Ultrathin Photonic Polymer Gel Films Templated by Non-Close-Packed Monolayer Colloidal Crystals to Enhance Colorimetric Sensing
Abstract
1. Introduction
2. Materials and Methods
2.1. Raw Material and Reactants
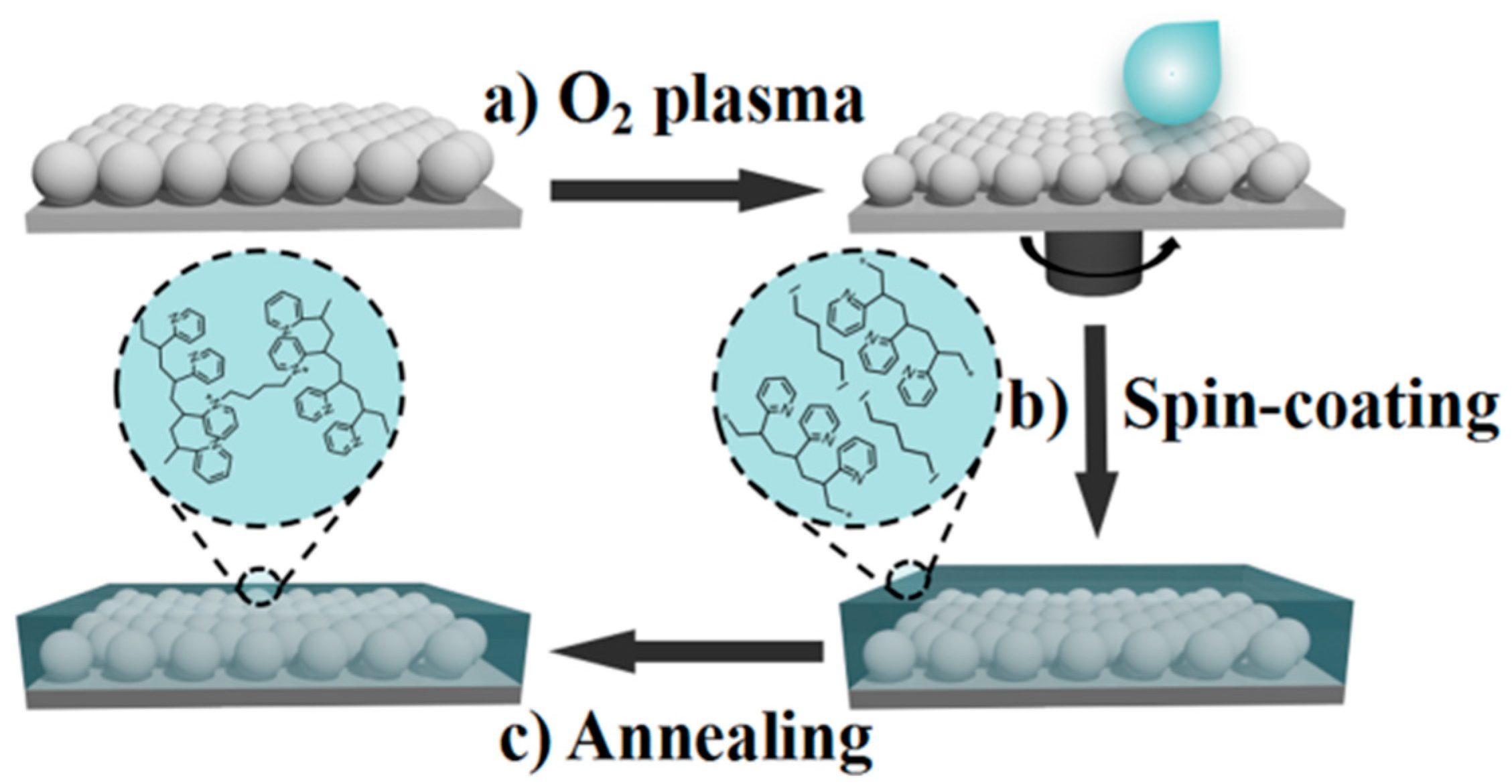
2.2. Preparation of Close-Packed Monolayer Colloidal Crystals
2.3. Preparation of UPPGF
2.4. Characterization
2.5. Sensor Test
3. Results and Discussion
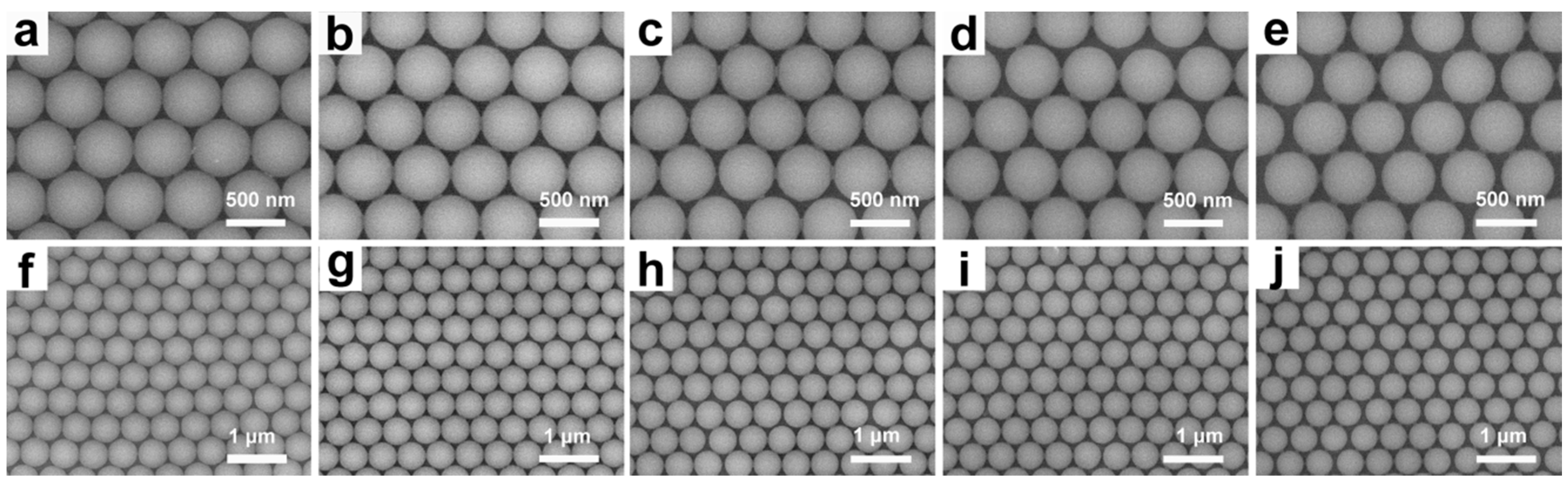
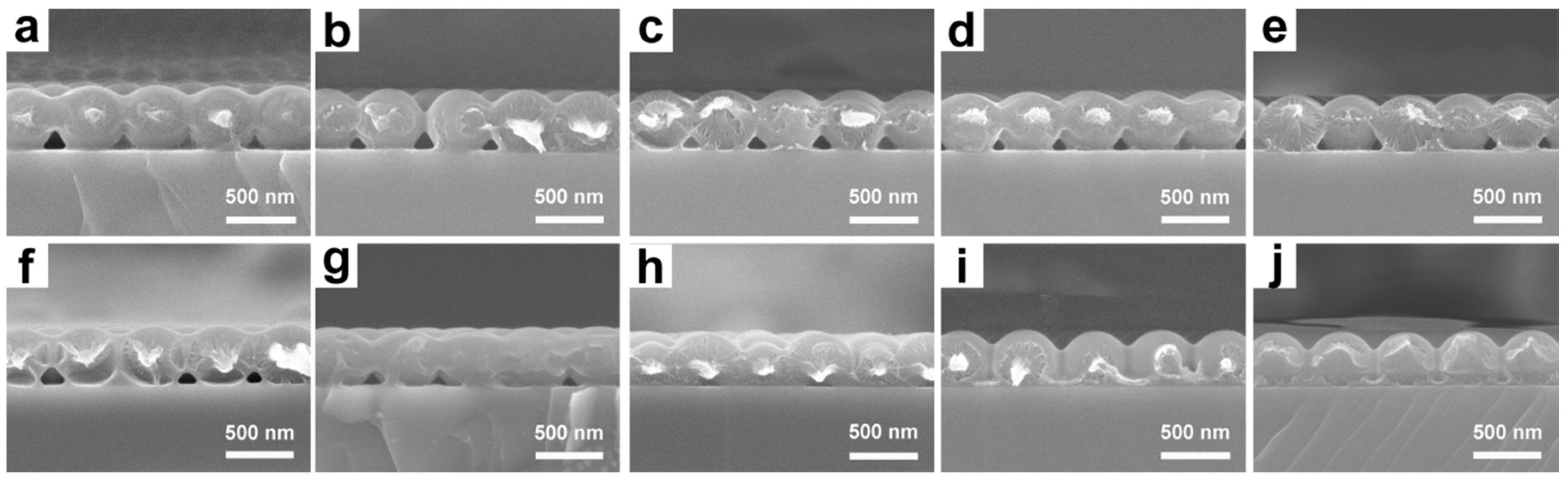
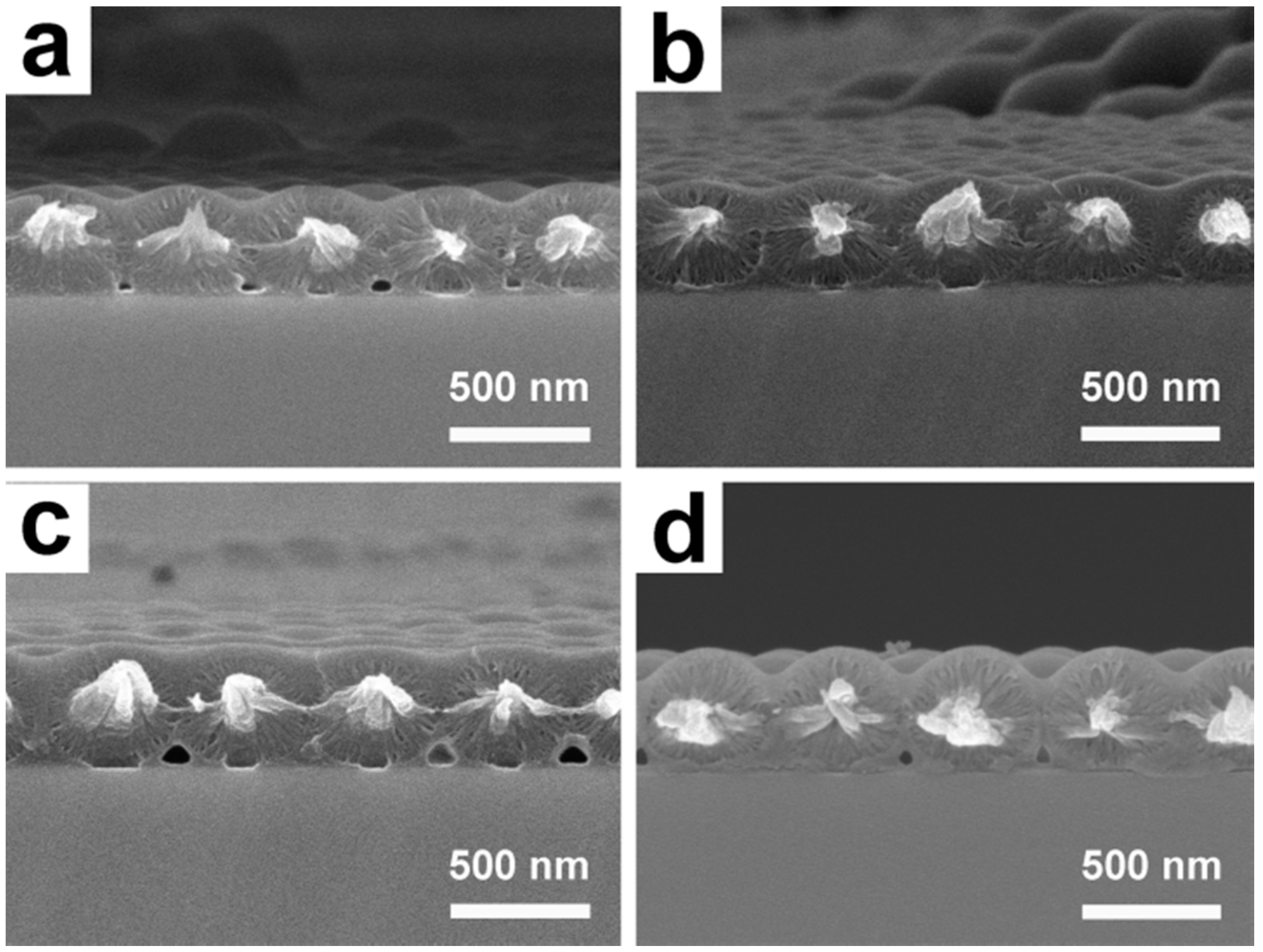
3.1. Preparation and Characterization of UPPGF
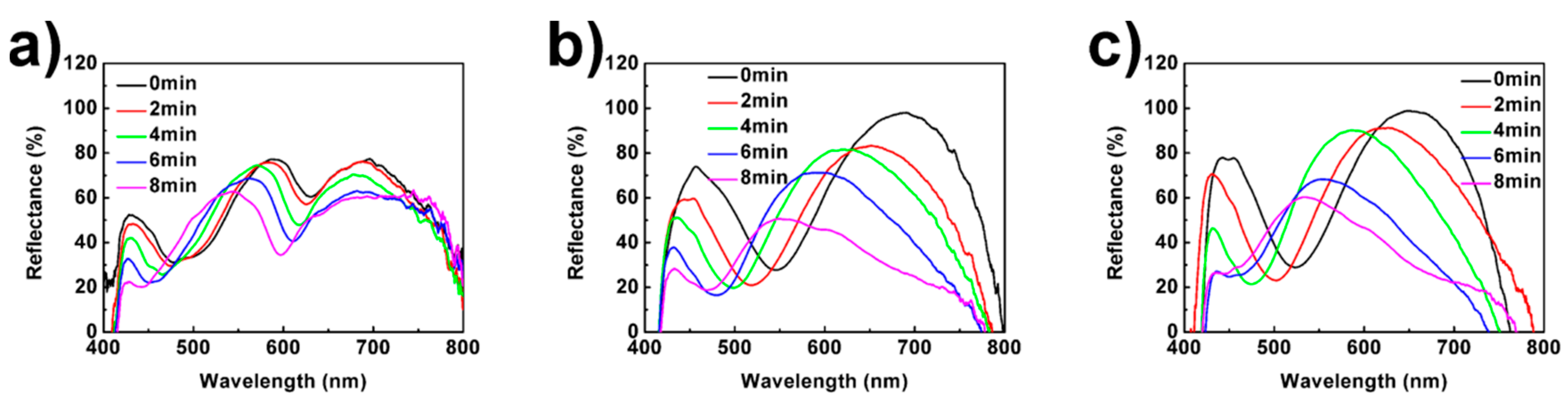
3.2. Optical Properties of UPPGF
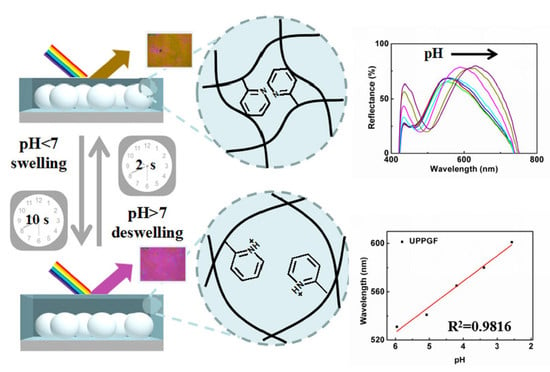
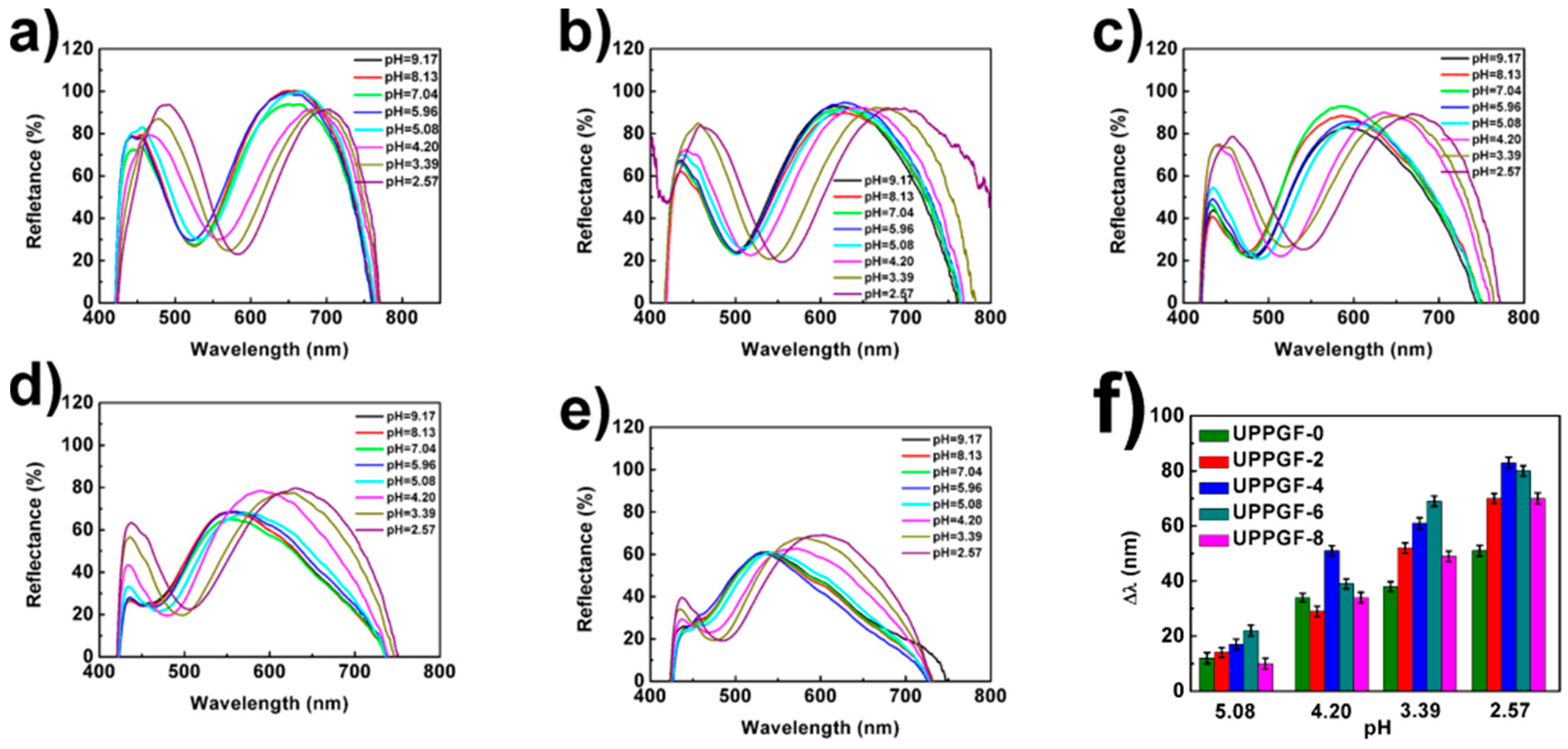
3.3. Responsiveness of the UPPGF to pH and Mechanism Research
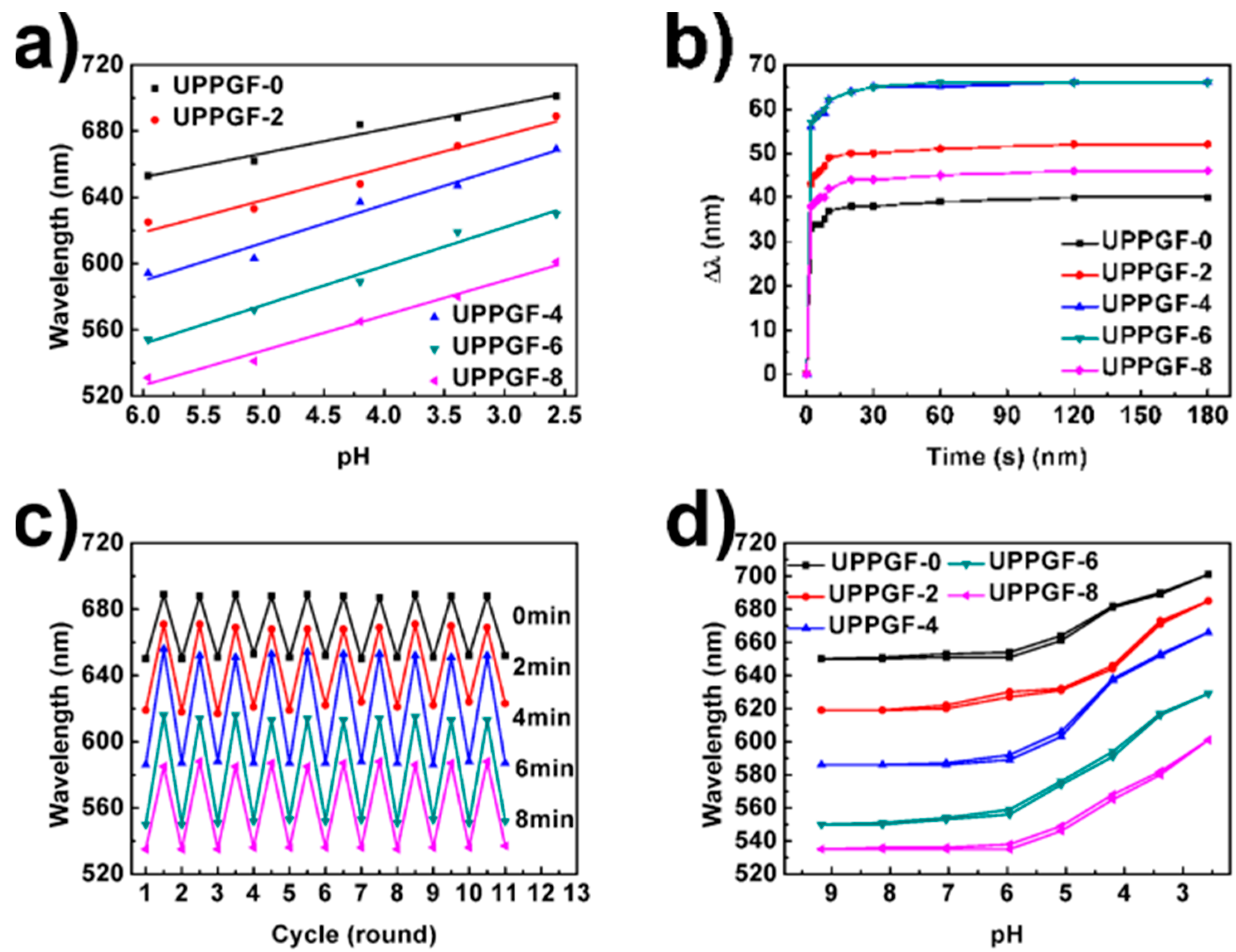
3.4. Linearity of the UPPGF to pH and Mechanism Research
3.5. Response Speed of the UPPGF to pH
3.6. Stability of the UPPGF to pH
3.7. Hysteresis Loops of the UPPGF to pH
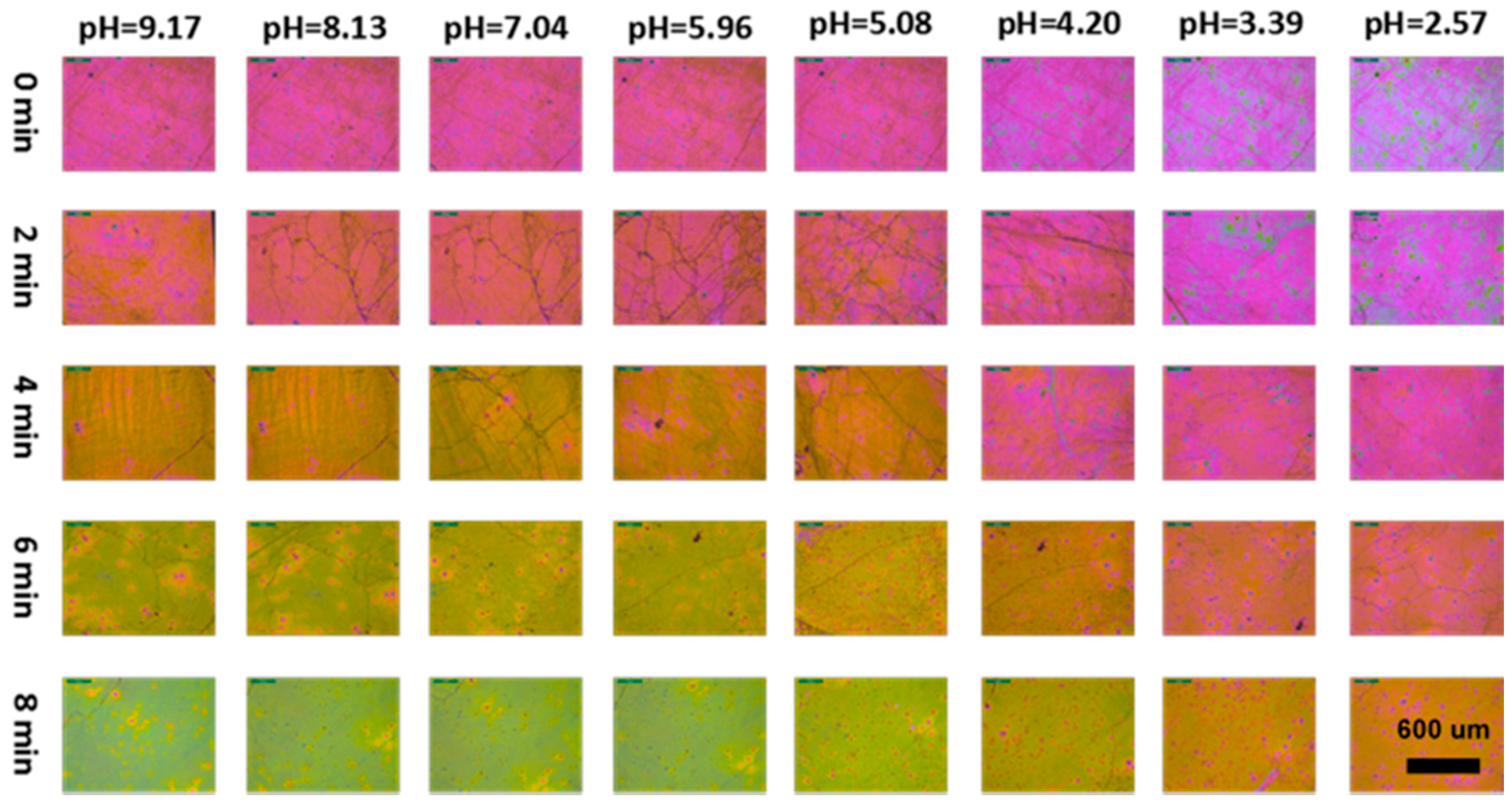
3.8. Visual Response of the UPPGF to pH
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Isapour, G.; Lattuada, M. Bioinspired Stimuli-Responsive Color-Changing Systems. Adv. Mater. 2018, 30, 1707069. [Google Scholar] [CrossRef]
