A Complexed Initiating System AlCl3·Phenetole/TiCl4·H2O with Dominant Synergistic Effect for Efficient Synthesis of High Molecular Weight Polyisobutylene
Abstract
1. Introduction
2. Materials and Methods
2.1. Raw Material
2.2. Catalyst Preparation and Polymerization
2.3. Polymer Characterization
3. Results and Discussion
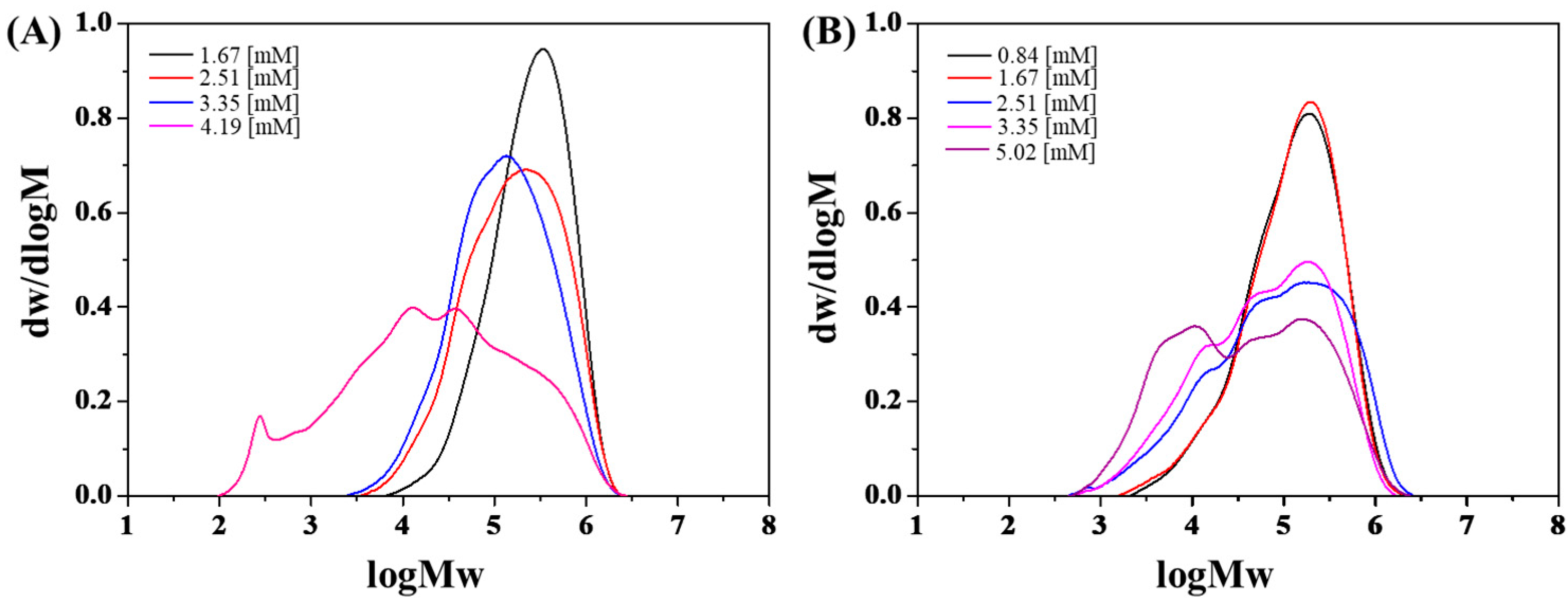
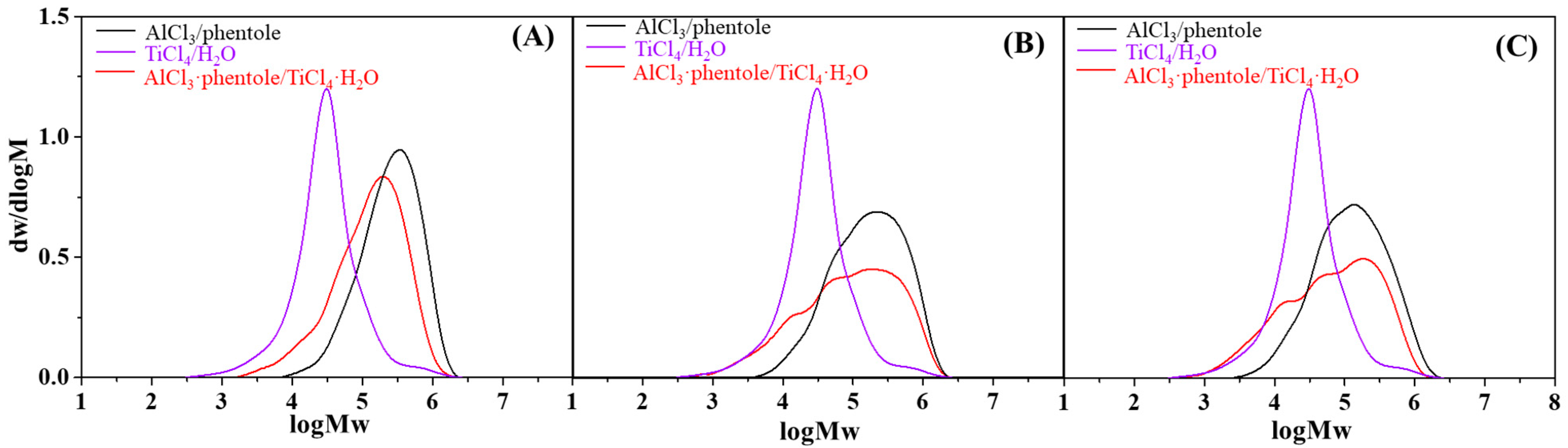
3.1. Effect of Coinitiator Concentration
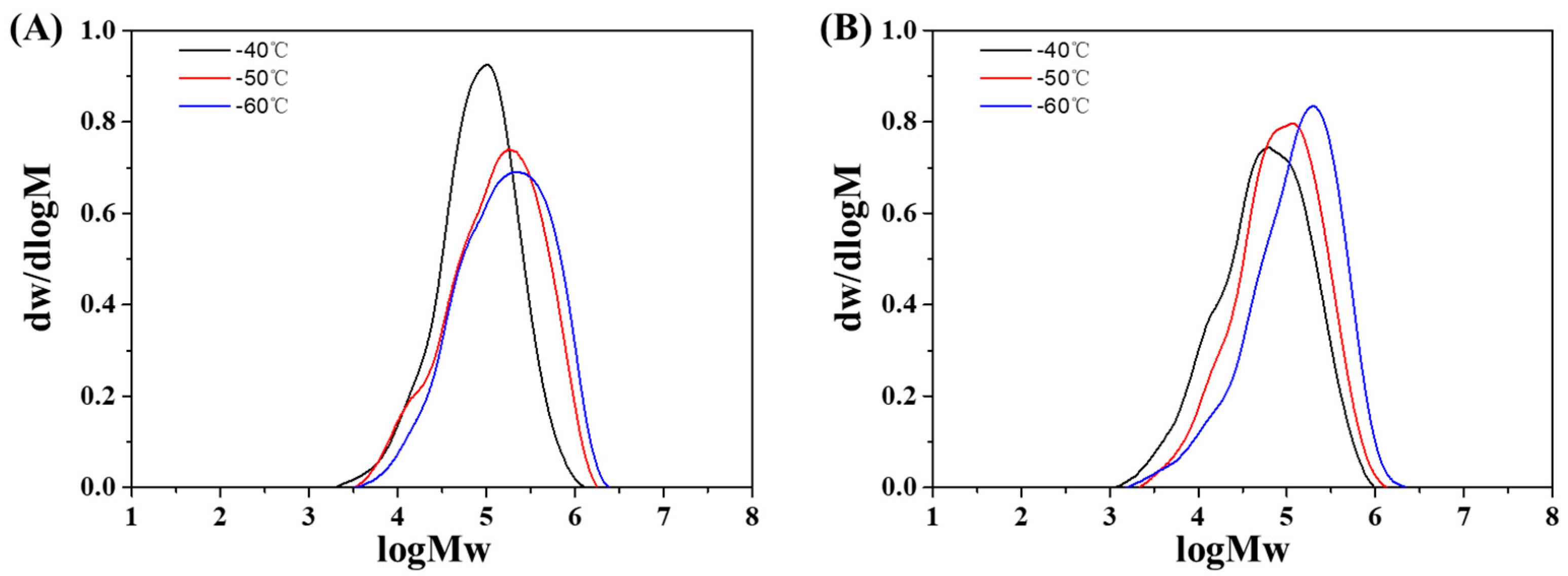
3.2. Effect of Reaction Temperature
3.3. Effect of Monomer Concentration
3.4. Effect of Polymerization Time
4. Conclusion
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Kunal, K.; Paluch, M.; Roland, C.M.; Puskas, J.E.; Chen, Y.; Sokolov, A.P. Polyisobutylene: A most unusual polymer. J. Polym. Sci. Pol. Phys. 2008, 46, 1390–1399. [Google Scholar] [CrossRef]
- Csihony, S.; Janssen, N.; Mühlbach, K. Controlling heat transfer for the manufacturing of high molecular weight polyisobutylene via formation of micelles. Chin. J. Polym. Sci. 2019, 37, 898–902. [Google Scholar] [CrossRef]
- Li, Y.; Wu, Y.; Liang, L.; Li, Y.; Wu, G. Cationic polymerization of isobutylene coinitiated by AlCl3 in the presence of ethyl benzoate. Chin. J. Polym. Sci. 2009, 28, 55–62. [Google Scholar] [CrossRef]
- Nuyken, O.; Vierle, M.; Kühn, F.E.; Zhang, Y. Solvent-ligated transition metal complexes as initiators for the polymerization of isobutene. Macromol. Symp. 2006, 236, 69–77. [Google Scholar] [CrossRef]
- Dimitrov, I.; Faust, R. Kinetic and mechanistic studies of the carbocationic precipitation polymerization of isobutylene in polar solvents. Macromolecules 2004, 37, 9753–9760. [Google Scholar] [CrossRef]
- Kennedy, J.P.; Squires, R.G. Fundamental studies on cationic polymerization IV—Homo- and co-polymerizations with various catalysts. Polymer 1965, 6, 579–587. [Google Scholar] [CrossRef]
- Bochmann, M. Highly electrophilic organometallics for carbocationic polymerizations: From anion engineering to new polymer materials. Account. Chem. Res. 2010, 43, 1267–1278. [Google Scholar] [CrossRef]
