudo-second-order adsorption kinetic models for the removal of methylene blue by activated carbon. Front. Environ. Sci. Eng. Chin. 2009, 3, 320–324. [Google Scholar] [CrossRef]
- Duong, D.D. Adsorption Analysis Equilibria and Kinetics; Imperial College Press: London, UK, 1998. [Google Scholar]
- Paderewski, M.L. Adsorption Processes in Chemical Engineering; WNT: Warszawa, Poland, 1999. [Google Scholar]
- Bursali, E.A.; Seki, Y.; Seyhan, S.; Delener, M.; Yurdakoc, M. Synthesis of Chitosan Beads as Boron Sorbents. J. Appl. Polym. Sci. 2011, 122, 657–665. [Google Scholar] [CrossRef]
- Gazi, M.; Shahmohammadi, S. Removal of trace boron from aqueous solution using iminobis-(propylene glycol) modified chitosan beads. React. Funct. Polym. 2012, 72, 680–686. [Google Scholar] [CrossRef]
- Wei, Y.T.; Zheng, Y.M.; Chen, J.P. Design and fabrication of an innovative and environmental friendly adsorbent for boron removal. Water Res. 2011, 45, 2297–2305. [Google Scholar] [CrossRef]
- Hall, K.R.; Eagleton, L.C.; Acrivos, A.; Vermeulen, T. Pore and solid diffusion kinetics in fixed bed adsorption order constant pattern conditions. Ind. Eng. Chem. Fundam. 1966, 5, 212–223. [Google Scholar] [CrossRef]
- Treybal, R.E. Mass Transfer Operations; McGraw Hill: New York, NY, USA, 1980. [Google Scholar]
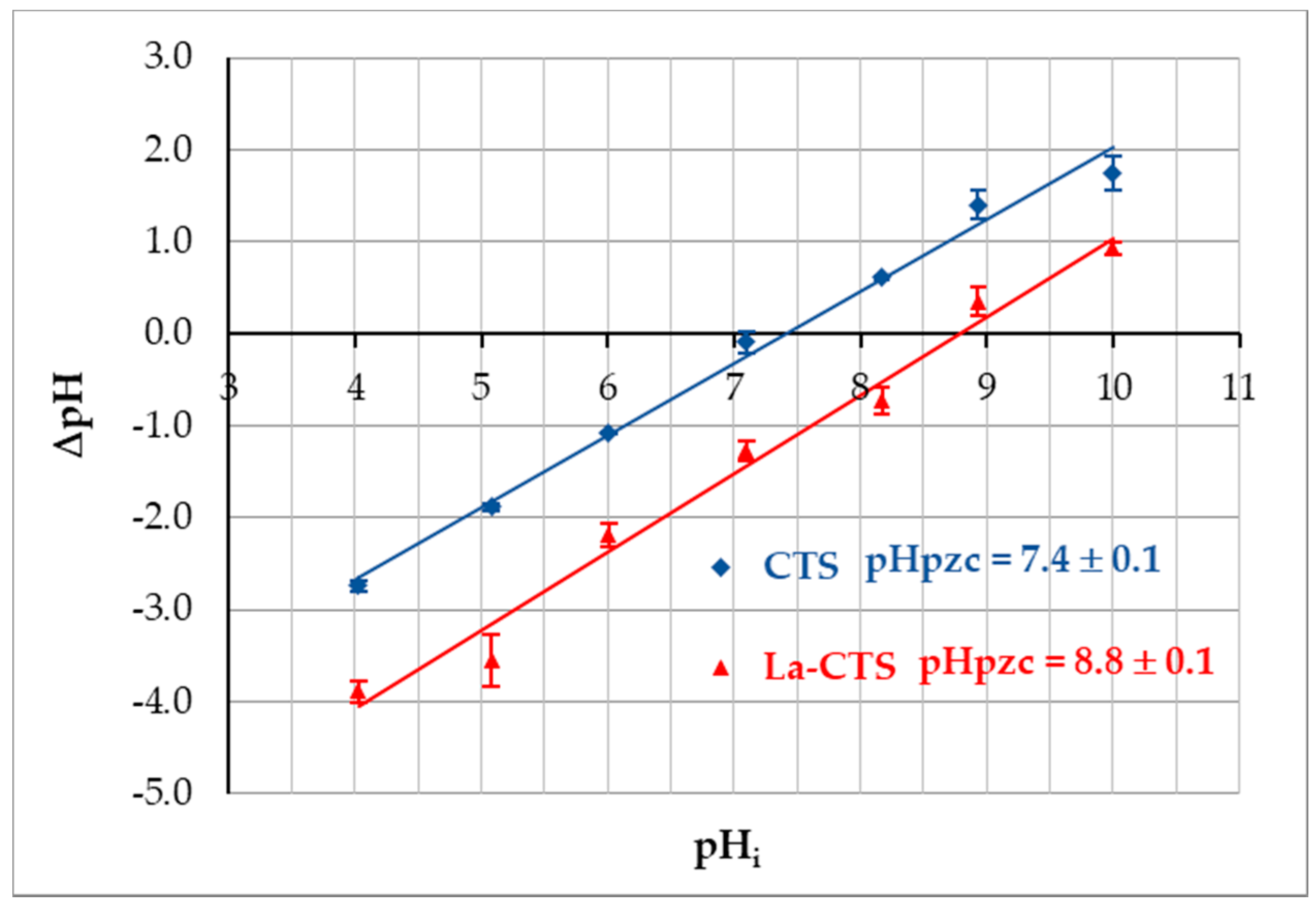
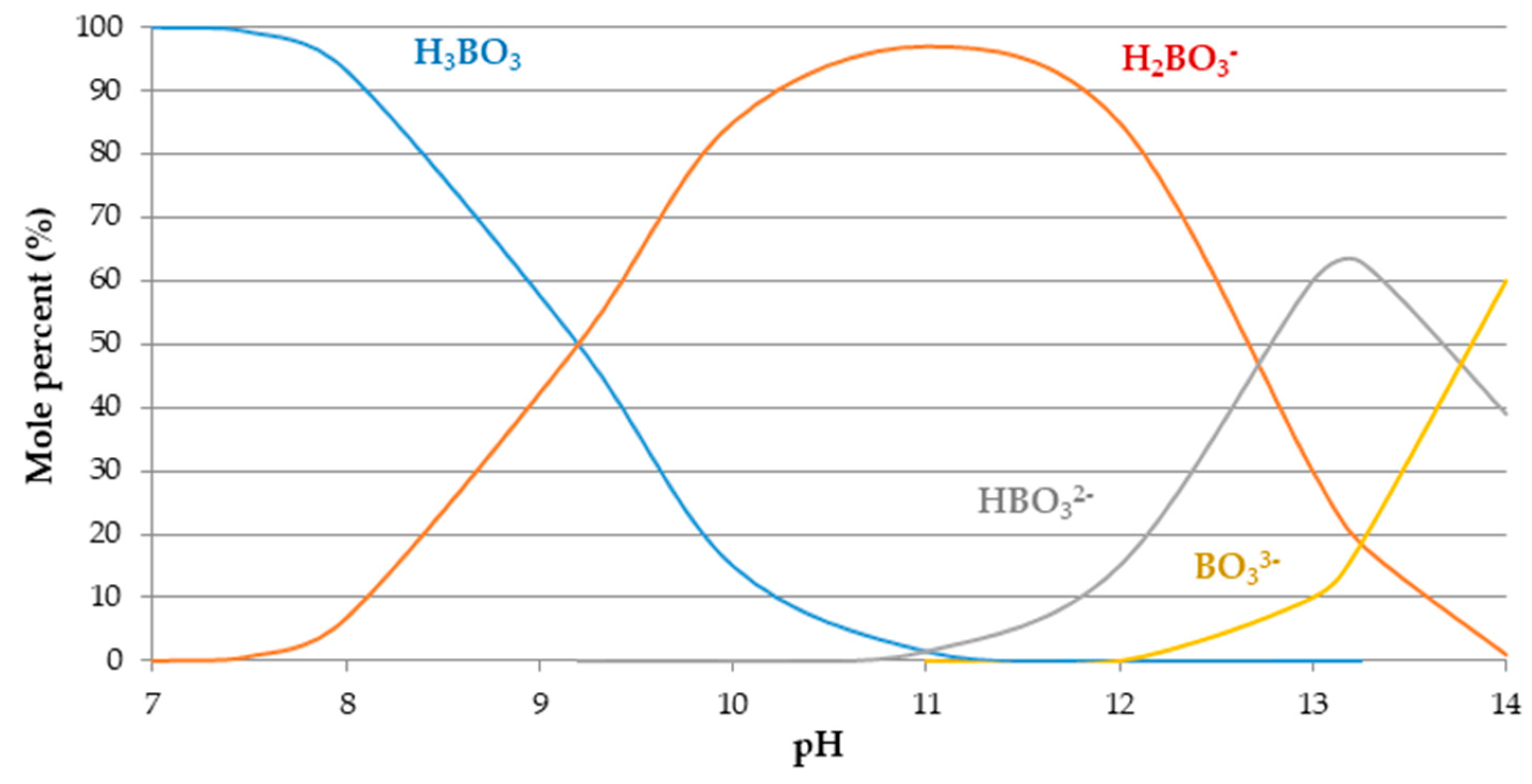
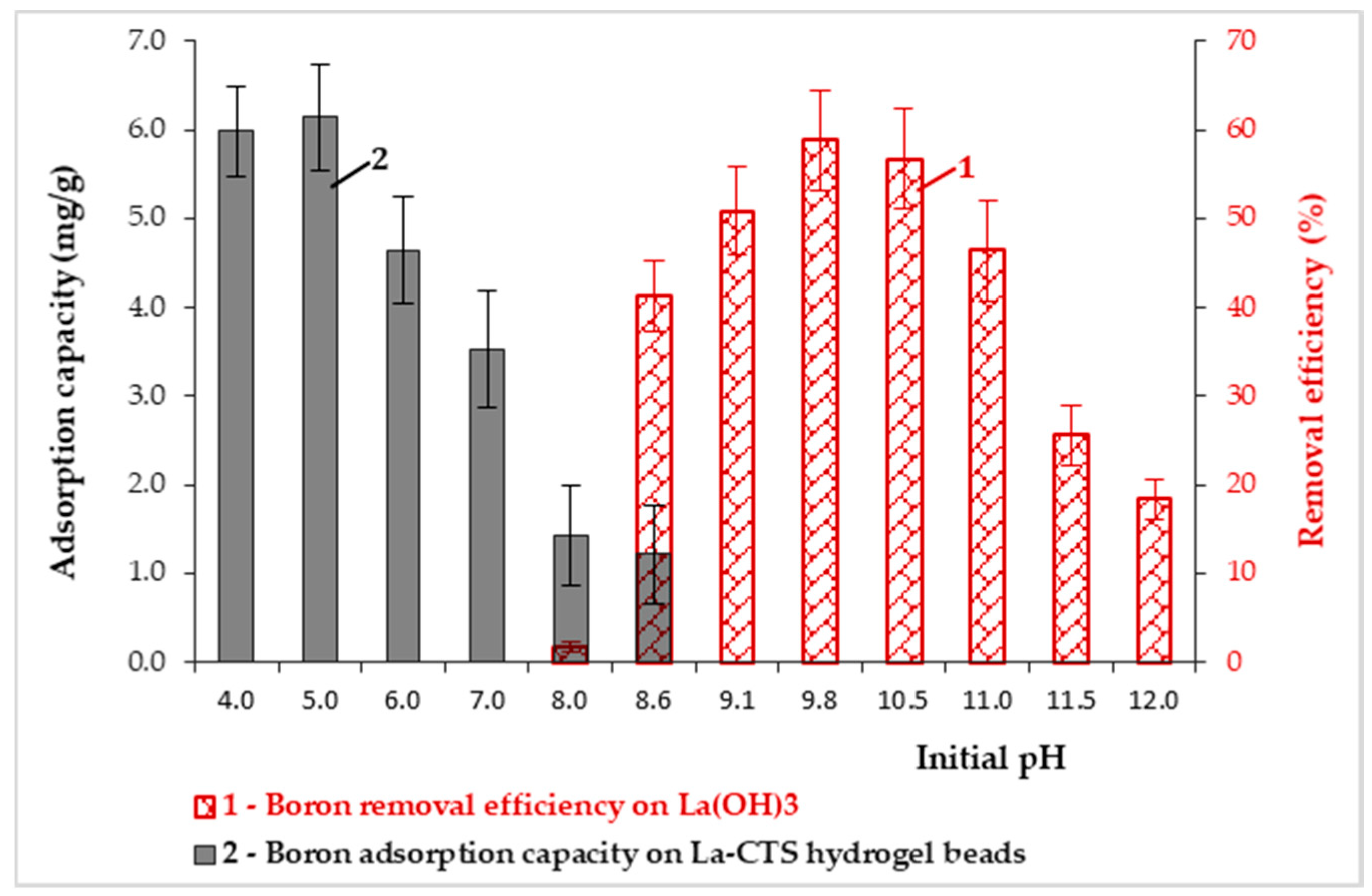
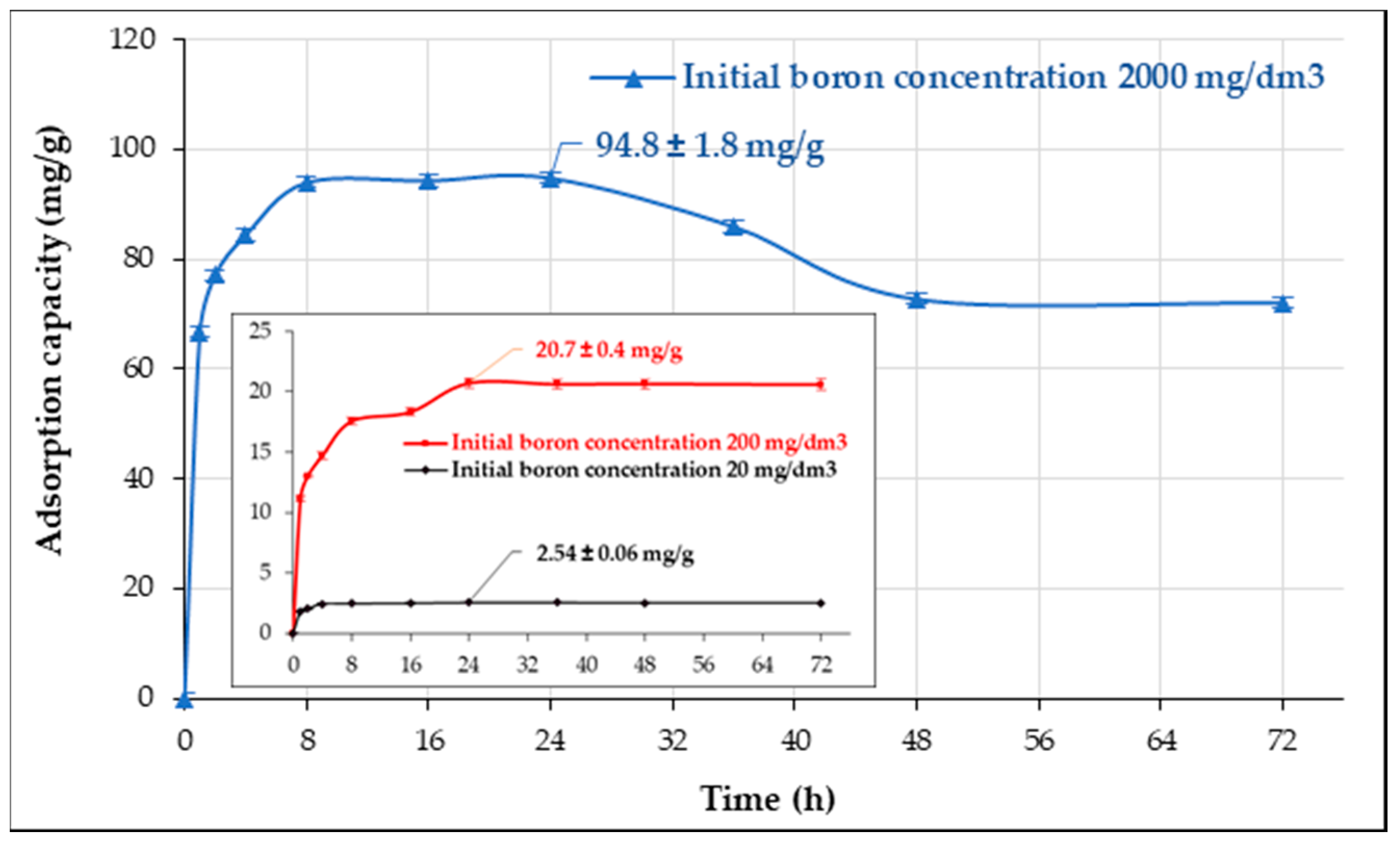


