A Porous Polymer-Based Solid Acid Catalyst with Excellent Amphiphilicity: An Active and Environmentally Friendly Catalyst for the Hydration of Alkynes
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
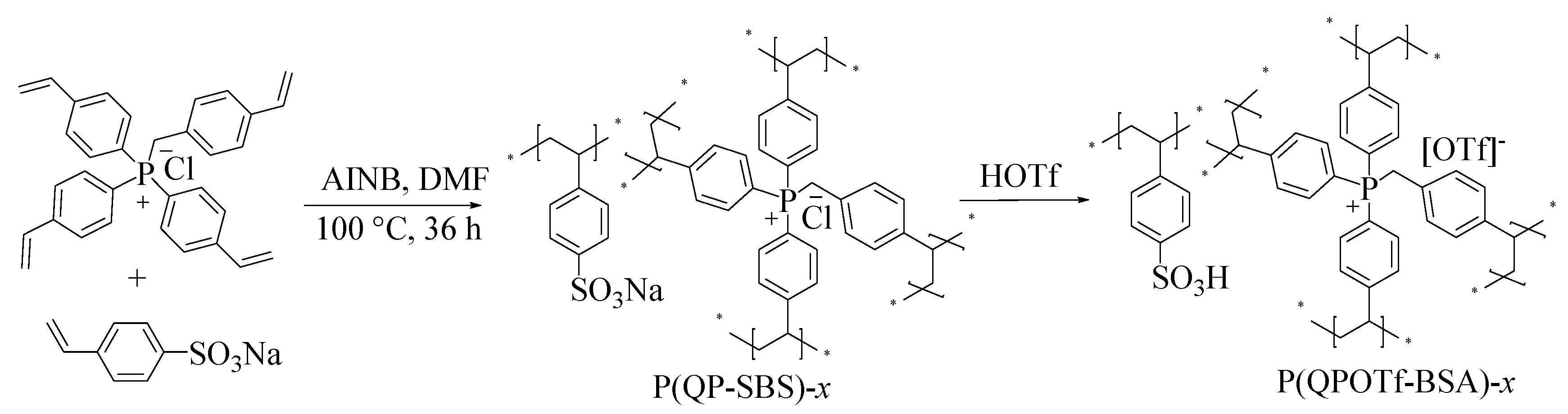
2.2. Catalyst Preparation
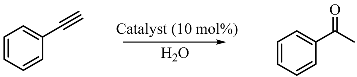
2.3. Catalytic Activity
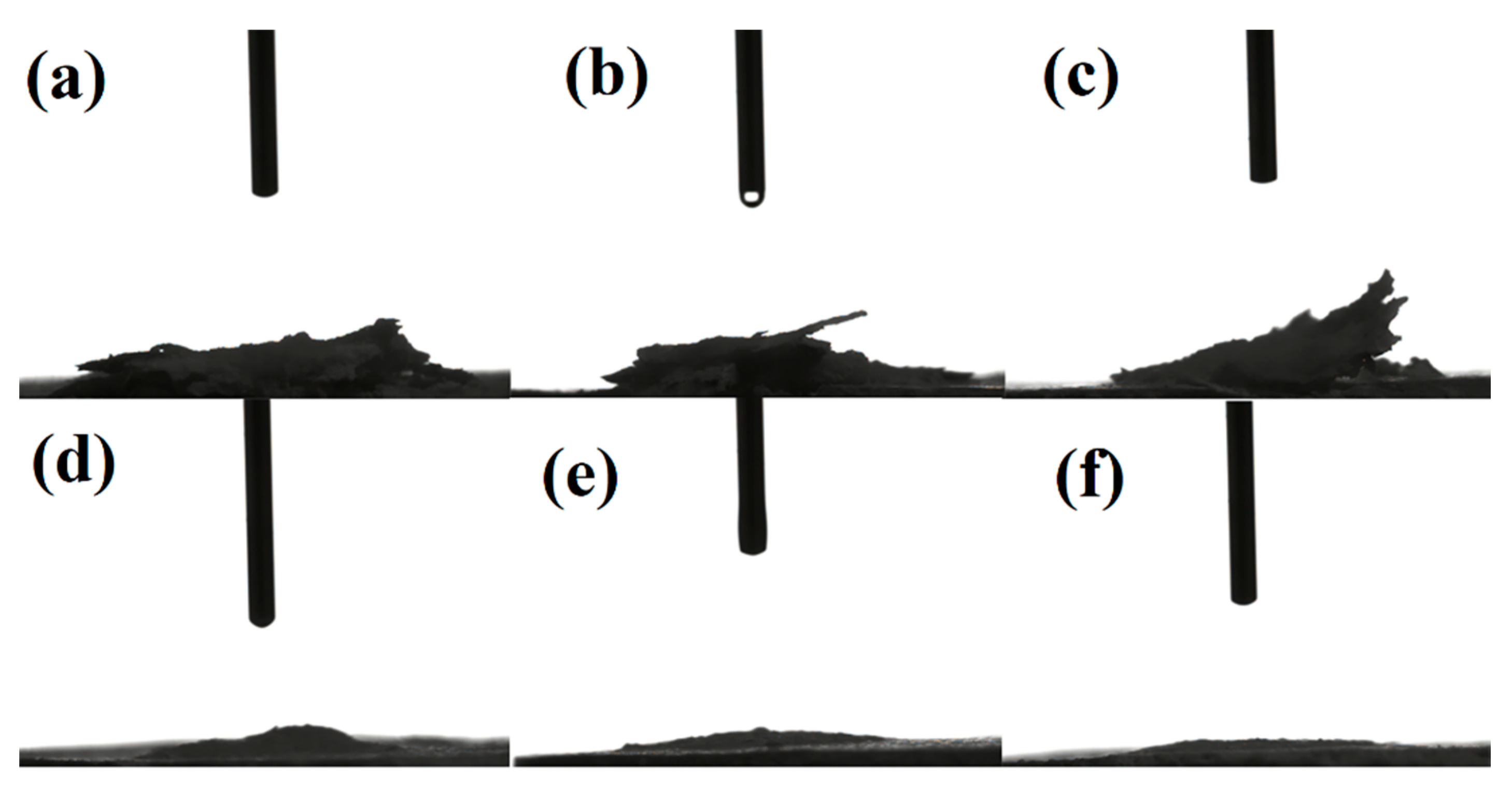
2.4. Adsorption Capacity
2.5. Characterization
3. Results
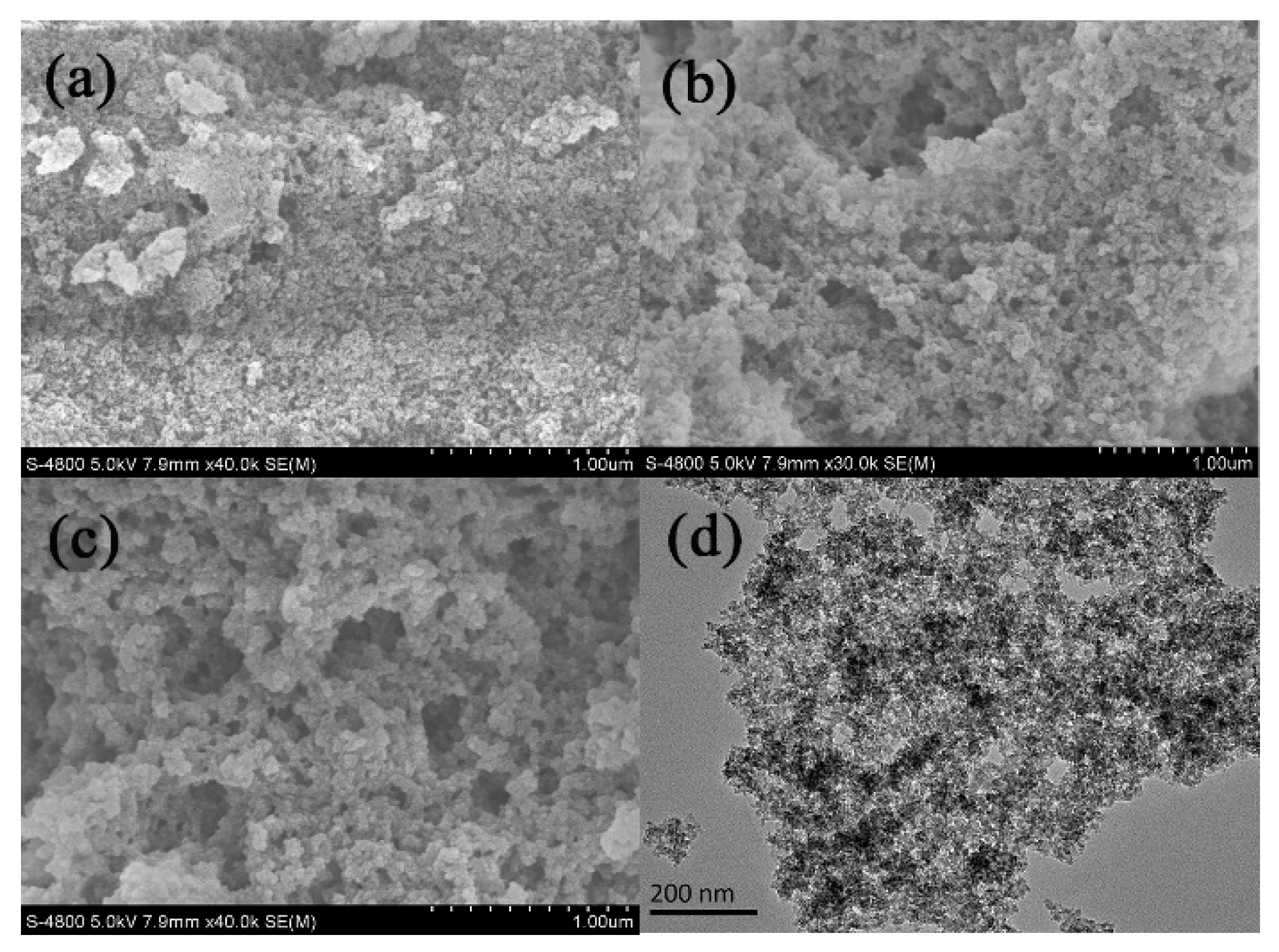
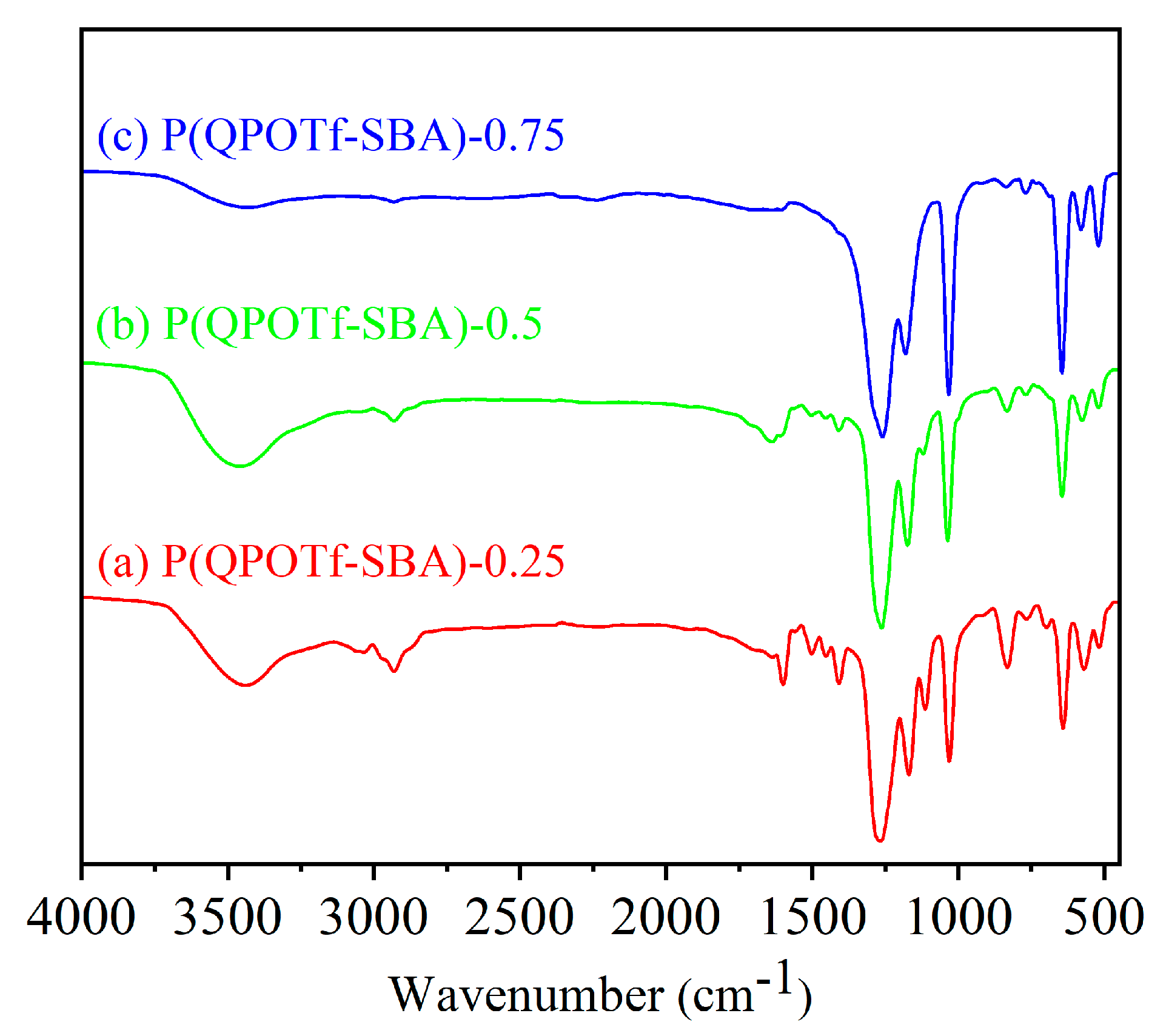
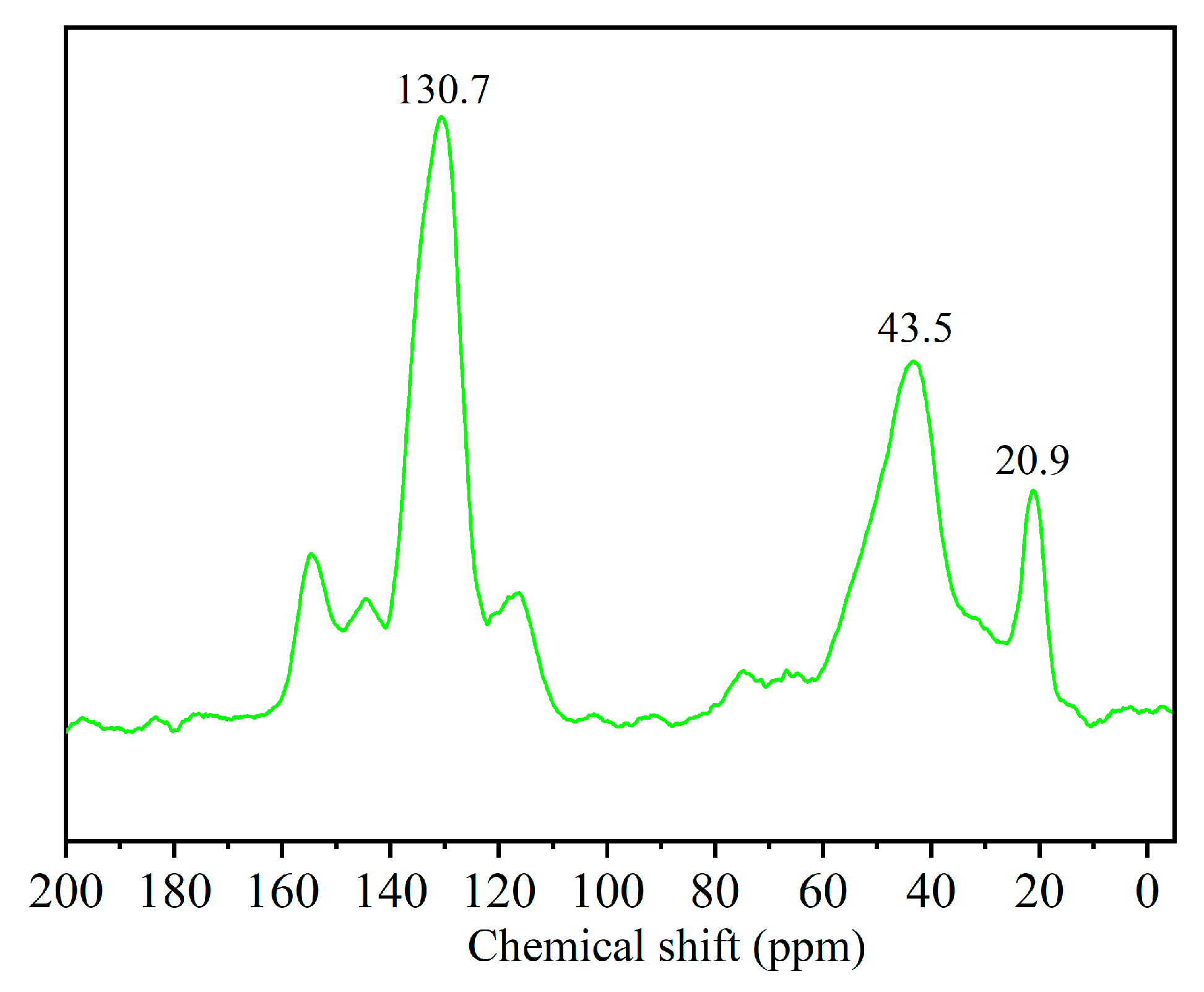
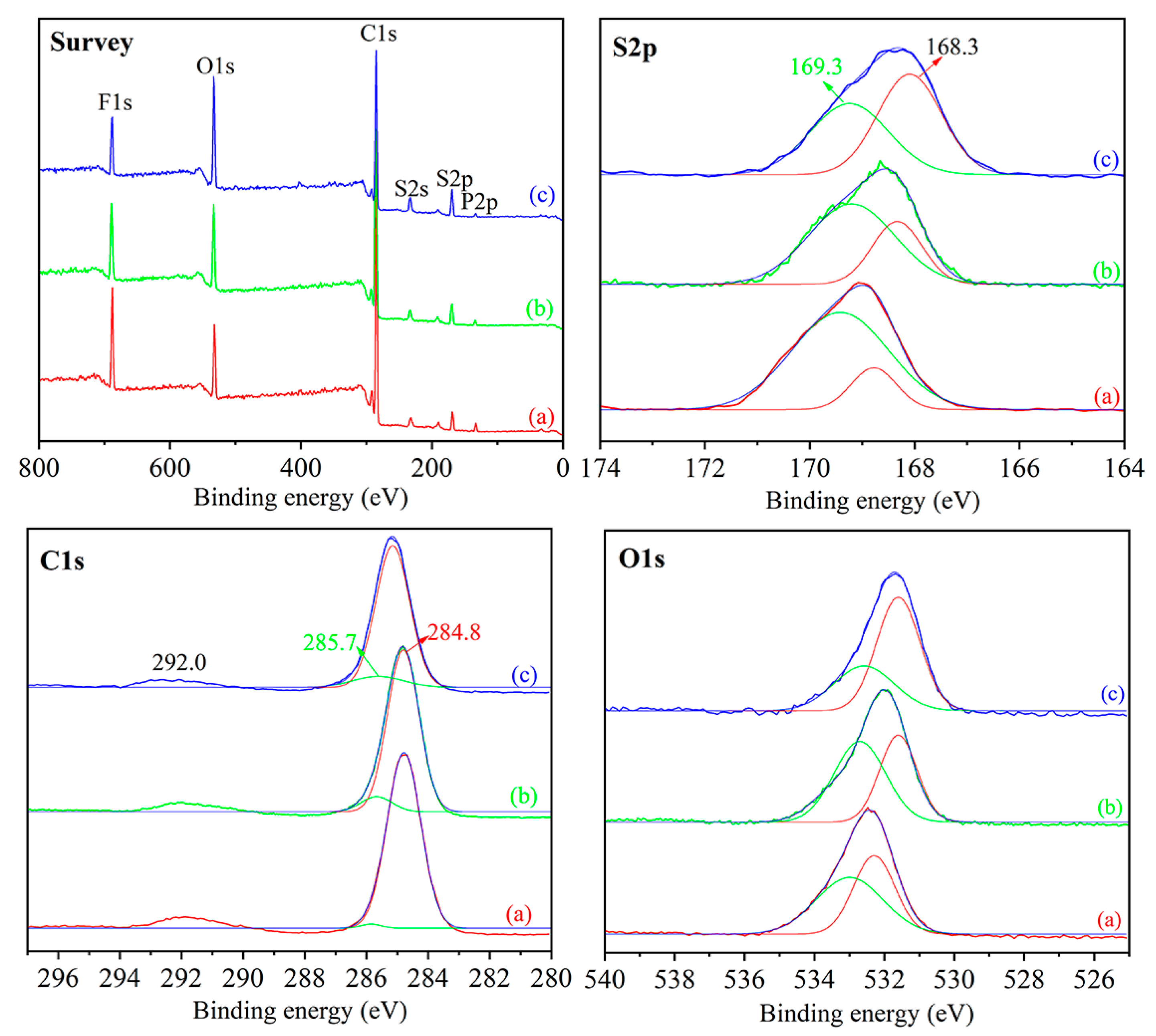
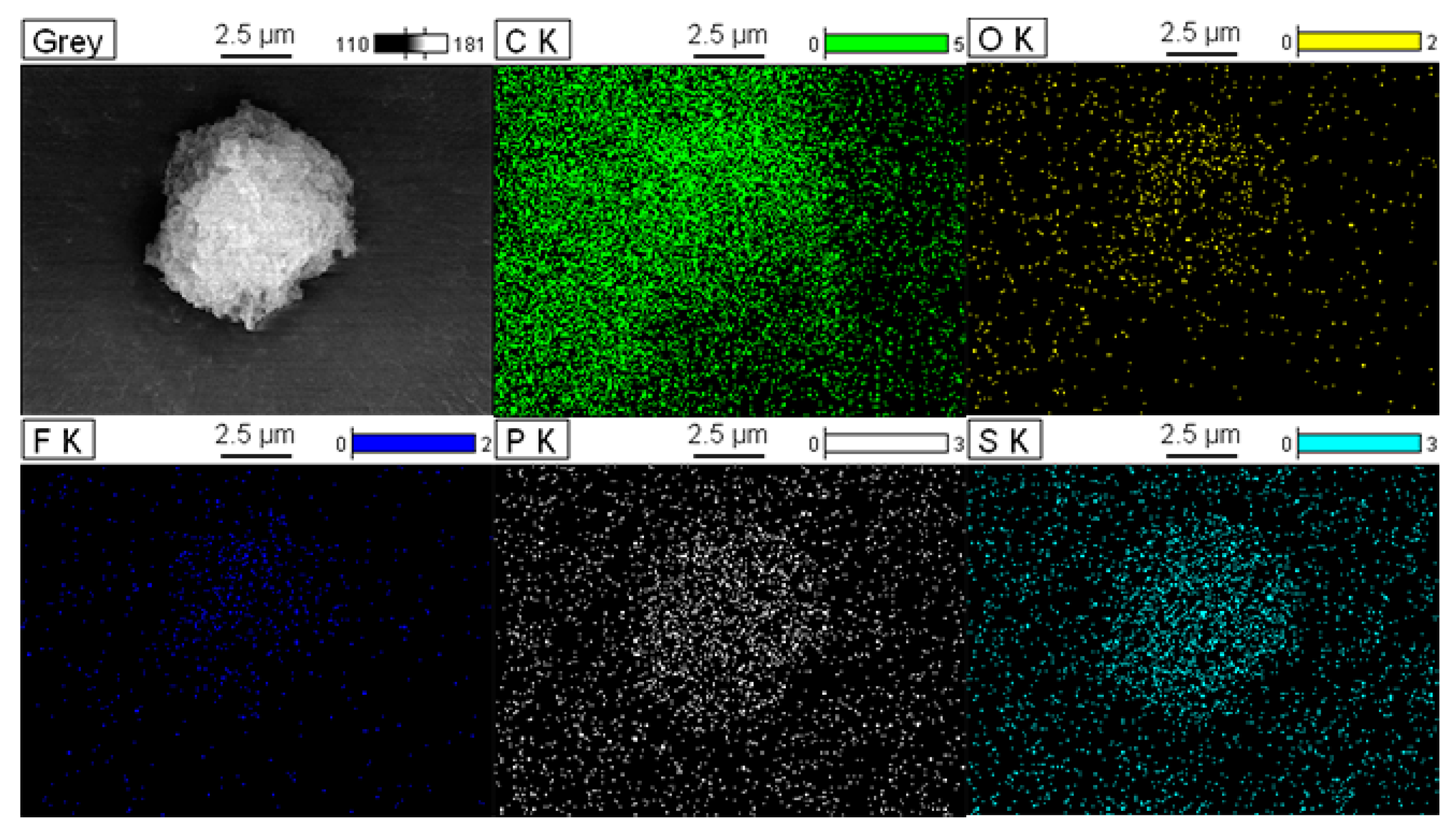
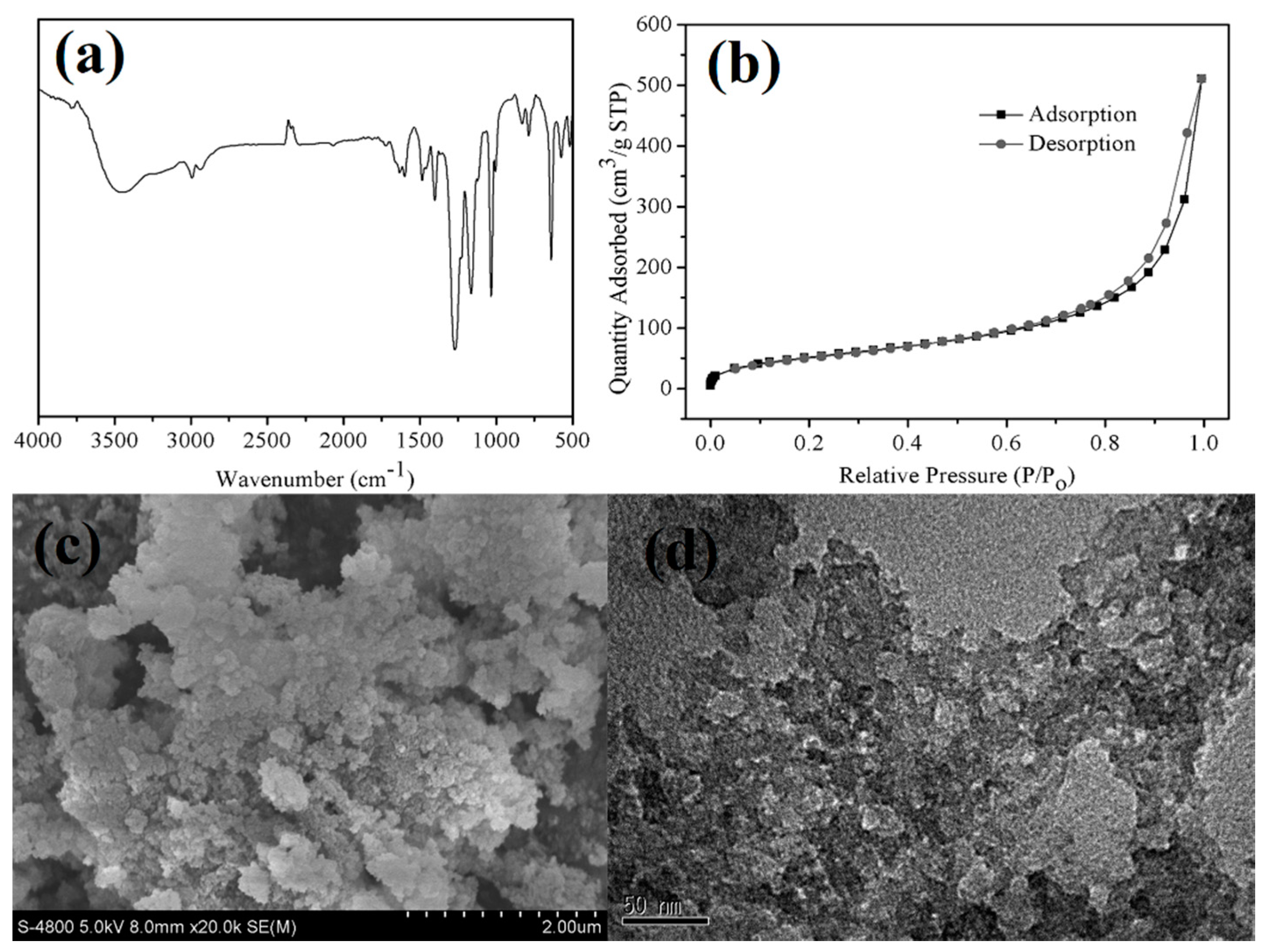
3.1. Characterization
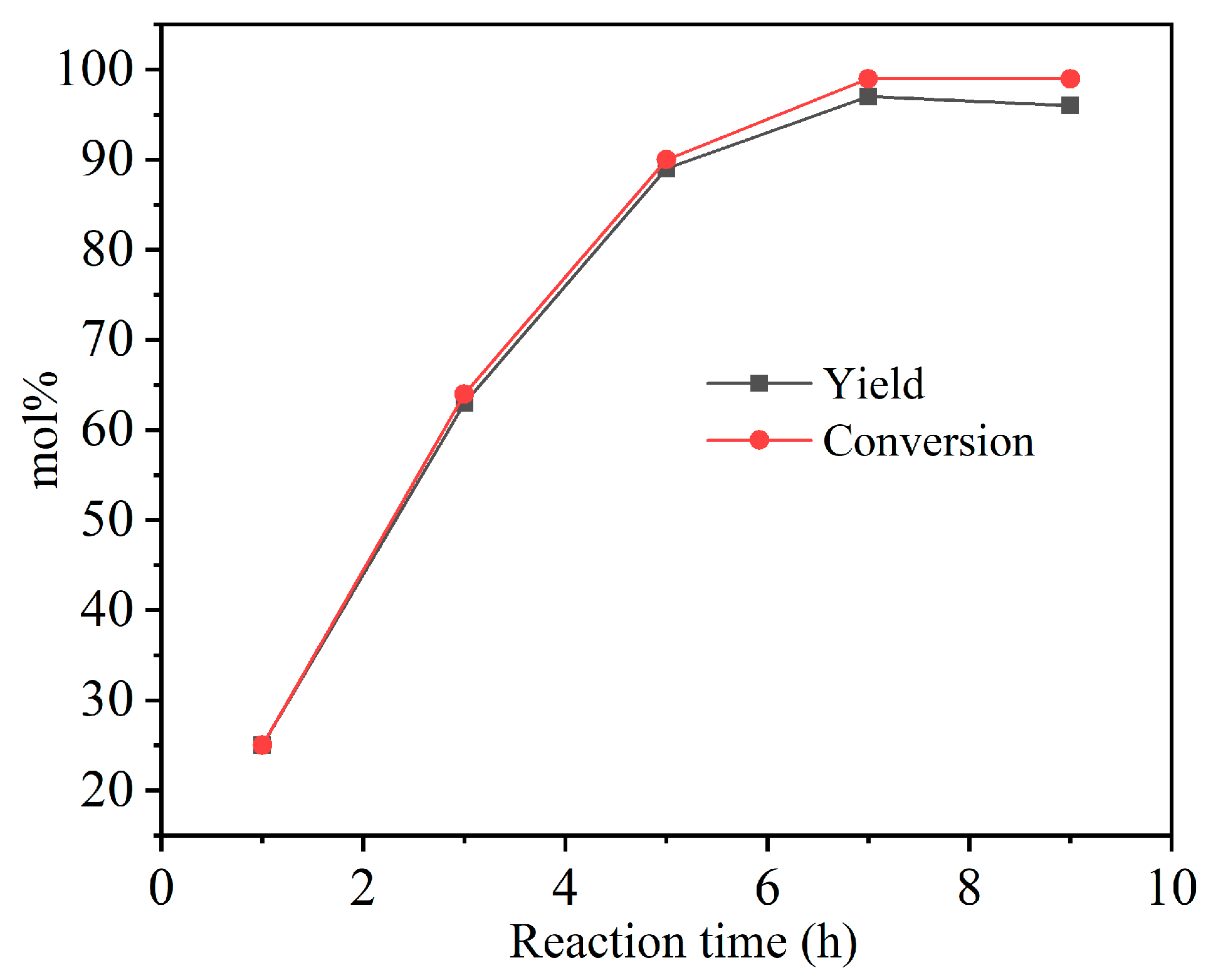
3.2. Catalytic Activity
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kitanosono, T.; Masuda, K.; Xu, P.; Kobayashi, S. Catalytic organic reactions in water toward sustainable society. Chem. Rev. 2017, 118, 679–746. [Google Scholar] [CrossRef]
- Simon, M.O.; Li, C.J. Green chemistry oriented organic synthesis in water. Chem. Soc. Rev. 2012, 41, 1415–1427. [Google Scholar] [CrossRef]
- Nairoukh, Z.; Avnir, D.; Blum, J. Acid-Catalyzed Hydration of Alkynes in Aqueous Microemulsions. ChemSusChem 2013, 6, 430–432. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, W.; Wang, L.; Su, M.; Liu, F. Efficient Hydrolysis of Cyclohexyl Acetate to Cyclohexanol Catalyzed by Dual-SO3H-Functionalized Heteropolyacid-Based Solid Acids. Ind. Eng. Chem. Res. 2018, 57, 5207–5214. [Google Scholar] [CrossRef]