- Carr, A.G.; Dawson, D.M.; Bochmann, M. Zirconocenes as initiators for carbocationic isobutene homo- and copolymerizations. Macromolecules 1998, 31, 2035–2040. [Google Scholar] [CrossRef]
- Saßmannshausen, J. Cationic and dicationic zirconocene compounds as initiators of carbocationic isobutene polymerisation. Dalton Trans. 2009, 41, 9026–9032. [Google Scholar] [CrossRef]
- Kumar, K.R.; Hall, C.; Penciu, A.; Drewitt, M.J.; McInenly, P.J.; Baird, M.C. Isobutene polymerization initiated by [CP*TiMe2]+ in the presence of a series of novel, weakly coordinating counteranions. J. Polym. Sci. Pol. Chem. 2002, 40, 3302–3311. [Google Scholar] [CrossRef]
- Li, Y.; Cokoja, M.; Kühn, F.E. Inorganic/organometallic catalysts and initiators involving weakly coordinating anions for isobutene polymerisation. Coordin. Chem. Rev. 2011, 255, 1541–1557. [Google Scholar] [CrossRef]
- Kostjuk, S.V. Recent progress in the Lewis acid co-initiated cationic polymerization of isobutylene and 1,3-dienes. RSC. Adv. 2015, 5, 13125–13144. [Google Scholar] [CrossRef]
- Liu, Q.; Wu, Y.; Zhang, Y.; Yan, P.; Xu, R. A cost-effective process for highly reactive polyisobutylenes via cationic polymerization coinitiated by AlCl3. Polymer 2010, 51, 5960–5969. [Google Scholar] [CrossRef]
- Dimitrov, P.; Emert, J.; Faust, R. Polymerization of isobutylene by AlCl3/Ether complexes in nonpolar solvent. Macromolecules 2012, 45, 3318–3325. [Google Scholar] [CrossRef]
- Kostjuk, S.V.; Vasilenko, I.V.; Shiman, D.I.; Frolov, A.N.; Gaponik, L.V. Highly reactive polyisobutylenes via cationic polymerization of isobutylene by AlCl3/Ether complexes in non-polar media: Scope and limitations. Macromol. Symp. 2015, 349, 94–103. [Google Scholar] [CrossRef]
- Zhu, S.; Lu, Y.; Wang, K.; Luo, G. Cationic polymerization of isobutylene catalysed by AlCl3 with multiple nucleophilic reagents. RSC. Adv. 2016, 6, 97983–97989. [Google Scholar] [CrossRef]
