Oxidative Depolymerization of Cellulolytic Enzyme Lignin over Silicotungvanadium Polyoxometalates
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
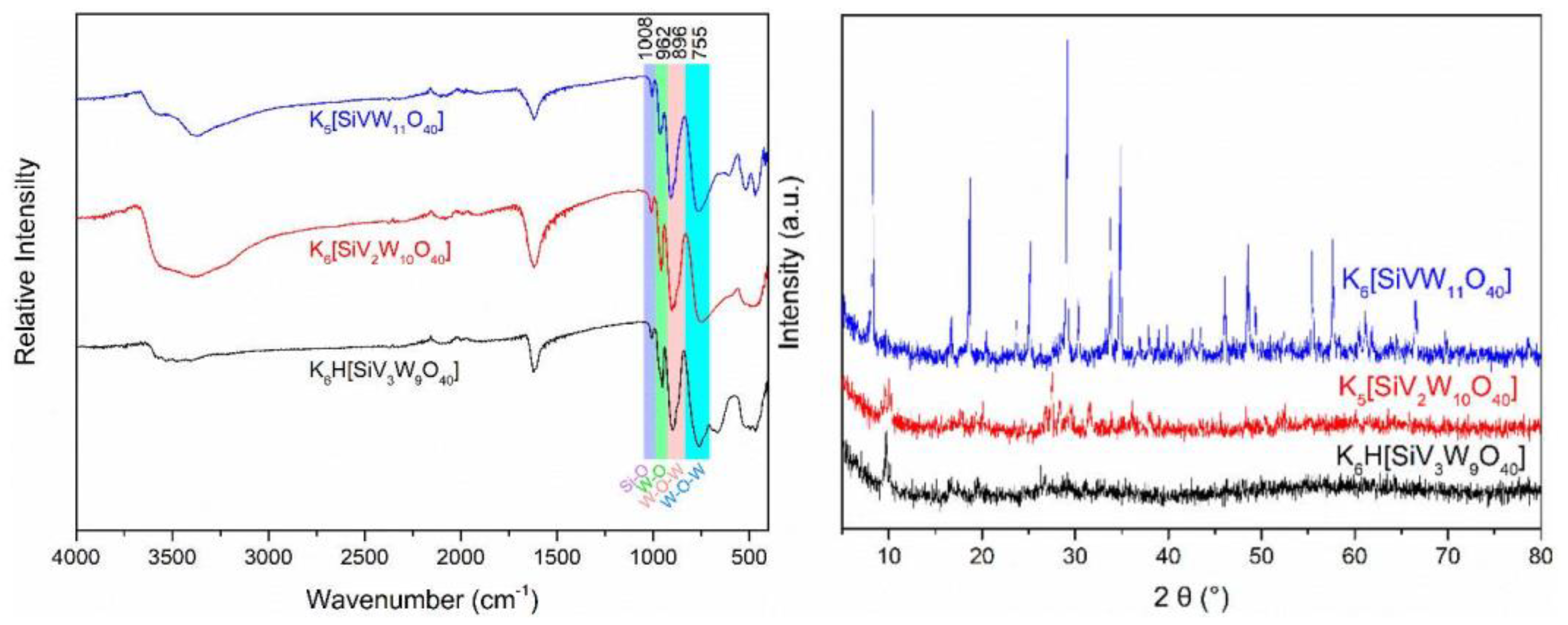
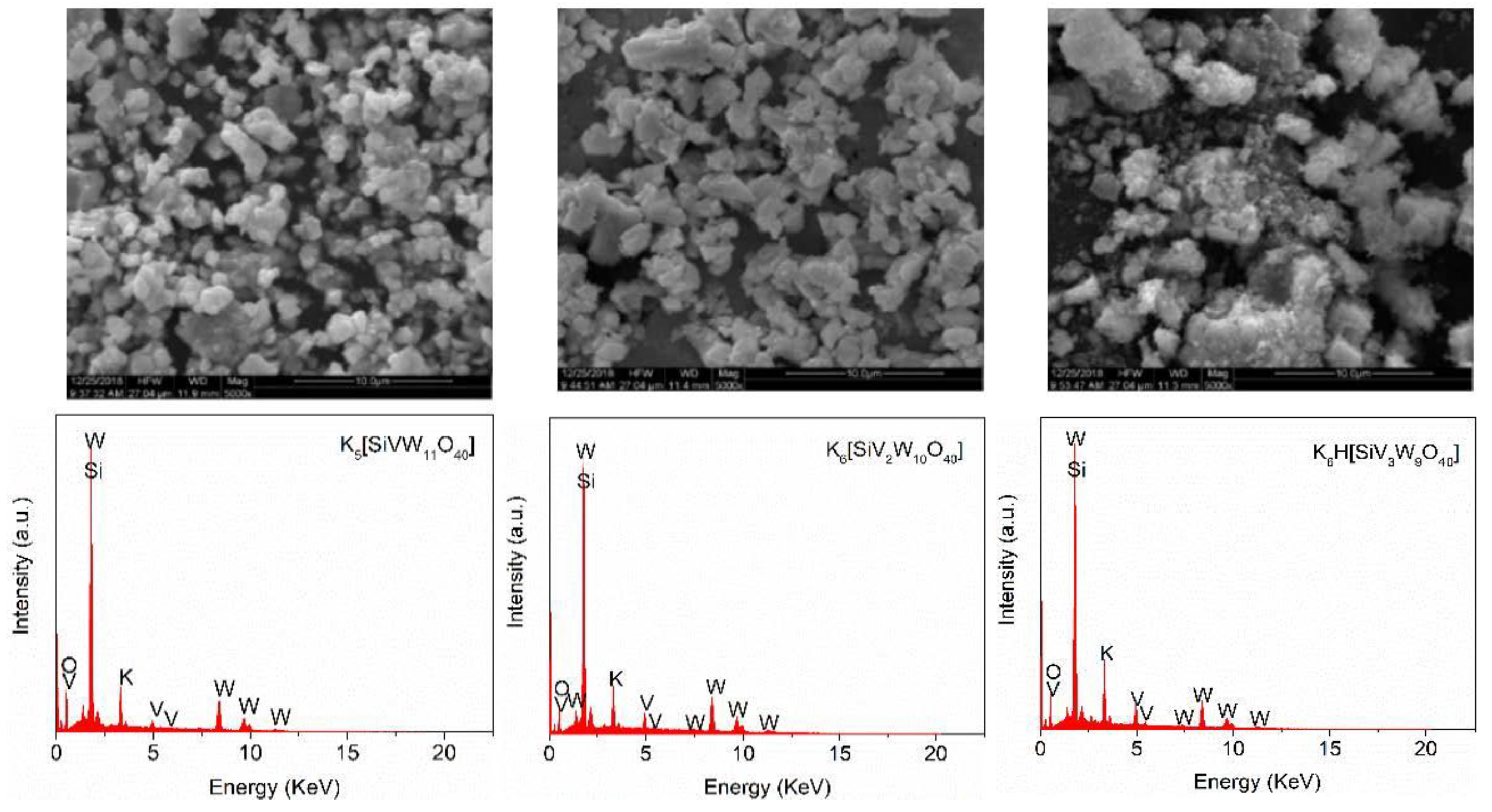
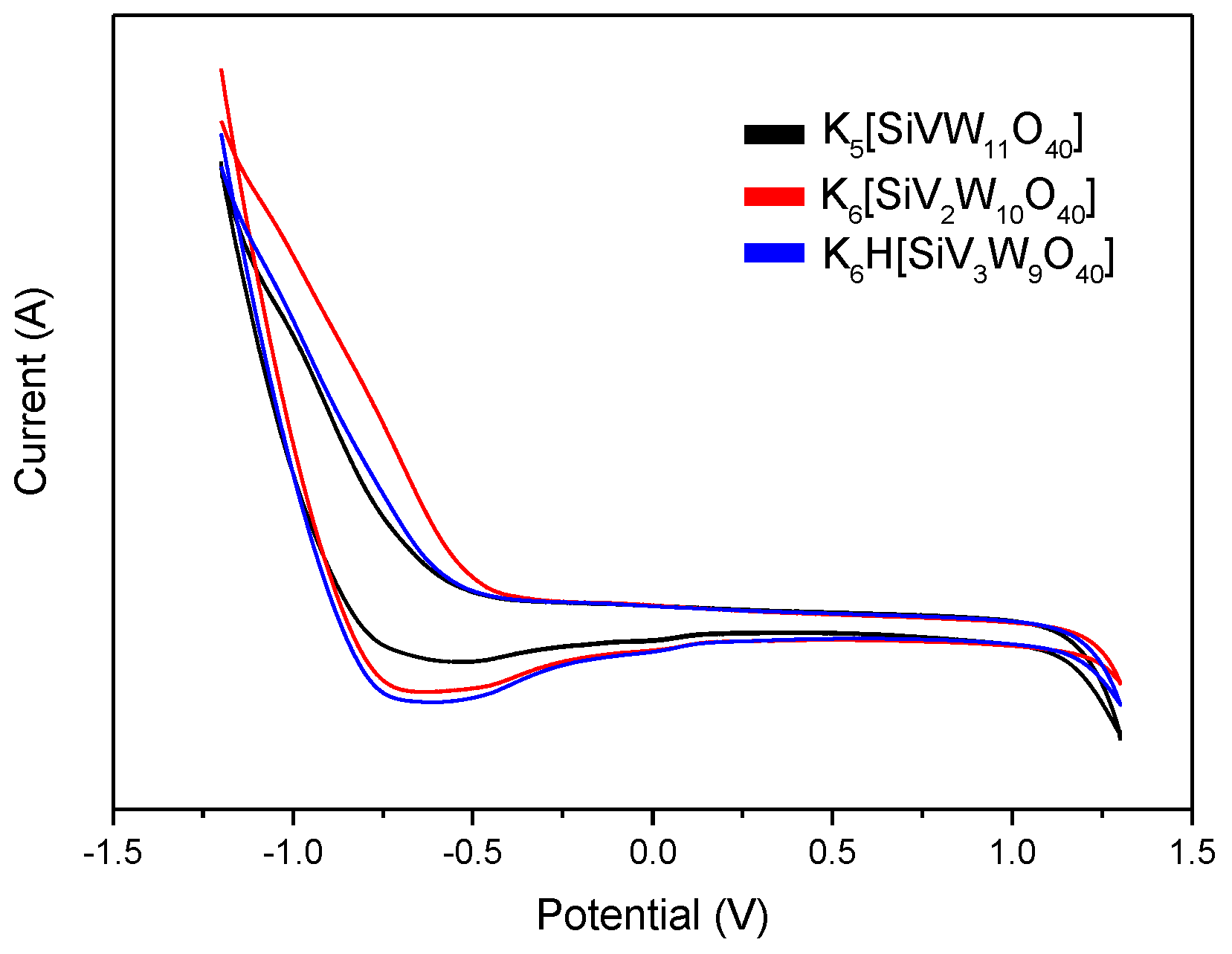
2.2. Preparation and Characterization of the Catalysts
2.3. Catalytic Decomposition of the CEL
2.4. Product Analysis
3. Results and Discussion
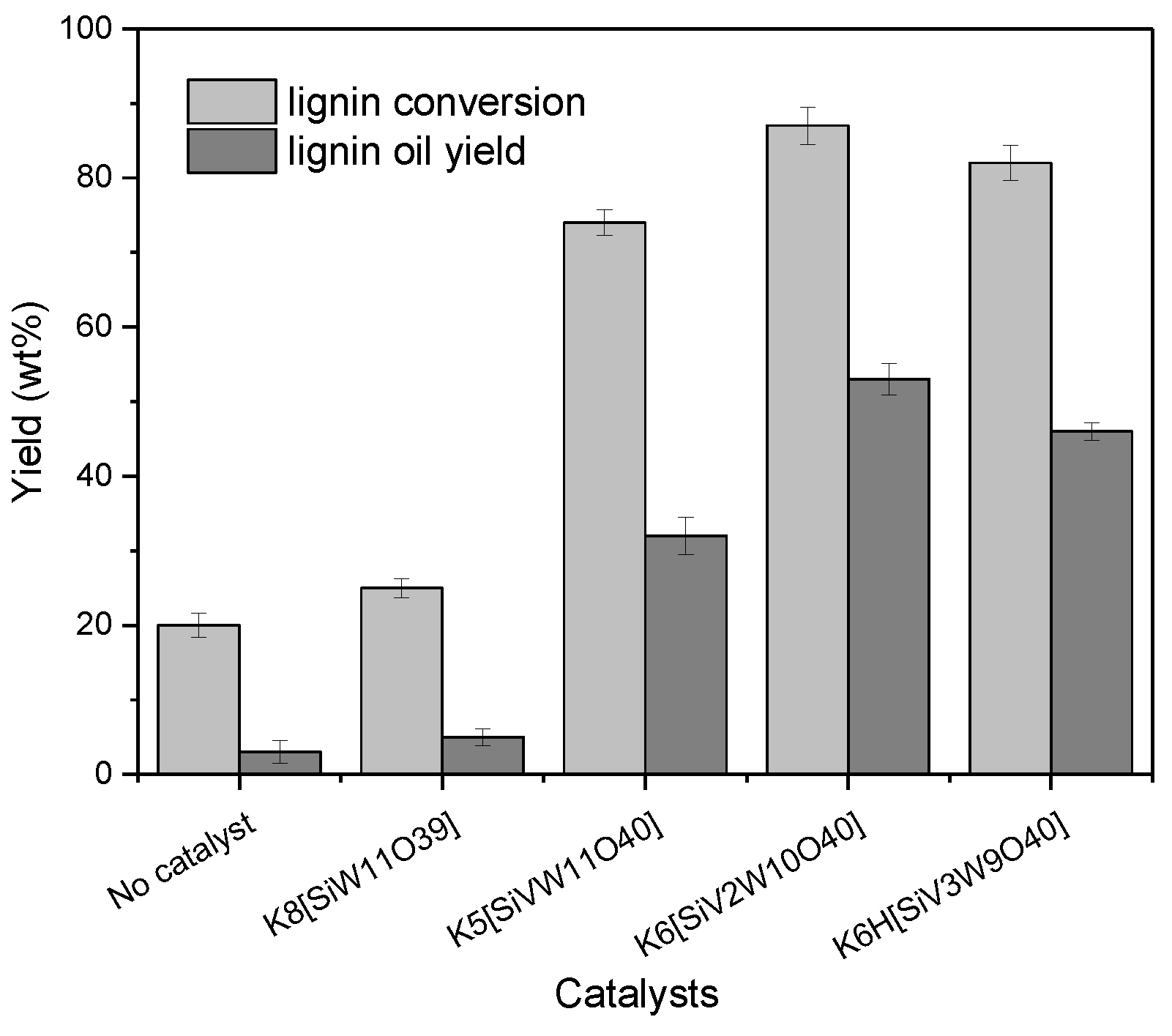
3.1. Catalytic Oxidation Depolymerization of CEL
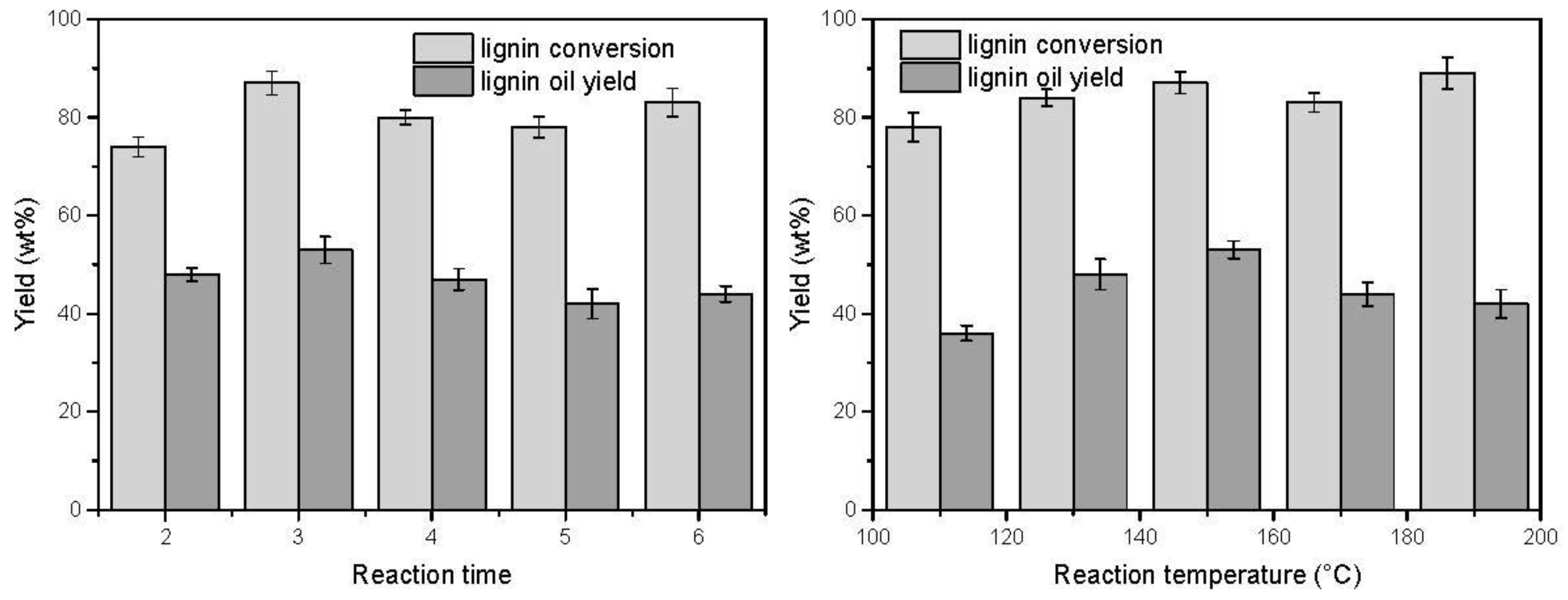
3.2. Effect of the Reaction Time and Temperature
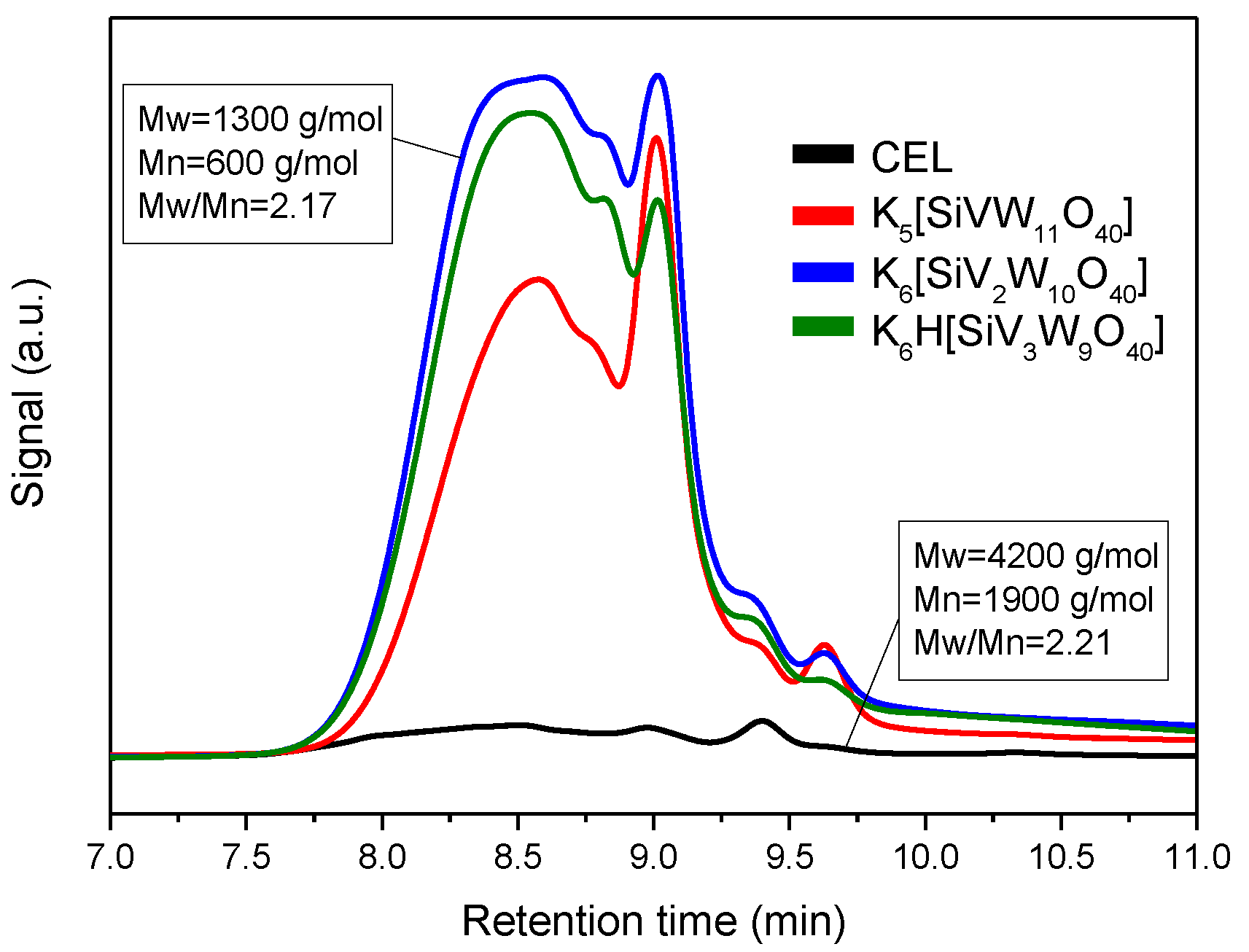
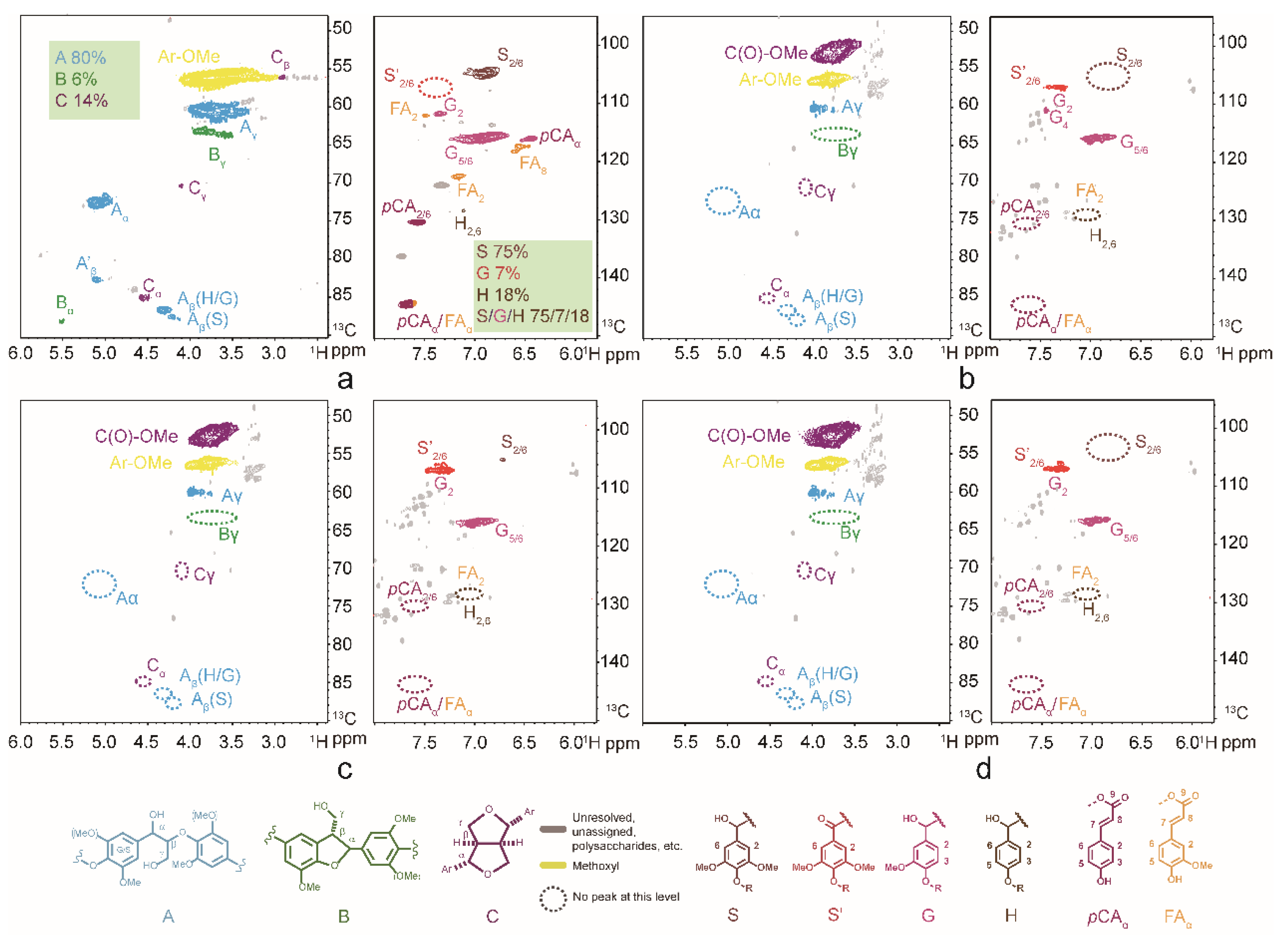
3.3. Depolymerization Products Analysis
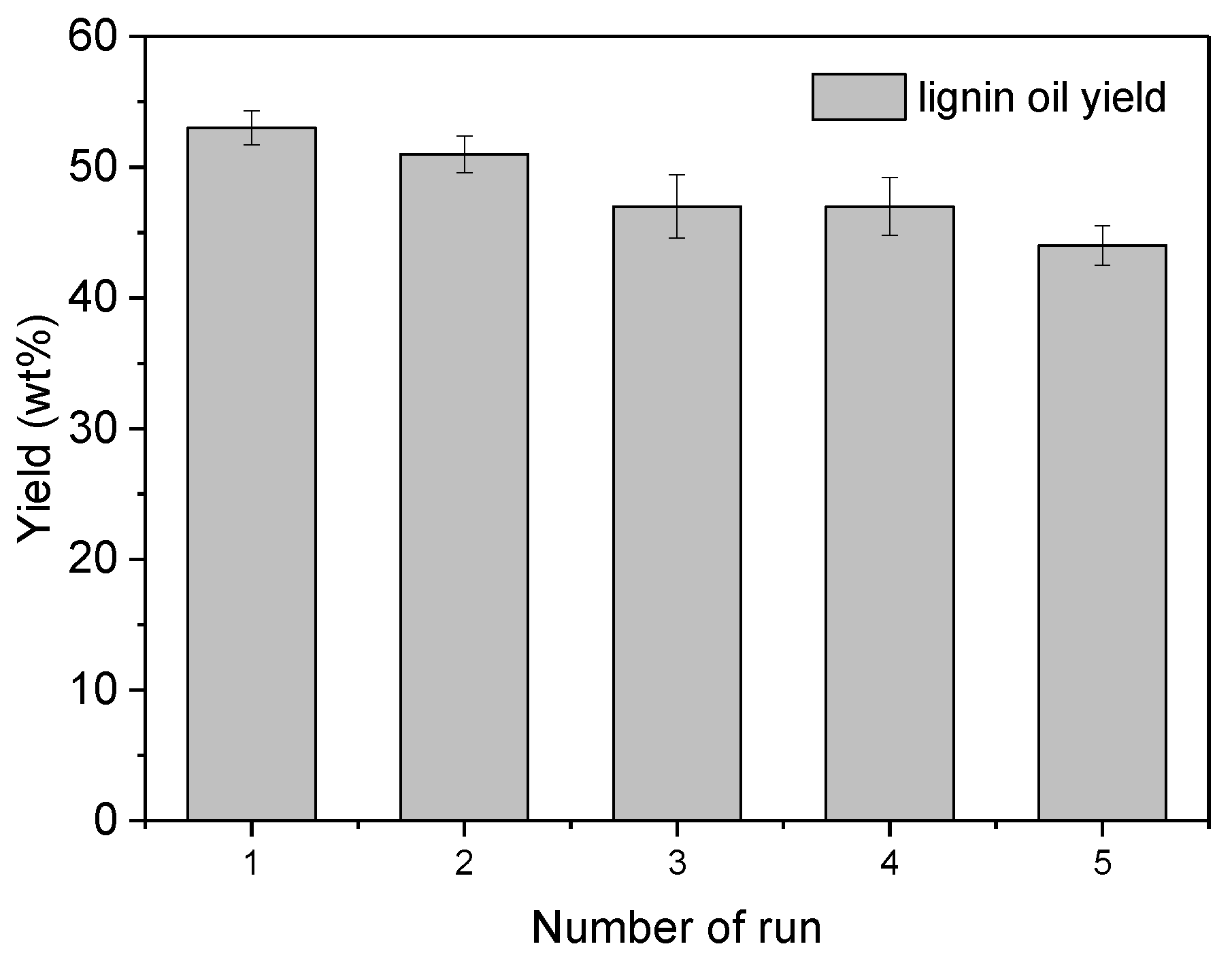
3.4. Recycling Experiments
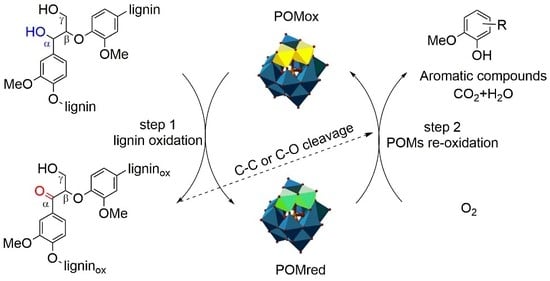
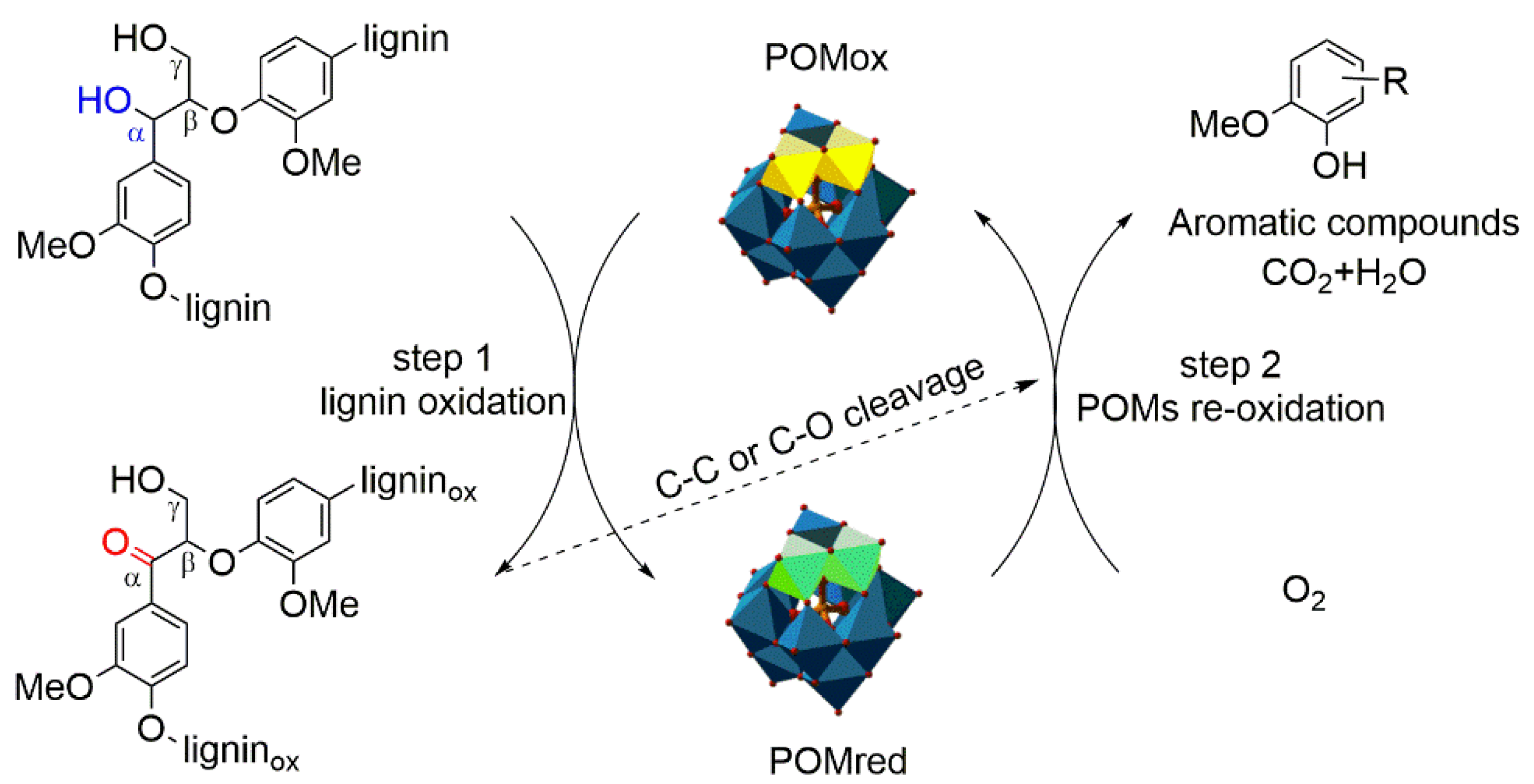
3.5. Possible Mechanisms of Lignin Depolymerization
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Rahimi, A.; Ulbrich, A.; Coon, J.J.; Stahl, S.S. Formic-acid-induced depolymerization of oxidized lignin to aromatics. Nature 2014, 515, 249–252. [Google Scholar] [CrossRef]
- Sun, Z.; Fridrich, B.; Santi, A.D.; Elangovan, S.; Barta, K. Bright Side of Lignin Depolymerization: Toward New Platform Chemicals. Chem. Rev. 2018, 118, 614–678. [Google Scholar] [CrossRef]
- Gregorio, G.F.D.; Prado, R.; Vriamont, C.; Erdocia, X.; Labidi, J.; Hallett, J.P.; Welton, T. Oxidative depolymerization of lignin using a novel polyoxometalate-protic ionic liquid system. ACS Sustain. Chem. 2016, 4, 6031–6036. [Google Scholar] [CrossRef]