- Shimizu, K.I.; Furukawa, H.; Kobayashi, N.; Itaya, Y.; Satsuma, A. Effects of Brønsted and Lewis acidities on activity and selectivity of heteropolyacid-based catalysts for hydrolysis of cellobiose and cellulose. Green Chem. 2009, 11, 1627–1632. [Google Scholar] [CrossRef]
- Guo, F.; Fang, Z.; Xu, C.C.; Smith, R.L., Jr. Solid acid mediated hydrolysis of biomass for producing biofuels. Prog. Energy Combust. Sci. 2012, 38, 672–690. [Google Scholar] [CrossRef]
- Vilcocq, L.; Castilho, P.C.; Carvalheiro, F.; Duarte, L.C. Hydrolysis of oligosaccharides over solid acid catalysts: A review. ChemSusChem 2014, 7, 1010–1019. [Google Scholar] [CrossRef]
- Zhang, M.; Wei, L.; Chen, H.; Du, Z.; Binks, B.P.; Yang, H. Compartmentalized droplets for continuous flow liquid–liquid interface catalysis. J. Am. Chem. Soc. 2016, 138, 10173–10183. [Google Scholar] [CrossRef]
- Tanemura, K.; Suzuki, T. Hydration of aromatic terminal alkynes catalyzed by sulfonated condensed polynuclear aromatic (S-COPNA) resin in water. Tetrahedron Lett. 2017, 58, 955–958. [Google Scholar] [CrossRef]
- Huang, Y.B.; Fu, Y. Hydrolysis of cellulose to glucose by solid acid catalysts. Green Chem. 2013, 15, 1095–1111. [Google Scholar] [CrossRef]
- Alonso, F.; Beletskaya, I.P.; Yus, M. Transition-metal-catalyzed addition of heteroatom−hydrogen bonds to alkynes. Chem. Rev. 2004, 104, 3079–3160. [Google Scholar] [CrossRef]
- Das, A.K.; Park, S.; Muthaiah, S.; Hong, S.H. Ligand-and Acid-Free Gold (I) Chloride Catalyzed Hydration of Terminal Alkynes. Synlett 2015, 26, 2517–2520. [Google Scholar]