- Jean-Pierre, V.; Nicolas, S. Industrial Cationic Polymerizations: An Overview. In Cationic Polymerizations: Mechanisms, Synthesis & Applications, 1st ed.; Matyjaszewski, K., Ed.; Taylor & Francis: Boca Raton, FL, USA, 1996; Volume 8, pp. 682–768. [Google Scholar]
- Zhang, L.B.; Wu, Y.X.; Zhou, P.; Xu, R.W. Synthesis of highly reactive polyisobutylene by selective polymerization with o-cresol/AlCl3 initiating system. Polym. Adv. Technol. 2012, 23, 522–528. [Google Scholar] [CrossRef]
- Kostjuk, S.V.; Dubovik, A.Y.; Vasilenkol, I.V.; Mardykin, V.P.; Gaponik, L.V.; Kaputsky, F.N.; Antipin, L.M. Novel initiating system based on AlCl3 etherate for quasiliving cationic polymerization of styrene. Polym. Bull. 2004, 52, 227–234. [Google Scholar] [CrossRef]
- Shiman, D.I.; Vasilenko, I.V.; Kostjuk, S.V. Cationic polymerization of isobutylene by AlCl3/ether complexes in non-polar solvents: Effect of ether structure on the selectivity of β-H elimination. Polymer 2013, 54, 2235–2242. [Google Scholar] [CrossRef]
- Zhu, S.; Lu, Y.; Wang, K.; Luo, G. Flow synthesis of medium molecular weight polyisobutylene coinitiated by AlCl3. Eur. Polym. J. 2016, 80, 219–226. [Google Scholar] [CrossRef]
- Li, Y.; Wu, Y.; Xu, X.; Liang, L.; Wu, G. Electron-pair-donor reaction order in the cationic polymerization of isobutylene coinitiated by AlCl3. J. Polym. Sci. Pol. Chem. 2007, 45, 3053–3061. [Google Scholar] [CrossRef]
- Huang, Q.; He, P.; Wang, J.; Wu, Y. Synthesis of high molecular weight polyisobutylene via cationic polymerization at elevated temperatures. Chin. J. Polym. Sci. 2013, 31, 1139–1147. [Google Scholar] [CrossRef]
- Vasilenko, I.V.; Nikishev, P.A.; Shiman, D.I.; Kostjuk, S.V. Cationic polymerization of isobutylene in toluene: Toward well-defined exo-olefin terminated medium molecular weight polyisobutylenes under mild conditions. Polym. Chem. 2017, 8, 1417–1425. [Google Scholar] [CrossRef]
- Shiman, D.I.; Vasilenko, I.V.; Kostjuk, S.V. Alkoxy aluminum chlorides in the cationic polymerization of isobutylene: A co-initiator, carbocation stabilizer and chain-transfer agent. Polym. Chem. 2019, 10, 5998–6002. [Google Scholar] [CrossRef]
