Thermal Conductivity of Protein-Based Materials: A Review
Abstract
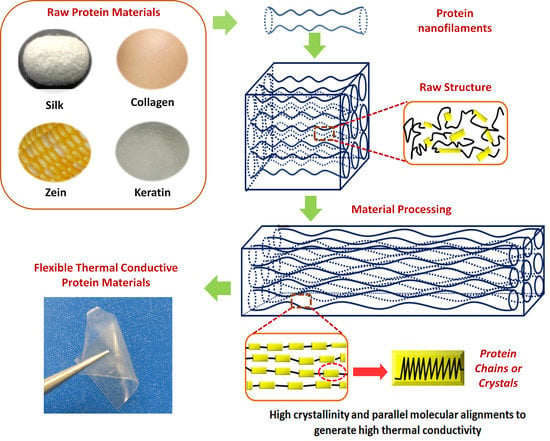
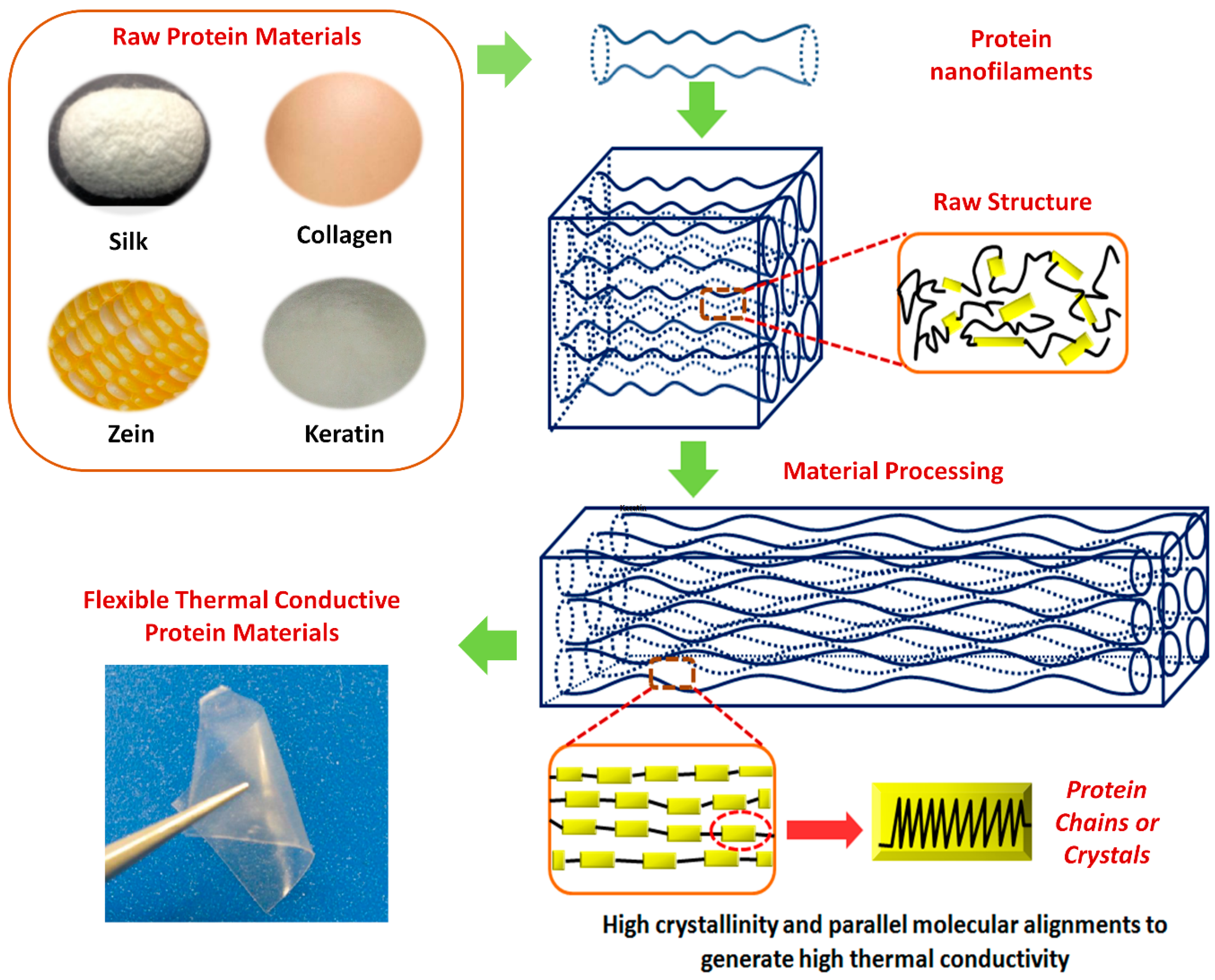
1. Introduction
2. Structure of Protein-Based Polymers
2.1. Silk
2.2. Collagen
2.3. Keratin
3. Parameters to Influence the Thermal Conductivity of Protein Polymers
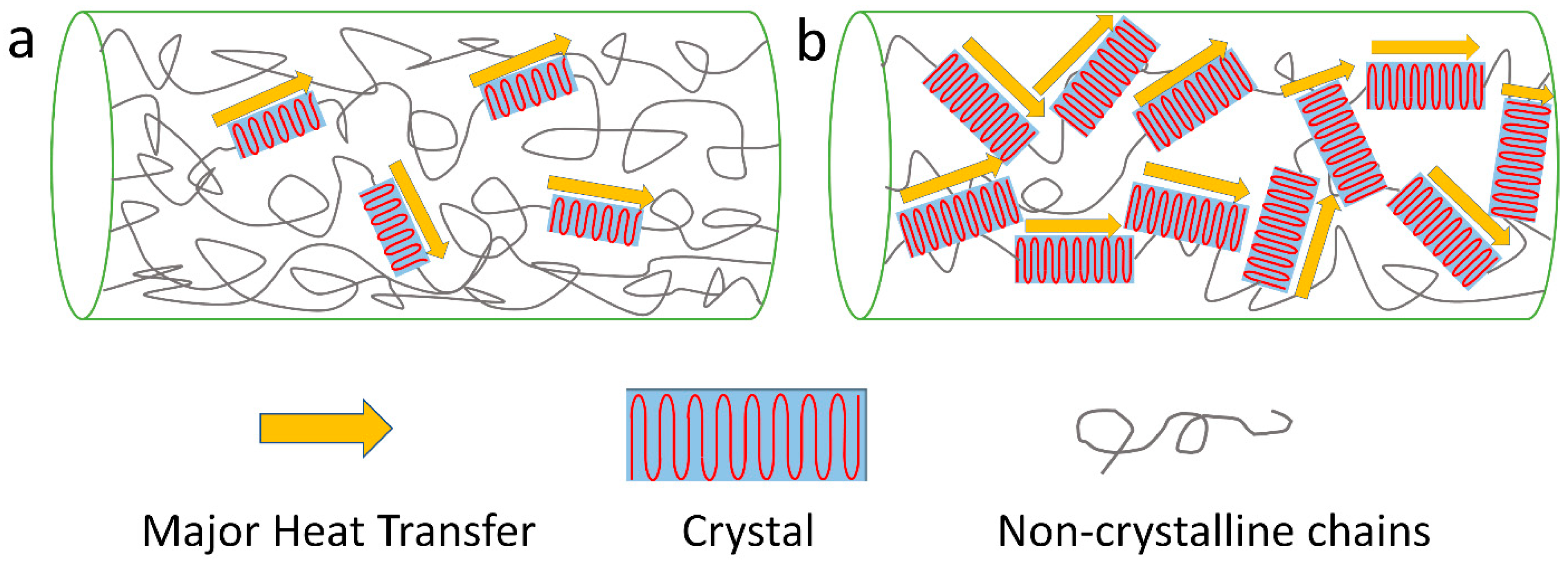
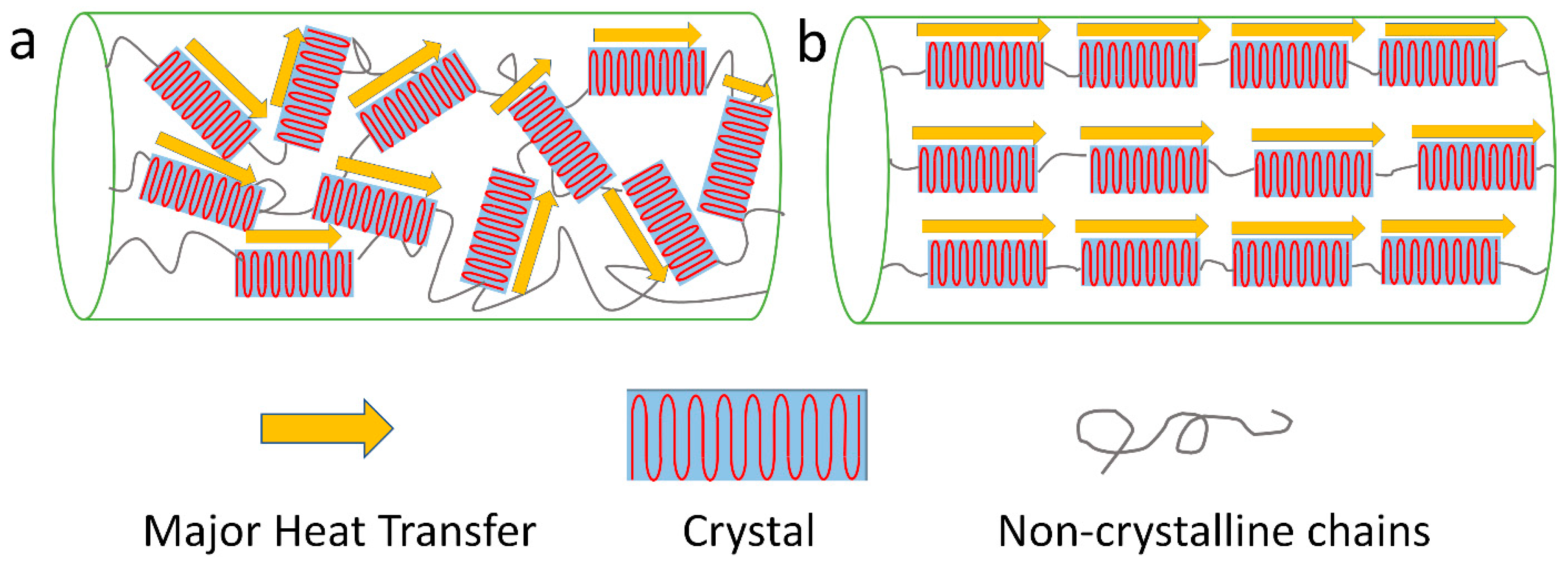
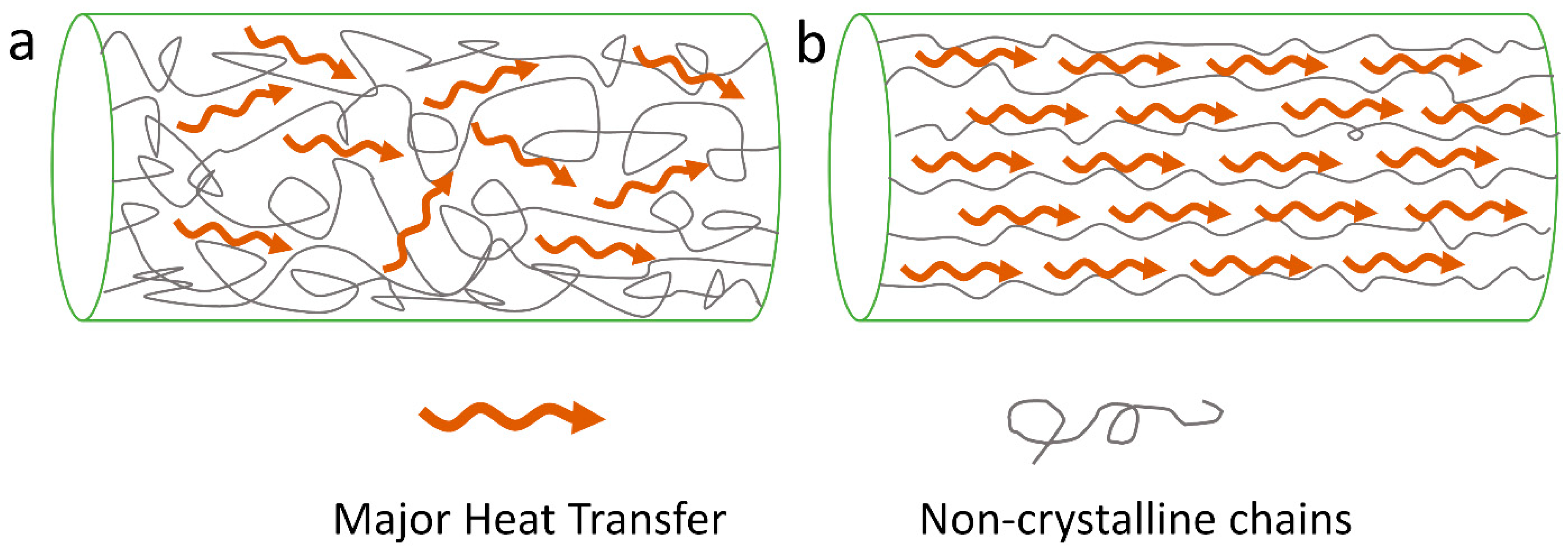
3.1. Crystallinity and Crystal Alignment
3.2. Chain Orientation
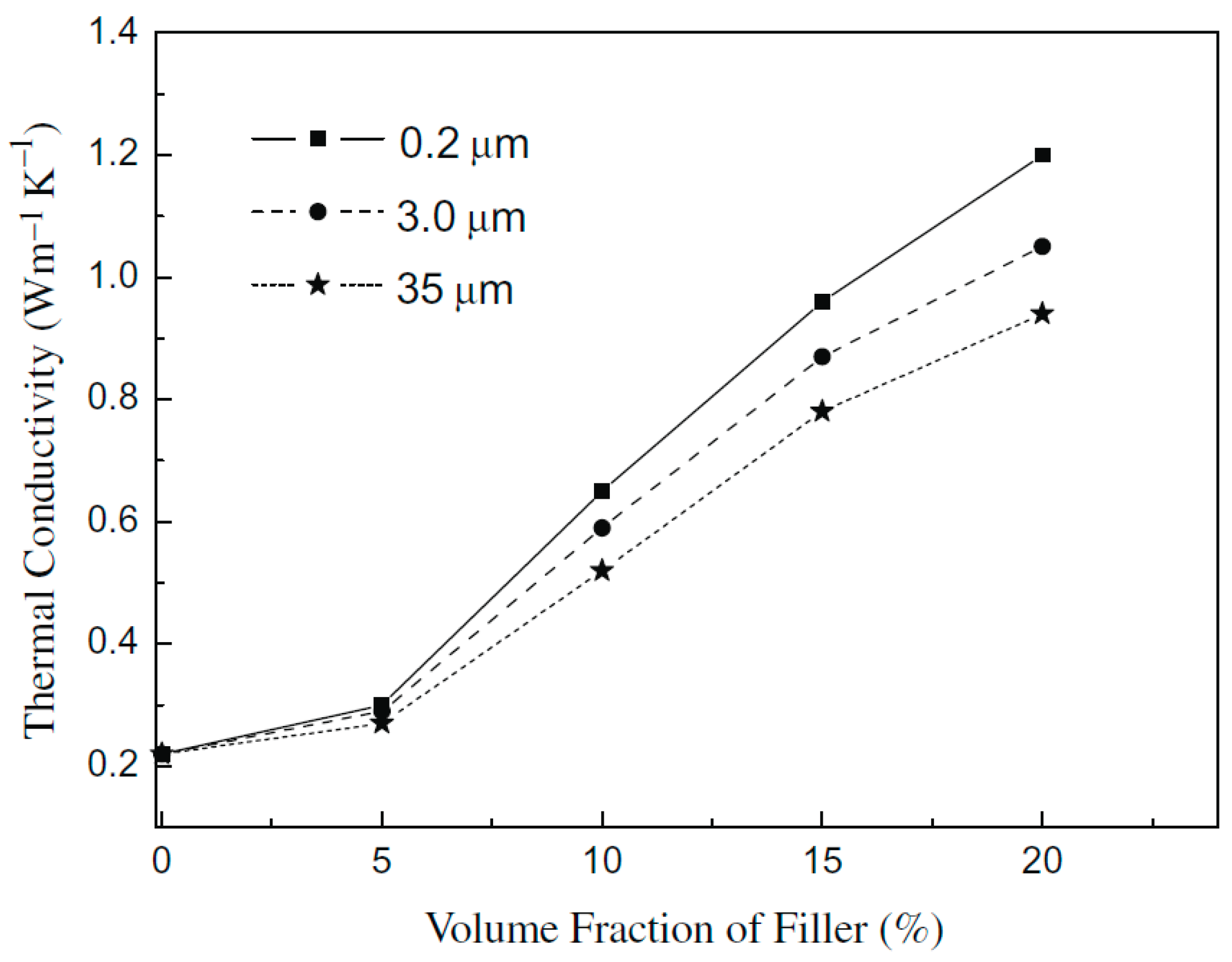
3.3. Composites
3.4. Other Parameters
4. Experimental Methods to Measure the Thermal Conductivity of Protein-Based Materials
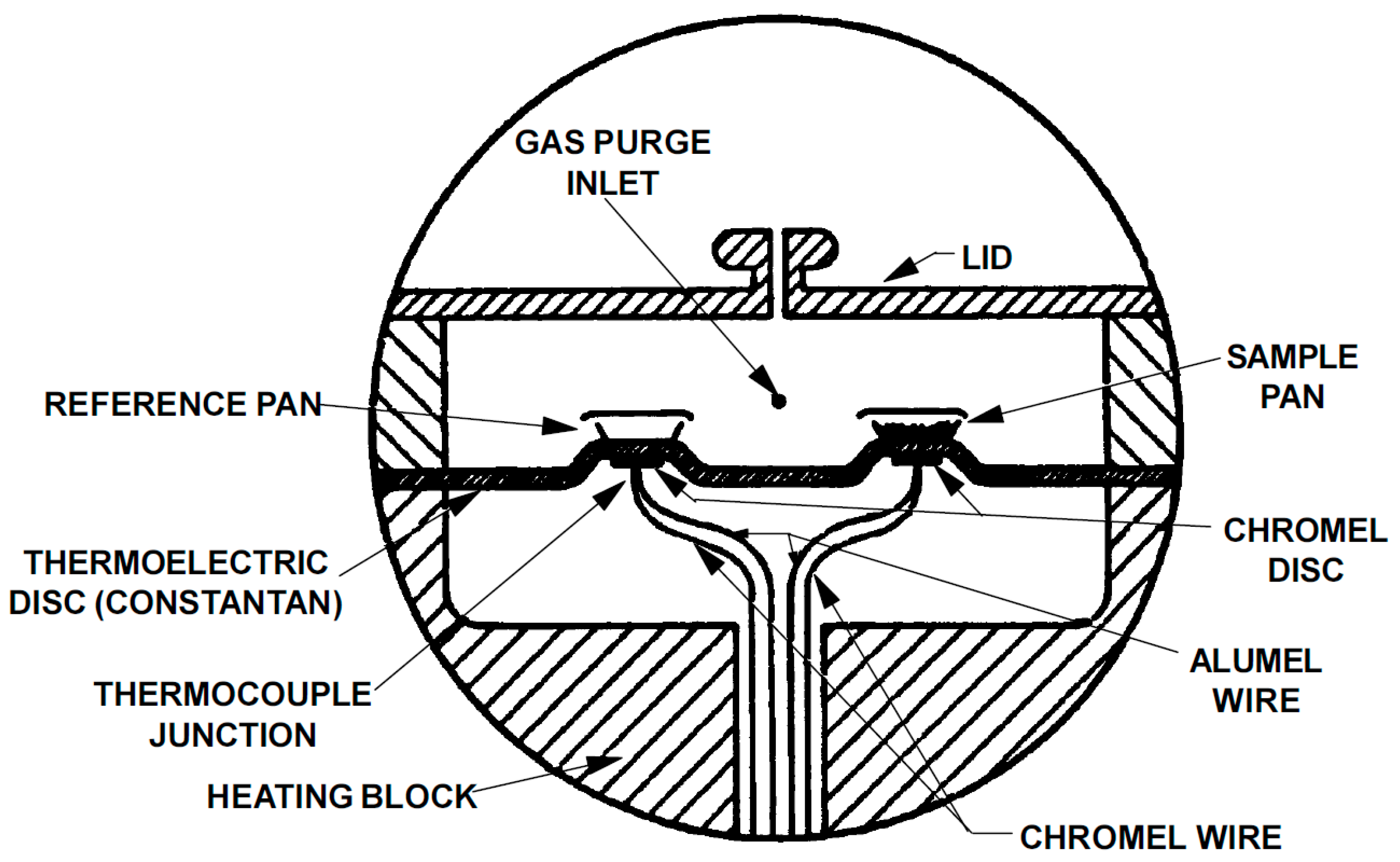
4.1. Temperature-Modulated Differential Scanning Calorimetry Method
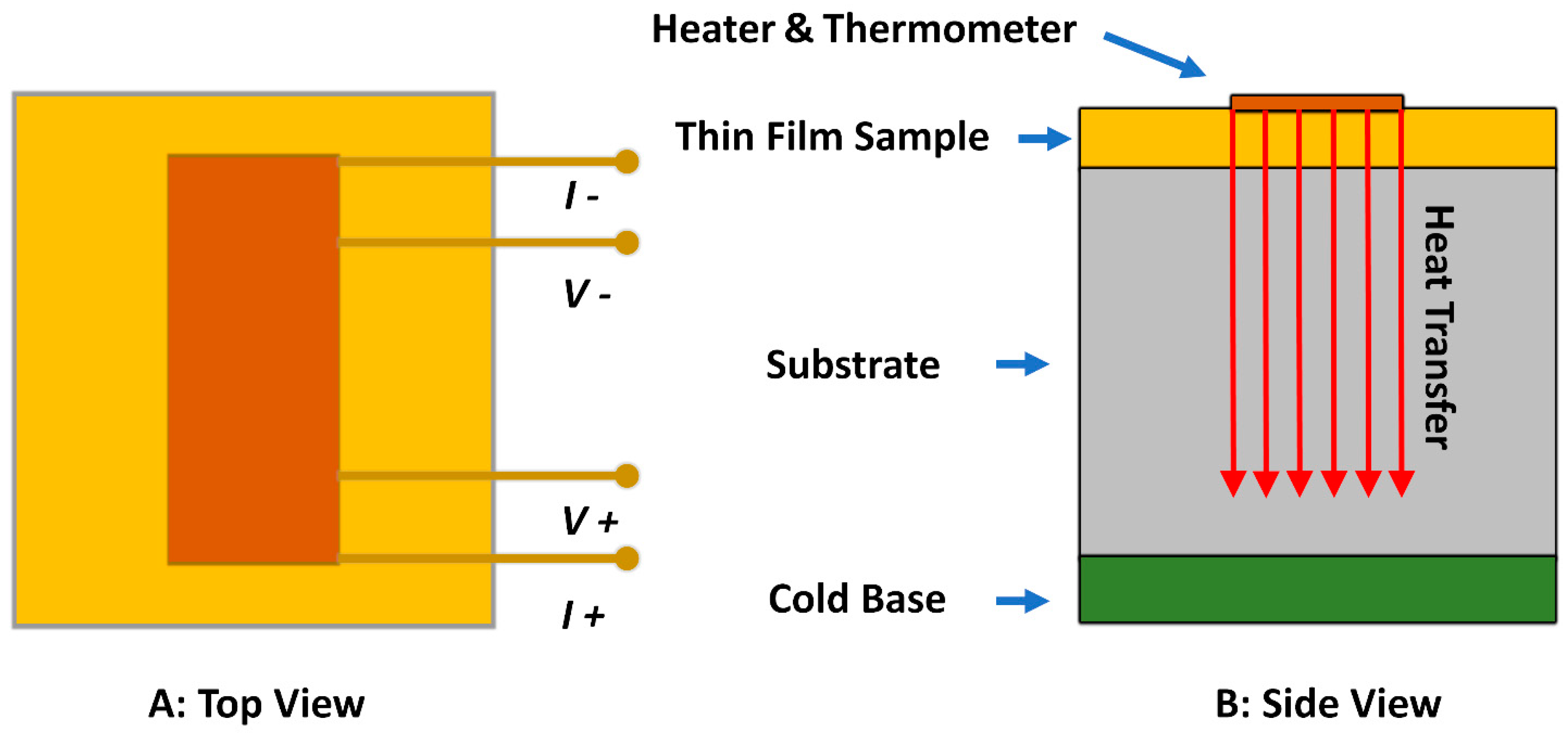
4.2. 3-ω Method (Transient Hot Wire Method)
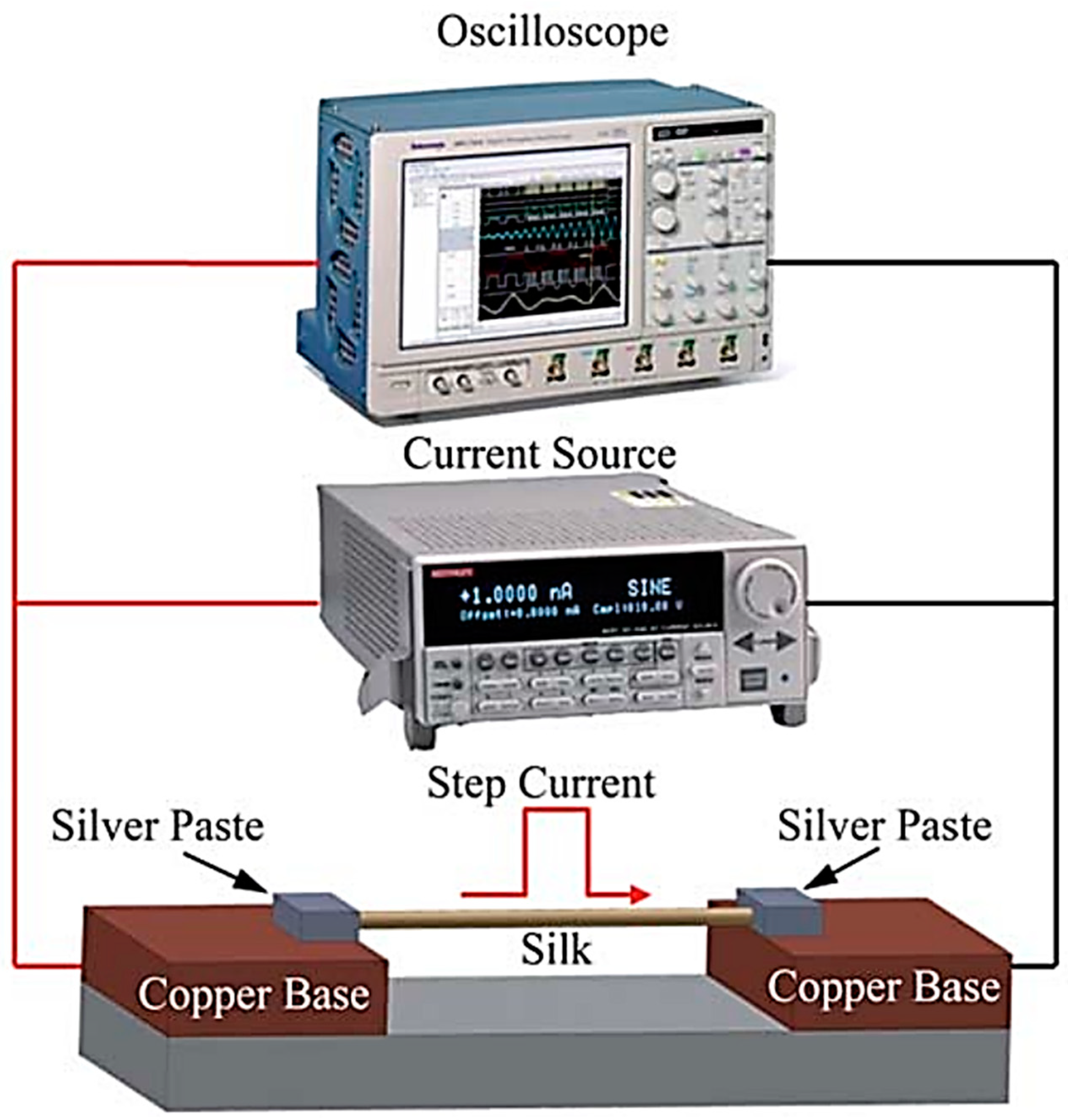
4.3. Transient Electrothermal Technique (TET) Method
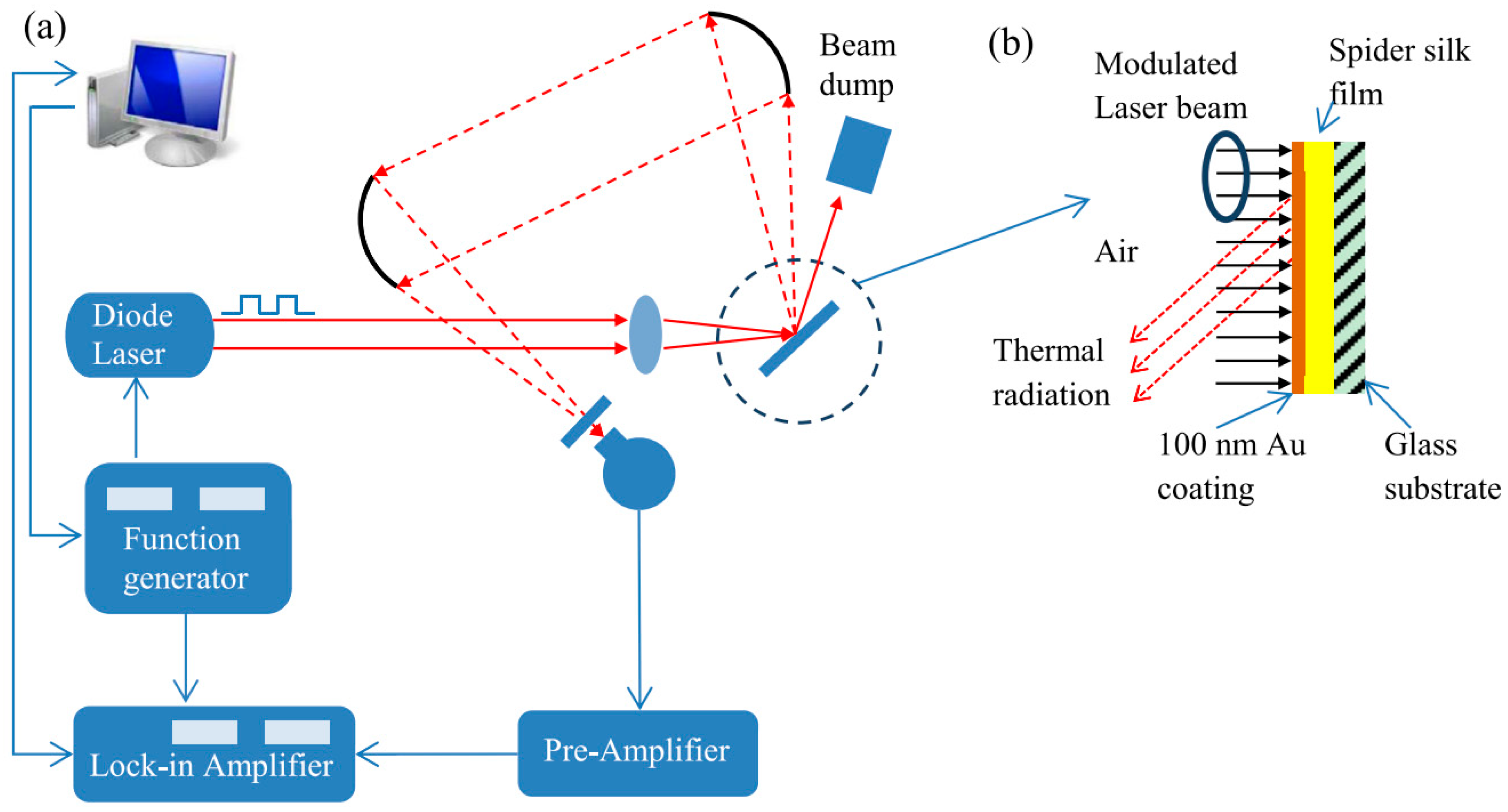
4.4. Photothermal Technique
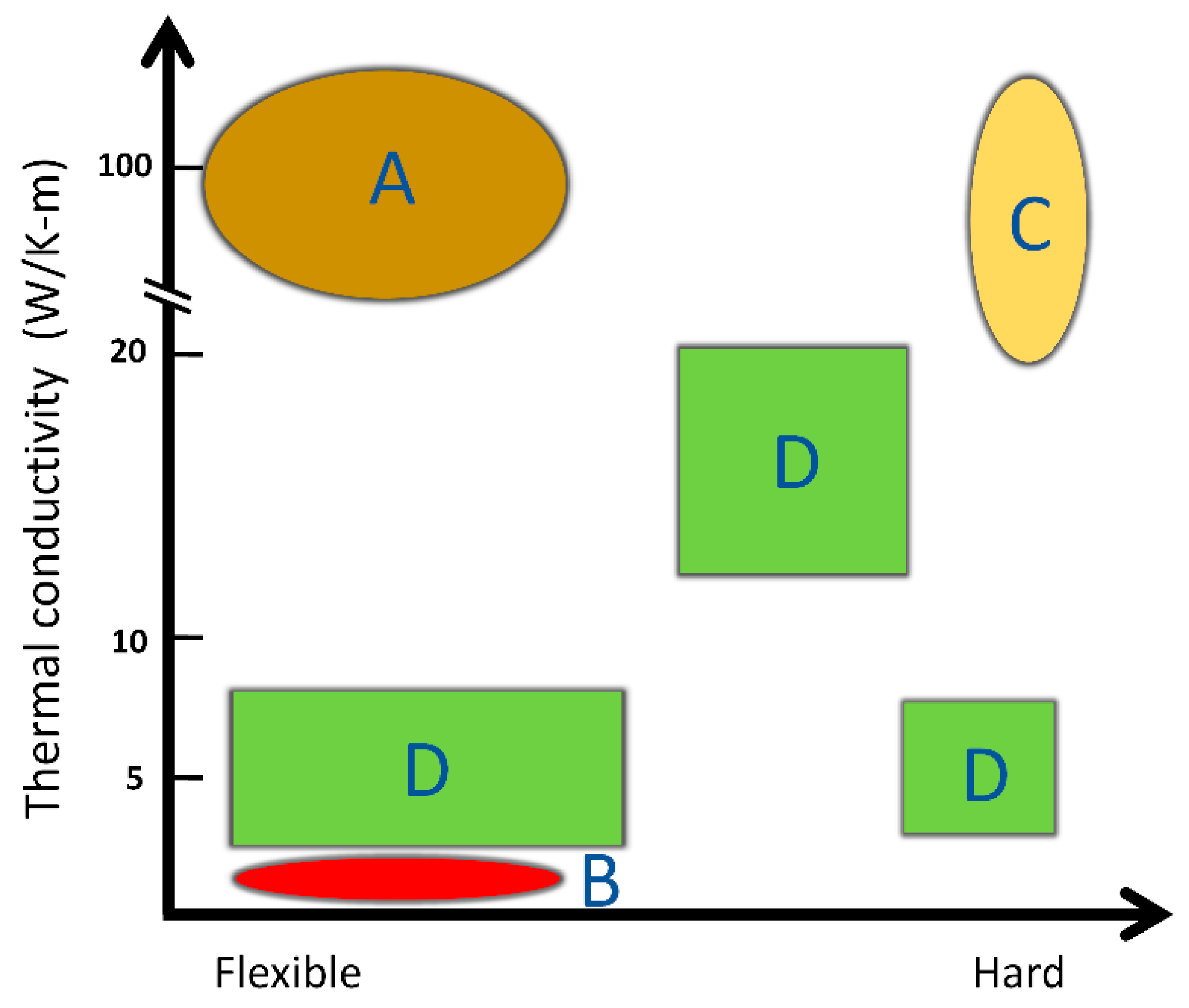
5. Thermal Conductivity of Different Types of Protein-Based Materials
6. Thermal Conductivity Differences between 1-D Fibers and 2-D Films
7. Conclusions and Future Development
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Mülhaupt, R. Green polymer chemistry and bio-based plastics: Dreams and reality. Macromol. Chem. Phys. 2013, 214, 159–174. [Google Scholar] [CrossRef]
- Shahil, K.M.; Balandin, A.A. Graphene–multilayer graphene nanocomposites as highly efficient thermal interface materials. Nano Lett. 2012, 12, 861–867. [Google Scholar] [CrossRef] [PubMed]
- Jin, X.; Zhang, J.; Gao, W.; Li, J.; Wang, X. Cocoon of the silkworm antheraea pernyi as an example of a thermally insulating biological interface. Biointerphases 2014, 9, 031013. [Google Scholar] [CrossRef] [PubMed]
- Haxaire, K.; Marechal, Y.; Milas, M.; Rinaudo, M. Hydration of polysaccharide hyaluronan observed by ir spectrometry. I. Preliminary experiments and band assignments. Biopolymers 2003, 72, 10–20. [Google Scholar] [CrossRef] [PubMed]
- Toberer, E.S.; Baranowski, L.L.; Dames, C. Advances in thermal conductivity. Annu. Rev. Mater. Res. 2012, 42, 179–209. [Google Scholar] [CrossRef]
- Anderson, D. Thermal conductivity of polymers. Chem. Rev. 1966, 66, 677–690. [Google Scholar] [CrossRef]
- Shen, S.; Henry, A.; Tong, J.; Zheng, R.; Chen, G. Polyethylene nanofibres with very high thermal conductivities. Nat. Nanotechnol. 2010, 5, 251–255. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Chen, X.; Shao, Z.; Huang, Y.; Knight, D.P. Effect of metallic ions on silk formation in the mulberry silkworm, bombyx m ori. J. Phys. Chem. B 2005, 109, 16937–16945. [Google Scholar] [CrossRef] [PubMed]
- Han, Z.; Fina, A. Thermal conductivity of carbon nanotubes and their polymer nanocomposites: A review. Prog. Polym. Sci. 2011, 36, 914–944. [Google Scholar] [CrossRef]
- Li, X.; Tabil, L.G.; Oguocha, I.N.; Panigrahi, S. Thermal diffusivity, thermal conductivity, and specific heat of flax fiber–hdpe biocomposites at processing temperatures. Compos. Sci. Technol. 2008, 68, 1753–1758. [Google Scholar] [CrossRef]
- Rajkumar, G.; Srinivasan, J.; Suvitha, L. Development of novel silk/wool hybrid fibre polypropylene composites. Iran. Polym. J. 2013, 22, 277–284. [Google Scholar] [CrossRef]
- Okabe, T.; Fujimura, T.; Okajima, J.; Aiba, S.; Maruyama, S. Non-invasive measurement of effective thermal conductivity of human skin with a guard-heated thermistor probe. Int. J. Heat Mass Transf. 2018, 126, 625–635. [Google Scholar] [CrossRef]