- Teyssier, J.; Saenko, S.V.; van der Marel, D.; Milinkovitch, M.C. Photonic crystals cause active colour change in chameleons. Nat. Commun. 2015, 6, 6368. [Google Scholar] [CrossRef] [PubMed]
- Rossiter, J.; Yap, B.; Conn, A. Biomimetic chromatophores for camouflage and soft active surfaces. Bioinspir. Biomim. 2012, 7, 036009. [Google Scholar] [CrossRef] [PubMed]
- Takeoka, Y.; Watanabe, M. Tuning Structural Color Changes of Porous Thermosensitive Gels through Quantitative Adjustment of the Cross-Linker in Pre-gel Solutions. Langmuir 2003, 19, 9104–9106. [Google Scholar] [CrossRef]
- Stumpel, J.E.; Broer, D.J.; Schenning, A.P.H.J. Stimuli-responsive photonic polymer coatings. Chem. Commun. 2014, 50, 15839–15848. [Google Scholar] [CrossRef]
- Lee, J.H.; Koh, C.Y.; Singer, J.P.; Jeon, S.J.; Maloovan, M.; Stein, O.; Thomas, E.L. 25th anniversary article: Ordered polymer structures for the engineering of photons and phonons. Adv. Mater. 2014, 26, 532–569. [Google Scholar] [CrossRef] [PubMed]
- Stuart, M.A.C.; Huck, W.T.S.; Genzer, J.; Marcus, M.; Ober, C.; Stamm, M.; Sukhorukov, G.B.; Szleifer, I.; Tsukruk, V.V.; Urban, M.; et al. Emerging applications of stimuli-responsive polymer materials. Nat. Mater. 2010, 9, 101–103. [Google Scholar] [CrossRef]