- Chen, L.; Zhu, Z.; Liu, Z.; Liu, B. Synthesis of MMPIB and HMPIB catalyzed by AlCl3/oxygen-containing initiator. China Synth. Resin. Plast. 2017, 34, 13–16. [Google Scholar]
- Jin, Y.; Dong, K.; Xu, L.; Guo, X.; Cheng, R.; Liu, B. Facile synthesis of medium molecular weight polyisobutylene with remarkable efficiency employing the complexed BF3·EtOH/TiCl4·H2O initiating system. Eur. Polym. J. 2019, 120, 109204. [Google Scholar] [CrossRef]
- Barsan, F.; Karam, A.R.; Parent, M.A.; Baird, M.C. Polymerization of isobutylene and the copolymerization of isobutylene and isoprene initiated by the metallocene derivative Cp*TiMe2(μ-Me)B(C6F5)3. Macromolecules 1998, 31, 8439–8447. [Google Scholar] [CrossRef]
- Gyor, M.; Wang, H.-C.; Faust, R. Living carbocationic polymerization of isobutylene with blocked bifunctional initiators in the presence of di-tert-butylpyridine as a proton trap. J. Macromol. Sci. 1992, 29, 639–653. [Google Scholar] [CrossRef]
- Imre, P.; Seymour, M. Anomalous carbon numbers in cationic oligomers of propylene and butylenes. J. Org. Chem. 1984, 49, 258–262. [Google Scholar]
- Marek, M.; Chmelíř, M. Influence of some friedel-crafts halides on the polymerization of isobutylene catalyzed by aluminum bromide. J. Polym. Sci. Pol. Symp. 1968, 23, 223–229. [Google Scholar] [CrossRef]
- Masure, M.; Anh-Hung, N.; Sauvet, G.; Sigwalt, P. Initiation of 1,1-diphenylethylene dimerization and isobutylene polymerization by TiCl4, AlBr3 and their mixtures in hydrocarbon solvents. Macromol. Chem. Phys. 1981, 182, 2695–2703. [Google Scholar] [CrossRef]
- Marek, M.; Pecka, J.; Halaška, V. Initiators of cationic polymerization of olefins arising from interaction between two lewis acids. Macromol. Symp. 1988, 13-14, 443–455. [Google Scholar] [CrossRef]
- Vasilenko, I.V.; Frolov, A.N.; Kostjuk, S.V. Cationic polymerization of isobutylene using AlCl3OBu2 as a coinitiator: Synthesis of highly reactive polyisobutylene. Macromolecules 2010, 43, 5503–5507. [Google Scholar] [CrossRef]
