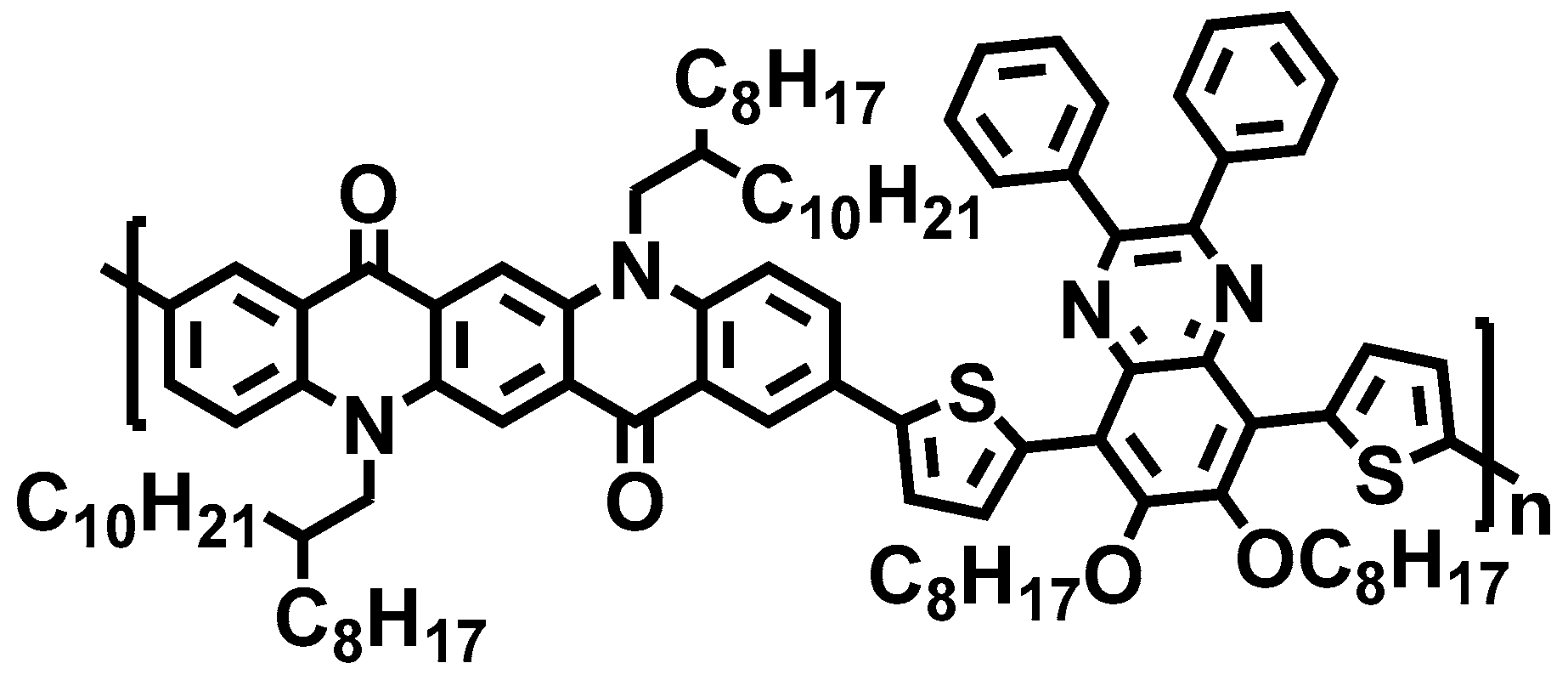
A Quinacridone-Diphenylquinoxaline-Based Copolymer for Organic Field-Effect Transistors
Abstract
:1. Introduction
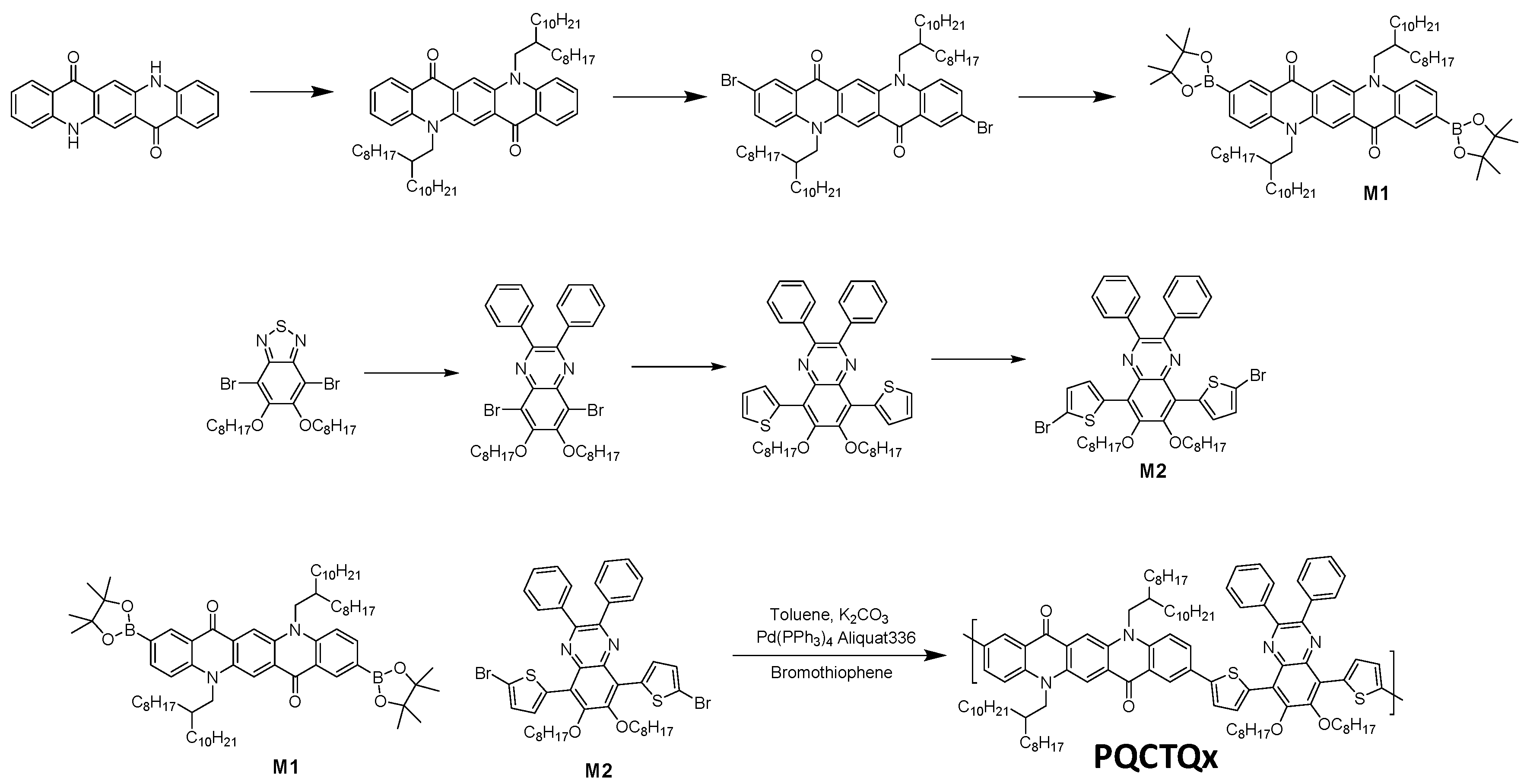
2. Experimental
2.1. Materials, Device Fabrication
2.2. Electrical Characterization of the OFET Devices
2.3. Morphological Characterization
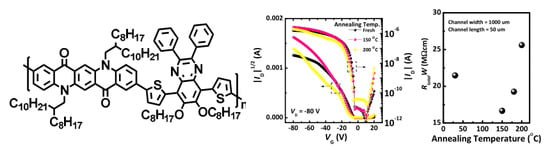
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ozdemir, R.; Choi, D.; Ozdemir, M.; Kwon, G.; Kim, H.; Sen, U.; Kim, C.; Usta, H. Ultralow bandgap molecular semiconductors for ambient-stable and solution-processable ambipolar organic field-effect transistors and inverters. J. Mater. Chem. C 2017, 5, 2368–2379. [Google Scholar] [CrossRef]
- Yu, S.H.; Park, K.H.; Kim, Y.-H.; Chung, D.S.; Kwon, S.-K. Fine Molecular Tuning of Diketopyrrolopyrrole-Based Polymer Semiconductors for Efficient Charge Transport: Effects of Intramolecular Conjugation Structure, Macromolecules. Macromolecules 2017, 50, 4227–4234. [Google Scholar] [CrossRef]