- Tomko, J.A.; Pena-Francesch, A.; Jung, H.; Tyagi, M.; Allen, B.D.; Demirel, M.C.; Hopkins, P.E. Tunable thermal transport and reversible thermal conductivity switching in topologically networked bio-inspired materials. Nat. Nanotechnol. 2018, 13, 959. [Google Scholar] [CrossRef] [PubMed]
- Willis, P.B.; Hsieh, C.-H. Space applications of polymeric materials. Kobunshi 2000, 49, 52–56. [Google Scholar] [CrossRef]
- Keller, T. Recent all-composite and hybrid fibre-reinforced polymer bridges and buildings. Prog. Struct. Eng. Mater. 2001, 3, 132–140. [Google Scholar] [CrossRef]
- Fuchs, E.R.; Field, F.R.; Roth, R.; Kirchain, R.E. Strategic materials selection in the automobile body: Economic opportunities for polymer composite design. Compos. Sci. Technol. 2008, 68, 1989–2002. [Google Scholar] [CrossRef]
- Zubkova, N. A highly effective domestic fire retardant for fireproofing fibrous textile materials. Fibre Chem. 1997, 29, 126–129. [Google Scholar] [CrossRef]
- Bäcklund, T.; Österbacka, R.; Stubb, H.; Bobacka, J.; Ivaska, A. Operating principle of polymer insulator organic thin-film transistors exposed to moisture. J. Appl. Phys. 2005, 98, 074504. [Google Scholar] [CrossRef]
- Henry, A.; Chen, G. High thermal conductivity of single polyethylene chains using molecular dynamics simulations. Phys. Rev. Lett. 2008, 101, 235502. [Google Scholar] [CrossRef] [PubMed]
- Marconnet, A.M.; Yamamoto, N.; Panzer, M.A.; Wardle, B.L.; Goodson, K.E. Thermal conduction in aligned carbon nanotube–polymer nanocomposites with high packing density. ACS Nano 2011, 5, 4818–4825. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y. Fiber and textile waste utilization. Waste Biomass Valoriz. 2010, 1, 135–143. [Google Scholar] [CrossRef]
- Shahinpoor, M.; Kim, K.J. Ionic polymer–metal composites: Iv. Industrial and medical applications. Smart Mater. Struct. 2005, 14, 197. [Google Scholar] [CrossRef]
- Ashori, A. Wood–plastic composites as promising green-composites for automotive industries! Bioresour. Technol. 2008, 99, 4661–4667. [Google Scholar] [CrossRef] [PubMed]
- Polishuk, P. Plastic optical fibers branch out. Commun. Mag. IEEE 2006, 44, 140–148. [Google Scholar] [CrossRef]
- Williams, G.; Trask, R.; Bond, I. A self-healing carbon fibre reinforced polymer for aerospace applications. Compos. Part A Appl. Sci. Manuf. 2007, 38, 1525–1532. [Google Scholar] [CrossRef]
- Tao, H.; Kaplan, D.L.; Omenetto, F.G. Silk materials–a road to sustainable high technology. Adv. Mater. 2012, 24, 2824–2837. [Google Scholar] [CrossRef] [PubMed]
- Wicklein, B.; Salazar-Alvarez, G. Functional hybrids based on biogenic nanofibrils and inorganic nanomaterials. J. Mater. Chem. A 2013, 1, 5469–5478. [Google Scholar] [CrossRef]
- Xue, Y.; Jao, D.; Hu, W.; Hu, X. Silk-silk blend materials. J. Therm. Anal. Calorim. 2017, 127, 915–921. [Google Scholar] [CrossRef]
- Najjar, R.; Luo, Y.; Jao, D.; Brennan, D.; Xue, Y.; Beachley, V.; Hu, X.; Xue, W. Biocompatible silk/polymer energy harvesters using stretched poly (vinylidene fluoride-co-hexafluoropropylene)(pvdf-hfp) nanofibers. Polymers 2017, 9, 479. [Google Scholar] [CrossRef]
- Rockwood, D.N.; Preda, R.C.; Yücel, T.; Wang, X.; Lovett, M.L.; Kaplan, D.L. Materials fabrication from bombyx mori silk fibroin. Nat. Protoc. 2011, 6, 1612. [Google Scholar] [CrossRef] [PubMed]
