Recent Innovation of Metal-Organic Frameworks for Carbon Dioxide Photocatalytic Reduction
Abstract
1. Introduction
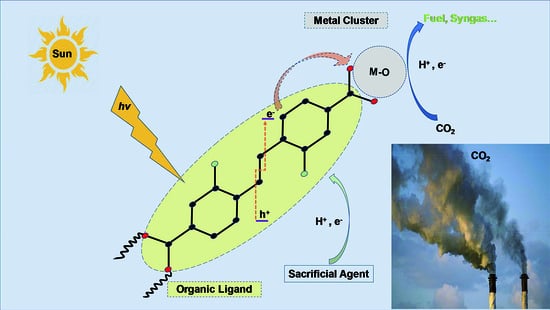
2. Big Picture of Metal-Organic Frameworks (MOFs)-Photoinduced CO2 Metamorphosis
2.1. Factors Affecting Sorbent Material Selection
2.2. Electronic Structure and Photosensitivity of MOFs for CO2 Photolytic Reaction
2.3. CO2 Saturation Capacity
3. Photocatalytic CO2 Reduction Process Steps
4. Photocatalyst MOFs Category
4.1. Zr-Based MOFs
4.2. Zn-Based MOFs
4.3. Ti/TiO2-Based MOFs
4.4. Fe-Based MOFs
4.5. Ni-Based MOF
4.6. Cu-Based MOF
4.7. Composite MOFs
4.7.1. Ti/TiO2 Composite
4.7.2. CdS-Based Composite MOF
4.7.3. Zr-Based Composite MOF
4.7.4. Co-Based Composite MOF
4.7.5. Graphene-Based Composite MOFs
5. MOFs-Photocatalytic Performance Enrichment
5.1. Organic Ligand Modification
5.2. Central Metal Site Modification
5.3. PS
6. Conclusion and Outlook
Author Contributions
Funding
Conflicts of Interest
Abbreviations/Acronyms
- TCPP = tetrakis(4-carboxyphenyl)porphyrine
- BH-(mim)3− = boron imidazolate
- bpy = 2,2′-bipyridine
- dpe = 1,2-di(4-pyridyl)ethylene
- bpydc = 2,2′-bipyridine-5,5′-dicarboxylate
- cptpy = (4′-(4-carboxyphenyl) -terpyridine)
- DHBDC = 2,5-dihydroxybenzene-1,4-dicarboxylic acid
- BDC = benzene-1,4-dicarboxylic acid
- H4DOBDC = 2,5-dioxide-1,4-benzenedicarboxylate
- THPP = 5,10,15,20-tetrakis(3,4,5-trihydroxyphenyl) porphyrin
- BA = butanedioic Acid
- IA = isobutyric Acid
- TCBPE = tetrabenzoatetraphenylethylene
- ptcda = perylene-3,4,9,10-tetracarboxylic dianhydride
- NTB = 4,4′,4″-nitrilotribenzoic acid
- NBDC = 2-nitro-1,4-benzenedicarboxylic acid
- BPDC = 4,4′-biphenyldicarboxylic acid
- TIPA = triisopropanolamine
- ATA = 2-aminoterephthalic acid
- HCHC = hexakis (4-carboxyphenyl) hexabenzocoronene
- HCBB = hexakis(4′-carboxy[1,1′-biphenyl]-4-yl) benzene
- TAA = thioacetamide
- TCA = 4,4′,4″-nitrilotribenzoic acid ligands
- dcbpy = 2,2′-bipyridine-5,5′-dicarboxylic acid
- HAD = adenine
- TPA = terephthalic acid
- BTC = 1,3,5-benzenetricarboxylat
- 2-mIM = 2-methylimidazole
- L = 4,4′-((((perfluoropropane-2,2-diyl)bis(4,1-phenylene))bis(oxy))bis(methylene))dibenzoate anion
- H2L1 = 2,2′-diamino-4,4′-stilbenedicarboxylic acid
- H2L = 4,4′-(anthracene-9,10-diylbis(ethyne-2,1-diyl))dibenzoic acid
- H3L = Ru-L metalloligand
- H4L = 2-amino-1, 4-benzenedicarboxylic acid (BDC-NH2)
- H2BTTA = 2,5-di(1H-1,2,4,-trazol-1-yl)terephthalic acid
- NDI = naphthalenediimide
- H2SDCA = 4,4′-stilbenedicarboxylic acid
- MeCN = acetonitrile
- H2O = water
- TEOA = triethanolamine
- TIPA = triisopropanolamine
- MeOH = metahnol
- NaHCO3 aqu. = sodium bicarbonate aqueous
- BET = Brunauer-Emmett-Teller
- TGA = thermogravimetric analysis
- TEOA = triethanolamine
- TIPA = triisopropanolamine
- EN = ethylenediamine
- DETA = diethylenetriamine
- TETA = triethylenetetramine
- ZrPP-1-Co = [Zr2(THPP-Co)]n
- ZIF-8 = Zn(MeIM)2
- ZIF-67 = Co(MeIM)2
- UiO-66 = [Zr6O4(OH)4(BDC)6]
- UiO-67 = [Zr6O4(OH)4(BPDC)6]
- UiO-bpy = Zr6(µ3-O)4(µ3-OH)4(µ1-OH)(H2O)-(bpydc)5(HCO2)
- Ir-CP Y = [Ir(ppy)2(dcbpy)]2[OH]
- HKUST-1 = [Cu3(btc)2(H2O)3]
- Hf12-Ru = Hf12(µ3-O)8(µ3-OH)8(µ2-OH)6(TFA)6(L-Ru)6
- L-Ru = bis(2,2′-bipyridine)[5,5′-di(4-carboxyl-phenyl)-2,2′-bipyridine]ruthenium(II) dichloride
- BIF-20 = Zn2(BH(MeIM)3)2(OBB)
- FJI-H14 = [Cu(BTTA)H2O]n·6H2O
- TCPP-Co = tetrakis(4-carboxyphenyl)porphyrin-Co
- PCN-222 = Zr6(µ3-OH)8(OH)8-(TCPP)2
- PCN-136 =(Zr6(μ3-O)4(μ3-OH)4(OH)6(H2O)6(HCHC)
- pbz-MOF-1 = (Zr6(μ3-O)6(μ3-OH)2(Ac)5(OH)(H2O)(HCBB))
- NH2-UiO-66 = [Zr6O4(OH)4(NH2-BDC)6
- MOF-525(Fe) = Zr6O4(OH)8(TCPP-Fe)2
- MOF-525 = Zr6O4(OH)8(TCPP)2
- MOF-525-Co = Zr6O4(OH)8(TCPP-Co)2
- MOF-525(Fe) = Zr6O4(OH)8(TCPP-Fe)2
- NH2-MIL-88B(Fe) = Fe3O(solvent)3Cl(NH2-BDC)3(solvent)m
- NH2-MIL-101(Fe) = Fe3F(H2O)2O(NH2-BDC)3
- MOF-253 = [Al(OH)(bpydc)]
- MOF-74-Co = [Co2(dobdc)]
- MOF-5 = [Zn4O(BDC)3]
- MIL-101 = Cr3F(H2O)2O(BDC)3·nH2O (n ~ 25)
- MIL-101(Fe) = Fe3F(H2O)2O(BDC)3
- MAF-X27-OH = [Co2(µ-OH)2(bbta)]
- MAF-X27-Cl = [Co2(µ-Cl)2(bbta)]
References
- Hansen, J.; Sato, M.; Ruedy, R.; Lo, K.; Lea, D.W.; Medina-Elizade, M. Global temperature change. Proc. Natl. Acad. Sci. USA 2006, 103, 14288–14293. [Google Scholar] [CrossRef] [PubMed]
- Chapin, F.S., III; Jefferies, R.L.; Reynolds, J.F.; Shaver, G.R.; Svoboda, J.; Chu, E.W. Arctic Ecosystems in a Changing Climate: An Ecophysiological Perspective; Academic Press: Cambridge, MA, USA, 2012. [Google Scholar]
- Mastalerz, M.; Schneider, M.W.; Oppel, I.M.; Presly, O. A salicylbisimine cage compound with high surface area and selective CO2/CH4 adsorption. Angew. Chem. Int. Ed. 2011, 50, 1046–1051. [Google Scholar] [CrossRef] [PubMed]
- D‘Alessandro, D.M.; Smit, B.; Long, J.R. Carbon dioxide capture: Prospects for new materials. Angew. Chem. Int. Ed. 2010, 49, 6058–6082. [Google Scholar] [CrossRef] [PubMed]
- Botkin, D.B.; Saxe, H.; Araujo, M.B.; Betts, R.; Bradshaw, R.H.; Cedhagen, T.; Chesson, P.; Dawson, T.P.; Etterson, J.R.; Faith, D.P. Forecasting the effects of global warming on biodiversity. Bioscience 2007, 57, 227–236. [Google Scholar] [CrossRef]
- Benbow, P.K. Chymists and Chymistry: Studies in the History of Alchemy and Early Modern Chemistry; Taylor & Francis Ltd.: Abingdon, UK, 2009. [Google Scholar]
- Hannaway, O. Laboratory design and the aim of science: Andreas Libavius versus Tycho Brahe. Isis 1986, 77, 585–610. [Google Scholar] [CrossRef]
- Bolin, B.; Doos, B.R. Greenhouse Effect; John Wiley and Sons Inc.: New York, NY, USA, 1989. [Google Scholar]
- Schlamadinger, B.; Apps, M.; Bohlin, F.; Gustavsson, L.; Jungmeier, G.; Marland, G.; Pingoud, K.; Savolainen, I. Towards a standard methodology for greenhouse gas balances of bioenergy systems in comparison with fossil energy systems. Biomass Bioenerg. 1997, 13, 359–375. [Google Scholar] [CrossRef]
- Lacis, A.; Hansen, J.; Lee, P.; Mitchell, T.; Lebedeff, S. Greenhouse effect of trace gases, 1970–1980. Geophys. Res. Lett. 1981, 8, 1035–1038. [Google Scholar] [CrossRef]
- O‘Neill, B.C.; Oppenheimer, M. Climate change impacts are sensitive to the concentration stabilization path. Proc. Natl. Acad. Sci. USA 2004, 101, 16411–16416. [Google Scholar] [CrossRef]
- Lüthi, D.; Le Floch, M.; Bereiter, B.; Blunier, T.; Barnola, J.-M.; Siegenthaler, U.; Raynaud, D.; Jouzel, J.; Fischer, H.; Kawamura, K. High-resolution carbon dioxide concentration record 650,000–800,000 years before present. Nature 2008, 453, 379. [Google Scholar] [CrossRef]
- Hansen, J.; Nazarenko, L.; Ruedy, R.; Sato, M.; Willis, J.; Del Genio, A.; Koch, D.; Lacis, A.; Lo, K.; Menon, S. Earth’s energy imbalance: Confirmation and implications. Science 2005, 308, 1431–1435. [Google Scholar] [CrossRef]
- Dlugokencky, E.; Hall, B.; Montzka, S.; Dutton, G.; Mühle, J.; Elkins, J. Atmospheric composition [in state of the climate in 2017]. B. Am. Meteorol. Soc. 2018, 99, S46–S49. [Google Scholar]
- Smith, P.; Martino, Z.; Cai, D. ‘Agriculture’. In Climate Change 2007: Mitigation; Cambridge University: Cambridge, UK, 2007. [Google Scholar]
- Solomon, S.; Qin, D.; Manning, M.; Chen, Z.; Marquis, M.; Averyt, K.; Tignor, M.; Miller, H. Climmate Change 2007: The Physical Science Basis; Cambridge University Press: Cambridge, UK; New York, NY, USA, 2007. [Google Scholar]
- Foster, G.; Rahmstorf, S. Global temperature evolution 1979–2010. Environ. Res. 2011, 6, 044022. [Google Scholar] [CrossRef]
- Al-Rashidi, T.B.; El-Gamily, H.I.; Amos, C.L.; Rakha, K.A. Sea surface temperature trends in Kuwait Bay, Arabian Gulf. Nat. Hazards 2009, 50, 73–82. [Google Scholar] [CrossRef]
- Echer, M.S.; Echer, E.; Rigozo, N.; Brum, C.; Nordemann, D.; Gonzalez, W. On the relationship between global, hemispheric and latitudinal averaged air surface temperature (GISS time series) and solar activity. J. Atmos. Sol. Terr. Phys. 2012, 74, 87–93. [Google Scholar] [CrossRef]
- Santer, B.; Wigley, T.; Taylor, K. The reproducibility of observational estimates of surface and atmospheric temperature change. Science 2011, 334, 1232–1233. [Google Scholar] [CrossRef]
- Blunden, J. Reporting on the State of the Climate in 2018. 2019. Available online: https://www.climate.gov/news-features/understanding-climate/reporting-state-climate-2018 (accessed on 12 August 2019).