- Mizushima, E.; Sato, K.; Hayashi, T.; Tanaka, M. Highly Efficient AuI-Catalyzed Hydration of Alkynes. Angew. Chem. Int. Ed. 2002, 41, 4563–4565. [Google Scholar] [CrossRef]
- Xu, Y.; Hu, X.; Shao, J.; Yang, G.; Wu, Y.; Zhang, Z. Hydration of alkynes at room temperature catalyzed by gold (I) isocyanide compounds. Green Chem. 2015, 17, 532–537. [Google Scholar] [CrossRef]
- Das, R.; Chakraborty, D. AgOTf catalyzed hydration of terminal alkynes. Appl. Organomet. Chem. 2012, 26, 722–726. [Google Scholar] [CrossRef]
- Mann, A.; Wagner, A. Mild chemo-selective hydration of terminal alkynes catalysed by AgSbF6. Chem. Commun. 2012, 48, 434–436. [Google Scholar]
- Hartman, J.W.; Hiscox, W.C.; Jennings, P.W. Catalytic hydration of alkynes with platinum (II) complexes. J. Org. Chem. 1993, 58, 7613–7614. [Google Scholar] [CrossRef]
- Hiscox, W.; Jennings, P.W. Catalytic hydration of alkynes with Zeise’s dimer. Organometallics 1990, 9, 1997–1999. [Google Scholar] [CrossRef]
- Tokunaga, M.; Wakatsuki, Y. The First Anti-Markovnikov Hydration of Terminal Alkynes: Formation of Aldehydes Catalyzed by a Ruthenium (II)/Phosphane Mixture. Angew. Chem. Int. Ed. 1998, 37, 2867–2869. [Google Scholar] [CrossRef]
- Liu, H.; Wei, Y.; Cai, C. A Combination System of p-Toluenesulfonic Acid and Acetic Acid for the Hydration of Alkynes. Synlett 2016, 27, 2378–2383. [Google Scholar]
- Liu, W.; Wang, H.; Li, C.J. Metal-free markovnikov-type alkyne hydration under mild conditions. Org. Lett. 2016, 9, 2184–2187. [Google Scholar] [CrossRef] [PubMed]