- Xia, X.-X.; Xu, Q.; Hu, X.; Qin, G.; Kaplan, D.L. Tunable self-assembly of genetically engineered silk–elastin-like protein polymers. Biomacromolecules 2011, 12, 3844–3850. [Google Scholar] [CrossRef] [PubMed]
- Keten, S.; Xu, Z.; Ihle, B.; Buehler, M.J. Nanoconfinement controls stiffness, strength and mechanical toughness of β-sheet crystals in silk. Nat. Mater. 2010, 9, 359. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Vepari, C.; Jin, H.-J.; Kim, H.J.; Kaplan, D.L. Electrospun silk-bmp-2 scaffolds for bone tissue engineering. Biomaterials 2006, 27, 3115–3124. [Google Scholar] [CrossRef] [PubMed]
- Lammel, A.S.; Hu, X.; Park, S.-H.; Kaplan, D.L.; Scheibel, T.R. Controlling silk fibroin particle features for drug delivery. Biomaterials 2010, 31, 4583–4591. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Fan, H.; Wang, Y.; Toh, S.L.; Goh, J.C. The interaction between a combined knitted silk scaffold and microporous silk sponge with human mesenchymal stem cells for ligament tissue engineering. Biomaterials 2008, 29, 662–674. [Google Scholar] [CrossRef] [PubMed]
- Marelli, B.; Brenckle, M.; Kaplan, D.L.; Omenetto, F.G. Silk fibroin as edible coating for perishable food preservation. Sci. Rep. 2016, 6, 25263. [Google Scholar] [CrossRef] [PubMed]
- Xing, C.; Munro, T.; White, B.; Ban, H.; Copeland, C.G.; Lewis, R.V. Thermophysical properties of the dragline silk of nephila clavipes spider. Polymer 2014, 55, 4226–4231. [Google Scholar] [CrossRef]
- Zhang, L.; Chen, T.; Ban, H.; Liu, L. Hydrogen bonding-assisted thermal conduction in β-sheet crystals of spider silk protein. Nanoscale 2014, 6, 7786–7791. [Google Scholar] [CrossRef] [PubMed]
- Fuente, R.; Mendioroz, A.; Salazar, A. Revising the exceptionally high thermal diffusivity of spider silk. Mater. Lett. 2014, 114, 1–3. [Google Scholar] [CrossRef]
- Ferreira, A.M.; Gentile, P.; Chiono, V.; Ciardelli, G. Collagen for bone tissue regeneration. Acta Biomater. 2012, 8, 3191–3200. [Google Scholar] [CrossRef] [PubMed]
- Balaji, S.; Kumar, R.; Sripriya, R.; Rao, U.; Mandal, A.; Kakkar, P.; Reddy, P.N.; Sehgal, P.K. Characterization of keratin–collagen 3d scaffold for biomedical applications. Polym. Adv. Technol. 2012, 23, 500–507. [Google Scholar] [CrossRef]
- Maynes, R. Structure and Function of Collagen Types; Elsevier: Amsterdam, The Netherlands, 2012. [Google Scholar]
- Li, Y.; Foss, C.A.; Summerfield, D.D.; Doyle, J.J.; Torok, C.M.; Dietz, H.C.; Pomper, M.G.; Yu, S.M. Targeting collagen strands by photo-triggered triple-helix hybridization. Proc. Natl. Acad. Sci. USA 2012, 109, 14767–14772. [Google Scholar] [CrossRef] [PubMed]
- Sherman, V.R.; Yang, W.; Meyers, M.A. The materials science of collagen. J. Mech. Behav. Biomed. Mater. 2015, 52, 22–50. [Google Scholar] [CrossRef] [PubMed]
- Fujimaki, H.; Uchida, K.; Inoue, G.; Miyagi, M.; Nemoto, N.; Saku, T.; Isobe, Y.; Inage, K.; Matsushita, O.; Yagishita, S. Oriented collagen tubes combined with basic fibroblast growth factor promote peripheral nerve regeneration in a 15 mm sciatic nerve defect rat model. J. Biomed. Mater. Res. Part A 2017, 105, 8–14. [Google Scholar] [CrossRef] [PubMed]
- Brougham, C.M.; Levingstone, T.J.; Shen, N.; Cooney, G.M.; Jockenhoevel, S.; Flanagan, T.C.; O’brien, F.J. Freeze-drying as a novel biofabrication method for achieving a controlled microarchitecture within large, complex natural biomaterial scaffolds. Adv. Healthc. Mater. 2017, 6, 1700598. [Google Scholar] [CrossRef] [PubMed]