- Hendrickson, R.G.H.; Lyon, L.A. Bioresponsive hydrogels for sensing applications. Soft Matter 2009, 5, 29–35. [Google Scholar] [CrossRef]
- Wei, M.; Gao, Y.F.; Li, X.; Serpe, M.J. Stimuli-responsive polymers and their applications. Polym. Chem. 2017, 8, 127–143. [Google Scholar] [CrossRef]
- Paquet, C.; Kumacheva, E. Nanostructured polymers for photonics. Mater. Today 2008, 11, 48–56. [Google Scholar] [CrossRef]
- Holtz, J.H.; Asher, S.A. Polymerized colloidal crystal hydrogel films as intelligent chemical sensing materials. Nature 1997, 389, 829–832. [Google Scholar] [CrossRef] [PubMed]
- Asher, S.A.; Alexeev, V.L.; Goponenko, A.V.; Sharma, A.C.; Lednev, I.K.; Wilcox, C.S.; Finegold, D.N. Photonic crystal carbohydrate sensors: Low ionic strength sugar sensing. J. Am. Chem. Soc. 2003, 125, 3322–3329. [Google Scholar] [CrossRef] [PubMed]
- Ben-moshe, M.; Alexeev, V.L.; Asher, S.A. Fast responsive crystalline colloidal array photonic crystal glucose sensors. Anal. Chem. 2006, 78, 5149–5157. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.L.; Goponenko, A.V.; Asher, S.A. Polymerized polyHEMA photonic crystals: pH and ethanol sensor materials. J. Am. Chem. Soc. 2008, 130, 3113–3119. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.T.; Wang, L.L.; Luo, J.; Tikhonov, A.; Kornienko, N.; Asher, S.A. Photonic crystal chemical sensors: pH and ionic strength. J. Am. Chem. Soc. 2011, 133, 9152–9155. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.T.; Chao, X.; Liu, X.L.; Asher, S.A. Two-dimensional array Debye ring diffraction protein recognition sensing. Chem. Commun. 2013, 49, 6337–6339. [Google Scholar] [CrossRef] [PubMed]
- Cai, Z.Y.; Zhang, J.T.; Xue, F.; Hong, Z.M.; Punihaole, D.; Asher, S.A. 2D photonic crystal protein hydrogel coulometer for sensing serum albumin ligand binding. Anal. Chem. 2014, 86, 4840–4847. [Google Scholar] [CrossRef]
- Cai, Z.Y.; Smith, N.L.; Zhang, J.T.; Asher, S.A. Two-dimensional photonic crystal chemical and biomolecular sensors. Anal. Chem. 2015, 87, 5013–5025. [Google Scholar] [CrossRef] [PubMed]
- Cai, Z.Y.; Luck, L.A.; Punihaole, D.; Madurac, J.D.; Asher, S.A. Photonic crystal protein hydrogel sensor materials enabled by conformationally induced volume phase transition. Chem. Sci. 2016, 7, 4557–4562. [Google Scholar] [CrossRef]