- Sipos, L.; De, P.; Faust, R. Effect of temperature, solvent polarity, and nature of Lewis acid on the rate constants in the carbocationic polymerization of isobutylene. Macromolecules 2003, 36, 8282–8290. [Google Scholar] [CrossRef]
- Frolov, A.N.; Kostjuk, S.V.; Vasilenko, I.V.; Kaputsky, F.N. Controlled cationic polymerization of styrene using AlCl3OBu2 as a coinitiator: Toward high molecular weight polystyrenes at elevated temperatures. J. Polym. Sci. Pol. Chem. 2010, 48, 3736–3743. [Google Scholar] [CrossRef]
- Toman, L.; Spěváček, J.; Vlček, P.; Holler, P. Thermally induced polymerization of isobutylene in the presence of SnCl4: Kinetic study of the polymerization and NMR structural investigation of low molecular weight products. J. Polym. Sci. Pol. Chem. 2000, 38, 1568–1579. [Google Scholar] [CrossRef]
- Vasilenko, I.V.; Shiman, D.I.; Kostjuk, S.V. Highly reactive polyisobutylenes via AlCl3OBu2-coinitiated cationic polymerization of isobutylene: Effect of solvent polarity, temperature, and initiator. J. Polym. Sci. Pol. Chem. 2012, 50, 750–758. [Google Scholar] [CrossRef]
| No | Catalysts | [AlCl3 + TiCl4] | Conv. | Act. c | Mw d | Mn d | MWD | Δ e |
|---|---|---|---|---|---|---|---|---|
| (mmol·L−1) | (%) | |||||||
| 1 | 1AlCl3·1phenetole | 0.84 | 0 | - | - | - | - | - |
| 2 | 1AlCl3·1phenetole/1TiCl4·1H2O | 0.84 | 34.9 | 186.14 | 20.89 | 5.50 | 3.8 | - |
| 3 | 1AlCl3·1phenetole | 1.67 | 16.0 | 42.67 | 35.78 | 14.31 | 2.5 | 1 |
| 4 | 1AlCl3·1phenetole/1TiCl4·1H2O | 1.67 | 53.0 | 141.34 | 20.88 | 5.09 | 4.1 | 3.31 |
| 5 | 1AlCl3·1phenetole | 2.51 | 40.5 | 72.00 | 28.86 | 7.59 | 3.8 | 1 |
| 6 | 1AlCl3·1phenetole/1TiCl4·1H2O | 2.51 | 95.0 | 168.90 | 20.03 | 0.96 | 20.9 | 2.35 |
| 7 | 1AlCl3·1phenetole | 3.35 | 100 | 133.34 | 20.42 | 5.24 | 3.9 | 1 |
| 8 | 1AlCl3·1phenetole/1TiCl4·1H2O | 3.35 | 98.7 | 131.61 | 14.37 | 0.98 | 14.7 | 0.99 |
| 9 | 1AlCl3·1phenetole | 4.19 | 100 | 106.67 | 12.20 | 0.26 | 46.1 | - |