- Tombolato, L.; Novitskaya, E.E.; Chen, P.-Y.; Sheppard, F.A.; McKittrick, J. Microstructure, elastic properties and deformation mechanisms of horn keratin. Acta Biomater. 2010, 6, 319–330. [Google Scholar] [CrossRef] [PubMed]
- Mercer, E. The heterogeneity of the keratin fibers. Text. Res. J. 1953, 23, 388–397. [Google Scholar] [CrossRef]
- Aboushwareb, T.; Eberli, D.; Ward, C.; Broda, C.; Holcomb, J.; Atala, A.; Van Dyke, M. A keratin biomaterial gel hemostat derived from human hair: Evaluation in a rabbit model of lethal liver injury. J. Biomed. Mater. Res. Part B Appl. Biomater. 2009, 90, 45–54. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Yang, W.; McKittrick, J.; Meyers, M.A. Keratin: Structure, mechanical properties, occurrence in biological organisms, and efforts at bioinspiration. Prog. Mater. Sci. 2016, 76, 229–318. [Google Scholar] [CrossRef]
- Lee, H.; Noh, K.; Lee, S.C.; Kwon, I.-K.; Han, D.-W.; Lee, I.-S.; Hwang, Y.-S. Human hair keratin and its-based biomaterials for biomedical applications. Tissue Eng. Regen. Med. 2014, 11, 255–265. [Google Scholar] [CrossRef]
- Li, R.; Wang, D. Preparation of regenerated wool keratin films from wool keratin–ionic liquid solutions. J. Appl. Polym. Sci. 2013, 127, 2648–2653. [Google Scholar] [CrossRef]
- Vu, T.; Xue, Y.; Vuong, T.; Erbe, M.; Bennet, C.; Palazzo, B.; Popielski, L.; Rodriguez, N.; Hu, X. Comparative study of ultrasonication-induced and naturally self-assembled silk fibroin-wool keratin hydrogel biomaterials. Int. J. Mol. Sci. 2016, 17, 1497. [Google Scholar] [CrossRef] [PubMed]
- Edwards, A.; Jarvis, D.; Hopkins, T.; Pixley, S.; Bhattarai, N. Poly (ε-caprolactone)/keratin-based composite nanofibers for biomedical applications. J. Biomed. Mater. Res. Part B Appl. Biomater. 2015, 103, 21–30. [Google Scholar] [CrossRef] [PubMed]
- Clarke, D.R. Materials selection guidelines for low thermal conductivity thermal barrier coatings. Surf. Coat. Technol. 2003, 163, 67–74. [Google Scholar] [CrossRef]
- Rouison, D.; Sain, M.; Couturier, M. Resin transfer molding of natural fiber reinforced composites: Cure simulation. Compos. Sci. Technol. 2004, 64, 629–644. [Google Scholar] [CrossRef]
- Berber, S.; Kwon, Y.-K.; Tománek, D. Unusually high thermal conductivity of carbon nanotubes. Phys. Rev. Lett. 2000, 84, 4613. [Google Scholar] [CrossRef] [PubMed]
- Majumdar, A. Thermoelectricity in semiconductor nanostructures. Science 2004, 303, 777–778. [Google Scholar] [CrossRef] [PubMed]
- Biswas, K.; He, J.; Blum, I.D.; Wu, C.-I.; Hogan, T.P.; Seidman, D.N.; Dravid, V.P.; Kanatzidis, M.G. High-performance bulk thermoelectrics with all-scale hierarchical architectures. Nature 2012, 489, 414–418. [Google Scholar] [CrossRef] [PubMed]
- Boukai, A.I.; Bunimovich, Y.; Tahir-Kheli, J.; Yu, J.-K.; Goddard Iii, W.A.; Heath, J.R. Silicon nanowires as efficient thermoelectric materials. Nature 2008, 451, 168–171. [Google Scholar] [CrossRef] [PubMed]
- Luckyanova, M.N.; Garg, J.; Esfarjani, K.; Jandl, A.; Bulsara, M.T.; Schmidt, A.J.; Minnich, A.J.; Chen, S.; Dresselhaus, M.S.; Ren, Z. Coherent phonon heat conduction in superlattices. Science 2012, 338, 936–939. [Google Scholar] [CrossRef] [PubMed]