- Shuai, L.; Amiri, M.T.; Questell-Santiago, Y.M.; Héroguel, F.; Li, Y.; Kim, H.; Meilan, R.; Chapple, C.; Ralph, J.; Luterbacher, J.S. Formaldehyde stabilization facilitates lignin monomer production during biomass depolymerization. Science 2016, 354, 329–333. [Google Scholar] [CrossRef]
- Sun, Z.; Bottari, G.; Afanasenko, A.; Stuart, M.C.A.; Deuss, P.J.; Fridrich, B.; Barta, K. Complete lignocellulose conversion with integrated catalyst recycling yielding valuable aromatics and fuels. Nat. Catal. 2018, 1, 82–92. [Google Scholar] [CrossRef]
- Li, C.; Zhao, X.; Wang, A.; Huber, G.W.; Zhang, T. Catalytic transformation of lignin for the production of chemicals and fuels. Chem. Rev. 2015, 115, 11559–11624. [Google Scholar] [CrossRef]
- Deuss, P.J.; Martin, S.; Fanny, T.; Westwood, N.J.; Vries, J.G.; De Katalin, B. Aromatic monomers by in situ conversion of reactive intermediates in the acid-catalyzed depolymerization of lignin. J. Am. Chem. Soc. 2015, 137, 7456–7467. [Google Scholar] [CrossRef]
- Kijima, M.; Hirukawa, T.; Hanawa, F.; Hata, T. Thermal conversion of alkaline lignin and its structured derivatives to porous carbonized materials. Bioresour. Technol. 2011, 102, 6279–6285. [Google Scholar] [CrossRef]
- Zhao, Y.; Deng, L.; Liao, B.; Fu, Y.; Guo, Q.X. Aromatics Production via Catalytic Pyrolysis of Pyrolytic Lignins from Bio-Oil. Energy Fuels 2010, 24, 5735–5740. [Google Scholar] [CrossRef]
- Yu, Y.; Li, X.; Lu, S.; Ying, Z.; Wang, Y.; Zhang, H. The role of shape selectivity in catalytic fast pyrolysis of lignin with zeolite catalysts. Appl. Catal. A Gen. 2012, 447, 115–123. [Google Scholar] [CrossRef]