- Chen, H.; Hurhangee, M.; Nikolka, M.; Zhang, W.; Kirkus, M.; Neophytou, M.; Cryer, S.J.; Harkin, D.; Hayoz, P.; Abdi-Jalebi, M.; et al. Dithiopheneindenofluorene (TIF) Semiconducting Polymers with Very High Mobility in Field-Effect Transistors. Adv. Mater. 2017, 29, 1702523. [Google Scholar] [CrossRef]
- Kim, K.; Cho, J.; Jhon, H.; Jeon, J.; Kang, M.; Park, C.E.; Lee, J.; An, T.K. Repurposing compact discs as master molds to fabricate high-performance organic nanowire field-effect transistors. Nanotechnology 2017, 28, 205304. [Google Scholar] [CrossRef]
- Chanwoo, Y.; Yoo, E.J.; Lee, S.W.; An, T.K.; Kim, S.H. Hybrid flexible ambipolar thin-film transistors based on pentacene and ZnO capable of low-voltage operation. Chin. J. Phys. 2016, 54, 471–474. [Google Scholar]
- Root, S.E.; Savagatrup, S.; Printz, A.D.; Rodriquez, D.; Lipomi, D.J. Mechanical properties of organic semiconductors for stretchable, highly flexible, and mechanically robust electronics. Chem. Rev. 2017, 117, 6467–6499. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Wang, S.; Wang, G.-J.N.; Zhu, C.; Luo, S.; Jin, L.; Gu, X.; Chen, S.; Feig, V.R.; To, J.W.F.; et al. Highly stretchable polymer semiconductor films through the nanoconfinement effect. Science 2017, 335, 59–64. [Google Scholar] [CrossRef]