- Rose, M. Nanoporous polymers: Bridging the gap between molecular and solid catalysts? ChemCatChem 2014, 6, 1166–1182. [Google Scholar] [CrossRef]
- Kramer, S.; Bennedsen, N.R.; Kegnæs, S. Porous organic polymers containing active metal centers as catalysts for synthetic organic chemistry. ACS Catal. 2018, 8, 6961–6982. [Google Scholar] [CrossRef]
- Sun, Q.; Dai, Z.; Meng, X.; Wang, L.; Xiao, F.S. Task-specific design of porous polymer heterogeneous catalysts beyond homogeneous counterparts. ACS Catal. 2015, 5, 4556–4567. [Google Scholar] [CrossRef]
- Tan, L.; Tan, B. Hypercrosslinked porous polymer materials: Design, synthesis, and applications. Chem. Soc. Rev. 2017, 46, 3322–3356. [Google Scholar] [CrossRef]
- Liu, F.; Wang, L.; Sun, Q.; Zhu, L.; Meng, X.; Xiao, F.S. Transesterification catalyzed by ionic liquids on superhydrophobic mesoporous polymers: Heterogeneous catalysts that are faster than homogeneous catalysts. J. Am. Chem. Soc. 2012, 134, 16948–16950. [Google Scholar] [CrossRef]
- Liu, F.; Kong, W.; Qi, C.; Zhu, L.; Xiao, F.S. Design and synthesis of mesoporous polymer-based solid acid catalysts with excellent hydrophobicity and extraordinary catalytic activity. ACS Catal. 2012, 2, 565–572. [Google Scholar] [CrossRef]
- Tao, D.J.; Liu, F.; Wang, L.; Jiang, L. A green and efficient hydration of alkynes catalyzed by hierarchically porous poly (ionic liquid) s solid strong acids. Appl. Catal. A Gen. 2018, 564, 56–63. [Google Scholar] [CrossRef]