- Loeser, J.D.; Treede, R.-D. The Kyoto protocol of IASP basic pain Terminology. Pain 2008, 137, 473–477. [Google Scholar] [CrossRef] [PubMed]
- Evans, A.; Strezov, V.; Evans, T.J. Assessment of sustainability indicators for renewable energy technologies. Renew. Sust. Energ. Rev. 2009, 13, 1082–1088. [Google Scholar] [CrossRef]
- Sahir, M.H.; Qureshi, A.H. Assessment of new and renewable energy resources potential and identification of barriers to their significant utilization in Pakistan. Renew. Sust. Energ. Rev. 2008, 12, 290–298. [Google Scholar] [CrossRef]
- Buckman, G.; Diesendorf, M. Design limitations in Australian renewable electricity policies. Energ. Policy 2010, 38, 3365–3376. [Google Scholar] [CrossRef]
- Al-Badi, A.; Malik, A.; Gastli, A. Assessment of renewable energy resources potential in Oman and identification of barrier to their significant utilization. Renew. Sust. Energ. Rev. 2009, 13, 2734–2739. [Google Scholar] [CrossRef]
- Boot-Handford, M.E.; Abanades, J.C.; Anthony, E.J.; Blunt, M.J.; Brandani, S.; Mac Dowell, N.; Fernández, J.R.; Ferrari, M.-C.; Gross, R.; Hallett, J.P. Carbon capture and storage update. Energ. Environ. Sci. 2014, 7, 130–189. [Google Scholar] [CrossRef]
- Hunt, A.J.; Sin, E.H.; Marriott, R.; Clark, J.H. Generation, capture, and utilization of industrial carbon dioxide. ChemSusChem 2010, 3, 306–322. [Google Scholar] [CrossRef]
- Romeo, L.M.; Abanades, J.C.; Escosa, J.M.; Paño, J.; Giménez, A.; Sánchez-Biezma, A.; Ballesteros, J.C. Oxyfuel carbonation/calcination cycle for low cost CO2 capture in existing power plants. Energ. Convers. Manag. 2008, 49, 2809–2814. [Google Scholar] [CrossRef]
- Ahrens, C.D. Meteorology Today: An Introduction to Weather, Climate, and the Environment; Cengage Learning: Boston, MA, USA, 2012. [Google Scholar]
- Serna-Guerrero, R.; Da’na, E.; Sayari, A. New insights into the interactions of CO2 with amine-functionalized silica. Ind. Eng. Chem. Res. 2008, 47, 9406–9412. [Google Scholar] [CrossRef]
- Rochelle, G.T. Amine scrubbing for CO2 capture. Science 2009, 325, 1652–1654. [Google Scholar] [CrossRef]
- Sartori, G.; Leder, F. Process for Removing Carbon Dioxide Containing Acidic Gases from Gaseous Mixtures using Aqueous Amine Scrubbing Solutions. Google Patents 4,112,052, 5 September 1978. [Google Scholar]
- Singh, D.; Croiset, E.; Douglas, P.L.; Douglas, M.A. Techno-economic study of CO2 capture from an existing coal-fired power plant: MEA scrubbing vs. O2/CO2 recycle combustion. Energ. Convers. Manag. 2003, 44, 3073–3091. [Google Scholar] [CrossRef]
- McElroy, P.L., Jr. Separation of Acidic Gas Constituents from Gaseous Mixtures Containing the Same. Google Patents 4,044,100, 23 August 1977. [Google Scholar]
- Sartori, G.; Leder, F. Process for Removing Carbon Dioxide Containing Acidic Gases from Gaseous Mixtures using a Basic Salt Activated with a Hindered Amine. Google Patents 4,112,050, 5 September 1978. [Google Scholar]
- Cummings, A.L.; Smith, G.D.; Nelsen, D.K. Advances in amine reclaiming–why there’s no excuse to operate a dirty amine system. In Proceedings of the Laurance Reid Gas Conditioning Conference, Citeseer, Norman, OK, USA, 25–28 February 2007. [Google Scholar]
- Vaidhyanathan, R.; Iremonger, S.S.; Shimizu, G.K.; Boyd, P.G.; Alavi, S.; Woo, T.K. Direct observation and quantification of CO2 binding within an amine-functionalized nanoporous solid. Science 2010, 330, 650–653. [Google Scholar] [CrossRef]
- Rochelle, G.T. Thermal degradation of amines for CO2 capture. Curr. Opin. Chem. Eng. 2012, 1, 183–190. [Google Scholar] [CrossRef]
- Adhoum, N.; Monser, L. Removal of cyanide from aqueous solution using impregnated activated carbon. Chem. Eng. Process. Process Intensif. 2002, 41, 17–21. [Google Scholar] [CrossRef]
- Davidson, P.J.; Lywood, W.J. Adsorption Process. Google Patents 4,758,253, 19 July 1988. [Google Scholar]
- Matviya, T.M. Method for Producing Self-Supporting Activated Carbon Structures. Google Patents 6,682,667, 27 January 2004. [Google Scholar]
- Duan, F.; Yang, Y.; Li, Y.; Cao, H.; Wang, Y.; Zhang, Y. Heterogeneous Fenton-like degradation of 4-chlorophenol using iron/ordered mesoporous carbon catalyst. J. Environ. Sci. 2014, 26, 1171–1179. [Google Scholar] [CrossRef]
- Huang, T.; Mei, J.; Liu, H. Preparation of MnOx Loaded Activated Carbon for SO2 Removal by Redox Deposition. Recent Innov. Chem. Eng. Former. Recent Pat. Chem. Eng. 2016, 9, 98–106. [Google Scholar] [CrossRef]
- Li, Y.; Zhao, C.; Chen, H.; Liang, C.; Duan, L.; Zhou, W. Modified CaO-based sorbent looping cycle for CO2 mitigation. Fuel 2009, 88, 697–704. [Google Scholar] [CrossRef]
- Gupta, H.; Fan, L.-S. Carbonation−calcination cycle using high reactivity calcium oxide for carbon dioxide separation from flue gas. Ind. Eng. Chem. Res. 2002, 41, 4035–4042. [Google Scholar] [CrossRef]
- Kutney, G. Carbon Politics and the Failure of the Kyoto Protocol; Routledge: Abingdon, UK, 2014. [Google Scholar]
- Rosen, A.M. The wrong solution at the right time: The failure of the kyoto protocol on climate change. Polit. Policy 2015, 43, 30–58. [Google Scholar] [CrossRef]
- Wang, J.; Huang, L.; Yang, R.; Zhang, Z.; Wu, J.; Gao, Y.; Wang, Q.; O’Hare, D.; Zhong, Z. Recent advances in solid sorbents for CO 2 capture and new development trends. Energ. Environ. Sci. 2014, 7, 3478–3518. [Google Scholar] [CrossRef]
- Tan, Y.; Nookuea, W.; Li, H.; Thorin, E.; Yan, J. Property impacts on Carbon Capture and Storage (CCS) processes: A review. Energ. Convers. Manag. 2016, 118, 204–222. [Google Scholar] [CrossRef]
- Patel, H.A.; Karadas, F.; Canlier, A.; Park, J.; Deniz, E.; Jung, Y.; Atilhan, M.; Yavuz, C.T. High capacity carbon dioxide adsorption by inexpensive covalent organic polymers. J. Mater. Chem. 2012, 22, 8431–8437. [Google Scholar] [CrossRef]
- Weston, M.H.; Farha, O.K.; Hauser, B.G.; Hupp, J.T.; Nguyen, S.T. Synthesis and metalation of catechol-functionalized porous organic polymers. Chem. Mater. 2012, 24, 1292–1296. [Google Scholar] [CrossRef]
- Yuan, S.; White, D.; Mason, A.; Liu, D.J. Porous organic polymers containing carborane for hydrogen storage. Int. J. Energ. Res. 2013, 37, 732–740. [Google Scholar] [CrossRef]
- Holst, J.R.; Cooper, A.I. Ultrahigh surface area in porous solids. Adv. Mater. 2010, 22, 5212–5216. [Google Scholar] [CrossRef]
- Zhang, Y.; Riduan, S.N. Functional porous organic polymers for heterogeneous catalysis. Chem. Soc. Rev. 2012, 41, 2083–2094. [Google Scholar] [CrossRef] [PubMed]
- Arab, P.; Rabbani, M.G.; Sekizkardes, A.K.; İslamoǧlu, T.; El-Kaderi, H.M. Copper (I)-catalyzed synthesis of nanoporous azo-linked polymers: Impact of textural properties on gas storage and selective carbon dioxide capture. Chem. Mater. 2014, 26, 1385–1392. [Google Scholar] [CrossRef]
- Chen, Q.; Luo, M.; Wang, T.; Wang, J.-X.; Zhou, D.; Han, Y.; Zhang, C.-S.; Yan, C.-G.; Han, B.-H. Porous organic polymers based on propeller-like hexaphenylbenzene building units. Macromolecules 2011, 44, 5573–5577. [Google Scholar] [CrossRef]
- Gomes, R.; Bhanja, P.; Bhaumik, A. A triazine-based covalent organic polymer for efficient CO2 adsorption. Chem. Commun. 2015, 51, 10050–10053. [Google Scholar] [CrossRef] [PubMed]
- Trewin, A.; Cooper, A.I. Porous organic polymers: Distinction from disorder? Angew. Chem. Int. Ed. 2010, 49, 1533–1535. [Google Scholar] [CrossRef] [PubMed]
- Weber, J.; Thomas, A. Toward stable interfaces in conjugated polymers: Microporous poly (p-phenylene) and poly (phenyleneethynylene) based on a spirobifluorene building block. J. Am. Chem. Soc. 2008, 130, 6334–6335. [Google Scholar] [CrossRef]
- Férey, G.; Serre, C.; Devic, T.; Maurin, G.; Jobic, H.; Llewellyn, P.L.; De Weireld, G.; Vimont, A.; Daturi, M.; Chang, J.-S. Why hybrid porous solids capture greenhouse gases? Chem. Soc. Rev. 2011, 40, 550–562. [Google Scholar] [CrossRef]
- Wang, S.; Yan, S.; Ma, X.; Gong, J. Recent advances in capture of carbon dioxide using alkali-metal-based oxides. Energ. Environ. Sci. 2011, 4, 3805–3819. [Google Scholar] [CrossRef]
- Tilford, R.W.; Mugavero, S.J., III; Pellechia, P.J.; Lavigne, J.J. Tailoring microporosity in covalent organic frameworks. Adv. Mater. 2008, 20, 2741–2746. [Google Scholar] [CrossRef]
- Gur, I.; Fromer, N.A.; Geier, M.L.; Alivisatos, A.P. Air-stable all-inorganic nanocrystal solar cells processed from solution. Science 2005, 310, 462–465. [Google Scholar] [CrossRef]
- Adachi, M.; Akishige, Y.; Asahi, T.; Deguchi, K.; Gesi, K.; Hasebe, K.; Hikita, T.; Ikeda, T.; Iwata, Y.; Komukae, M. CaNb2O6-SrNb2O6 BaNb2O6, 6C-d3, Oxides; Springer: New York, NY, USA, 2002; pp. 1–6. [Google Scholar]
- De Moraes, N.P.; Bacani, R.; da Silva, M.L.C.P.; Campos, T.M.B.; Thim, G.P.; Rodrigues, L.A. Effect of Nb/C ratio in the morphological, structural, optical and photocatalytic properties of novel and inexpensive Nb2O5/carbon xerogel composites. Ceram. Int. 2018, 44, 6645–6652. [Google Scholar] [CrossRef]
- Yang, P.; Zhao, D.; Margolese, D.I.; Chmelka, B.F.; Stucky, G.D. Generalized syntheses of large-pore mesoporous metal oxides with semicrystalline frameworks. Nature 1998, 396, 152. [Google Scholar] [CrossRef]
- Tanaka, D.; Kitagawa, S. Template effects in porous coordination polymers. Chem. Mater. 2007, 20, 922–931. [Google Scholar] [CrossRef]
- Rajeshwar, K.; Thomas, A.; Janáky, C. Photocatalytic Activity of Inorganic Semiconductor Surfaces: Myths, Hype, and Reality; ACS Publications: Washington, DC, USA, 2015. [Google Scholar]
- Batten, S.R.; Champness, N.R.; Chen, X.-M.; Garcia-Martinez, J.; Kitagawa, S.; Öhrström, L.; O’Keeffe, M.; Suh, M.P.; Reedijk, J. Terminology of metal–organic frameworks and coordination polymers (IUPAC Recommendations 2013). Pure Appl. Chem. 2013, 85, 1715–1724. [Google Scholar] [CrossRef]
- Batten, S.R.; Champness, N.R.; Chen, X.-M.; Garcia-Martinez, J.; Kitagawa, S.; Öhrström, L.; O’Keeffe, M.; Suh, M.P.; Reedijk, J. Coordination polymers, metal–organic frameworks and the need for terminology guidelines. CrystEngComm 2012, 14, 3001–3004. [Google Scholar] [CrossRef]
- Janiak, C.; Vieth, J.K. MOFs, MILs and more: Concepts, properties and applications for porous coordination networks (PCNs). New J. Chem. 2010, 34, 2366–2388. [Google Scholar] [CrossRef]
- Rosi, N.L.; Eckert, J.; Eddaoudi, M.; Vodak, D.T.; Kim, J.; O’Keeffe, M.; Yaghi, O.M. Hydrogen storage in microporous metal-organic frameworks. Science 2003, 300, 1127–1129. [Google Scholar] [CrossRef]
- Zhou, H.-C.; Long, J.R.; Yaghi, O.M. Introduction to Metal–Organic Frameworks; ACS Publications: Washington, DC, USA, 2012. [Google Scholar]
- Stock, N.; Biswas, S. Synthesis of metal-organic frameworks (MOFs): Routes to various MOF topologies, morphologies, and composites. Chem. Rev. 2011, 112, 933–969. [Google Scholar] [CrossRef]