| Initial Boron Concentration | Experimental Capacity | PFO Model | PSO Model | |||||
|---|---|---|---|---|---|---|---|---|
| C0 (mg/dm3) | qexpt (mg/g) | q1 (mg/g) | k1 (1/h) | R12 | q2 (mg/g) | k2 (g/mg∙h) | R22 | r (mg/g∙h) |
| 20 | 2.53 ± 0.06 | 2.49 ± 0.04 | 1.14 ± 0.11 | 0.987 | 2.57 ± 0.11 | 0.93 ± 0.10 | 0.995 | 6.14 |
| 200 | 20.7 ± 0.4 | 19.5 ± 0.8 | 0.57 ± 0.11 | 0.938 | 20.7 ± 0.5 | 0.0427 ± 0.0068 | 0.982 | 18.3 |
| 2000 | 94.8 ± 1.8 | 85.3 ± 3.3 | 1.44 ± 0.43 | 0.915 | 86.5 ± 4.0 | 0.0489 ± 0.0346 | 0.955 | 366 |
| Isotherm Model | Parameters of the Isotherm Models qexpt = 11.1 ± 0.3 mg/g for C0 = 100 mg/dm3 | |||||
|---|---|---|---|---|---|---|
| Langmuir | qm(mg/g) | B (dm3/mg) | R2 | |||
| 26.7 ± 3.6 | 0.0110 ± 0.0023 | 0.997 | ||||