- Kraemer, D.; Poudel, B.; Feng, H.-P.; Caylor, J.C.; Yu, B.; Yan, X.; Ma, Y.; Wang, X.; Wang, D.; Muto, A. High-performance flat-panel solar thermoelectric generators with high thermal concentration. Nat. Mater. 2011, 10, 532–538. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Liu, G.; Wang, X. New secrets of spider silk: Exceptionally high thermal conductivity and its abnormal change under stretching. Adv. Mater. 2012, 24, 1482–1486. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Huang, X.; Wang, Y.; Zhang, Y.-Q.; Wang, X. Thermal transport in single silkworm silks and the behavior under stretching. Soft Matter 2012, 8, 9792–9799. [Google Scholar] [CrossRef]
- Zhang, J.; Rajkhowa, R.; Li, J.; Liu, X.; Wang, X. Silkworm cocoon as natural material and structure for thermal insulation. Mater. Des. 2013, 49, 842–849. [Google Scholar] [CrossRef]
- Chen, S.; Wu, Q.; Mishra, C.; Kang, J.; Zhang, H.; Cho, K.; Cai, W.; Balandin, A.A.; Ruoff, R.S. Thermal conductivity of isotopically modified graphene. Nat. Mater. 2012, 11, 203–207. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.-K.; Mitrovic, S.; Tham, D.; Varghese, J.; Heath, J.R. Reduction of thermal conductivity in phononic nanomesh structures. Nat. Nanotechnol. 2010, 5, 718–721. [Google Scholar] [CrossRef] [PubMed]
- Fan, L.; Khodadadi, J. Thermal conductivity enhancement of phase change materials for thermal energy storage: A review. Renew. Sustain. Energy Rev. 2011, 15, 24–46. [Google Scholar] [CrossRef]
- Hu, Y.; Zeng, L.; Minnich, A.J.; Dresselhaus, M.S.; Chen, G. Spectral mapping of thermal conductivity through nanoscale ballistic transport. Nat. Nanotechnol. 2015, 10, 701. [Google Scholar] [CrossRef] [PubMed]
- Maldovan, M. Phonon wave interference and thermal bandgap materials. Nat. Mater. 2015, 14, 667–674. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.; Xu, Z.; Starrett, J.; Hayashi, C.; Wang, X. Cross-plane thermal transport in micrometer-thick spider silk films. Polymer 2014, 55, 1845–1853. [Google Scholar] [CrossRef]
- Hu, M.; Yu, D.; Wei, J. Thermal conductivity determination of small polymer samples by differential scanning calorimetry. Polym. Test. 2007, 26, 333–337. [Google Scholar] [CrossRef]
- Price, D.M.; Jarratt, M. Thermal conductivity of ptfe and ptfe composites. Thermochim. Acta 2002, 392, 231–236. [Google Scholar] [CrossRef]
- Peterlin, A. Drawing and extrusion of semi-crystalline polymers. Colloid Polym. Sci. 1987, 265, 357–382. [Google Scholar] [CrossRef]
- Van Aerle, N.; Braam, A. A structural study on solid state drawing of solution-crystallized ultra-high molecular weight polyethylene. J. Mater. Sci. 1988, 23, 4429–4436. [Google Scholar] [CrossRef]
- Choy, C. Thermal conductivity of polymers. Polymer 1977, 18, 984–1004. [Google Scholar] [CrossRef]
- Kanamoto, T.; Tsuruta, A.; Tanaka, K.; Takeda, M.; Porter, R.S. Super-drawing of ultrahigh molecular weight polyethylene. 1. Effect of techniques on drawing of single crystal mats. Macromolecules 1988, 21, 470–477. [Google Scholar] [CrossRef]
- Choy, C.; Wong, Y.; Yang, G.; Kanamoto, T. Elastic modulus and thermal conductivity of ultradrawn polyethylene. J. Polym. Sci. Part B Polym. Phys. 1999, 37, 3359–3367. [Google Scholar] [CrossRef]
- Terao, T.; Zhi, C.; Bando, Y.; Mitome, M.; Tang, C.; Golberg, D. Alignment of boron nitride nanotubes in polymeric composite films for thermal conductivity improvement. J. Phys. Chem. C 2010, 114, 4340–4344. [Google Scholar] [CrossRef]
- Singh, V.; Bougher, T.L.; Weathers, A.; Cai, Y.; Bi, K.; Pettes, M.T.; McMenamin, S.A.; Lv, W.; Resler, D.P.; Gattuso, T.R. High thermal conductivity of chain-oriented amorphous polythiophene. Nat. Nanotechnol. 2014, 9, 384–390. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Yang, R. Length-dependent thermal conductivity of single extended polymer chains. Phys. Rev. B 2012, 86, 104307. [Google Scholar] [CrossRef]