- Yan, N.; Zhao, C.; Dyson, P.J.; Wang, C.; Liu, L.T.; Yuan, K. Selective Degradation of Wood Lignin over Noble-Metal Catalysts in a Two-Step Process. ChemSusChem 2008, 1, 626–629. [Google Scholar] [CrossRef]
- Zhang, J.; Deng, H.; Lu, L.; Yong, S.; Pan, C.; Liu, S. Isolation and characterization of wheat straw lignin with a formic acid process. Bioresour. Technol. 2010, 101, 2311–2316. [Google Scholar] [CrossRef]
- Gaspar, A.R.; Gamelas, J.A.F.; Evtuguin, D.V.; Neto, C.P. Alternatives for Lignocellulosic Pulp Delignification Using Polyoxometalates and Oxygen: A Review. Green Chem. 2007, 38, 717–730. [Google Scholar] [CrossRef]
- Voitl, T.; Rohr, P.R.V. Demonstration of a Process for the Conversion of Kraft Lignin into Vanillin and Methyl Vanillate by Acidic Oxidation in Aqueous Methanol. Ind. Eng. Chem. Res 2009, 49, 520–525. [Google Scholar] [CrossRef]
- Hanson, S.K.; Wu, R.; Pete, S.L.A. C-C or C-O bond cleavage in a phenolic lignin model compound: Selectivity depends on vanadium catalyst. Angew. Chem. Int. Ed. 2012, 51, 3410–3413. [Google Scholar] [CrossRef]
- Xu, C.; Arancon, R.A.D.; Labidi, J.; Luque, R. Lignin depolymerisation strategies: Towards valuable chemicals and fuels. Chem. Soc. Rev. 2014, 43, 7485–7500. [Google Scholar] [CrossRef]
- Schutyser, W.; Renders, T.; Van den Bosch, S.; Koelewijn, S.-F.; Beckham, G.T.; Sels, B.F. Chemicals from lignin: An interplay of lignocellulose fractionation, depolymerisation, and upgrading. Chem. Soc. Rev. 2018, 47, 852–908. [Google Scholar] [CrossRef]