- Pierre, A.; Sadeghi, M.; Payne, M.M.; Facchetti, A.; Anthony, J.E.; Arias, A.C. All-Printed Flexible Organic Transistors Enabled by Surface Tension-Guided Blade Coating. Adv. Mater. 2014, 26, 5722–5727. [Google Scholar] [CrossRef] [PubMed]
- Jeong, N.Y.; Jang, M.S.; Park, S.M.; Chung, D.S.; Kim, Y.H.; Kwon, S.K. Synthesis and characterization of highly soluble phenanthro[1,10,9,8-c,d,e,f,g]carbazole-based copolymer: Effects of thermal treatment on crystalline order and charge carrier mobility. Dyes Pigment. 2018, 149, 560–565. [Google Scholar] [CrossRef]
- Choi, D.; Kim, H.; Persson, N.; Chu, P.H.; Chang, M.; Kang, J.H.; Graham, S.; Reichmanis, E. Elastomer–Polymer Semiconductor Blends for High-Performance Stretchable Charge Transport Networks. Chem. Mater. 2016, 28, 1196–1204. [Google Scholar] [CrossRef]
- Osaka, I.; Akita, M.; Koganezawa, T.; Takimiya, K. Quinacridone-Based Semiconducting Polymers: Implication of Electronic Structure and Orientational Order for Charge Transport Property. Chem. Mater. 2012, 24, 1235–1243. [Google Scholar] [CrossRef]