- Karimi, B.; Mobaraki, A.; Mirzaei, H.M.; Zareyee, D.; Vali, H. Improving the Selectivity toward Three-Component Biginelli versus Hantzsch Reactions by Controlling the Catalyst Hydrophobic/Hydrophilic Surface Balance. ChemCatChem 2014, 6, 212–219. [Google Scholar] [CrossRef]
- Lei, Y.; Wan, Y.; Li, G.; Zhou, X.Y.; Gu, Y.; Feng, J.; Wang, R. Palladium supported on an amphiphilic porous organic polymer: A highly efficient catalyst for aminocarbonylation reactions in water. Mater. Chem. Front. 2017, 1, 1541–1549. [Google Scholar] [CrossRef]
- Lai, B.; Mei, F.; Gu, Y. Bifunctional Solid Catalyst for Organic Reactions in Water: Simultaneous Anchoring of Acetylacetone Ligands and Amphiphilic Ionic Liquid “Tags” by Using a Dihydropyran Linker. Chem. Asian J. 2018, 13, 2529–2542. [Google Scholar] [CrossRef] [PubMed]
- Dawson, R.; Laybourn, A.; Clowes, R.; Khimyak, Y.Z.; Adams, D.J.; Cooper, A.I. Functionalized conjugated microporous polymers. Macromolecules 2009, 42, 8809–8816. [Google Scholar] [CrossRef]
- Xu, D.; Guo, J.; Yan, F. Porous ionic polymers: Design, synthesis, and applications. Prog. Polym. Sci. 2018, 79, 121–143. [Google Scholar] [CrossRef]
- Zhang, S.; Dokko, K.; Watanabe, M. Porous ionic liquids: Synthesis and application. Chem. Sci. 2015, 6, 3684–3691. [Google Scholar] [CrossRef]
- Wang, Q.; Cai, X.; Liu, Y.; Xie, J.; Zhou, Y.; Wang, J. Pd nanoparticles encapsulated into mesoporous ionic copolymer: Efficient and recyclable catalyst for the oxidation of benzyl alcohol with O2 balloon in water. Appl. Catal. B Environ. 2016, 189, 242–251. [Google Scholar] [CrossRef]