- Collinson, S.R.; Thielemans, W. The catalytic oxidation of biomass to new materials focusing on starch, cellulose and lignin. Coord. Chem. Rev. 2010, 254, 1854–1870. [Google Scholar] [CrossRef]
- Wang, S.S.; Yang, G.Y. Recent Advances in Polyoxometalate-Catalyzed Reactions. Chem. Rev. 2015, 115, 1544–1548. [Google Scholar] [CrossRef]
- Voitl, T.; Rudolf von Rohr, P. Oxidation of lignin using aqueous polyoxometalates in the presence of alcohols. ChemSusChem 2008, 1, 763–769. [Google Scholar] [CrossRef]
- Voitl, T.; Nagel, M.V.; Rohr, P.R.V. Analysis of products from the oxidation of technical lignins by oxygen and H3PMo12O40 in water and aqueous methanol by size-exclusion chromatography. Holzforschung 2010, 64, 13–19. [Google Scholar] [CrossRef]
- Werhan, H.; Mir, J.M.; Voitl, T.; Von Rohr, P.R. Acidic oxidation of kraft lignin into aromatic monomers catalyzed by transition metal salts. Holzforschung 2011, 65, 703–709. [Google Scholar] [CrossRef]
- Baguc, I.B.; Saglam, S.; Ertas, I.E.; Keles, M.N.; Celebi, M.; Kaya, M.; Zahmakiran, M. Keggin Type-Polyoxometalate Decorated Ruthenium Nanoparticles: Highly Active and Selective Nanocatalyst for the Oxidation of Veratryl Alcohol as a Lignin Model Compound. ChemistrySelect 2017, 2, 2487–2494. [Google Scholar] [CrossRef]
- Anderson, E.; Crisci, A.; Murugappan, K.; Román-Leshkov, Y. Bifunctional Molybdenum Polyoxometalates for the Combined Hydrodeoxygenation and Alkylation of Lignin-Derived Model Phenolics. ChemSusChem 2017, 10, 2226–2234. [Google Scholar] [CrossRef]