| RL for initial boron concentration (mg/dm3): | ||||||
| 2 | 5 | 10 | 20 | 50 | 100 | |
| 0.978 | 0.948 | 0.901 | 0.820 | 0.645 | 0.476 | |
| Freundlich | KF (mg/g) | n | R2 | |||
| 0.508 ± 0.013 | 1.35 ± 0.02 | 0.999 | ||||
| Dubinin–Radushkevich | Xm (mg/g) | E (kJ/mol) | R2 | |||
| 10.2 ± 1.5 | 0.12 ± 0.03 | 0.924 | ||||
| Temkin | AT | bT | R2 | |||
| 0.766 ± 0.389 | 1072 ± 242 | 0.836 | ||||
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kluczka, J.; Dudek, G.; Kazek-Kęsik, A.; Gnus, M.; Krzywiecki, M.; Mitko, K.; Krukiewicz, K. The Use of Lanthanum Ions and Chitosan for Boron Elimination from Aqueous Solutions. Polymers 2019, 11, 718. https://doi.org/10.3390/polym11040718
Kluczka J, Dudek G, Kazek-Kęsik A, Gnus M, Krzywiecki M, Mitko K, Krukiewicz K. The Use of Lanthanum Ions and Chitosan for Boron Elimination from Aqueous Solutions. Polymers. 2019; 11(4):718. https://doi.org/10.3390/polym11040718
Chicago/Turabian StyleKluczka, Joanna, Gabriela Dudek, Alicja Kazek-Kęsik, Małgorzata Gnus, Maciej Krzywiecki, Krzysztof Mitko, and Katarzyna Krukiewicz. 2019. "The Use of Lanthanum Ions and Chitosan for Boron Elimination from Aqueous Solutions" Polymers 11, no. 4: 718. https://doi.org/10.3390/polym11040718
APA StyleKluczka, J., Dudek, G., Kazek-Kęsik, A., Gnus, M., Krzywiecki, M., Mitko, K., & Krukiewicz, K. (2019). The Use of Lanthanum Ions and Chitosan for Boron Elimination from Aqueous Solutions. Polymers, 11(4), 718. https://doi.org/10.3390/polym11040718