- Kang, H.S.; Lee, J.; Cho, S.M.; Park, T.H.; Kim, M.J.; Park, C.; Lee, S.W.; Kim, K.L.; Ryu, D.Y.; Huh, J. Printable and rewritable full block copolymer structural color. Adv. Mater. 2017, 29, 1700084. [Google Scholar] [CrossRef]
- Park, T.H.; Yu, S.; Cho, S.H.; Kang, H.S.; Kim, Y.; Kim, M.J.; Eoh, H.; Park, C.; Jeong, B.; Lee, S.W.; et al. Block copolymer structural color strain sensor. NPG Asia Mater. 2018, 10, 328–339. [Google Scholar] [CrossRef]
- Cassagneau, T.; Caruso, F. Conjugated polymer inverse opals for potentiometric biosensing. Adv. Mater. 2002, 14, 1837–1841. [Google Scholar] [CrossRef]
- Lee, Y.J.; Braun, P.V. Tunable inverse opal hydrogel pH sensors. Adv. Mater. 2003, 15, 563–566. [Google Scholar] [CrossRef]
- Xu, X.B.; An, Q.; Li, G.T.; Tao, S.Y.; Liu, J. Imprinted photonic polymers for chiral recognition. Angew. Chem. Int. Ed. 2006, 45, 8145–8148. [Google Scholar]
- Hong, W.; Hu, X.B.; Zhao, B.Y.; Zhang, F.; Zhang, D. Tunable photonic polyelectrolyte colorimetric sensing for anions, cations and zwitterions. Adv. Mater. 2010, 22, 5043–5047. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.J.; Cano, G.G.; Braun, P.V. Linear and fast hydrogel glucose sensor materials enabled by volume resetting agents. Adv. Mater. 2014, 26, 5678–5683. [Google Scholar] [CrossRef] [PubMed]