- Park, H.W.; Kim, J.K.; Hong, U.G.; Lee, Y.J.; Song, J.H.; Song, I.K. Catalytic Decomposition of Lignin Model Compounds to Aromatics over Acidic Catalysts. Catal. Surv. Asia 2013, 17, 119–131. [Google Scholar] [CrossRef]
- Bujanovic, B.; Ralph, S.; Reiner, R.; Hirth, K.; Atalla, R. Polyoxometalates in oxidative delignification of chemical pulps: Effect on lignin. Materials 2010, 3, 1888–1903. [Google Scholar] [CrossRef]
- Jung, K.A.; Woo, S.H.; Lim, S.R.; Park, J.M. Pyrolytic production of phenolic compounds from the lignin residues of bioethanol processes. Chem. Eng. J. 2015, 259, 107–116. [Google Scholar] [CrossRef]
- Tian, D.; Hu, J.; Chandra, R.P.; Saddler, J.N.; Lu, C. Valorizing Recalcitrant Cellulolytic Enzyme Lignin via Lignin Nanoparticles Fabrication in an Integrated Biorefinery. ACS Sustain. Chem. Eng. 2017, 5, 2702–2710. [Google Scholar] [CrossRef]
- Xu, W.; Li, X.; Shi, J. Activation of Cellulosic Ethanol Lignin by Laccase and its Application as Plywood Adhesive. BioResources 2018, 13, 3005–3016. [Google Scholar] [CrossRef]
- Deng, H.; Lin, L.; Sun, Y.; Pang, C.; Zhuang, J.; Ouyang, P.; Li, Z.; Liu, S. Perovskite-type Oxide LaMnO3: An Efficient and Recyclable Heterogeneous Catalyst for the Wet Aerobic Oxidation of Lignin to Aromatic Aldehydes. Catal. Lett. 2008, 126, 106–111. [Google Scholar] [CrossRef]
- Deng, H.; Lin, L.; Sun, Y.; Pang, C.; Zhuang, J.; Ouyang, P.; Li, J.; Liu, S. Activity and Stability of Perovskite-Type Oxide LaCoO3 Catalyst in Lignin Catalytic Wet Oxidation to Aromatic Aldehydes Process. Energy Fuels 2008, 23, 19–24. [Google Scholar] [CrossRef]