- Li, H.; Gu, C.; Jiang, L.; Wei, L.; Hu, W.; Fu, H. Donor-acceptor copolymers containing quinacridone and benzothiadiazole for thin film transistors. J. Mater. Chem. C 2013, 1, 2021–2027. [Google Scholar] [CrossRef]
- Liu, J.; Gao, B.; Cheng, Y.; Xie, Z.; Geng, Y.; Wang, L.; Jing, X.; Wang, F. Novel White Electroluminescent Single Polymer Derived from Fluorene and Quinacridone. Macromolecules 2008, 41, 1162–1167. [Google Scholar] [CrossRef]
- Jeong, Y.J.; Jeon, J.; Lee, S.; Kang, M.; Jhon, H.; Song, H.J.; Park, C.E.; An, T.K. Development of Organic Semiconductors Based on Quinacridone Derivatives for Organic Field-Effect Transistors: High-Voltage Logic Circuit Applications. IEEE J. Electron Devices Soc. 2017, 5, 209–213. [Google Scholar] [CrossRef]
- Jeon, J.; Jhon, H.; Kang, M.; Song, H.J.; An, T.K. Quinacridone-quinoxaline-based copolymer for organic field-effect transistors and its high-voltage logic circuit operations. Org. Electron. 2018, 56, 1–4. [Google Scholar] [CrossRef]