- Couturier, J.P.; Sutterlin, M.; Laschewsky, A.; Hettrich, C.; Wischerhoff, E. Responsive inverse opal hydrogels for the sensing of macromolecules. Angew. Chem. Int. Ed. 2015, 54, 6641–6644. [Google Scholar] [CrossRef] [PubMed]
- Shin, J.; Han, S.G.; Lee, W. Dually tunable inverse opal hydrogel colorimetric sensor with fast and reversible color changes. Sens. Actuators B 2012, 168, 20–26. [Google Scholar] [CrossRef]
- Tanaka, T.; Fillmore, D.J. Kinetics of swelling of gels. J. Chem. Phys. 1979, 70, 1214–1218. [Google Scholar] [CrossRef]
- Zhang, X.; Guan, Y.; Zhang, Y.J. Ultrathin hydrogel films for rapid optical biosensing. Biomacromolecules 2012, 13, 92–97. [Google Scholar] [CrossRef]
- Li, C.; Lotsch, B.V. Stimuli-responsive 2D polyelectrolyte photonic crystals for optically encoded pH sensing. Chem. Commun. 2012, 48, 6169–6171. [Google Scholar] [CrossRef]
- Yu, S.M.; Han, Z.M.; Jiao, X.L.; Chen, D.R.; Li, C. Ultrathin polymer gel-infiltrated monolayer colloidal crystal films for rapid colorimetric chemical sensing. RSC Adv. 2016, 6, 66191–66196. [Google Scholar] [CrossRef]
- Hu, L.; Serpe, M.J. Controlling the response of color tunable poly (N-isopropylacrylamide) microgel-based etalons with hysteresis. Chem. Commun. 2013, 49, 2649–2651. [Google Scholar] [CrossRef]