- Weinstock, I.A.; Atalla, R.H.; Reiner, R.S.; Moen, M.A.; Hammel, K.E.; Houtman, C.J.; Hill, C.L.; Harrup, M.K. A new environmentally benign technology for transforming wood pulp into paper. Engineering polyoxometalates as catalysts for multiple processes. J. Mol. Catal. A Chem. 1997, 116, 59–84. [Google Scholar] [CrossRef]
- Li, C.; Yan, Z.; O’Halloran, K.P.; Zhang, J.; Ma, H. Electrochemical behavior of vanadium-substituted Keggin-type polyoxometalates in aqueous solution. J. Appl. Electrochem. 2009, 39, 421–427. [Google Scholar] [CrossRef]
- Tao, M.; Zhang, D.; Guan, H.; Huang, G.; Wang, X. Designation of highly efficient catalysts for one pot conversion of glycerol to lactic acid. Sci. Rep. 2016, 6, 29840. [Google Scholar] [CrossRef]
- Sun, Y.; Wen, J.; Xu, F.; Sun, R. Fractional and structural characterization of organosolv and alkaline lignins from Tamarix austromogoliac. Sci. Res. Essays 2010, 5, 3850–3864. [Google Scholar] [CrossRef]
- Chen, C.; Jin, D.; Ouyang, X.; Zhao, L.; Qiu, X.; Wang, F. Effect of structural characteristics on the depolymerization of lignin into phenolic monomers. Fuel 2018, 223, 366–372. [Google Scholar] [CrossRef]
- Villaverde, J.J.; Li, J.; Ek, M.; Ligero, P.; de Vega, A. Native lignin structure of Miscanthus x giganteus and its changes during acetic and formic acid fractionation. J. Agric. Food Chem. 2009, 57, 6262–6270. [Google Scholar] [CrossRef]