| 10 | 1AlCl3·1phenetole/1TiCl4·1H2O | 5.02 | 97.1 | 86.32 | 12.87 | 0.67 | 19.2 | - |
| 11 b | 1TiCl4·1H2O | 50.00 | 71.5 | 4.86 | 6.41 | 1.78 | 3.6 | - |
| 12 | 1AlCl3·1H2O | 3.35 | 59.8 | 79.76 | 21.21 | 3.98 | 5.3 | - |
| 13 | 1TiCl4·1phenetole | 1.67 | N.D f | N.D f | N.D f | N.D f | N.D f | - |
| 14 | 1AlCl3·1phenetole·1H2O | 1.67 | 0.6 | 1.47 | - | - | - | - |
| 15 | 1TiCl4·1phenetole·1H2O | 1.67 | 0.4 | 0.90 | - | - | - | - |
| 16 | 1AlCl3·2phenetole/1TiCl4 | 1.67 | 0.1 | 0.21 | - | - | - | - |
| No | Coinitiator | Tp | Conv. | Act. d | Mw | Mn | MWD | Δ e |
|---|---|---|---|---|---|---|---|---|
| (°C) | (%) | (×104 g·mol−1) | (×104 g·mol−1) | |||||
| 1 b | AlCl3 | −60 | 40.5 | 72.07 | 28.86 | 7.59 | 3.8 | 1 |
| 2 c | AlCl3/TiCl4 | −60 | 53.0 | 140.91 | 20.88 | 5.09 | 4.1 | 2.96 |
| 3 b | AlCl3 | −50 | 61.0 | 108.55 | 22.80 | 5.70 | 4.0 | 1 |
| 4 c | AlCl3/TiCl4 | −50 | 88.8 | 236.10 | 13.72 | 4.04 | 3.4 | 2.17 |
| 5 b | AlCl3 | −40 | 98.0 | 174.40 | 12.70 | 4.54 | 2.8 | 1 |
| 6 c | AlCl3/TiCl4 | −40 | 97.2 | 258.43 | 10.03 | 2.51 | 4.0 | 1.48 |
| No | Coinitiator | [IB] | Conv. | Act. d | Mw | Mn | MWD | Δ e |
|---|---|---|---|---|---|---|---|---|
| (mol·L−1) | (%) | (×104 g·mol−1) | (×104 g·mol−1) | |||||
| 1 b | AlCl3 | 2.4 | 73.8 | 131.27 | 17.90 | 4.97 | 3.6 | 1 |
| 2 c | AlCl3/TiCl4 | 91.7 | 163.10 | 17.90 | 1.24 | 14.4 | 1.24 | |
| 3 b | AlCl3 | 3.3 | 52.5 | 93.38 | 25.47 | 6.70 | 3.8 | 1 |
| 4 c | AlCl3/TiCl4 | 91.1 | 162.03 | 18.47 | 1.00 | 18.6 | 1.74 | |
| 5 b | AlCl3 | 4.0 | 40.5 | 72.04 | 28.86 | 7.59 | 3.8 | 1 |
| 6 c | AlCl3/TiCl4 | 95.0 | 168.97 | 20.03 | 0.96 | 20.9 | 2.35 | |
| 7 b | AlCl3 | 4.6 | 36.4 | 64.74 | 27.23 | 5.79 | 4.7 | 1 |
| 8 c | AlCl3/TiCl4 | 57.6 | 102.45 | 21.22 | 5.18 | 4.1 | 1.58 | |
| 9 b | AlCl3 | 5.1 | 15.4 | 27.39 | 30.30 | 11.22 | 2.7 | 1 |
| 10 c | AlCl3/TiCl4 | 24.1 | 42.87 | 31.58 | 13.73 | 2.3 | 1.56 |
| No | Coinitiator | tp | Conv. | Act. d | Mw | Mn | MWD | Δ e |
|---|---|---|---|---|---|---|---|---|