- Marx, S.; Kleist, W.; Huang, J.; Maciejewski, M.; Baiker, A. Tuning functional sites and thermal stability of mixed-linker MOFs based on MIL-53 (Al). Dalton T. 2010, 39, 3795–3798. [Google Scholar] [CrossRef]
- Kitagawa, S. Metal–organic frameworks (MOFs). Chem. Soc. Rev. 2014, 43, 5415–5418. [Google Scholar]
- Vilhelmsen, L.B.; Walton, K.S.; Sholl, D.S. Structure and mobility of metal clusters in MOFs: Au, Pd, and AuPd clusters in MOF-74. J. Am. Chem. Soc. 2012, 134, 12807–12816. [Google Scholar] [CrossRef] [PubMed]
- Steiner, P.; Kinsinger, V.; Sander, I.; Siegwart, B.; Hüfner, S.; Politis, C.; Hoppe, R.; Müller, H. The Cu valence in the high Tc superconductors and in monovalent, divalent and trivalent copper oxides determined from XPS core level spectroscopy. Z. Phys. B Con. Mat. 1987, 67, 497–502. [Google Scholar] [CrossRef]
- Song, J.; Zhang, B.; Jiang, T.; Yang, G.; Han, B. Synthesis of cyclic carbonates and dimethyl carbonate using CO2 as a building block catalyzed by MOF-5/KI and MOF-5/KI/K2CO3. Front. Chem. China 2011, 6, 21–30. [Google Scholar] [CrossRef]
- Furukawa, H.; Cordova, K.E.; O’Keeffe, M.; Yaghi, O.M. The chemistry and applications of metal-organic frameworks. Science 2013, 341, 1230444. [Google Scholar] [CrossRef]
- Czaja, A.U.; Trukhan, N.; Müller, U. Industrial applications of metal–organic frameworks. Chem. Soc. Rev. 2009, 38, 1284–1293. [Google Scholar] [CrossRef]
- Panella, B.; Hirscher, M.; Pütter, H.; Müller, U. Hydrogen adsorption in metal–organic frameworks: Cu-MOFs and Zn-MOFs compared. Adv. Funct. Mater. 2006, 16, 520–524. [Google Scholar] [CrossRef]
- Xia, B.Y.; Yan, Y.; Li, N.; Wu, H.B.; Lou, X.W.D.; Wang, X. A metal–organic framework-derived bifunctional oxygen electrocatalyst. Nat. Energy 2016, 1, 15006. [Google Scholar] [CrossRef]
- Sabetghadam, A.; Seoane, B.; Keskin, D.; Duim, N.; Rodenas, T.; Shahid, S.; Sorribas, S.; Guillouzer, C.L.; Clet, G.; Tellez, C. Metal organic framework crystals in mixed-matrix membranes: Impact of the filler morphology on the gas separation performance. Adv. Funct. Mater. 2016, 26, 3154–3163. [Google Scholar] [CrossRef]
- Haldoupis, E.; Nair, S.; Sholl, D.S. Efficient calculation of diffusion limitations in metal organic framework materials: A tool for identifying materials for kinetic separations. J. Am. Chem. Soc. 2010, 132, 7528–7539. [Google Scholar] [CrossRef]
- Khan, N.A.; Hasan, Z.; Jhung, S.H. Adsorptive removal of hazardous materials using metal-organic frameworks (MOFs): A review. J. Hazard. Mater. 2013, 244, 444–456. [Google Scholar] [CrossRef]
- Li, H.; Eddaoudi, M.; O’Keeffe, M.; Yaghi, O.M. Design and synthesis of an exceptionally stable and highly porous metal-organic framework. Nature 1999, 402, 276. [Google Scholar] [CrossRef]
- Farha, O.K.; Hupp, J.T. Rational design, synthesis, purification, and activation of metal− organic framework materials. Acc. Chem. Res. 2010, 43, 1166–1175. [Google Scholar] [CrossRef] [PubMed]
- Schlichte, K.; Kratzke, T.; Kaskel, S. Improved synthesis, thermal stability and catalytic properties of the metal-organic framework compound Cu3 (BTC) 2. Micropor. Mesopor. Mat. 2004, 73, 81–88. [Google Scholar] [CrossRef]
- Fu, Y.; Sun, D.; Chen, Y.; Huang, R.; Ding, Z.; Fu, X.; Li, Z. An amine-functionalized titanium metal–organic framework photocatalyst with visible-light-induced activity for CO2 reduction. Angew. Chem. Int. Ed. 2012, 51, 3364–3367. [Google Scholar] [CrossRef]
- Huang, W.; Liu, N.; Zhang, X.; Wu, M.; Tang, L. Metal organic framework g-C3N4/MIL-53 (Fe) heterojunctions with enhanced photocatalytic activity for Cr (VI) reduction under visible light. Appl. Surf. Sci. 2017, 425, 107–116. [Google Scholar] [CrossRef]
- Rao, X.; Cai, J.; Yu, J.; He, Y.; Wu, C.; Zhou, W.; Yildirim, T.; Chen, B.; Qian, G. A microporous metal–organic framework with both open metal and Lewis basic pyridyl sites for high C2H2 and CH4 storage at room temperature. Chem. Commun. 2013, 49, 6719–6721. [Google Scholar] [CrossRef]
- Qian, J.; Li, Q.; Liang, L.; Li, T.-T.; Hu, Y.; Huang, S. A microporous MOF with open metal sites and Lewis basic sites for selective CO2 capture. Dalton T. 2017, 46, 14102–14106. [Google Scholar] [CrossRef]
- Liang, X.; Quan, B.; Ji, G.; Liu, W.; Cheng, Y.; Zhang, B.; Du, Y. Novel nanoporous carbon derived from metal–organic frameworks with tunable electromagnetic wave absorption capabilities. Inorg. Chem. Commun. Front. 2016, 3, 1516–1526. [Google Scholar] [CrossRef]
- Klontzas, E.; Mavrandonakis, A.; Tylianakis, E.; Froudakis, G.E. Improving hydrogen storage capacity of MOF by functionalization of the organic linker with lithium atoms. Nano Lett. 2008, 8, 1572–1576. [Google Scholar] [CrossRef]
- Zhang, Y.-B.; Furukawa, H.; Ko, N.; Nie, W.; Park, H.J.; Okajima, S.; Cordova, K.E.; Deng, H.; Kim, J.; Yaghi, O.M. Introduction of functionality, selection of topology, and enhancement of gas adsorption in multivariate metal–organic framework-177. J. Am. Chem. Soc. 2015, 137, 2641–2650. [Google Scholar] [CrossRef]
- Bureekaew, S.; Amirjalayer, S.; Tafipolsky, M.; Spickermann, C.; Roy, T.K.; Schmid, R. MOF-FF–A flexible first-principles derived force field for metal-organic frameworks. Phys. Status Solidi B 2013, 250, 1128–1141. [Google Scholar] [CrossRef]
- Bunz, U.H. Poly (aryleneethynylene) s: Syntheses, properties, structures, and applications. Chem. Rev. 2000, 100, 1605–1644. [Google Scholar] [CrossRef] [PubMed]
- Park, H.B.; Jung, C.H.; Lee, Y.M.; Hill, A.J.; Pas, S.J.; Mudie, S.T.; Van Wagner, E.; Freeman, B.D.; Cookson, D.J. Polymers with cavities tuned for fast selective transport of small molecules and ions. Science 2007, 318, 254–258. [Google Scholar] [CrossRef] [PubMed]
- Britt, D.; Tranchemontagne, D.; Yaghi, O.M. Metal-organic frameworks with high capacity and selectivity for harmful gases. Proc. Natl. Acad. Sci. USA 2008, 105, 11623–11627. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Xie, Z.; deKrafft, K.E.; Lin, W. Doping metal–organic frameworks for water oxidation, carbon dioxide reduction, and organic photocatalysis. J. Am. Chem. Soc. 2011, 133, 13445–13454. [Google Scholar] [CrossRef] [PubMed]
- Wu, G.; Xing, W. Facile Preparation of Semiconductor Silver Phosphate Loaded on Multi-walled Carbon Nanotube Surface and Its Enhanced Catalytic Performance. J. Inorg. Organomet. P. 2019, 29, 617–627. [Google Scholar] [CrossRef]
- Demessence, A.; D’Alessandro, D.M.; Foo, M.L.; Long, J.R. Strong CO2 binding in a water-stable, triazolate-bridged metal− organic framework functionalized with ethylenediamine. J. Am. Chem. Soc. 2009, 131, 8784–8786. [Google Scholar] [CrossRef]
- Zhang, T.; Lin, W. Metal–organic frameworks for artificial photosynthesis and photocatalysis. Chem. Soc. Rev. 2014, 43, 5982–5993. [Google Scholar] [CrossRef]
- Liu, J.; Zhou, W.; Liu, J.; Howard, I.; Kilibarda, G.; Schlabach, S.; Coupry, D.; Addicoat, M.; Yoneda, S.; Tsutsui, Y. Photoinduced Charge-Carrier Generation in Epitaxial MOF Thin Films: High Efficiency as a Result of an Indirect Electronic Band Gap? Angew. Chem. Int. Ed. 2015, 54, 7441–7445. [Google Scholar] [CrossRef]
- Maza, W.A.; Haring, A.J.; Ahrenholtz, S.R.; Epley, C.C.; Lin, S.; Morris, A.J. Ruthenium (II)-polypyridyl zirconium (IV) metal–organic frameworks as a new class of sensitized solar cells. Chem. Sci. 2016, 7, 719–727. [Google Scholar] [CrossRef]
- Zeng, Y.; Fu, Z.; Chen, H.; Liu, C.; Liao, S.; Dai, J. Photo-and thermally induced coloration of a crystalline MOF accompanying electron transfer and long-lived charge separation in a stable host–guest system. Chem. Commun. 2012, 48, 8114–8116. [Google Scholar] [CrossRef] [PubMed]
- Horiuchi, Y.; Toyao, T.; Saito, M.; Mochizuki, K.; Iwata, M.; Higashimura, H.; Anpo, M.; Matsuoka, M. Visible-light-promoted photocatalytic hydrogen production by using an amino-functionalized Ti (IV) metal–organic framework. J. Phys. Chem. C 2012, 116, 20848–20853. [Google Scholar] [CrossRef]
- Wang, C.; Volotskova, O.; Lu, K.; Ahmad, M.; Sun, C.; Xing, L.; Lin, W. Synergistic assembly of heavy metal clusters and luminescent organic bridging ligands in metal–organic frameworks for highly efficient X-ray scintillation. J. Am. Chem. Soc. 2014, 136, 6171–6174. [Google Scholar] [CrossRef] [PubMed]
- Silva, C.G.; Corma, A.; García, H. Metal–organic frameworks as semiconductors. J. Mater. Chem. 2010, 20, 3141–3156. [Google Scholar] [CrossRef]
- Alvaro, M.; Carbonell, E.; Ferrer, B.; Llabrés i Xamena, F.X.; Garcia, H. Semiconductor behavior of a metal-organic framework (MOF). Chem. Eur. J. 2007, 13, 5106–5112. [Google Scholar] [CrossRef] [PubMed]
- Klontzas, E.; Tylianakis, E.; Froudakis, G.E. Hydrogen storage in 3D covalent organic frameworks. A multiscale theoretical investigation. J. Phys. Chem. C 2008, 112, 9095–9098. [Google Scholar] [CrossRef]
- Tylianakis, E.; Klontzas, E.; Froudakis, G.E. The effect of structural and energetic parameters of MOFs and COFs towards the improvement of their hydrogen storage properties. Nanotechnology 2009, 20, 204030. [Google Scholar] [CrossRef]
- Germain, J.; Svec, F.; Fréchet, J.M. Preparation of size-selective nanoporous polymer networks of aromatic rings: Potential adsorbents for hydrogen storage. Chem. Mater. 2008, 20, 7069–7076. [Google Scholar] [CrossRef]
- Ahmed, D.S.; El-Hiti, G.A.; Yousif, E.; Ali, A.A.; Hameed, A.S. Design and synthesis of porous polymeric materials and their applications in gas capture and storage: A review. J. Polym. Res. 2018, 25, 75. [Google Scholar] [CrossRef]
- Sun, H.; Yang, B.; Li, A. Biomass derived porous carbon for efficient capture of carbon dioxide, organic contaminants and volatile iodine with exceptionally high uptake. Chem. Eng. J. 2019, 372, 65–73. [Google Scholar] [CrossRef]
- Gregory, P. Solar Cells, High-Technology Applications of Organic Colorants; Springer: New York, NY, USA, 1991; pp. 45–52. [Google Scholar]
- Fondriest Environmental, Inc. “Solar Radiation and Photosynethically Active Radiation.” Fundamentals of Environmental Measurements. 21 March 2014. Available online: https://www.fondriest.com/environmental-measurements/parameters/weather/photosynthetically-active-radiation/#cite (accessed on 12 March 2019).
- Taniguchi, I. Electrochemical and Photoelectrochemical Reduction of Carbon Dioxide, Modern Aspects of Electrochemistry No 20; Springer: New York, NY, USA, 1989; pp. 327–400. [Google Scholar]
- Deria, P.; Yu, J.; Smith, T.; Balaraman, R.P. Ground-state versus excited-state interchromophoric interaction: Topology dependent excimer contribution in metal–organic framework photophysics. J. Am. Chem. Soc. 2017, 139, 5973–5983. [Google Scholar] [CrossRef] [PubMed]
- Borse, P.H.; Kim, J.Y.; Lee, J.S.; Lim, K.T.; Jeong, E.D.; Bae, J.S.; Yoon, J.H.; Yu, S.M.; Kim, H.G. Ti-dopant-enhanced photocatalytic activity of a CaFe2O4/MgFe2O4 bulk heterojunction under visible-light irradiation. J. Korean Phys. Soc. 2012, 61, 73–79. [Google Scholar] [CrossRef]