- Song, H.J.; Kim, D.H.; Lee, E.J.; Moon, D.K. Conjugated polymers consisting of quinacridone and quinoxaline as donor materials for organic photovoltaics: Orientation and charge transfer properties of polymers formed by phenyl structures with a quinoxaline derivative. J. Mater. Chem. A 2013, 1, 6010–6020. [Google Scholar] [CrossRef]
- Yum, S.; An, T.K.; Wang, X.; Lee, W.; Uddin, M.A.; Kim, Y.J.; Nguyen, T.L.; Xu, S.; Hwang, S.; Park, C.E.; et al. Benzotriazole-Containing Planar Conjugated Polymers with Noncovalent Conformational Locks for Thermally Stable and Efficient Polymer Field-Effect Transistors. Chem. Mater. 2014, 26, 2147–2154. [Google Scholar] [CrossRef]
- Raj, M.R.; Kim, Y.; Park, C.E.; An, T.K.; Park, T. Effect of the length of a symmetric branched side chain on charge transport in thienoisoindigo-based polymer field-effect transistors. Org. Electron. 2019, 65, 251–258. [Google Scholar] [CrossRef]
- Kawabata, K.; Saito, M.; Takemura, N.; Osaka, I.; Takimiya, K. Effects of branching position of alkyl side chains on ordering structure and charge transport property in thienothiophenedione-and quinacridone-based semiconducting polymers. Polym. J. 2017, 49, 169–176. [Google Scholar] [CrossRef]