- Manojkumar, K.; Sivaramakrishna, A.; Vijayakrishna, K. A short review on stable metal nanoparticles using ionic liquids, supported ionic liquids, and poly (ionic liquids). J. Nanopart. Res. 2016, 18, 103. [Google Scholar] [CrossRef]
- Qian, W.; Texter, J.; Yan, F. Frontiers in poly (ionic liquid) s: Syntheses and applications. Chem. Soc. Rev. 2017, 46, 1124–1159. [Google Scholar] [CrossRef]
- Huang, M.; Cao, J.; Hong, Y.; Su, Y.; Wang, H.; Li, J. Sorption and Separation of CO2 from Syngas by a Quaternary Ammonium-based Poly (ionic liquid). Ind. Eng. Chem. Res. 2019, 58, 8317–8322. [Google Scholar] [CrossRef]
- Wan, Y.; Song, F.; Ye, T.; Li, G.; Liu, D.; Lei, Y. Carbonylative Suzuki coupling and alkoxycarbonylation of aryl halides using palladium supported on phosphorus-doped porous organic polymer as an active and robust catalyst. Appl. Organometal. Chem. 2019, 33, e4714. [Google Scholar] [CrossRef]
- Sun, Q.; Ma, S.; Dai, Z.; Meng, X.; Xiao, F.S. A hierarchical porous ionic organic polymer as a new platform for heterogeneous phase transfer catalysis. J. Mater. Chem. A 2015, 3, 23871–23875. [Google Scholar] [CrossRef]
- Sun, Q.; Dai, Z.; Meng, X.; Xiao, F.S. Porous polymer catalysts with hierarchical structures. Chem. Soc. Rev. 2015, 44, 6018–6034. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Jiang, Z.; Ma, F.; Su, F.; Chen, L.; Zhang, S.; Guo, Y. Design of mesoporous SO42−/ZrO2–SiO2(Et) hybrid material as an efficient and reusable heterogeneous acid catalyst for biodiesel production. Green Chem. 2010, 12, 2135–2138. [Google Scholar] [CrossRef]