- Justi, E.W. The Photolytic Production of Hydrogen, A Solar—Hydrogen Energy System; Springer: New York, NY, USA, 1987; pp. 157–174. [Google Scholar]
- Joshi, U.A.; Palasyuk, A.M.; Maggard, P.A. Photoelectrochemical Investigation and Electronic Structure of ap-Type CuNbO3 Photocathode. J. Phys. Chem. C 2011, 115, 13534–13539. [Google Scholar] [CrossRef]
- Canepa, P.; Arter, C.A.; Conwill, E.M.; Johnson, D.H.; Shoemaker, B.A.; Soliman, K.Z.; Thonhauser, T. High-throughput screening of small-molecule adsorption in MOF. J. Mater. Chem. A 2013, 1, 13597–13604. [Google Scholar] [CrossRef]
- Su, X.; Bromberg, L.; Martis, V.; Simeon, F.; Huq, A.; Hatton, T.A. Postsynthetic functionalization of Mg-MOF-74 with tetraethylenepentamine: Structural characterization and enhanced CO2 adsorption. ACS Appl. Mater. Interfaces 2017, 9, 11299–11306. [Google Scholar] [CrossRef]
- Wang, X.; Thiel, I.; Fedorov, A.; Copéret, C.; Mougel, V.; Fontecave, M. Site-isolated manganese carbonyl on bipyridine-functionalities of periodic mesoporous organosilicas: Efficient CO2 photoreduction and detection of key reaction intermediates. Chem. Sci. 2017, 8, 8204–8213. [Google Scholar] [CrossRef]
- Aresta, M.; Dibenedetto, A.; Baran, T.; Wojtyła, S.; Macyk, W. Solar energy utilization in the direct photocarboxylation of 2, 3-dihydrofuran using CO2. Faraday Discuss. 2015, 183, 413–427. [Google Scholar] [CrossRef]
- Bezuidenhout, C.X.; Smith, V.J.; Bhatt, P.M.; Esterhuysen, C.; Barbour, L.J. Extreme Carbon Dioxide Sorption Hysteresis in Open-Channel Rigid Metal–Organic Frameworks. Angew. Chem. Int. Ed. 2015, 54, 2079–2083. [Google Scholar] [CrossRef]
- Masoumi, S.; Nabiyouni, G.; Ghanbari, D. Photo-degradation of azo dyes: Photo catalyst and magnetic investigation of CuFe2O4–TiO2 nanoparticles and nanocomposites. J. Mater. Sci-Mater. El.J. Mater. Sci-Mater. El. 2016, 27, 9962–9975. [Google Scholar] [CrossRef]
- Tang, J.; Yamauchi, Y. Carbon materials: MOF morphologies in control. Nat. Chem. 2016, 8, 638. [Google Scholar] [CrossRef]
- Chaudhari, A.K.; Han, I.; Tan, J.C. Multifunctional Supramolecular Hybrid Materials Constructed from Hierarchical Self-Ordering of In Situ Generated Metal-Organic Framework (MOF) Nanoparticles. Adv. Mater. 2015, 27, 4438–4446. [Google Scholar] [CrossRef] [PubMed]
- Ghorbanloo, M.; Safarifard, V.; Morsali, A. Heterogeneous catalysis with a coordination modulation synthesized MOF: Morphology-dependent catalytic activity. New J. Chem. 2017, 41, 3957–3965. [Google Scholar] [CrossRef]
- Deria, P.; Gómez-Gualdrón, D.A.; Hod, I.; Snurr, R.Q.; Hupp, J.T.; Farha, O.K. Framework-topology-dependent catalytic activity of zirconium-based (porphinato) zinc (II) MOFs. J. Am. Chem. Soc. 2016, 138, 14449–14457. [Google Scholar] [CrossRef] [PubMed]
- Deria, P.; Yu, J.; Balaraman, R.P.; Mashni, J.; White, S.N. Topology-dependent emissive properties of zirconium-based porphyrin MOFs. Chem. Commun. 2016, 52, 13031–13034. [Google Scholar] [CrossRef]
- Tu, J.; Zeng, X.; Xu, F.; Wu, X.; Tian, Y.; Hou, X.; Long, Z. Microwave-induced fast incorporation of titanium into UiO-66 metal–organic frameworks for enhanced photocatalytic properties. Chem. Commun. 2017, 53, 3361–3364. [Google Scholar] [CrossRef]
- Sun, D.; Liu, W.; Qiu, M.; Zhang, Y.; Li, Z. Introduction of a mediator for enhancing photocatalytic performance via post-synthetic metal exchange in metal–organic frameworks (MOFs). Chem. Commun. 2015, 51, 2056–2059. [Google Scholar] [CrossRef]
- Farrusseng, D.; Aguado, S.; Pinel, C. Metal–organic frameworks: Opportunities for catalysis. Angew. Chem. Int. Ed. 2009, 48, 7502–7513. [Google Scholar] [CrossRef]
- Chen, E.X.; Qiu, M.; Zhang, Y.F.; Zhu, Y.S.; Liu, L.Y.; Sun, Y.Y.; Bu, X.; Zhang, J.; Lin, Q. Acid and Base Resistant Zirconium Polyphenolate-Metalloporphyrin Scaffolds for Efficient CO2 Photoreduction. Adv. Mater. 2018, 30, 1704388. [Google Scholar] [CrossRef]
- Qin, J.-S.; Yuan, S.; Zhang, L.; Li, B.; Du, D.-Y.; Huang, N.; Guan, W.; Drake, H.F.; Pang, J.; Lan, Y.-Q. Creating well-defined hexabenzocoronene in zirconium metal–organic framework by postsynthetic annulation. J. Am. Chem. Soc. 2019, 141, 2054–2060. [Google Scholar] [CrossRef]
- Wan, S.; Ou, M.; Zhong, Q.; Wang, X. Perovskite-type CsPbBr3 quantum dots/UiO-66 (NH2) nanojunction as efficient visible-light-driven photocatalyst for CO2 reduction. Chem. Eng. J. 2019, 358, 1287–1295. [Google Scholar] [CrossRef]
- Deng, X.; Albero, J.; Xu, L.; García, H.; Li, Z. Construction of a Stable Ru–Re Hybrid System Based on Multifunctional MOF-253 for Efficient Photocatalytic CO2 Reduction. Inorg. Chem. 2018, 57, 8276–8286. [Google Scholar] [CrossRef] [PubMed]
- Sadeghi, N.; Sharifnia, S.; Do, T.-O. Enhanced CO2 photoreduction by a graphene–porphyrin metal–organic framework under visible light irradiation. J. Mater. Chem. A 2018, 6, 18031–18035. [Google Scholar] [CrossRef]
- Dao, X.-Y.; Guo, J.-H.; Wei, Y.-P.; Guo, F.; Liu, Y.; Sun, W.-Y. Solvent-Free Photoreduction of CO2 to CO Catalyzed by Fe-MOFs with Superior Selectivity. Inorg. Chem. 2019, 58, 8517–8524. [Google Scholar] [CrossRef] [PubMed]
- Elcheikh Mahmoud, M.; Audi, H.; Assoud, A.; Ghaddar, T.H.; Hmadeh, M. Metal–Organic Framework Photocatalyst Incorporating Bis (4′-(4-carboxyphenyl)-terpyridine) ruthenium (II) for Visible-Light-Driven Carbon Dioxide Reduction. J. Am. Chem. Soc. 2019, 141, 7115–7121. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.-Q.; Hu, J.; Wang, D.; Li, Z.; Zhang, Q.; Luo, Y.; Yu, S.-H.; Jiang, H.-L. Visible-light photoreduction of CO2 in a metal–organic framework: Boosting electron–hole separation via electron trap states. J. Am. Chem. Soc. 2015, 137, 13440–13443. [Google Scholar] [CrossRef] [PubMed]
- Yan, Z.-H.; Du, M.-H.; Liu, J.; Jin, S.; Wang, C.; Zhuang, G.-L.; Kong, X.-J.; Long, L.-S.; Zheng, L.-S. Photo-generated dinuclear {Eu(II)}2 active sites for selective CO2 reduction in a photosensitizing metal-organic framework. Nat. Commun. 2018, 9, 3353. [Google Scholar] [CrossRef]
- Wang, D.; Huang, R.; Liu, W.; Sun, D.; Li, Z. Fe-based MOFs for photocatalytic CO2 reduction: Role of coordination unsaturated sites and dual excitation pathways. ACS Catal. 2014, 4, 4254–4260. [Google Scholar] [CrossRef]
- Sun, D.; Gao, Y.; Fu, J.; Zeng, X.; Chen, Z.; Li, Z. Construction of a supported Ru complex on bifunctional MOF-253 for photocatalytic CO2 reduction under visible light. Chem. Commun. 2015, 51, 2645–2648. [Google Scholar] [CrossRef]
- Sun, M.; Yan, S.; Sun, Y.; Yang, X.; Guo, Z.; Du, J.; Chen, D.; Chen, P.; Xing, H. Enhancement of visible-light-driven CO2 reduction performance using an amine-functionalized zirconium metal–organic framework. Dalton T. 2018, 47, 909–915. [Google Scholar] [CrossRef]
- Chen, D.; Xing, H.; Wang, C.; Su, Z. Highly efficient visible-light-driven CO2 reduction to formate by a new anthracene-based zirconium MOF via dual catalytic routes. J. Mater. Chem. A 2016, 4, 2657–2662. [Google Scholar] [CrossRef]
- Li, L.; Zhang, S.; Xu, L.; Wang, J.; Shi, L.-X.; Chen, Z.-N.; Hong, M.; Luo, J. Effective visible-light driven CO2 photoreduction via a promising bifunctional iridium coordination polymer. Chem. Sci. 2014, 5, 3808–3813. [Google Scholar] [CrossRef]
- Sun, D.; Fu, Y.; Liu, W.; Ye, L.; Wang, D.; Yang, L.; Fu, X.; Li, Z. Studies on Photocatalytic CO2 Reduction over NH2-Uio-66 (Zr) and Its Derivatives: Towards a Better Understanding of Photocatalysis on Metal–Organic Frameworks. Chem. Eur. J. 2013, 19, 14279–14285. [Google Scholar] [CrossRef] [PubMed]
- Ye, L.; Gao, Y.; Cao, S.; Chen, H.; Yao, Y.; Hou, J.; Sun, L. Assembly of highly efficient photocatalytic CO2 conversion systems with ultrathin two-dimensional metal–organic framework nanosheets. Appl. Catal. B-Environ. 2018, 227, 54–60. [Google Scholar] [CrossRef]
- Yan, S.; Yu, Y.; Cao, Y. Synthesis of porous ZnMn2O4 flower-like microspheres by using MOF as precursors and its application on photoreduction of CO2 into CO. Appl. Surf. Sci. 2019, 465, 383–388. [Google Scholar] [CrossRef]
- Zhao, J.; Wang, Q.; Sun, C.; Zheng, T.; Yan, L.; Li, M.; Shao, K.; Wang, X.; Su, Z. A hexanuclear cobalt metal–organic framework for efficient CO2 reduction under visible light. J. Mater. Chem. A 2017, 5, 12498–12505. [Google Scholar] [CrossRef]
- Wang, L.; Wan, J.; Zhao, Y.; Yang, N.; Wang, D. Hollow multi-shelled structures of Co3O4 dodecahedron with unique crystal orientation for enhanced photocatalytic CO2 reduction. J. Am. Chem. Soc. 2019, 141, 2238–2241. [Google Scholar] [CrossRef]
- Hong, Q.-L.; Zhang, H.-X.; Zhang, J. Synthesis of boron imidazolate frameworks with cobalt clusters for efficient visible-light driven CO2 reduction. J. Mater. Chem. A 2019, 7, 17272–17276. [Google Scholar] [CrossRef]
- Han, Y.; Xu, H.; Su, Y.; Xu, Z.-L.; Wang, K.; Wang, W. Noble metal (Pt, Au@ Pd) nanoparticles supported on metal organic framework (MOF-74) nanoshuttles as high-selectivity CO2 conversion catalysts. J. Catal. 2019, 370, 70–78. [Google Scholar] [CrossRef]
- Xu, Y.; Mo, J.; Xie, G.; Ding, D.; Ding, S.; Wang, X.; Li, C. MOF-derived Co1.11Te2 with half-metallic characteristic for efficient photochemical conversion of CO2 under visible-light irradiation. Chem. Commun. 2019, 55, 6862–6865. [Google Scholar] [CrossRef]
- Han, B.; Ou, X.; Deng, Z.; Song, Y.; Tian, C.; Deng, H.; Xu, Y.J.; Lin, Z. Nickel Metal–Organic Framework Monolayers for Photoreduction of Diluted CO2: Metal-Node-Dependent Activity and Selectivity. Angew. Chem. Int. Ed. 2018, 57, 16811–16815. [Google Scholar] [CrossRef]
- Zhao, H.; Wang, X.; Feng, J.; Chen, Y.; Yang, X.; Gao, S.; Cao, R. Synthesis and characterization of Zn2 GeO4/Mg-MOF-74 composites with enhanced photocatalytic activity for CO2 reduction. Catal. Sci. Technol. 2018, 8, 1288–1295. [Google Scholar] [CrossRef]
- Qiu, Y.-C.; Yuan, S.; Li, X.-X.; Du, D.-Y.; Wang, C.; Qin, J.-S.; Drake, H.F.; Lan, Y.-Q.; Jiang, L.; Zhou, H.-C. Face-Sharing Archimedean Solids Stacking for the Construction of Mixed-Ligand Metal–Organic Frameworks. J. Am. Chem. Soc. 2019, 141, 13841–13848. [Google Scholar] [CrossRef] [PubMed]
- Zhu, W.; Zhang, C.; Li, Q.; Xiong, L.; Chen, R.; Wan, X.; Wang, Z.; Chen, W.; Deng, Z.; Peng, Y. Selective reduction of CO2 by conductive MOF nanosheets as an efficient co-catalyst under visible light illumination. Appl. Catal. B- Environ. 2018, 238, 339–345. [Google Scholar] [CrossRef]
- Meng, J.; Chen, Q.; Lu, J.; Liu, H. Z-Scheme Photocatalytic CO2 Reduction on a Heterostructure of Oxygen-Defective ZnO/Reduced Graphene Oxide/UiO-66-NH2 under Visible Light. ACS Appl. Mater. Interfaces 2018, 11, 550–562. [Google Scholar] [CrossRef] [PubMed]
- Crake, A.; Christoforidis, K.C.; Kafizas, A.; Zafeiratos, S.; Petit, C. CO2 capture and photocatalytic reduction using bifunctional TiO2/MOF nanocomposites under UV–vis irradiation. Appl. Catal. B- Environ. 2017, 210, 131–140. [Google Scholar] [CrossRef]