- Guo, X.; Puniredd, S.R.; Baumgarten, M.; Pisula, W.; Müllen, K. Rational Design of Benzotrithiophene-Diketopyrrolopyrrole-Containing Donor-Acceptor Polymers for Improved Charge Carrier Transport. Adv. Mater. 2013, 25, 5467–5472. [Google Scholar] [CrossRef] [PubMed]
- Chesterfield, R.J.; McKeen, J.C.; Newman, C.R.; Frisbie, C.D.; Ewbank, P.C.; Mann, K.R.; Miller, L.L. Variable temperature film and contact resistance measurements on operating n-channel organic thin film transistors. J. Appl. Phys. 2004, 95, 6396–6405. [Google Scholar] [CrossRef]
- Jeong, Y.J.; Yun, D.J.; Jang, J.; Park, S.; An, T.K.; Kim, L.H.; Kim, S.H.; Park, C.E. Solution-processed n-type fullerene field-effect transistors prepared using CVD-grown graphene electrodes: Improving performance with thermal annealing. PCCP 2015, 17, 6635–6643. [Google Scholar] [CrossRef] [PubMed]
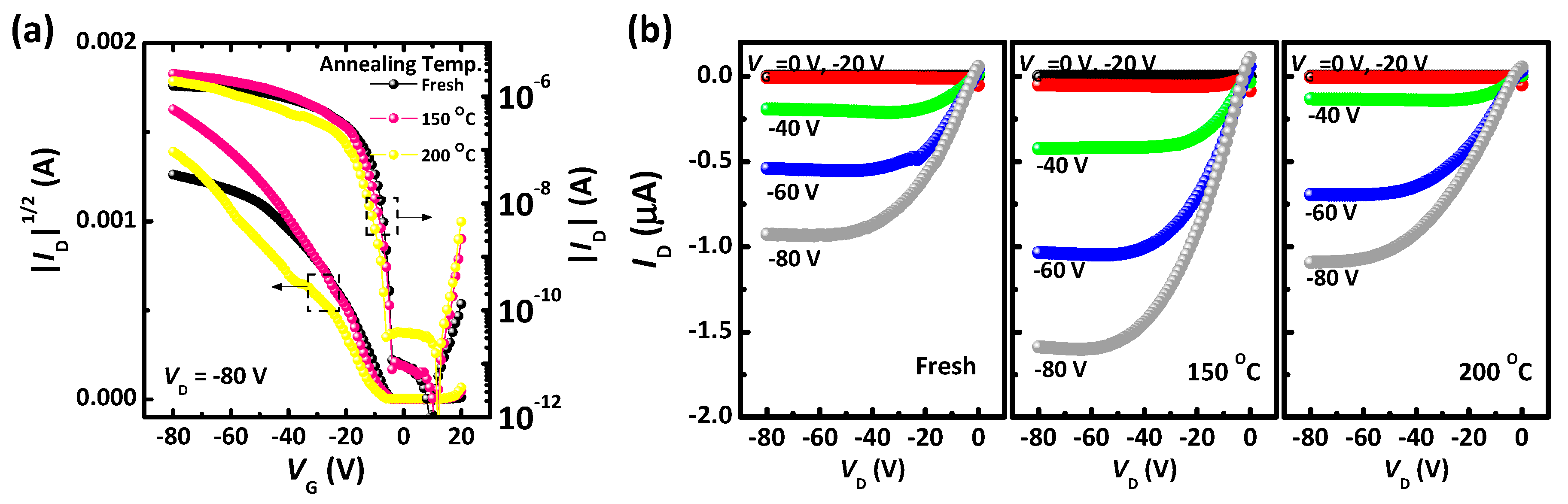
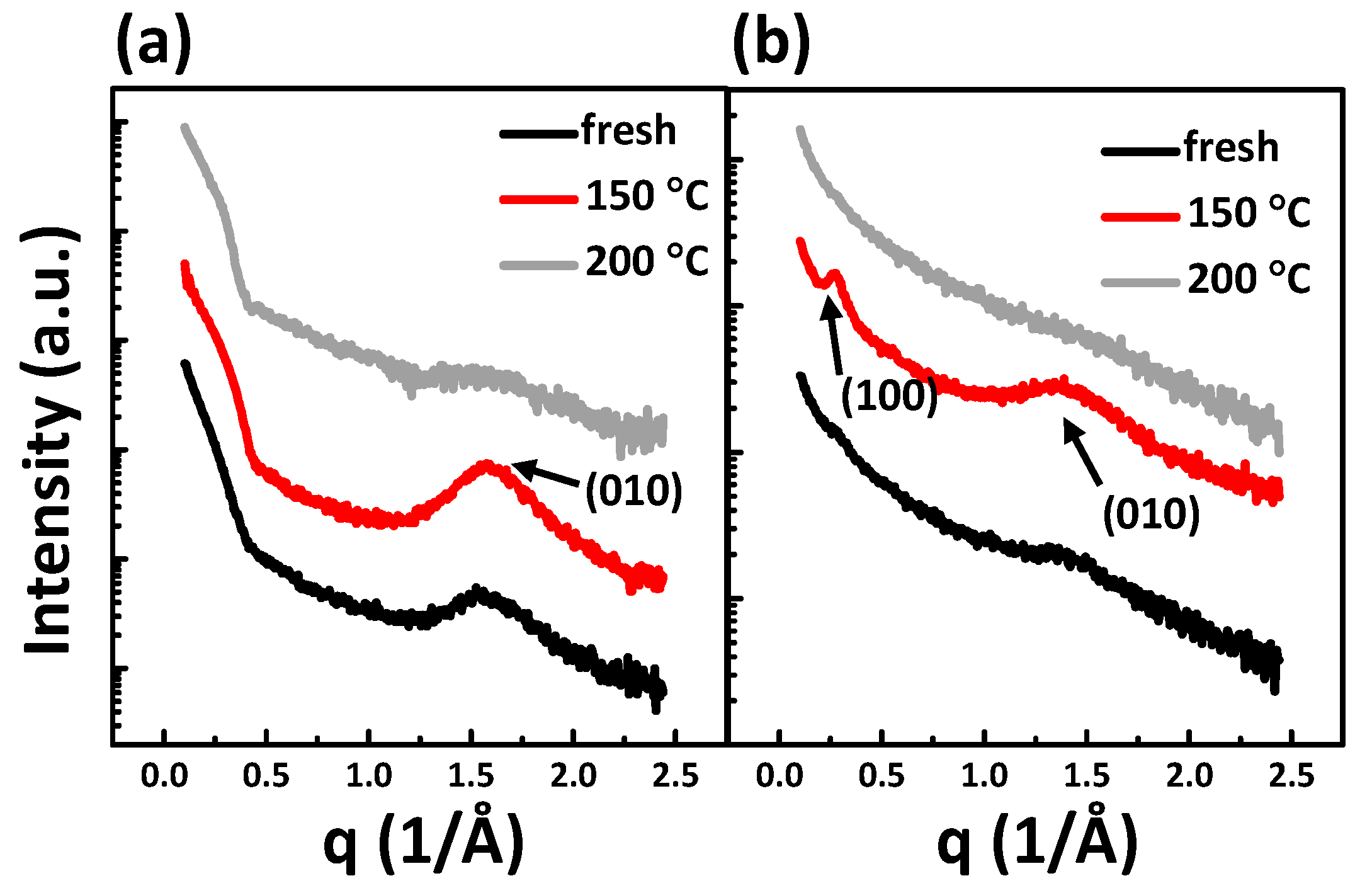
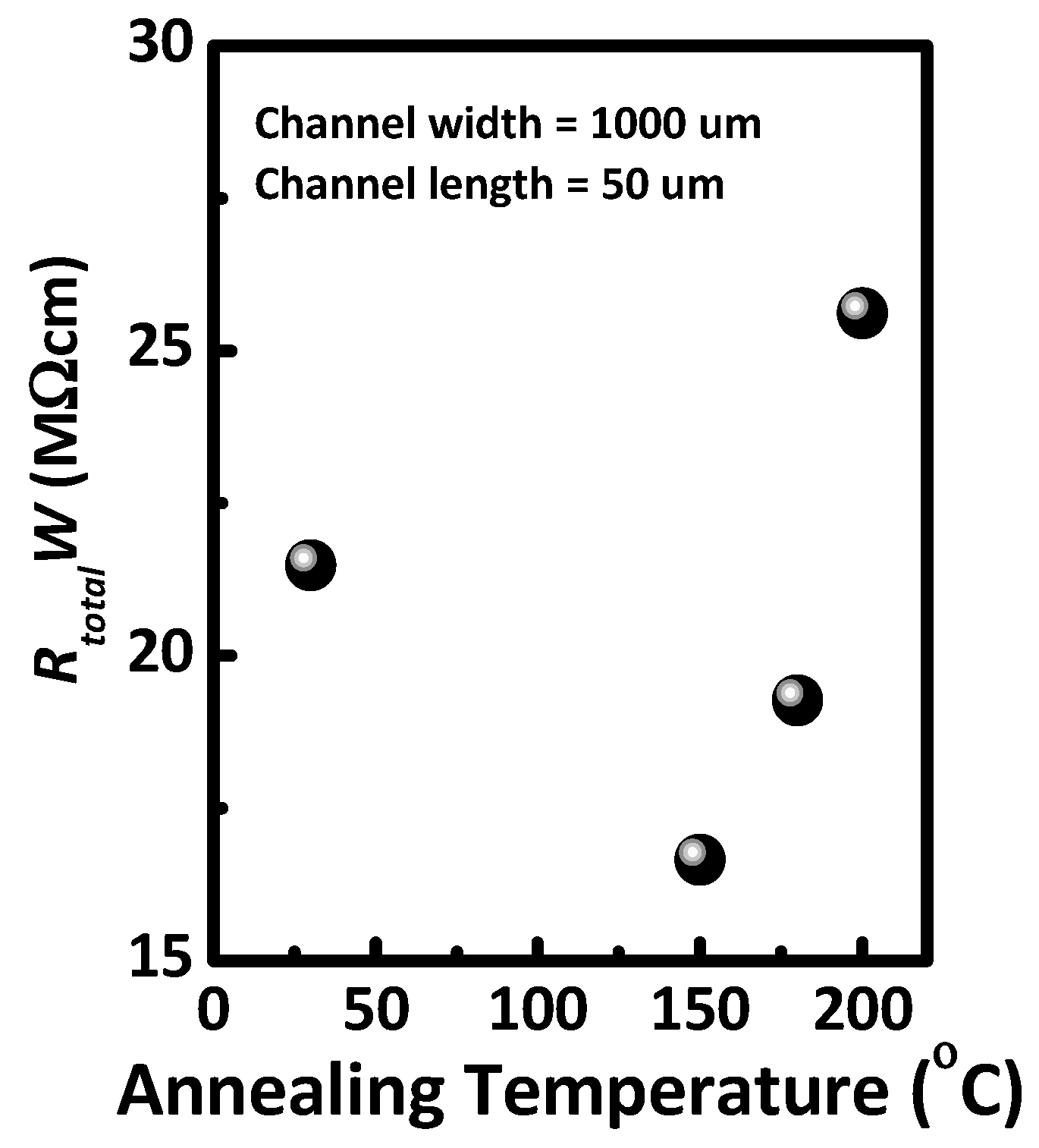
| PQCTQx film | Mobility (cm2/Vs) | On/off | Vth (V) | RtotalW (MΩcm) |