- Holland, B.T.; Blanford, C.F.; Do, T.; Stein, A. Synthesis of highly ordered, three-dimensional, macroporous structures of amorphous or crystalline inorganic oxides, phosphates, and hybrid composites. Chem. Mater. 1999, 11, 795–805. [Google Scholar] [CrossRef]
- Li, C.; Hong, G.; Wang, P.; Yu, D.; Qi, L. Wet chemical approaches to patterned arrays of well-aligned ZnO nanopillars assisted by monolayer colloidal crystals. Chem. Mater. 2009, 21, 891–897. [Google Scholar] [CrossRef]
- Tokarev, I.; Orlov, M.; Minko, S. Responsive polyelectrolyte gel membranes. Adv. Mater. 2006, 18, 2458–2460. [Google Scholar] [CrossRef]
- Romanov, S.G.; Vogel, N.; Bley, K.; Landfester, K.; Weiss, C.K.; Orlov, S.; Korovin, A.V.; Chuiko, G.P.; Regensourger, A.; Romanova, A.S.; et al. Probing guided modes in a monolayer colloidal crystal on a flat metal film. Phys. Rev. B 2012, 86, 195145. [Google Scholar] [CrossRef]
- Lu, G.; Hupp, J.T. Metal-Organic Frameworks as Sensors: A ZIF-8 Based Fabry-Pérot Device as a Selective Sensor for Chemical Vapors and Gases. J. Am. Chem. Soc. 2010, 132, 7832–7833. [Google Scholar] [CrossRef]