- Zhang, H.; Wei, J.; Dong, J.; Liu, G.; Shi, L.; An, P.; Zhao, G.; Kong, J.; Wang, X.; Meng, X. Efficient Visible-Light-Driven Carbon Dioxide Reduction by a Single-Atom Implanted Metal–Organic Framework. Angew. Chem. Int. Ed. 2016, 55, 14310–14314. [Google Scholar] [CrossRef]
- Hu, C.Y.; Zhou, J.; Sun, C.Y.; Chen, M.M.; Wang, X.L.; Su, Z.M. HKUST-1 Derived Hollow C-Cu2−xS Nanotube/g-C3N4 Composites for Visible-Light CO2 Photoreduction with H2O Vapor. Chem. Eur. J. 2019, 25, 379–385. [Google Scholar] [CrossRef]
- Xu, G.; Zhang, H.; Wei, J.; Zhang, H.-X.; Wu, X.; Li, Y.; Li, C.; Zhang, J.; Ye, J. Integrating the g-C3N4 Nanosheet with B–H Bonding Decorated Metal–Organic Framework for CO2 Activation and Photoreduction. ACS Nano 2018, 12, 5333–5340. [Google Scholar] [CrossRef]
- Li, N.; Liu, J.; Liu, J.J.; Dong, L.Z.; Xin, Z.F.; Teng, Y.L.; Lan, Y.Q. Adenine Components in Biomimetic Metal–Organic Frameworks for Efficient CO2 Photoconversion. Angew. Chem. 2019, 131, 5280–5285. [Google Scholar] [CrossRef]
- Xie, Y.; Fang, Z.; Li, L.; Yang, H.; Liu, T.-F. Creating Chemisorption Sites for Enhanced CO2 Photoreduction Activity through Alkylamine Modification of MIL-101-Cr. ACS Appl. Mater. Interfaces 2019, 11, 27017–27023. [Google Scholar] [CrossRef]
- Li, X.-X.; Liu, J.; Zhang, L.; Dong, L.-Z.; Xin, Z.-F.; Li, S.-L.; Huang-Fu, X.-Q.; Huang, K.; Lan, Y.-Q. Hydrophobic Polyoxometalate-Based Metal-Organic Framework for Efficient CO2 Photoconversion. ACS Appl. Mater. Interfaces 2019, 11, 25790–25795. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.-K.; Liu, J.; Zhang, L.; Dong, L.-Z.; Li, S.-L.; Kan, Y.-H.; Li, D.-S.; Lan, Y.-Q. Monometallic Catalytic Models Hosted in Stable Metal–Organic Frameworks for Tunable CO2 Photoreduction. ACS Catal. 2019, 9, 1726–1732. [Google Scholar] [CrossRef]
- Wang, L.; Jin, P.; Huang, J.; She, H.; Wang, Q. Integration of Copper (II)-Porphyrin Zirconium Metal–Organic Framework and Titanium Dioxide to Construct Z-Scheme System for Highly Improved Photocatalytic CO2 Reduction. ACS Sustain. Chem. Eng. 2019, 7, 15660–15670. [Google Scholar] [CrossRef]
- Liu, M.; Mu, Y.-F.; Yao, S.; Guo, S.; Guo, X.-W.; Zhang, Z.-M.; Lu, T.-B. Photosensitizing single-site metal− organic framework enabling visible-light-driven CO2 reduction for syngas production. Appl. Catal. B- Environ. 2019, 245, 496–501. [Google Scholar] [CrossRef]
- Ding, D.; Jiang, Z.; Jin, J.; Li, J.; Ji, D.; Zhang, Y.; Zan, L. Impregnation of semiconductor CdS NPs in MOFs cavities via double solvent method for effective photocatalytic CO2 conversion. J. Catal. 2019, 375, 21–31. [Google Scholar] [CrossRef]
- Chen, C.; Wu, T.; Wu, H.; Liu, H.; Qian, Q.; Liu, Z.; Yang, G.; Han, B. Highly effective photoreduction of CO2 to CO promoted by integration of CdS with molecular redox catalysts through metal–organic frameworks. Chem. Sci. 2018, 9, 8890–8894. [Google Scholar] [CrossRef]
- Kong, Z.-C.; Liao, J.-F.; Dong, Y.-J.; Xu, Y.-F.; Chen, H.-Y.; Kuang, D.-B.; Su, C.-Y. Core@ Shell CsPbBr3@ Zeolitic imidazolate framework nanocomposite for efficient photocatalytic CO2 reduction. ACS Energy Lett. 2018, 3, 2656–2662. [Google Scholar] [CrossRef]
- Reinsch, H.; van der Veen, M.A.; Gil, B.; Marszalek, B.; Verbiest, T.; De Vos, D.; Stock, N. Structures, sorption characteristics, and nonlinear optical properties of a new series of highly stable aluminum MOFs. Chem. Mater. 2012, 25, 17–26. [Google Scholar] [CrossRef]
- Lan, G.; Li, Z.; Veroneau, S.S.; Zhu, Y.-Y.; Xu, Z.; Wang, C.; Lin, W. Photosensitizing metal–organic layers for efficient sunlight-driven carbon dioxide reduction. J. Am. Chem. Soc. 2018, 140, 12369–12373. [Google Scholar] [CrossRef]
- Liao, W.-M.; Zhang, J.-H.; Wang, Z.; Yin, S.-Y.; Pan, M.; Wang, H.-P.; Su, C.-Y. Post-synthetic exchange (PSE) of UiO-67 frameworks with Ru/Rh half-sandwich units for visible-light-driven H2 evolution and CO2 reduction. J. Mater. Chem. A 2018, 6, 11337–11345. [Google Scholar] [CrossRef]
- Hu, X.; Wang, Z.; Lin, B.; Zhang, C.; Cao, L.; Wang, T.; Zhang, J.; Wang, C.; Lin, W. Two-Dimensional Metal-Organic Layers as a Bright and Processable Phosphor for Fast White-Light Communication. Chem. Eur. J. 2017, 23, 8390–8394. [Google Scholar] [CrossRef] [PubMed]
- Xue, F.; Kumar, P.; Xu, W.; Mkhoyan, K.A.; Tsapatsis, M. Direct Synthesis of 7 nm-Thick Zinc (II)–Benzimidazole–Acetate Metal–Organic Framework Nanosheets. Chem. Mater. 2017, 30, 69–73. [Google Scholar] [CrossRef]
- Hu, X.; Chen, P.; Zhang, C.; Wang, Z.; Wang, C. Energy transfer on a two-dimensional antenna enhances the photocatalytic activity of CO2 reduction by metal–organic layers. Chem. Commun. 2019, 55, 9657–9660. [Google Scholar] [CrossRef] [PubMed]
- Jo, Y.; Kim, J.; Cho, S.; Kim, H.; Im, H. Coulomb Interaction Induced Gap in an Al/SiO2/Si: P tunnelling Device. Appl. Sci. Converg. Technol. 2017, 26, 50–51. [Google Scholar] [CrossRef]
- Kou, Y.; Nabetani, Y.; Masui, D.; Shimada, T.; Takagi, S.; Tachibana, H.; Inoue, H. Direct detection of key reaction intermediates in photochemical CO2 reduction sensitized by a rhenium bipyridine complex. J. Am. Chem. Soc. 2014, 136, 6021–6030. [Google Scholar] [CrossRef]
- Morimoto, T.; Nakajima, T.; Sawa, S.; Nakanishi, R.; Imori, D.; Ishitani, O. CO2 Capture by a Rhenium (I) Complex with the Aid of Triethanolamine. J. Am. Chem. Soc. 2013, 135, 16825–16828. [Google Scholar] [CrossRef]
- Morimoto, T.; Nishiura, C.; Tanaka, M.; Rohacova, J.; Nakagawa, Y.; Funada, Y.; Koike, K.; Yamamoto, Y.; Shishido, S.; Kojima, T. Ring-shaped Re (I) multinuclear complexes with unique photofunctional properties. J. Am. Chem. Soc. 2013, 135, 13266–13269. [Google Scholar] [CrossRef]
- Dou, Y.; Zhou, J.; Zhou, A.; Li, J.-R.; Nie, Z. Visible-light responsive MOF encapsulation of noble-metal-sensitized semiconductors for high-performance photoelectrochemical water splitting. J. Mater. Chem. A 2017, 5, 19491–19498. [Google Scholar] [CrossRef]
- Wang, D.; Wu, H.; Zhou, J.; Xu, P.; Wang, C.; Shi, R.; Wang, H.; Wang, H.; Guo, Z.; Chen, Q. In Situ One-Pot Synthesis of MOF–Polydopamine Hybrid Nanogels with Enhanced Photothermal Effect for Targeted Cancer Therapy. Adv. Sci. 2018, 5, 1800287. [Google Scholar] [CrossRef]
- Cao, F.; Zhao, M.; Yu, Y.; Chen, B.; Huang, Y.; Yang, J.; Cao, X.; Lu, Q.; Zhang, X.; Zhang, Z. Synthesis of two-dimensional CoSi. 097/nitrogen-doped carbon nanocomposites using metal–organic framework nanosheets as precursors for supercapacitor application. J. Am. Chem. Soc. 2016, 138, 6924–6927. [Google Scholar] [CrossRef]
- Zhao, M.; Wang, Y.; Ma, Q.; Huang, Y.; Zhang, X.; Ping, J.; Zhang, Z.; Lu, Q.; Yu, Y.; Xu, H. Ultrathin 2D metal–organic framework nanosheets. Adv. Mater. 2015, 27, 7372–7378. [Google Scholar] [CrossRef] [PubMed]
- Li, P.-Z.; Maeda, Y.; Xu, Q. Top-down fabrication of crystalline metal–organic framework nanosheets. Chem. Commun. 2011, 47, 8436–8438. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Li, X.; Zhao, J. Fabrication, characterization and photocatalytic activity of cubic-like ZnMn2O4. Appl. Surf. Sci. 2013, 268, 274–277. [Google Scholar] [CrossRef]
- Bessekhouad, Y.; Robert, D.; Weber, J.-V. Photocatalytic activity of Cu2O/TiO2, Bi2O3/TiO2 and ZnMn2O4/TiO2 heterojunctions. Catal. Today 2005, 101, 315–321. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, T.; Wang, J.; Liu, H.; Dao, T.D.; Li, M.; Liu, G.; Meng, X.; Chang, K.; Shi, L. Surface-plasmon-enhanced photodriven CO2 reduction catalyzed by metal–organic-framework-derived iron nanoparticles encapsulated by ultrathin carbon layers. Adv. Mater. 2016, 28, 3703–3710. [Google Scholar] [CrossRef]
- Yu, H.; Wang, X.; Sun, H.; Huo, M. Photocatalytic degradation of malathion in aqueous solution using an Au–Pd–TiO2 nanotube film. J. Hazard. Mater. 2010, 184, 753–758. [Google Scholar] [CrossRef]
- Navalon, S.; Dhakshinamoorthy, A.; Álvaro, M.; Garcia, H. Photocatalytic CO2 reduction using non-titanium metal oxides and sulfides. ChemSusChem 2013, 6, 562–577. [Google Scholar] [CrossRef]
- Nguyen, N.T.; Altomare, M.; Yoo, J.E.; Taccardi, N.; Schmuki, P. Noble Metals on Anodic TiO2 Nanotube Mouths: Thermal Dewetting of Minimal Pt Co-Catalyst Loading Leads to Significantly Enhanced Photocatalytic H2 Generation. Adv. Energy Mater. 2016, 6, 1501926. [Google Scholar] [CrossRef]
- Altomare, M.; Nguyen, N.T.; Schmuki, P. Templated dewetting: Designing entirely self-organized platforms for photocatalysis. Chem. Sci. 2016, 7, 6865–6886. [Google Scholar] [CrossRef]
- Li, D.; Chen, Y.; Abanades, S.; Zhang, Z. Enhanced activity of TiO2 by concentrating light for photoreduction of CO2 with H2O to CH4. Catal. Commun. 2018, 113, 6–9. [Google Scholar] [CrossRef]
- Neaţu, S.T.; Maciá-Agulló, J.A.; Concepción, P.; Garcia, H. Gold–copper nanoalloys supported on TiO2 as photocatalysts for CO2 reduction by water. J. Am. Chem. Soc. 2014, 136, 15969–15976. [Google Scholar] [CrossRef] [PubMed]
- Xie, S.; Wang, Y.; Zhang, Q.; Deng, W.; Wang, Y. MgO-and Pt-promoted TiO2 as an efficient photocatalyst for the preferential reduction of carbon dioxide in the presence of water. ACS Catal. 2014, 4, 3644–3653. [Google Scholar] [CrossRef]
- Zhang, N.; Liu, S.; Fu, X.; Xu, Y.-J. Synthesis of M@ TiO2 (M = Au, Pd, Pt) core–shell nanocomposites with tunable photoreactivity. J. Phys. Chem. C 2011, 115, 9136–9145. [Google Scholar] [CrossRef]
- Kuo, Y.-L.; Chen, H.-W.; Ku, Y. Analysis of silver particles incorporated on TiO2 coatings for the photodecomposition of o-cresol. Thin Solid Films 2007, 515, 3461–3468. [Google Scholar] [CrossRef]
- Ma, X.; Dai, Y.; Yu, L.; Huang, B. Noble-metal-free plasmonic photocatalyst: Hydrogen doped semiconductors. Sci. Rep. 2014, 4, 3986. [Google Scholar] [CrossRef]
- Kar, P.; Zeng, S.; Zhang, Y.; Vahidzadeh, E.; Manuel, A.; Kisslinger, R.; Alam, K.M.; Thakur, U.K.; Mahdi, N.; Kumar, P. High rate CO2 photoreduction using flame annealed TiO2 nanotubes. Appl. Catal. B- Environ. 2019, 243, 522–536. [Google Scholar] [CrossRef]
- Lam, S.-M.; Sin, J.-C.; Mohamed, A.R. A newly emerging visible light-responsive BiFeO3 perovskite for photocatalytic applications: A mini review. Mater. Res. Bull. 2017, 90, 15–30. [Google Scholar] [CrossRef]
- Liu, Q.; Zeng, C.; Ai, L.; Hao, Z.; Jiang, J. Boosting visible light photoreactivity of photoactive metal-organic framework: Designed plasmonic Z-scheme Ag/AgCl@ MIL-53-Fe. Appl. Catal. B- Environ. 2018, 224, 38–45. [Google Scholar] [CrossRef]
- Qiao, Z.; Wang, N.; Jiang, J.; Zhou, J. Design of amine-functionalized metal–organic frameworks for CO2 separation: The more amine, the better? Chem. Commun. 2016, 52, 974–977. [Google Scholar] [CrossRef]
- Windle, C.D.; Perutz, R.N. Advances in molecular photocatalytic and electrocatalytic CO2 reduction. Coord. Chem. Rev. 2012, 256, 2562–2570. [Google Scholar] [CrossRef]