- Zhu, H.; Li, Y.; Fang, Z.; Xu, J.; Cao, F.; Wan, J.; Preston, C.; Yang, B.; Hu, L. Highly thermally conductive papers with percolative layered boron nitride nanosheets. ACS Nano 2014, 8, 3606–3613. [Google Scholar] [CrossRef] [PubMed]
- Balandin, A.A.; Ghosh, S.; Bao, W.; Calizo, I.; Teweldebrhan, D.; Miao, F.; Lau, C.N. Superior thermal conductivity of single-layer graphene. Nano Lett. 2008, 8, 902–907. [Google Scholar] [CrossRef] [PubMed]
- Nan, C.-W.; Liu, G.; Lin, Y.; Li, M. Interface effect on thermal conductivity of carbon nanotube composites. Appl. Phys. Lett. 2004, 85, 3549–3551. [Google Scholar] [CrossRef]
- Che, J.; Cagin, T.; Goddard, W.A., III. Thermal conductivity of carbon nanotubes. Nanotechnology 2000, 11, 65. [Google Scholar] [CrossRef]
- Eastman, J.; Choi, S.; Li, S.; Yu, W.; Thompson, L. Anomalously increased effective thermal conductivities of ethylene glycol-based nanofluids containing copper nanoparticles. Appl. Phys. Lett. 2001, 78, 718–720. [Google Scholar] [CrossRef]
- Kuang, J.; Zhang, C.; Zhou, X.; Wang, S. Synthesis of high thermal conductivity nano-scale aluminum nitride by a new carbothermal reduction method from combustion precursor. J. Cryst. Growth 2003, 256, 288–291. [Google Scholar] [CrossRef]
- Ghosh, S.; Calizo, I.; Teweldebrhan, D.; Pokatilov, E.; Nika, D.; Balandin, A.; Bao, W.; Miao, F.; Lau, C. Extremely high thermal conductivity of graphene: Prospects for thermal management applications in nanoelectronic circuits. Appl. Phys. Lett. 2008, 92, 151911. [Google Scholar] [CrossRef]
- Hu, J.; Ruan, X.; Chen, Y.P. Thermal conductivity and thermal rectification in graphene nanoribbons: A molecular dynamics study. Nano Lett. 2009, 9, 2730–2735. [Google Scholar] [CrossRef] [PubMed]
- Ishida, H.; Rimdusit, S. Very high thermal conductivity obtained by boron nitride-filled polybenzoxazine. Thermochim. Acta 1998, 320, 177–186. [Google Scholar] [CrossRef]
- Golberg, D.; Bando, Y.; Huang, Y.; Terao, T.; Mitome, M.; Tang, C.; Zhi, C. Boron nitride nanotubes and nanosheets. ACS Nano 2010, 4, 2979–2993. [Google Scholar] [CrossRef] [PubMed]
- Stankovich, S.; Dikin, D.A.; Dommett, G.H.; Kohlhaas, K.M.; Zimney, E.J.; Stach, E.A.; Piner, R.D.; Nguyen, S.T.; Ruoff, R.S. Graphene-based composite materials. Nature 2006, 442, 282–286. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Xue, Y.; Qin, S.; Liu, D.; Wang, X.; Hu, X.; Li, J.; Wang, X.; Bando, Y.; Golberg, D. Bn nanosheet/polymer films with highly anisotropic thermal conductivity for thermal management applications. ACS Appl. Mater. Interfaces 2017, 9, 43163–43170. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Wu, Y.; Xue, Y.; Liu, D.; Wang, X.; Hu, X.; Bando, Y.; Lei, W. Super-compatible functional boron nitride nanosheets/polymer films with excellent mechanical properties and ultra-high thermal conductivity for thermal management. J. Mater. Chem. C 2018, 6, 1363–1369. [Google Scholar] [CrossRef]
- Zhou, W.; Wang, C.; Ai, T.; Wu, K.; Zhao, F.; Gu, H. A novel fiber-reinforced polyethylene composite with added silicon nitride particles for enhanced thermal conductivity. Compos. Part A Appl. Sci. Manuf. 2009, 40, 830–836. [Google Scholar] [CrossRef]
- Hung, M.-T.; Choi, O.; Ju, Y.S.; Hahn, H. Heat conduction in graphite-nanoplatelet-reinforced polymer nanocomposites. Appl. Phys. Lett. 2006, 89, 023117. [Google Scholar] [CrossRef]