- López-Garcia, M.; Galisteo-Lóper, J.F.; López, C.; Garcia-Martín, A. Light confinement by two-dimensional arrays of dielectric spheres. Phys. Rev. B 2012, 85, 235145. [Google Scholar] [CrossRef]

© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, S.; Dong, S.; Jiao, X.; Li, C.; Chen, D. Ultrathin Photonic Polymer Gel Films Templated by Non-Close-Packed Monolayer Colloidal Crystals to Enhance Colorimetric Sensing. Polymers 2019, 11, 534. https://doi.org/10.3390/polym11030534
Yu S, Dong S, Jiao X, Li C, Chen D. Ultrathin Photonic Polymer Gel Films Templated by Non-Close-Packed Monolayer Colloidal Crystals to Enhance Colorimetric Sensing. Polymers. 2019; 11(3):534. https://doi.org/10.3390/polym11030534
Chicago/Turabian StyleYu, Shimo, Shun Dong, Xiuling Jiao, Cheng Li, and Dairong Chen. 2019. "Ultrathin Photonic Polymer Gel Films Templated by Non-Close-Packed Monolayer Colloidal Crystals to Enhance Colorimetric Sensing" Polymers 11, no. 3: 534. https://doi.org/10.3390/polym11030534
APA StyleYu, S., Dong, S., Jiao, X., Li, C., & Chen, D. (2019). Ultrathin Photonic Polymer Gel Films Templated by Non-Close-Packed Monolayer Colloidal Crystals to Enhance Colorimetric Sensing. Polymers, 11(3), 534. https://doi.org/10.3390/polym11030534