- Zhang, M.; Yao, W.; Lv, Y.; Bai, X.; Liu, Y.; Jiang, W.; Zhu, Y. Enhancement of mineralization ability of C3 N4 via a lower valence position by a tetracyanoquinodimethane organic semiconductor. J. Mater. Chem. A 2014, 2, 11432–11438. [Google Scholar] [CrossRef]
- Alkhatib, I.I.; Garlisi, C.; Pagliaro, M.; Al-Ali, K.; Palmisano, G. Metal-organic frameworks for photocatalytic CO2 reduction under visible radiation: A review of strategies and applications. Catal. Today 2018, 340, 209–224. [Google Scholar] [CrossRef]
- Zhao, S.; Wang, Y.; Dong, J.; He, C.-T.; Yin, H.; An, P.; Zhao, K.; Zhang, X.; Gao, C.; Zhang, L. Ultrathin metal–organic framework nanosheets for electrocatalytic oxygen evolution. Nat. Energy 2016, 1, 16184. [Google Scholar] [CrossRef]
- Duan, J.; Chen, S.; Zhao, C. Ultrathin metal-organic framework array for efficient electrocatalytic water splitting. Nat. Commun. 2017, 8, 15341. [Google Scholar] [CrossRef] [PubMed]
- Sheberla, D.; Sun, L.; Blood-Forsythe, M.A.; Er, S.L.; Wade, C.R.; Brozek, C.K.; Aspuru-Guzik, A.N.; Dincă, M. High electrical conductivity in Ni3 (2, 3, 6, 7, 10, 11-hexaiminotriphenylene)2, a semiconducting metal–organic graphene analogue. J. Am. Chem. Soc. 2014, 136, 8859–8862. [Google Scholar] [CrossRef]
- Kambe, T.; Sakamoto, R.; Hoshiko, K.; Takada, K.; Miyachi, M.; Ryu, J.-H.; Sasaki, S.; Kim, J.; Nakazato, K.; Takata, M. π-Conjugated nickel bis (dithiolene) complex nanosheet. J. Am. Chem. Soc. 2013, 135, 2462–2465. [Google Scholar] [CrossRef]
- Wu, G.; Huang, J.; Zang, Y.; He, J.; Xu, G. Porous field-effect transistors based on a semiconductive metal–organic framework. J. Am. Chem. Soc. 2016, 139, 1360–1363. [Google Scholar] [CrossRef]
- Liang, L.; Liu, C.; Jiang, F.; Chen, Q.; Zhang, L.; Xue, H.; Jiang, H.-L.; Qian, J.; Yuan, D.; Hong, M. Carbon dioxide capture and conversion by an acid-base resistant metal-organic framework. Nat. Commun. 2017, 8, 1233. [Google Scholar] [CrossRef]
- Goyal, S.; Shaharun, M.; Kait, C.; Abdullah, B.; Ameen, M. Photoreduction of Carbon Dioxide to Methanol over Copper Based Zeolitic Imidazolate Framework-8: A New Generation Photocatalyst. Catalysts 2018, 8, 581. [Google Scholar] [CrossRef]
- Larsen, R.W.; Wojtas, L. Fixed distance photoinduced electron transfer between Fe and Zn porphyrins encapsulated within the Zn HKUST-1 metal organic framework. Dalton T. 2015, 44, 2959–2963. [Google Scholar] [CrossRef]
- Tian, N.; Gao, Y.; Wu, J.; Luo, S.; Dai, W. Water-resistant HKUST-1 functionalized with polydimethylsiloxane for efficient rubidium ion capture. New J. Chem. 2019, 43, 15539–15547. [Google Scholar] [CrossRef]
- Mosleh, S.; Rahimi, M.; Ghaedi, M.; Dashtian, K.; Hajati, S.; Wang, S. Ag3PO4/AgBr/Ag-HKUST-1-MOF composites as novel blue LED light active photocatalyst for enhanced degradation of ternary mixture of dyes in a rotating packed bed reactor. Chem. Eng. Process. Process Intensif. 2017, 114, 24–38. [Google Scholar] [CrossRef]
- Guan, C.; Liu, X.; Elshahawy, A.M.; Zhang, H.; Wu, H.; Pennycook, S.J.; Wang, J. Metal–organic framework derived hollow CoS2 nanotube arrays: An efficient bifunctional electrocatalyst for overall water splitting. Nanoscale Horiz. 2017, 2, 342–348. [Google Scholar] [CrossRef]
- Pérez-Mayoral, E.; Čejka, J. [Cu3(BTC)2]: A metal–organic framework catalyst for the Friedländer reaction. Chemcatchem 2011, 3, 157–159. [Google Scholar] [CrossRef]
- Chen, Y.; Lykourinou, V.; Vetromile, C.; Hoang, T.; Ming, L.-J.; Larsen, R.W.; Ma, S. How can proteins enter the interior of a MOF? Investigation of cytochrome c translocation into a MOF consisting of mesoporous cages with microporous windows. J. Am. Chem. Soc. 2012, 134, 13188–13191. [Google Scholar] [CrossRef]
- Chen, Y.; Lykourinou, V.; Hoang, T.; Ming, L.-J.; Ma, S. Size-selective biocatalysis of myoglobin immobilized into a mesoporous metal–organic framework with hierarchical pore sizes. Inorg. Chem. 2012, 51, 9156–9158. [Google Scholar] [CrossRef]
- Feng, D.; Liu, T.-F.; Su, J.; Bosch, M.; Wei, Z.; Wan, W.; Yuan, D.; Chen, Y.-P.; Wang, X.; Wang, K. Stable metal-organic frameworks containing single-molecule traps for enzyme encapsulation. Nat. Commun. 2015, 6, 5979. [Google Scholar] [CrossRef]
- Li, X.-X.; Ma, X.; Zheng, W.-X.; Qi, Y.-J.; Zheng, S.-T.; Yang, G.-Y. Composite hybrid cluster built from the integration of polyoxometalate and a metal halide cluster: Synthetic strategy, structure, and properties. Inorg. Chem. 2016, 55, 8257–8259. [Google Scholar] [CrossRef]
- Lykourinou, V.; Chen, Y.; Wang, X.-S.; Meng, L.; Hoang, T.; Ming, L.-J.; Musselman, R.L.; Ma, S. Immobilization of MP-11 into a mesoporous metal–organic framework, MP-11@ mesoMOF: A new platform for enzymatic catalysis. J. Am. Chem. Soc. 2011, 133, 10382–10385. [Google Scholar] [CrossRef]
- Lu, G.; Farha, O.K.; Kreno, L.E.; Schoenecker, P.M.; Walton, K.S.; Van Duyne, R.P.; Hupp, J.T. Fabrication of Metal-Organic Framework-Containing Silica-Colloidal Crystals for Vapor Sensing. Adv. Mater. 2011, 23, 4449–4452. [Google Scholar] [CrossRef]
- Lu, G.; Hupp, J.T. Metal− organic frameworks as sensors: A ZIF-8 based Fabry− Pérot device as a selective sensor for chemical vapors and gases. J. Am. Chem. Soc 2010, 132, 7832–7833. [Google Scholar] [CrossRef] [PubMed]
- Ameloot, R.; Stappers, L.; Fransaer, J.; Alaerts, L.; Sels, B.F.; De Vos, D.E. Patterned growth of metal-organic framework coatings by electrochemical synthesis. Chem. Mater 2009, 21, 2580–2582. [Google Scholar] [CrossRef]
- Gerfin, T.; Grätzel, M.; Walder, L. Molecular and Supramolecular Surface Modification of Nanocrystalline TiO2 Films: Charge-Separating and Charge-Injecting Devices. In Progress in Inorganic Chemistry: Molecular Level Artificial Photosynthetic Materials; Karlin, K.D., Ed.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 1996; Volume 44. [Google Scholar]
- Anitha, V.; Banerjee, A.N.; Joo, S.W. Recent developments in TiO2 as n-and p-type transparent semiconductors: Synthesis, modification, properties, and energy-related applications. J. Mater. Sci. 2015, 50, 7495–7536. [Google Scholar] [CrossRef]
- Abedi, S.; Morsali, A. Ordered mesoporous metal–organic frameworks incorporated with amorphous TiO2 as photocatalyst for selective aerobic oxidation in sunlight irradiation. ACS Catal. 2014, 4, 1398–1403. [Google Scholar] [CrossRef]
- Ramasubbu, V.; Alwin, S.; Mothi, E.; Shajan, X.S. TiO2 aerogel–Cu-BTC metal-organic framework composites for enhanced photon absorption. Mater. Lett. 2017, 197, 236–240. [Google Scholar] [CrossRef]
- Cardoso, J.; Stulp, S.; de Brito, J.; Flor, J.; Frem, R.; Zanoni, M. MOFs based on ZIF-8 deposited on TiO2 nanotubes increase the surface adsorption of CO2 and its photoelectrocatalytic reduction to alcohols in aqueous media. Appl. Catal. B- Environ. 2018, 225, 563–573. [Google Scholar] [CrossRef]
- Yaghi, O.M.; O’Keeffe, M.; Ockwig, N.W.; Chae, H.K.; Eddaoudi, M.; Kim, J. Reticular synthesis and the design of new materials. Nature 2003, 423, 705–714. [Google Scholar] [CrossRef]
- Huang, Z.; Dong, P.; Zhang, Y.; Nie, X.; Wang, X.; Zhang, X. A ZIF-8 decorated TiO2 grid-like film with high CO2 adsorption for CO2 photoreduction. J. CO2 Util. 2018, 24, 369–375. [Google Scholar] [CrossRef]
- Wang, Y.; Hou, P.; Wang, Z.; Kang, P. Zinc Imidazolate Metal–Organic Frameworks (ZIF-8) for Electrochemical Reduction of CO2 to CO. Chemphyschem 2017, 18, 3142–3147. [Google Scholar] [CrossRef]
- Chandra, R.; Mukhopadhyay, S.; Nath, M. TiO2@ ZIF-8: A novel approach of modifying micro-environment for enhanced photo-catalytic dye degradation and high usability of TiO2 nanoparticles. Mater. Lett. 2016, 164, 571–574. [Google Scholar] [CrossRef]
- Park, K.S.; Ni, Z.; Côté, A.P.; Choi, J.Y.; Huang, R.; Uribe-Romo, F.J.; Chae, H.K.; O’Keeffe, M.; Yaghi, O.M. Exceptional chemical and thermal stability of zeolitic imidazolate frameworks. Proc. Natl. Acad. Sci. USA 2006, 103, 10186–10191. [Google Scholar] [CrossRef]
- Ding, Y.-H.; Zhang, X.-L.; Zhang, N.; Zhang, J.-Y.; Zhang, R.; Liu, Y.-F.; Fang, Y.-Z. A visible-light driven Bi2S3@ ZIF-8 core–shell heterostructure and synergistic photocatalysis mechanism. Dalton T. 2018, 47, 684–692. [Google Scholar] [CrossRef] [PubMed]
- Zeng, X.; Huang, L.; Wang, C.; Wang, J.; Li, J.; Luo, X. Sonocrystallization of ZIF-8 on electrostatic spinning TiO2 nanofibers surface with enhanced photocatalysis property through synergistic effect. ACS Appl. Mater. Interfaces 2016, 8, 20274–20282. [Google Scholar] [CrossRef]
- Maina, J.W.; Schütz, J.R.A.; Grundy, L.; Des Ligneris, E.; Yi, Z.; Kong, L.; Pozo-Gonzalo, C.; Ionescu, M.; Dumée, L.F. Inorganic nanoparticles/metal organic framework hybrid membrane reactors for efficient photocatalytic conversion of CO2. ACS Appl. Mater. Interfaces 2017, 9, 35010–35017. [Google Scholar] [CrossRef]
- Chen, X.; Mao, S.S. Titanium dioxide nanomaterials: Synthesis, properties, modifications, and applications. Chem. Rev. 2007, 107, 2891–2959. [Google Scholar] [CrossRef]
- Asahi, R.; Morikawa, T.; Ohwaki, T.; Aoki, K.; Taga, Y. Visible-light photocatalysis in nitrogen-doped titanium oxides. Science 2001, 293, 269–271. [Google Scholar] [CrossRef] [PubMed]
- Yan, S.; Ouyang, S.; Xu, H.; Zhao, M.; Zhang, X.; Ye, J. Co-ZIF-9/TiO2 nanostructure for superior CO2 photoreduction activity. J. Mater. Chem. A 2016, 4, 15126–15133. [Google Scholar] [CrossRef]
- Crake, A.; Christoforidis, K.C.; Gregg, A.; Moss, B.; Kafizas, A.; Petit, C. The Effect of Materials Architecture in TiO2/MOF Composites on CO2 Photoreduction and Charge Transfer. Small 2019, 15, 1805473. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Yuan, J.; Yu, Y.; Dong, Q.; Xiong, Z.; Yu, H.; Zhu, X.; Shen, H.; Xie, Y. Synthesis and photocatalytic activities of H2Ti6O13 nanofibers and anatase TiO2 nanofibers with high-density nanocavities. J. Alloy. Compd. 2017, 712, 549–554. [Google Scholar] [CrossRef]
- Wen, M.; Mori, K.; Kamegawa, T.; Yamashita, H. Amine-functionalized MIL-101 (Cr) with imbedded platinum nanoparticles as a durable photocatalyst for hydrogen production from water. Chem. Commun. 2014, 50, 11645–11648. [Google Scholar] [CrossRef]
- Etgar, L.; Gao, P.; Xue, Z.; Peng, Q.; Chandiran, A.K.; Liu, B.; Nazeeruddin, M.K.; Grätzel, M. Mesoscopic CH3NH3PbI3/TiO2 heterojunction solar cells. J. Am. Chem. Soc. 2012, 134, 17396–17399. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Chai, B.; Peng, T.; Mao, J.; Zan, L. Preparation of AgIn5S8/TiO2 heterojunction nanocomposite and its enhanced photocatalytic H2 production property under visible light. ACS Catal. 2013, 3, 170–177. [Google Scholar] [CrossRef]
- Brahimi, R.; Bessekhouad, Y.; Bouguelia, A.; Trari, M. CuAlO2/TiO2 heterojunction applied to visible light H2 production. J. Photoch. Photobio. A 2007, 186, 242–247. [Google Scholar] [CrossRef]