- Wang, X.; Ho, V.; Segalman, R.A.; Cahill, D.G. Thermal conductivity of high-modulus polymer fibers. Macromolecules 2013, 46, 4937–4943. [Google Scholar] [CrossRef]
- Cassignol, C.; Olivier, P.; Ricard, A. Influence of the dopant on the polypyrrole moisture content: Effects on conductivity and thermal stability. J. Appl. Polym. Sci. 1998, 70, 1567–1577. [Google Scholar] [CrossRef]
- Yano, O.; Yamaoka, H. Cryogenic properties of polymers. Prog. Polym. Sci. 1995, 20, 585–613. [Google Scholar] [CrossRef]
- Chen, Y.-M.; Ting, J.-M. Ultra high thermal conductivity polymer composites. Carbon 2002, 40, 359–362. [Google Scholar] [CrossRef]
- Hu, X.; Wang, X.; Rnjak, J.; Weiss, A.S.; Kaplan, D.L. Biomaterials derived from silk–tropoelastin protein systems. Biomaterials 2010, 31, 8121–8131. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Lu, Q.; Sun, L.; Cebe, P.; Wang, X.; Zhang, X.; Kaplan, D.L. Biomaterials from ultrasonication-induced silk fibroin− hyaluronic acid hydrogels. Biomacromolecules 2010, 11, 3178–3188. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharya, A.; Mahajan, R.L. Temperature dependence of thermal conductivity of biological tissues. Physiol. Meas. 2003, 24, 769–783. [Google Scholar] [CrossRef] [PubMed]
- Baxter, S. The thermal conductivity of textiles. Proc. Phys. Soc. 1946, 58, 105. [Google Scholar] [CrossRef]
- Das, S.K.; Putra, N.; Thiesen, P.; Roetzel, W. Temperature dependence of thermal conductivity enhancement for nanofluids. J. Heat Transf. 2003, 125, 567–574. [Google Scholar] [CrossRef]
- Ju, Y.; Goodson, K. Process-dependent thermal transport properties of silicon-dioxide films deposited using low-pressure chemical vapor deposition. J. Appl. Phys. 1999, 85, 7130–7134. [Google Scholar] [CrossRef]
- Morgen, M.; Ryan, E.T.; Zhao, J.-H.; Hu, C.; Cho, T.; Ho, P.S. Low dielectric constant materials for ulsi interconnects. Annu. Rev. Mater. Sci. 2000, 30, 645–680. [Google Scholar] [CrossRef]
- Hu, H.; Wang, X.; Xu, X. Generalized theory of the photoacoustic effect in a multilayer material. J. Appl. Phys. 1999, 86, 3953–3958. [Google Scholar] [CrossRef]
- Cahill, D.G.; Pohl, R.O. Thermal conductivity of amorphous solids above the plateau. Phys. Rev. B 1987, 35, 4067. [Google Scholar] [CrossRef]
- Delan, A.; Rennau, M.; Schulz, S.; Gessner, T. Thermal conductivity of ultra low-k dielectrics. Microelectron. Eng. 2003, 70, 280–284. [Google Scholar] [CrossRef]
- Rood, E.S. Thermal conductivity of some wearing materials. Phys. Rev. 1921, 18, 356. [Google Scholar] [CrossRef]
- Cebe, P.; Hu, X.; Kaplan, D.L.; Zhuravlev, E.; Wurm, A.; Arbeiter, D.; Schick, C. Beating the heat-fast scanning melts silk beta sheet crystals. Sci. Rep. 2013, 3, 1130. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.; Li, Z.; Wang, J.; Gao, H.; Zhou, D.; Tang, Y.; Hu, W. Combining tmdsc measurements between chip-calorimeter and molecular simulation to study reversible melting of polymer crystals. Thermochim. Acta 2015, 603, 79–84. [Google Scholar] [CrossRef]
- Wang, J.; Li, Z.; Pérez, R.A.; Müller, A.J.; Zhang, B.; Grayson, S.M.; Hu, W. Comparing crystallization rates between linear and cyclic poly (epsilon-caprolactones) via fast-scan chip-calorimeter measurements. Polymer 2015, 63, 34–40. [Google Scholar] [CrossRef]
- Foreman, J.; Marcus, S.; Blaine, R. Thermal Conductivity of Polymers, Glasses & Ceramics by Modulated DSC; SOC OF PLASTICS ENGINEERS: Brookfield, CT, USA, 1994. [Google Scholar]