| (min) | (%) | (×104 g·mol−1) | (×104 g·mol−1) | |||||
| 1 | AlCl3 b | 1.0 | 68.8 | 2200.29 | 27.21 | 6.80 | 4.0 | 1.00 |
| 2 | AlCl3/TiCl4 c | 51.4 | 2744.06 | 33.52 | 7.62 | 4.4 | 1.25 | |
| 3 | AlCl3 b | 3.0 | 77.7 | 1242.46 | 21.21 | 4.42 | 4.8 | 1.00 |
| 4 | AlCl3/TiCl4 c | 63.7 | 1700.36 | 34.43 | 13.24 | 2.6 | 1.37 | |
| 5 | AlCl3 b | 5.0 | 92.3 | 590.37 | 11.97 | 0.44 | 27.2 | 1.00 |
| 6 | AlCl3/TiCl4 c | 93.2 | 995.12 | 22.79 | 3.17 | 7.2 | 1.69 | |
| 7 | AlCl3 b | 10.0 | 95.8 | 306.38 | 11.03 | 0.53 | 20.8 | 1.00 |
| 8 | AlCl3/TiCl4 c | 90.1 | 481.02 | 23.60 | 4.37 | 5.4 | 1.57 | |
| 9 | AlCl3 b | 30.0 | 100.0 | 106.67 | 12.20 | 0.26 | 46.1 | 1.00 |
| 10 | AlCl3/TiCl4 c | 95.0 | 168.90 | 20.03 | 0.96 | 20.9 | 1.58 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jin, Y.; Chen, L.; Guo, X.; Xu, L.; Zhu, Z.; Liu, Z.; Cheng, R.; Liu, B. A Complexed Initiating System AlCl3·Phenetole/TiCl4·H2O with Dominant Synergistic Effect for Efficient Synthesis of High Molecular Weight Polyisobutylene. Polymers 2019, 11, 2121. https://doi.org/10.3390/polym11122121
Jin Y, Chen L, Guo X, Xu L, Zhu Z, Liu Z, Cheng R, Liu B. A Complexed Initiating System AlCl3·Phenetole/TiCl4·H2O with Dominant Synergistic Effect for Efficient Synthesis of High Molecular Weight Polyisobutylene. Polymers. 2019; 11(12):2121. https://doi.org/10.3390/polym11122121
Chicago/Turabian StyleJin, Yulong, Liang Chen, Xing Guo, Linfeng Xu, Zhihua Zhu, Zhen Liu, Ruihua Cheng, and Boping Liu. 2019. "A Complexed Initiating System AlCl3·Phenetole/TiCl4·H2O with Dominant Synergistic Effect for Efficient Synthesis of High Molecular Weight Polyisobutylene" Polymers 11, no. 12: 2121. https://doi.org/10.3390/polym11122121
APA StyleJin, Y., Chen, L., Guo, X., Xu, L., Zhu, Z., Liu, Z., Cheng, R., & Liu, B. (2019). A Complexed Initiating System AlCl3·Phenetole/TiCl4·H2O with Dominant Synergistic Effect for Efficient Synthesis of High Molecular Weight Polyisobutylene. Polymers, 11(12), 2121. https://doi.org/10.3390/polym11122121