- He, X.; Wang, W.-N. MOF-based ternary nanocomposites for better CO2 photoreduction: Roles of heterojunctions and coordinatively unsaturated metal sites. J. Mater. Chem. A 2018, 6, 932–940. [Google Scholar] [CrossRef]
- George, S.M. Atomic layer deposition: An overview. Chem. Rev. 2009, 110, 111–131. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Zheng, X.; Feng, X.; Li, Y. CO2 reduction by plasmonic Au nanoparticle-decorated TiO2 photocatalyst with an ultrathin Al2O3 Interlayer. J. Phys. Chem. C 2018, 122, 18949–18956. [Google Scholar] [CrossRef]
- Malola, S.; Lehtovaara, L.; Enkovaara, J.; Häkkinen, H. Birth of the localized surface plasmon resonance in monolayer-protected gold nanoclusters. ACS Nano 2013, 7, 10263–10270. [Google Scholar] [CrossRef]
- Feng, X.; Pan, F.; Zhao, H.; Deng, W.; Zhang, P.; Zhou, H.-C.; Li, Y. Atomic layer deposition enabled MgO surface coating on porous TiO2 for improved CO2 photoreduction. Appl. Catal. B- Environ. 2018, 238, 274–283. [Google Scholar] [CrossRef]
- Liu, L.; Zhao, C.; Zhao, H.; Pitts, D.; Li, Y. Porous microspheres of MgO-patched TiO2 for CO2 photoreduction with H2O vapor: Temperature-dependent activity and stability. Chem. Commun. 2013, 49, 3664–3666. [Google Scholar] [CrossRef]
- Liu, L.; Zhao, C.; Pitts, D.; Zhao, H.; Li, Y. CO2 photoreduction with H2O vapor by porous MgO–TiO2 microspheres: Effects of surface MgO dispersion and CO2 adsorption–desorption dynamics. Catal. Sci. Technol. 2014, 4, 1539–1546. [Google Scholar] [CrossRef]
- Manzanares, M.; Fàbrega, C.; Ossó, J.O.; Vega, L.F.; Andreu, T.; Morante, J.R. Engineering the TiO2 outermost layers using magnesium for carbon dioxide photoreduction. Appl. Catal. B- Environ. 2014, 150, 57–62. [Google Scholar] [CrossRef]
- Liu, L.; Zhao, C.; Li, Y. Spontaneous dissociation of CO2 to CO on defective surface of Cu (I)/TiO2−x nanoparticles at room temperature. J. Phys. Chem. C 2012, 116, 7904–7912. [Google Scholar] [CrossRef]
- Pipornpong, W.; Wanbayor, R.; Ruangpornvisuti, V. Adsorption CO2 on the perfect and oxygen vacancy defect surfaces of anatase TiO2 and its photocatalytic mechanism of conversion to CO. Appl. Surf. Sci. 2011, 257, 10322–10328. [Google Scholar] [CrossRef]
- Indrakanti, V.P.; Kubicki, J.D.; Schobert, H.H. Photoinduced activation of CO2 on TiO2 surfaces: Quantum chemical modeling of CO2 adsorption on oxygen vacancies. Fuel Process. Technol. 2011, 92, 805–811. [Google Scholar] [CrossRef]
- Uda, H.; Yonezawa, H.; Ohtsubo, Y.; Kosaka, M.; Sonomura, H. Thin CdS films prepared by metalorganic chemical vapor deposition. Sol. Energ. Mat. Sol. C 2003, 75, 219–226. [Google Scholar] [CrossRef]
- Gao, Z.; Liu, N.; Wu, D.; Tao, W.; Xu, F.; Jiang, K. Graphene–CdS composite, synthesis and enhanced photocatalytic activity. Appl. Surf. Sci. 2012, 258, 2473–2478. [Google Scholar] [CrossRef]
- Zhou, Y. Recent advances in ionic liquids for synthesis of inorganic nanomaterials. Curr. Nanosci. 2005, 1, 35–42. [Google Scholar] [CrossRef]
- Hutson, A.; McFee, J.; White, D. Ultrasonic amplification in CdS. Phys. Rev. Lett. 1961, 7, 237. [Google Scholar] [CrossRef]
- Kuang, X.; Ma, Y.; Zhang, C.; Su, H.; Zhang, J.; Tang, B. A new synthesis strategy for chiral CdS nanotubes based on a homochiral MOF template. Chem. Commun. 2015, 51, 5955–5958. [Google Scholar] [CrossRef][Green Version]
- Zhao, H.; Yang, X.; Xu, R.; Li, J.; Gao, S.; Cao, R. CdS/NH2-UiO-66 hybrid membrane reactors for the efficient photocatalytic conversion of CO2. J. Mater. Chem. A 2018, 6, 20152–20160. [Google Scholar] [CrossRef]
- Casado-Coterillo, C.; Fernández-Barquín, A.; Zornoza, B.; Téllez, C.; Coronas, J.; Irabien, Á. Synthesis and characterisation of MOF/ionic liquid/chitosan mixed matrix membranes for CO2/N2 separation. Rsc Adv. 2015, 5, 102350–102361. [Google Scholar] [CrossRef]
- Lingampalli, S.; Ayyub, M.M.; Magesh, G.; Rao, C. Photocatalytic reduction of CO2 by employing ZnO/Ag1−xCux/CdS and related heterostructures. Chem. Phys. Lett. 2018, 691, 28–32. [Google Scholar] [CrossRef]
- Zhou, W.; Yan, L.; Wang, Y.; Zhang, Y. SiC nanowires: A photocatalytic nanomaterial. Appl. Phys. Lett. 2006, 89, 013105. [Google Scholar] [CrossRef]
- Wang, S.; Wang, X. Photocatalytic CO2 reduction by CdS promoted with a zeolitic imidazolate framework. Appl. Catal. B- Environ. 2015, 162, 494–500. [Google Scholar] [CrossRef]
- Zhou, M.; Wang, S.; Yang, P.; Huang, C.; Wang, X. Boron carbon nitride semiconductors decorated with CdS nanoparticles for photocatalytic reduction of CO2. ACS Catal. 2018, 8, 4928–4936. [Google Scholar] [CrossRef]
- Yang, X.; Xin, W.; Yin, X.; Shao, X. Enhancement of photocatalytic activity in reducing CO2 over CdS/g-C3N4 composite catalysts under UV light irradiation. Chem. Phys. Lett. 2016, 651, 127–132. [Google Scholar] [CrossRef]
- Ijaz, S.; Ehsan, M.F.; Ashiq, M.N.; Karamt, N.; Najam-ul-Haq, M.; He, T. Flower-like CdS/CdV2O6 composite for visible-light photoconversion of CO2 into CH4. Mater. Design 2016, 107, 178–186. [Google Scholar] [CrossRef]
- Ijaz, S.; Ehsan, M.F.; Ashiq, M.N.; Karamat, N.; He, T. Preparation of CdS@ CeO2 core/shell composite for photocatalytic reduction of CO2 under visible-light irradiation. Appl. Surf. Sci. 2016, 390, 550–559. [Google Scholar] [CrossRef]
- Praus, P.; Svoboda, L.; Čížek, J.; Słowik, G. Precipitation of zinc oxide nanoparticles under UV-irradiation. J. Nanosci. Nanotechno. 2017, 17, 4805–4811. [Google Scholar] [CrossRef]
- Li, X.; Chen, J.; Li, H.; Li, J.; Xu, Y.; Liu, Y.; Zhou, J. Photoreduction of CO2 to methanol over Bi2S3/CdS photocatalyst under visible light irradiation. J. Nat. Gas. Sci. Chem. 2011, 20, 413–417. [Google Scholar] [CrossRef]
- Chen, Y.; Wang, D.; Deng, X.; Li, Z. Metal–organic frameworks (MOFs) for photocatalytic CO2 reduction. Catal. Sci. Technol. 2017, 7, 4893–4904. [Google Scholar] [CrossRef]
- Cavka, J.H.; Jakobsen, S.; Olsbye, U.; Guillou, N.; Lamberti, C.; Bordiga, S.; Lillerud, K.P. A new zirconium inorganic building brick forming metal organic frameworks with exceptional stability. J. Am. Chem. Soc. 2008, 130, 13850–13851. [Google Scholar] [CrossRef] [PubMed]
- Kandiah, M.; Nilsen, M.H.; Usseglio, S.; Jakobsen, S.; Olsbye, U.; Tilset, M.; Larabi, C.; Quadrelli, E.A.; Bonino, F.; Lillerud, K.P. Synthesis and stability of tagged UiO-66 Zr-MOFs. Chem. Mater. 2010, 22, 6632–6640. [Google Scholar] [CrossRef]
- Hasan, Z.; Tong, M.; Jung, B.K.; Ahmed, I.; Zhong, C.; Jhung, S.H. Adsorption of pyridine over amino-functionalized metal–organic frameworks: Attraction via hydrogen bonding versus base–base repulsion. J. Phys. Chem. C 2014, 118, 21049–21056. [Google Scholar] [CrossRef]
- Bárcia, P.S.; Guimarães, D.; Mendes, P.A.; Silva, J.A.; Guillerm, V.; Chevreau, H.; Serre, C.; Rodrigues, A.E. Reverse shape selectivity in the adsorption of hexane and xylene isomers in MOF UiO-66. Micropor. Mesopor. Mat. 2011, 139, 67–73. [Google Scholar] [CrossRef]
- Timofeeva, M.N.; Panchenko, V.N.; Jun, J.W.; Hasan, Z.; Matrosova, M.M.; Jhung, S.H. Effects of linker substitution on catalytic properties of porous zirconium terephthalate UiO-66 in acetalization of benzaldehyde with methanol. Appl. Catal. A-Gen. 2014, 471, 91–97. [Google Scholar] [CrossRef]
- Rungtaweevoranit, B.; Baek, J.; Araujo, J.R.; Archanjo, B.S.; Choi, K.M.; Yaghi, O.M.; Somorjai, G.A. Copper nanocrystals encapsulated in Zr-based metal–organic frameworks for highly selective CO2 hydrogenation to methanol. Nano Lett. 2016, 16, 7645–7649. [Google Scholar] [CrossRef]
- Wang, J.; Xia, Y.; Zhao, H.; Wang, G.; Xiang, L.; Xu, J.; Komarneni, S. Oxygen defects-mediated Z-scheme charge separation in g-C3N4/ZnO photocatalysts for enhanced visible-light degradation of 4-chlorophenol and hydrogen evolution. Appl. Catal. B- Environ. 2017, 206, 406–416. [Google Scholar] [CrossRef]
- Zhang, Z.; Huang, J.; Fang, Y.; Zhang, M.; Liu, K.; Dong, B. A nonmetal plasmonic Z-scheme photocatalyst with UV-to NIR-driven photocatalytic protons reduction. Adv. Mater. 2017, 29, 1606688. [Google Scholar] [CrossRef]
- Bradshaw, D.; Warren, J.E.; Rosseinsky, M.J. Reversible concerted ligand substitution at alternating metal sites in an extended solid. Science 2007, 315, 977–980. [Google Scholar] [CrossRef]
- Kongkanand, A.; Tvrdy, K.; Takechi, K.; Kuno, M.; Kamat, P.V. Quantum dot solar cells. Tuning photoresponse through size and shape control of CdSe− TiO2 architecture. J. Am. Chem. Soc. 2008, 130, 4007–4015. [Google Scholar] [CrossRef] [PubMed]
- Robel, I.; Subramanian, V.; Kuno, M.; Kamat, P.V. Quantum dot solar cells. Harvesting light energy with CdSe nanocrystals molecularly linked to mesoscopic TiO2 films. J. Am. Chem. Soc. 2006, 128, 2385–2393. [Google Scholar] [CrossRef] [PubMed]
- Kamat, P.V. Quantum dot solar cells. Semiconductor nanocrystals as light harvesters. J. Phys. Chem. C 2008, 112, 18737–18753. [Google Scholar] [CrossRef]
- Jin, S.; Son, H.-J.; Farha, O.K.; Wiederrecht, G.P.; Hupp, J.T. Energy transfer from quantum dots to metal–organic frameworks for enhanced light harvesting. J. Am. Chem. Soc. 2013, 135, 955–958. [Google Scholar] [CrossRef] [PubMed]
- Aresta, M.; Dibenedetto, A.; Quaranta, E. One-and Multi-electron Pathways for the Reduction of CO2 into C1 and C1+ Energy-Richer Molecules: Some Thermodynamic and Kinetic Facts. In Reaction Mechanisms in Carbon Dioxide Conversion; Springer: New York, NY, USA, 2016; pp. 311–345. [Google Scholar]
- Zeng, S.; Kar, P.; Thakur, U.K.; Shankar, K. A review on photocatalytic CO2 reduction using perovskite oxide nanomaterials. Nanotechnology 2018, 29, 052001. [Google Scholar] [CrossRef]
- Hou, J.; Cao, S.; Wu, Y.; Liang, F.; Ye, L.; Lin, Z.; Sun, L. Perovskite-based nanocubes with simultaneously improved visible-light absorption and charge separation enabling efficient photocatalytic CO2 reduction. Nano Energy 2016, 30, 59–68. [Google Scholar] [CrossRef]
- Shen, L.; Liang, S.; Wu, W.; Liang, R.; Wu, L. CdS-decorated UiO–66 (NH2) nanocomposites fabricated by a facile photodeposition process: An efficient and stable visible-light-driven photocatalyst for selective oxidation of alcohols. J. Mater. Chem. A 2013, 1, 11473–11482. [Google Scholar] [CrossRef]
- Hou, J.; Cao, S.; Wu, Y.; Gao, Z.; Liang, F.; Sun, Y.; Lin, Z.; Sun, L. Inorganic colloidal perovskite quantum dots for robust solar CO2 reduction. Chem-Eur. J. 2017, 23, 9481–9485. [Google Scholar] [CrossRef]
- Shen, L.; Liang, S.; Wu, W.; Liang, R.; Wu, L. Multifunctional NH2-mediated zirconium metal–organic framework as an efficient visible-light-driven photocatalyst for selective oxidation of alcohols and reduction of aqueous Cr (vi). Dalton T. 2013, 42, 13649–13657. [Google Scholar] [CrossRef]
- Dou, S.; Li, X.; Tao, L.; Huo, J.; Wang, S. Cobalt nanoparticle-embedded carbon nanotube/porous carbon hybrid derived from MOF-encapsulated Co3O4 for oxygen electrocatalysis. Chem. Commun. 2016, 52, 9727–9730. [Google Scholar] [CrossRef]