- Wan, Y.; Lei, Y.; Lan, G.; Liu, D.; Li, G.; Bai, R. Synthesis of glycerol carbonate from glycerol and dimethyl carbonate over DABCO embedded porous organic polymer as a bifunctional and robust catalyst. Appl. Catal. A Gen. 2018, 562, 267–275. [Google Scholar] [CrossRef]
- Heo, Y.; Im, H.; Kim, J. The effect of sulfonated graphene oxide on sulfonated poly (ether ether ketone) membrane for direct methanol fuel cells. J. Membr. Sci. 2013, 425, 11–22. [Google Scholar] [CrossRef]
- Setiawan, L.D.; Baumann, H.; Gribbin, D. Surface studies of keratin fibers and related model compounds using ESCA. I—intermediate oxidation products of the model compound 1-cystine and their hydrolytical behaviour. Surf. Interface Anal. 1985, 7, 188–195. [Google Scholar] [CrossRef]
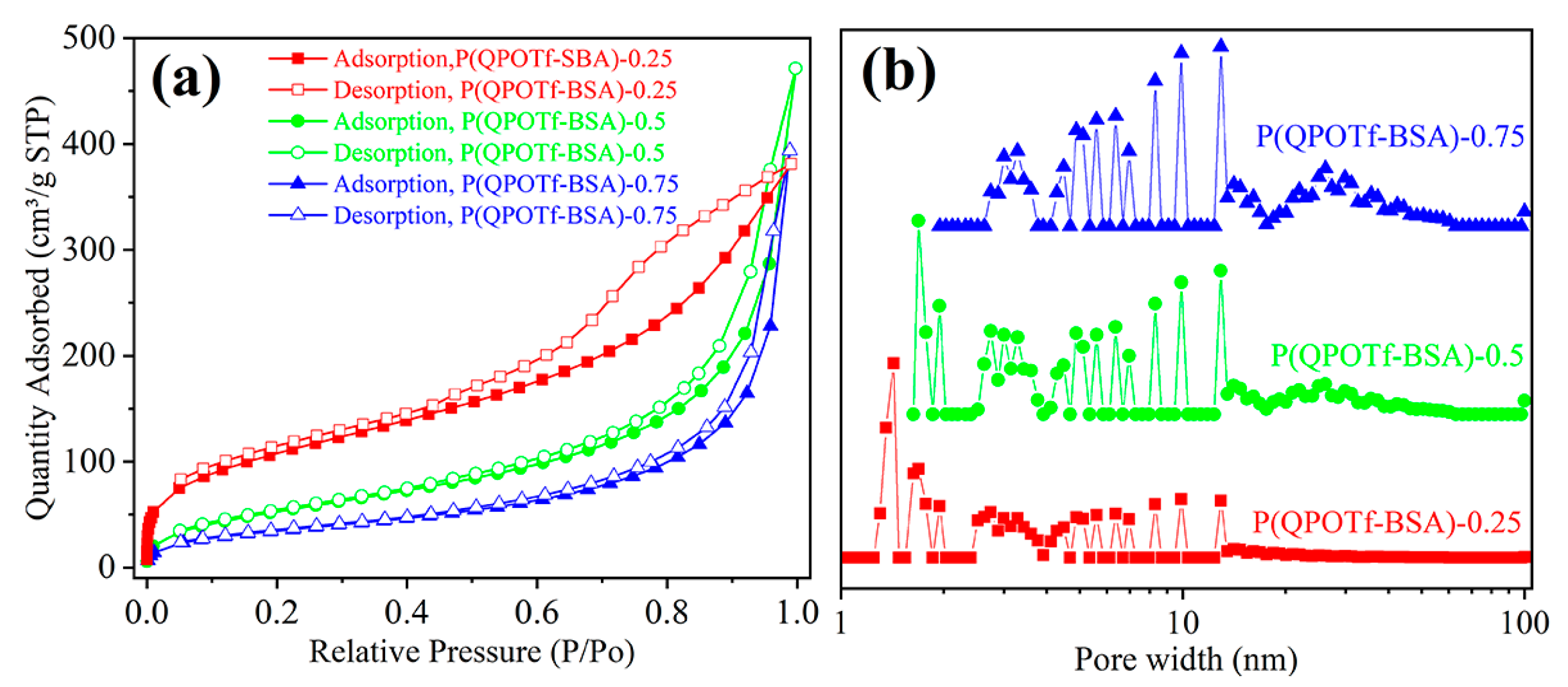


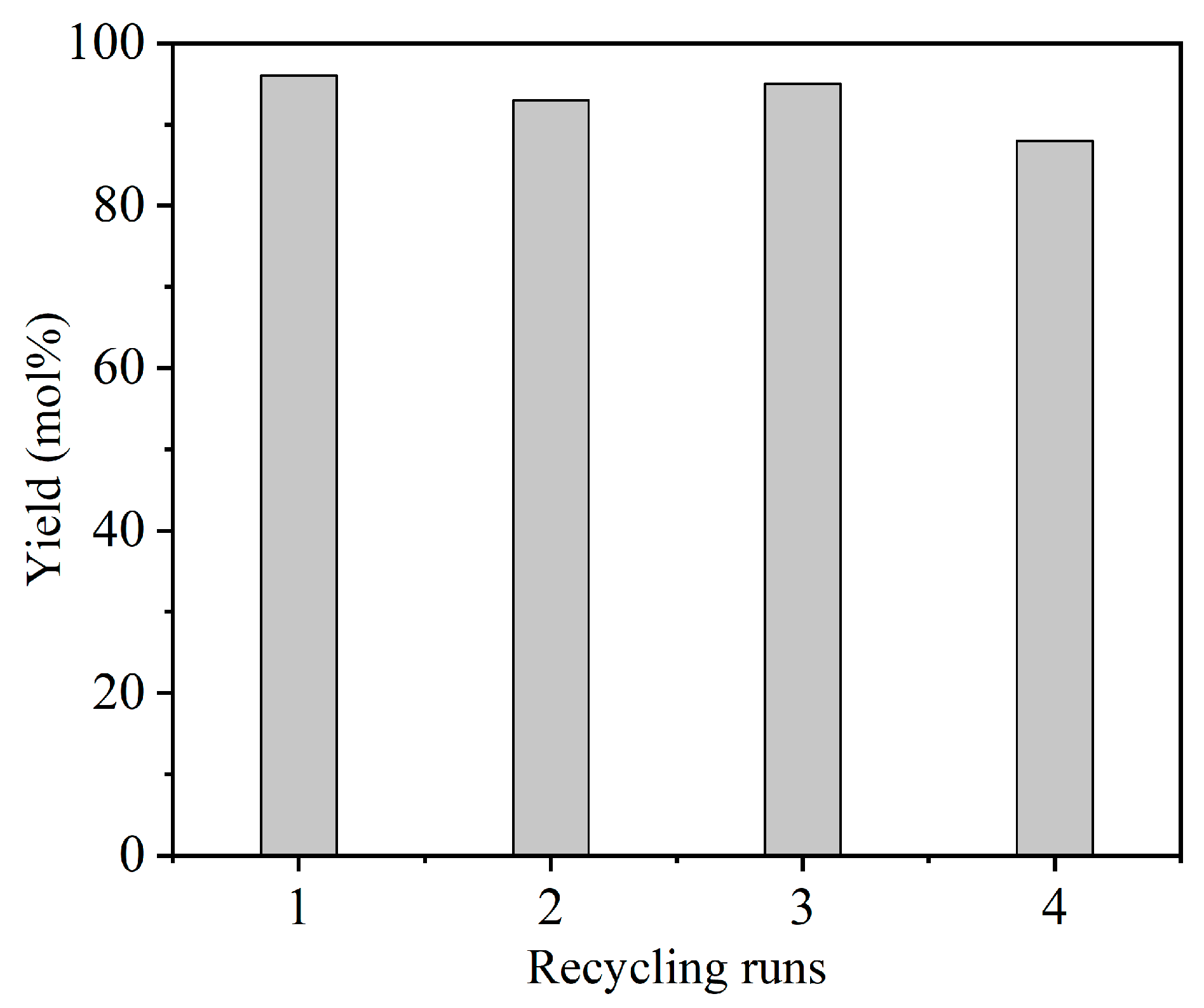
| Samples | SBET (m2 g−1) a | Vp (cm3 g−1) b | Dave (nm) c | Acid Content (mmol/g) d | Adsorption Capability (g/g) e |
|---|---|---|---|---|---|
| P(QPOTf-BSA)-0.25 | 392 | 0.56 | 3.2 | 0.45 | 8.6 |
| P(QPOTf-BSA)-0.5 | 204 | 0.68 | 5.1 | 1.17 | 6.5 |
| P(QPOTf-BSA)-0.75 | 130 | 0.61 | 8.8 | 2.34 | 4.1 |
| Entry | Catalyst | Conversion (mol%) b | Yield (mol%) b |
|---|---|---|---|
| 1 | P(QPOTf-BSA)-0.25 | 75 | 75 |
| 2 | P(QPOTf-BSA)-0.5 | 84 | 83 |
| 3 | P(QPOTf-BSA)-0.75 | 67 | 67 |
| 4 | Amberlyst-15 | 32 | 30 |
| 5 c | H2SO4 | 5 | 5 |
| 6 | PhSO3H | 14 | 13 |
| Entry | Alkyne | Product | Yield (mol%) b |
|---|---|---|---|
| 1 | 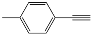 | 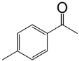 | 95 |