|---|---|---|---|---|
| As-cast | 6.1 × 10−3 | 1.7 × 105 | −9.2 | 21.5 |
| Annealed at 150 °C | 1.2 × 10−2 | 3.0 × 106 | −6.3 | 16.7 |
| Annealed at 200 °C | 6.0 × 10−3 | 5.1 × 104 | −6.1 | 25.6 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jeong, Y.J.; Oh, J.H.; Song, H.J.; An, T.K. A Quinacridone-Diphenylquinoxaline-Based Copolymer for Organic Field-Effect Transistors. Polymers 2019, 11, 563. https://doi.org/10.3390/polym11030563
Jeong YJ, Oh JH, Song HJ, An TK. A Quinacridone-Diphenylquinoxaline-Based Copolymer for Organic Field-Effect Transistors. Polymers. 2019; 11(3):563. https://doi.org/10.3390/polym11030563
Chicago/Turabian StyleJeong, Yong Jin, Jeong Hyun Oh, Ho Jun Song, and Tae Kyu An. 2019. "A Quinacridone-Diphenylquinoxaline-Based Copolymer for Organic Field-Effect Transistors" Polymers 11, no. 3: 563. https://doi.org/10.3390/polym11030563
APA StyleJeong, Y. J., Oh, J. H., Song, H. J., & An, T. K. (2019). A Quinacridone-Diphenylquinoxaline-Based Copolymer for Organic Field-Effect Transistors. Polymers, 11(3), 563. https://doi.org/10.3390/polym11030563