- Marcus, S.M.; Blaine, R.L. Thermal conductivity of polymers, glasses and ceramics by modulated dsc. Thermochim. Acta 1994, 243, 231–239. [Google Scholar] [CrossRef]
- Chiu, J.; Fair, P. Determination of thermal conductivity by differential scanning calorimetry. Thermochim. Acta 1979, 34, 267–273. [Google Scholar] [CrossRef]
- Keating, M.; McLaren, C. Thermal conductivity of polymer melts. Thermochim. Acta 1990, 166, 69–76. [Google Scholar] [CrossRef]
- Sircar, A.K.; Wells, J.L. Thermal conductivity of elastomer vulcanizates by differential scanning calorimetry. Rubber Chem. Technol. 1982, 55, 191–207. [Google Scholar] [CrossRef]
- Cahill, D.G. Thermal conductivity measurement from 30 to 750 k: The 3ω method. Rev. Sci. Instrum. 1990, 61, 802–808. [Google Scholar] [CrossRef]
- Koh, Y.K.; Singer, S.L.; Kim, W.; Zide, J.M.; Lu, H.; Cahill, D.G.; Majumdar, A.; Gossard, A.C. Comparison of the 3w method and time-domain thermoreflectance for measurements of the cross-plane thermal conductivity of epitaxial semiconductors. J. Appl. Phys. 2009, 105, 54303. [Google Scholar] [CrossRef]
- Wang, X.; Zhong, Z.; Xu, J. Noncontact thermal characterization of multiwall carbon nanotubes. J. Appl. Phys. 2005, 97, 064302. [Google Scholar] [CrossRef]
- Wang, T.; Wang, X.; Zhang, Y.; Liu, L.; Xu, L.; Liu, Y.; Zhang, L.; Luo, Z.; Cen, K. Effect of zirconium (IV) propoxide concentration on the thermophysical properties of hybrid organic-inorganic films. J. Appl. Phys. 2008, 104, 013528. [Google Scholar] [CrossRef]
- Chen, X.; He, Y.; Zhao, Y.; Wang, X. Thermophysical properties of hydrogenated vanadium-doped magnesium porous nanostructures. Nanotechnology 2010, 21, 055707. [Google Scholar] [CrossRef] [PubMed]
- Balderas-López, J.; Mandelis, A. Self-normalized photothermal technique for accurate thermal diffusivity measurements in thin metal layers. Rev. Sci. Instrum. 2003, 74, 5219–5225. [Google Scholar] [CrossRef]
- Arvidson, K.; Abdallah, B.; Applegate, L.; Baldini, N.; Cenni, E.; Gomez-Barrena, E.; Granchi, D.; Kassem, M.; Konttinen, Y.; Mustafa, K. Bone regeneration and stem cells. J. Cell. Mol. Med. 2011, 15, 718–746. [Google Scholar] [CrossRef] [PubMed]
| Material | Thermal Conductivity (W/m∙K) |
|---|---|
| Low density polyethylene (LDPE) | 0.3 |
| High density polyethylene (HDPE) | 0.44 |
| Polycarbonate | 0.22 |
| Polyvinyl chloride (PVC) | 0.19 |
| Nylon-6 (PA6) | 0.25 |
| Polythiophene nanofibers (amorphous) | ~4.4 |
| Polyethylene nanofibers | ~104 |
| Silkworm silk (axial direction) | ~6.53 |
| Flax fiber | 0.1187 |
| Squid protein | 0.3–1.3 |
| Silk/wool hybrid | 0.000397–0.000663 |
| Human skin | 0.23–0.488 |
| Aluminum | 235 |
| Copper | 400 |
| Nickel | 158 |
| Gold | 345 |
| Aluminum | 235 |
| Diamond | 1000 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xue, Y.; Lofland, S.; Hu, X. Thermal Conductivity of Protein-Based Materials: A Review. Polymers 2019, 11, 456. https://doi.org/10.3390/polym11030456
Xue Y, Lofland S, Hu X. Thermal Conductivity of Protein-Based Materials: A Review. Polymers. 2019; 11(3):456. https://doi.org/10.3390/polym11030456
Chicago/Turabian StyleXue, Ye, Samuel Lofland, and Xiao Hu. 2019. "Thermal Conductivity of Protein-Based Materials: A Review" Polymers 11, no. 3: 456. https://doi.org/10.3390/polym11030456
APA StyleXue, Y., Lofland, S., & Hu, X. (2019). Thermal Conductivity of Protein-Based Materials: A Review. Polymers, 11(3), 456. https://doi.org/10.3390/polym11030456