- Xiao, L.P.; Wang, S.Z.; Li, H.L.; Li, Z.W.; Shi, Z.J.; Xiao, L.; Sun, R.G.; Fang, Y.M.; Song, G.Y. Catalytic Hydrogenolysis of Lignins into Phenolic Compounds over Carbon Nanotube Supported Molybdenum Oxide. ACS Catal. 2017, 7, 7535–7542. [Google Scholar] [CrossRef]
- Dabral, S.; Hernandez, J.G.; Kamer, P.C.J.; Bolm, C. Organocatalytic Chemoselective Primary Alcohol Oxidation and Subsequent Cleavage of Lignin Model Compounds and Lignin. ChemSusChem 2017, 10, 2707–2713. [Google Scholar] [CrossRef]
- Lancefield, C.S.; Ojo, O.S.; Tran, F.; Westwood, N.J. Isolation of Functionalized Phenolic Monomers through Selective Oxidation and C-O Bond Cleavage of the β-O-4 Linkages in Lignin. Angew. Chem. Int. Ed. 2015, 54, 258–262. [Google Scholar] [CrossRef]
- Pacek, A.W.; Ping, D.; Garrett, M.; Sheldrake, G.; Nienow, A.W. Catalytic Conversion of Sodium Lignosulfonate to Vanillin: Engineering Aspects. Part 1. Effects of Processing Conditions on Vanillin Yield and Selectivity. Ind. Eng. Chem. Res. 2013, 52, 8361–8372. [Google Scholar] [CrossRef]
- Fache, M.; Darroman, E.; Besse, V.; Auvergne, R.; Caillol, S.; Boutevin, B. Vanillin, a promising biobased building-block for monomer synthesis. Green Chem. 2014, 16, 1987–1998. [Google Scholar] [CrossRef]
- Zhao, S.; Wang, X.; Huo, M. Catalytic wet air oxidation of phenol with air and micellar molybdovanadophosphoric polyoxometalates under room condition. Appl. Catal. B Environ. 2010, 97, 127–134. [Google Scholar] [CrossRef]
- Ralph, J.; Lundquist, K.; Brunow, G.; Lu, F.; Kim, H.; Schatz, P.F.; Marita, J.M.; Hatfield, R.D.; Ralph, S.A.; Christensen, J.H. Lignins: Natural polymers from oxidative coupling of 4-hydroxyphenyl-propanoids. Phytochem. Rev. 2004, 3, 29–60. [Google Scholar] [CrossRef]