| 2 | 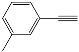 | 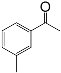 | 95 |
| 3 | 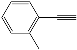 | 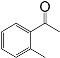 | 86 |
| 4 |  |  | 91 |
| 5 |  | 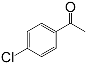 | 92 |
| 6 c |  | 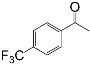 | 82 |
| 7 c |  |  | 73 |
| 8 c |  | 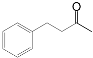 | 67 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lei, Y.; Zhang, M.; Li, Q.; Xia, Y.; Leng, G. A Porous Polymer-Based Solid Acid Catalyst with Excellent Amphiphilicity: An Active and Environmentally Friendly Catalyst for the Hydration of Alkynes. Polymers 2019, 11, 2091. https://doi.org/10.3390/polym11122091
Lei Y, Zhang M, Li Q, Xia Y, Leng G. A Porous Polymer-Based Solid Acid Catalyst with Excellent Amphiphilicity: An Active and Environmentally Friendly Catalyst for the Hydration of Alkynes. Polymers. 2019; 11(12):2091. https://doi.org/10.3390/polym11122091
Chicago/Turabian StyleLei, Yizhu, Maomin Zhang, Qian Li, Yu Xia, and Guojun Leng. 2019. "A Porous Polymer-Based Solid Acid Catalyst with Excellent Amphiphilicity: An Active and Environmentally Friendly Catalyst for the Hydration of Alkynes" Polymers 11, no. 12: 2091. https://doi.org/10.3390/polym11122091
APA StyleLei, Y., Zhang, M., Li, Q., Xia, Y., & Leng, G. (2019). A Porous Polymer-Based Solid Acid Catalyst with Excellent Amphiphilicity: An Active and Environmentally Friendly Catalyst for the Hydration of Alkynes. Polymers, 11(12), 2091. https://doi.org/10.3390/polym11122091