- Kleine, T.; Buendia, J.; Bolm, C. Mechanochemical degradation of lignin and wood by solvent-free grinding in a reactive medium. Green Chem. 2013, 15, 160–166. [Google Scholar] [CrossRef]
| Entry | Retention Time | Phenolic Compounds | Structure | Yield (%) |
|---|---|---|---|---|
| 1 | 7.569 | 2-Methoxy-4-vinylphenol |  | 3.43 |
| 2 | 8.363 | 4-Hydroxybenzaldehyde | 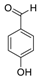 | 0.39 |
| 3 | 9.040 | Vanillin | 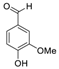 | 5.46 |
| 4 | 9.975 | 4-(Methoxycarbonyl)phenol | 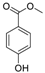 | 0.78 |
| 5 | 15.145 | Acetosyringone | 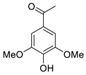 | 0.18 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, W.; Li, X.; Shi, J. Oxidative Depolymerization of Cellulolytic Enzyme Lignin over Silicotungvanadium Polyoxometalates. Polymers 2019, 11, 564. https://doi.org/10.3390/polym11030564
Xu W, Li X, Shi J. Oxidative Depolymerization of Cellulolytic Enzyme Lignin over Silicotungvanadium Polyoxometalates. Polymers. 2019; 11(3):564. https://doi.org/10.3390/polym11030564
Chicago/Turabian StyleXu, Wenbiao, Xiangyu Li, and Junyou Shi. 2019. "Oxidative Depolymerization of Cellulolytic Enzyme Lignin over Silicotungvanadium Polyoxometalates" Polymers 11, no. 3: 564. https://doi.org/10.3390/polym11030564
APA StyleXu, W., Li, X., & Shi, J. (2019). Oxidative Depolymerization of Cellulolytic Enzyme Lignin over Silicotungvanadium Polyoxometalates. Polymers, 11(3), 564. https://doi.org/10.3390/polym11030564