- Hou, Y.; Wen, Z.; Cui, S.; Ci, S.; Mao, S.; Chen, J. An advanced nitrogen-doped graphene/cobalt-embedded porous carbon polyhedron hybrid for efficient catalysis of oxygen reduction and water splitting. Adv. Funct. Mater. 2015, 25, 872–882. [Google Scholar] [CrossRef]
- Liu, K.; Song, C.; Subramani, V. Hydrogen and Syngas Production and Purification Technologies; John Wiley & Sons: Hoboken, NJ, USA, 2010. [Google Scholar]
- Chen, M.; Han, L.; Zhou, J.; Sun, C.; Hu, C.; Wang, X.; Su, Z. Photoreduction of carbon dioxide under visible light by ultra-small Ag nanoparticles doped into Co-ZIF-9. Nanotechnology 2018, 29, 284003. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Chen, D.; Li, N.; Xu, Q.; Li, H.; He, J.; Lu, J. ZIF-67-Derived 3D Hollow Mesoporous Crystalline Co3O4 Wrapped by 2D g-C3N4 Nanosheets for Photocatalytic Removal of Nitric Oxide. Small 2019, 15, 1902291. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Zhang, W.; Li, S.; Cui, C.; Wu, J.; Chen, H.; Huo, F. Designable yolk–shell nanoparticle@ MOF petalous heterostructures. Chem. Mater. 2014, 26, 1119–1125. [Google Scholar] [CrossRef]
- Liu, W.; Huang, J.; Yang, Q.; Wang, S.; Sun, X.; Zhang, W.; Liu, J.; Huo, F. Multi-shelled Hollow Metal–Organic Frameworks. Angew. Chem. Int. Ed. 2017, 56, 5512–5516. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.; Liu, W.; Wang, B.; Zhang, W.; Zeng, X.; Zhang, C.; Qin, Y.; Sun, X.; Wu, T.; Liu, J. Regulating the spatial distribution of metal nanoparticles within metal-organic frameworks to enhance catalytic efficiency. Nat. Commun. 2017, 8, 14429. [Google Scholar] [CrossRef]
- Deng, X.; Yang, L.; Huang, H.; Yang, Y.; Feng, S.; Zeng, M.; Li, Q.; Xu, D. Shape-Defined Hollow Structural Co-MOF-74 and Metal Nanoparticles@ Co-MOF-74 Composite through a Transformation Strategy for Enhanced Photocatalysis Performance. Small 2019, 15, 1902287. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, T.; Yu, D.; Xiao, K.; Hong, Y. Transition from ZIF-L-Co to ZIF-67: A new insight into the structural evolution of zeolitic imidazolate frameworks (ZIFs) in aqueous systems. CrystEngComm 2015, 17, 8212–8215. [Google Scholar] [CrossRef]
- Wang, S.; Hou, Y.; Wang, X. Development of a stable MnCO2O4 cocatalyst for photocatalytic CO2 reduction with visible light. ACS Appl. Mater. Interfaces 2015, 7, 4327–4335. [Google Scholar] [CrossRef]
- Lehn, J.-M.; Ziessel, R. Photochemical generation of carbon monoxide and hydrogen by reduction of carbon dioxide and water under visible light irradiation. Proc. Natl. Acad. Sci. USA 1982, 79, 701–704. [Google Scholar] [CrossRef]
- Ziessel, R.; Hawecker, J.; Lehn, J.M. Photogeneration of Carbon Monoxide and of Hydrogen via Simultaneous Photochemical Reduction of Carbon Dioxide and Water by Visible-Light Irradiation of Organic Solutions Containing Tris (2, 2′-bipyridine) ruthenium (II) and Cobalt (II) Species as Homogeneous Catalysts. Helv. Chim. Acta 1986, 69, 1065–1084. [Google Scholar]
- Zhang, J.; Wang, H.; Dalai, A.K. Development of stable bimetallic catalysts for carbon dioxide reforming of methane. J. Catal. 2007, 249, 300–310. [Google Scholar] [CrossRef]
- Richardson, R.D.; Holland, E.J.; Carpenter, B.K. A renewable amine for photochemical reduction of CO2. Nat. Chem. 2011, 3, 301. [Google Scholar] [CrossRef] [PubMed]
- Dhara, B.; Nagarkar, S.S.; Kumar, J.; Kumar, V.; Jha, P.K.; Ghosh, S.K.; Nair, S.; Ballav, N. Increase in electrical conductivity of MOF to billion-fold upon filling the nanochannels with conducting polymer. J Phys. Chem. Lett. 2016, 7, 2945–2950. [Google Scholar] [CrossRef]
- Talin, A.A.; Centrone, A.; Ford, A.C.; Foster, M.E.; Stavila, V.; Haney, P.; Kinney, R.A.; Szalai, V.; El Gabaly, F.; Yoon, H.P. Tunable electrical conductivity in metal-organic framework thin-film devices. Science 2014, 343, 66–69. [Google Scholar] [CrossRef]
- Galanakis, I.; Özdoğan, K.; Şaşıoğlu, E.; Aktaş, B. Doping of Mn2VAl and Mn2VSi Heusler alloys as a route to half-metallic antiferromagnetism. Phys. Rev. B 2007, 75, 092407. [Google Scholar] [CrossRef]
- Wang, X.; Huang, X.; Gao, W.; Tang, Y.; Jiang, P.; Lan, K.; Yang, R.; Wang, B.; Li, R. Metal–organic framework derived CoTe2 encapsulated in nitrogen-doped carbon nanotube frameworks: A high-efficiency bifunctional electrocatalyst for overall water splitting. J. Mater. Chem. A 2018, 6, 3684–3691. [Google Scholar] [CrossRef]
- Paszkiewicz, S.; Szymczyk, A.; Sui, X.; Wagner, H.; Linares, A.; Ezquerra, T.A.; Rosłaniec, Z. Synergetic effect of single-walled carbon nanotubes (SWCNT) and graphene nanoplatelets (GNP) in electrically conductive PTT-block-PTMO hybrid nanocomposites prepared by in situ polymerization. Compos. Sci. Technol. 2015, 118, 72–77. [Google Scholar] [CrossRef]
- Moisala, A.; Li, Q.; Kinloch, I.; Windle, A. Thermal and electrical conductivity of single-and multi-walled carbon nanotube-epoxy composites. Compos. Sci. Technol. 2006, 66, 1285–1288. [Google Scholar] [CrossRef]
- Moriche, R.; Prolongo, S.; Sánchez, M.; Jiménez-Suárez, A.; Chamizo, F.; Ureña, A. Thermal conductivity and lap shear strength of GNP/epoxy nanocomposites adhesives. Int. J. Adhes. Adhes. 2016, 68, 407–410. [Google Scholar] [CrossRef]
- Prolongo, S.; Redondo, O.; Campo, M.; Ureña, A. Heat dissipation on electrical conductor composites by combination of carbon nanotubes and graphene nanoplatelets. J. Coat. Technol. Res. 2019, 16, 491–498. [Google Scholar] [CrossRef]
- Wang, Y.; Xia, Q.; Bai, X.; Ge, Z.; Yang, Q.; Yin, C.; Kang, S.; Dong, M.; Li, X. Carbothermal activation synthesis of 3D porous g-C3N4/carbon nanosheets composite with superior performance for CO2 photoreduction. Appl. Catal. B- Environ. 2018, 239, 196–203. [Google Scholar] [CrossRef]
- Chen, S.; Yu, J.; Zhang, J. Enhanced photocatalytic CO2 reduction activity of MOF-derived ZnO/NiO porous hollow spheres. J. CO2 Util. 2018, 24, 548–554. [Google Scholar] [CrossRef]
- Bhanja, P.; Modak, A.; Bhaumik, A. Porous organic polymers for CO2 storage and conversion reactions. Chemcatchem 2019, 11, 244–257. [Google Scholar] [CrossRef]
- Logan, M.W.; Adamson, J.D.; Le, D.; Uribe-Romo, F.J. Structural Stability of N-Alkyl-Functionalized Titanium Metal–Organic Frameworks in Aqueous and Humid Environments. ACS Appl. Mater. Interfaces 2017, 9, 44529–44533. [Google Scholar] [CrossRef]
- Rossin, A.; Ienco, A.; Costantino, F.; Montini, T.; Di Credico, B.; Caporali, M.; Gonsalvi, L.; Fornasiero, P.; Peruzzini, M. Phase transitions and CO2 adsorption properties of polymeric magnesium formate. Cryst. Growth Des. 2008, 8, 3302–3308. [Google Scholar] [CrossRef]
- Di Credico, B.; Redaelli, M.; Bellardita, M.; Calamante, M.; Cepek, C.; Cobani, E.; D’Arienzo, M.; Evangelisti, C.; Marelli, M.; Moret, M. Step-by-step growth of hkust-1 on functionalized TiO2 surface: An efficient material for CO2 capture and solar photoreduction. Catalysts 2018, 8, 353. [Google Scholar] [CrossRef]
- Lin, Y.; Kong, C.; Chen, L. Direct synthesis of amine-functionalized MIL-101 (Cr) nanoparticles and application for CO2 capture. RSC Adv. 2012, 2, 6417–6419. [Google Scholar] [CrossRef]
- Hu, Y.; Verdegaal, W.M.; Yu, S.H.; Jiang, H.L. Alkylamine-Tethered Stable Metal–Organic Framework for CO2 Capture from Flue Gas. ChemSusChem 2014, 7, 734–737. [Google Scholar] [CrossRef]
- Zhao, Y.; Liu, Z. Recent Advances in Photocatalytic CO2 Reduction Using Earth-Abundant Metal Complexes-Derived Photocatalysts. Chin. J. Chem. 2018, 36, 455–460. [Google Scholar] [CrossRef]
- Pratik, S.M.; Cramer, C.J. Predicted Efficient Visible-Light Driven Water Splitting and Carbon Dioxide Reduction Using Photoredox-Active UiO-NDI Metal Organic Framework. J. Phys. Chem. C 2019, 123, 19778–19785. [Google Scholar] [CrossRef]
- Hod, I.; Sampson, M.D.; Deria, P.; Kubiak, C.P.; Farha, O.K.; Hupp, J.T. Fe-porphyrin-based metal–organic framework films as high-surface concentration, heterogeneous catalysts for electrochemical reduction of CO2. ACS Catal. 2015, 5, 6302–6309. [Google Scholar] [CrossRef]
- Nakajima, T.; Tamaki, Y.; Ueno, K.; Kato, E.; Nishikawa, T.; Ohkubo, K.; Yamazaki, Y.; Morimoto, T.; Ishitani, O. Photocatalytic reduction of low concentration of CO2. J. Am. Chem. Soc. 2016, 138, 13818–13821. [Google Scholar] [CrossRef] [PubMed]
- Xiao, D.J.; Bloch, E.D.; Mason, J.A.; Queen, W.L.; Hudson, M.R.; Planas, N.; Borycz, J.; Dzubak, A.L.; Verma, P.; Lee, K. Oxidation of ethane to ethanol by N2O in a metal–organic framework with coordinatively unsaturated iron (II) sites. Nat. Chem. 2014, 6, 590. [Google Scholar] [CrossRef]
- Stavila, V.; Parthasarathi, R.; Davis, R.W.; El Gabaly, F.; Sale, K.L.; Simmons, B.A.; Singh, S.; Allendorf, M.D. MOF-based catalysts for selective hydrogenolysis of carbon–oxygen ether bonds. ACS Catal. 2015, 6, 55–59. [Google Scholar] [CrossRef]
- Lin, J.; Wang, A.; Qiao, B.; Liu, X.; Yang, X.; Wang, X.; Liang, J.; Li, J.; Liu, J.; Zhang, T. Remarkable performance of Ir1/FeOx single-atom catalyst in water gas shift reaction. J. Am. Chem. Soc. 2013, 135, 15314–15317. [Google Scholar] [CrossRef]
- Lippi, R.; Howard, S.; Barron, H.; Easton, C.; Madsen, I.C.; Waddington, L.; Vogt, C.; Hill, M.; Sumby, C.; Doonan, C.J. Highly active catalyst for CO 2 methanation derived from a metal organic framework template. J. Mater. Chem. A 2017, 5, 12990–12997. [Google Scholar] [CrossRef]
- Kurisingal, J.F.; Babu, R.; Kim, S.-H.; Li, Y.X.; Chang, J.-S.; Cho, S.J.; Park, D.-W. Microwave-induced synthesis of a bimetallic charge-transfer metal organic framework: A promising host for the chemical fixation of CO2. Catal. Sci. Technol. 2018, 8, 591–600. [Google Scholar] [CrossRef]
- Miralda, C.M.; Macias, E.E.; Zhu, M.; Ratnasamy, P.; Carreon, M.A. Zeolitic imidazole framework-8 catalysts in the conversion of CO2 to chloropropene carbonate. ACS Catal. 2011, 2, 180–183. [Google Scholar] [CrossRef]
- Rogge, S.M.; Wieme, J.; Vanduyfhuys, L.; Vandenbrande, S.; Maurin, G.; Verstraelen, T.; Waroquier, M.; Van Speybroeck, V. Thermodynamic insight in the high-pressure behavior of UiO-66: Effect of linker defects and linker expansion. Chem. Mater. 2016, 28, 5721–5732. [Google Scholar] [CrossRef]
- Son, H.-J.; Jin, S.; Patwardhan, S.; Wezenberg, S.J.; Jeong, N.C.; So, M.; Wilmer, C.E.; Sarjeant, A.A.; Schatz, G.C.; Snurr, R.Q. Light-harvesting and ultrafast energy migration in porphyrin-based metal–organic frameworks. J. Am. Chem. Soc. 2013, 135, 862–869. [Google Scholar] [CrossRef] [PubMed]
- Kent, C.A.; Liu, D.; Ma, L.; Papanikolas, J.M.; Meyer, T.J.; Lin, W. Light harvesting in microscale metal–organic frameworks by energy migration and interfacial electron transfer quenching. J. Am. Chem. Soc. 2011, 133, 12940–12943. [Google Scholar] [CrossRef] [PubMed]
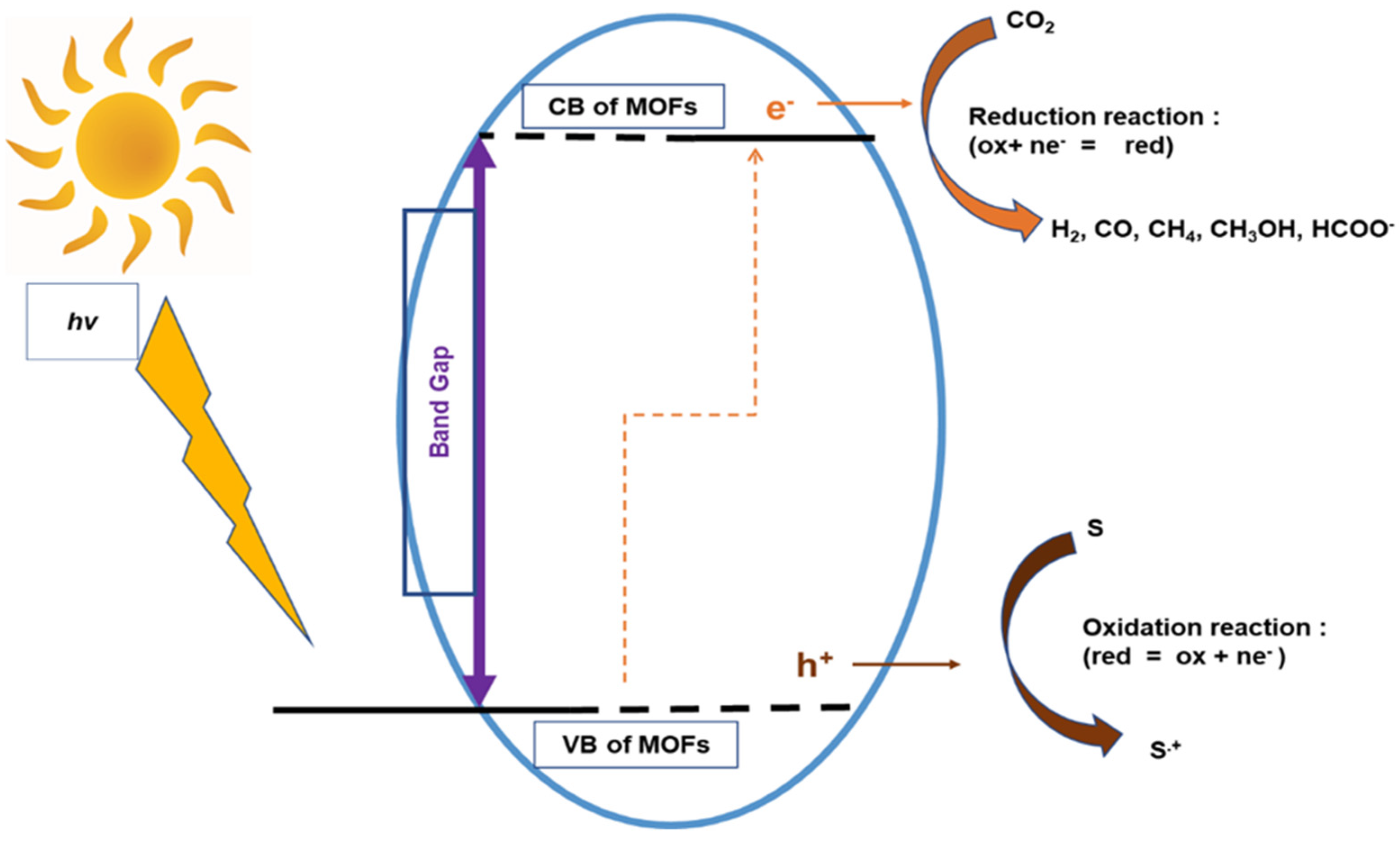
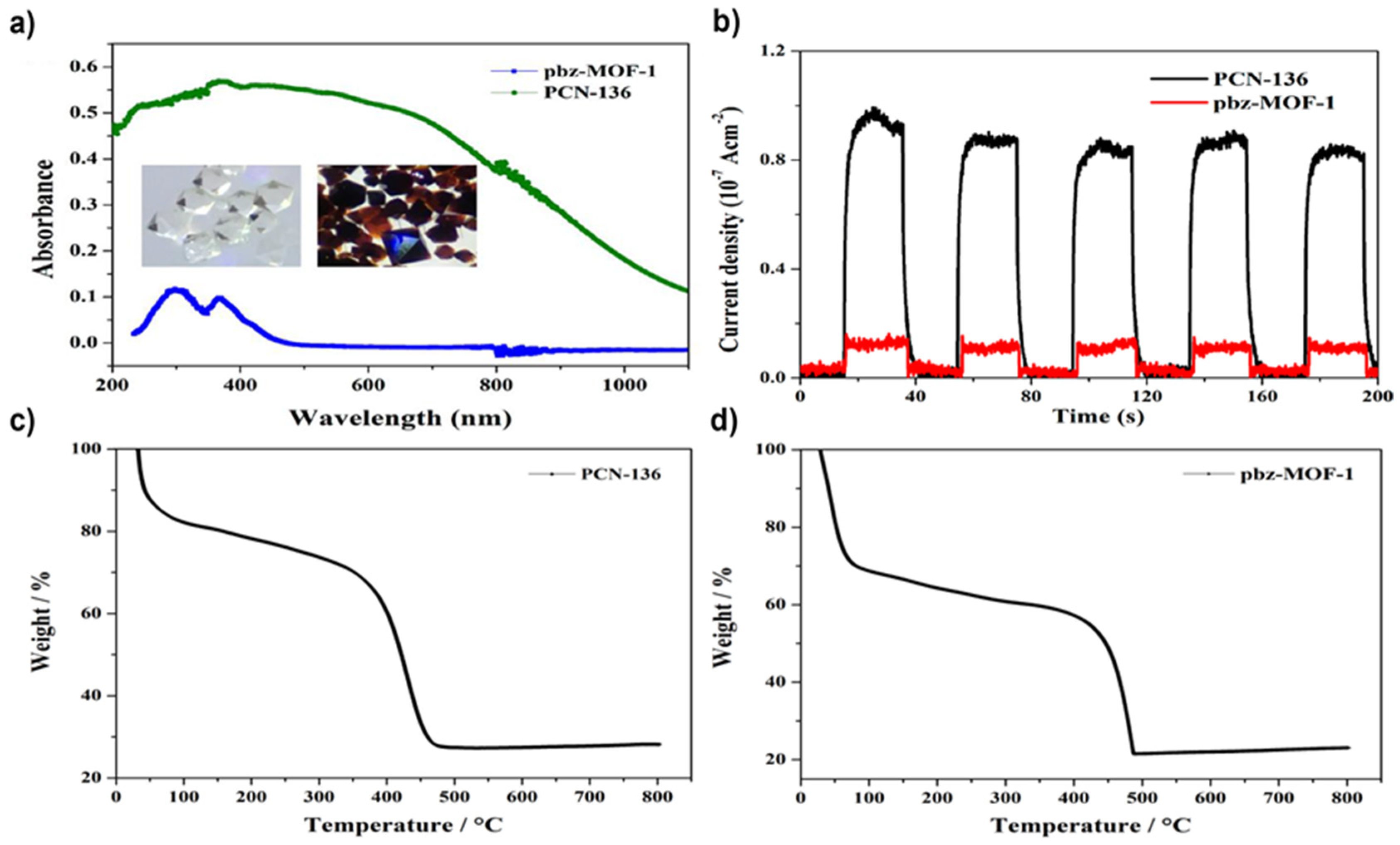
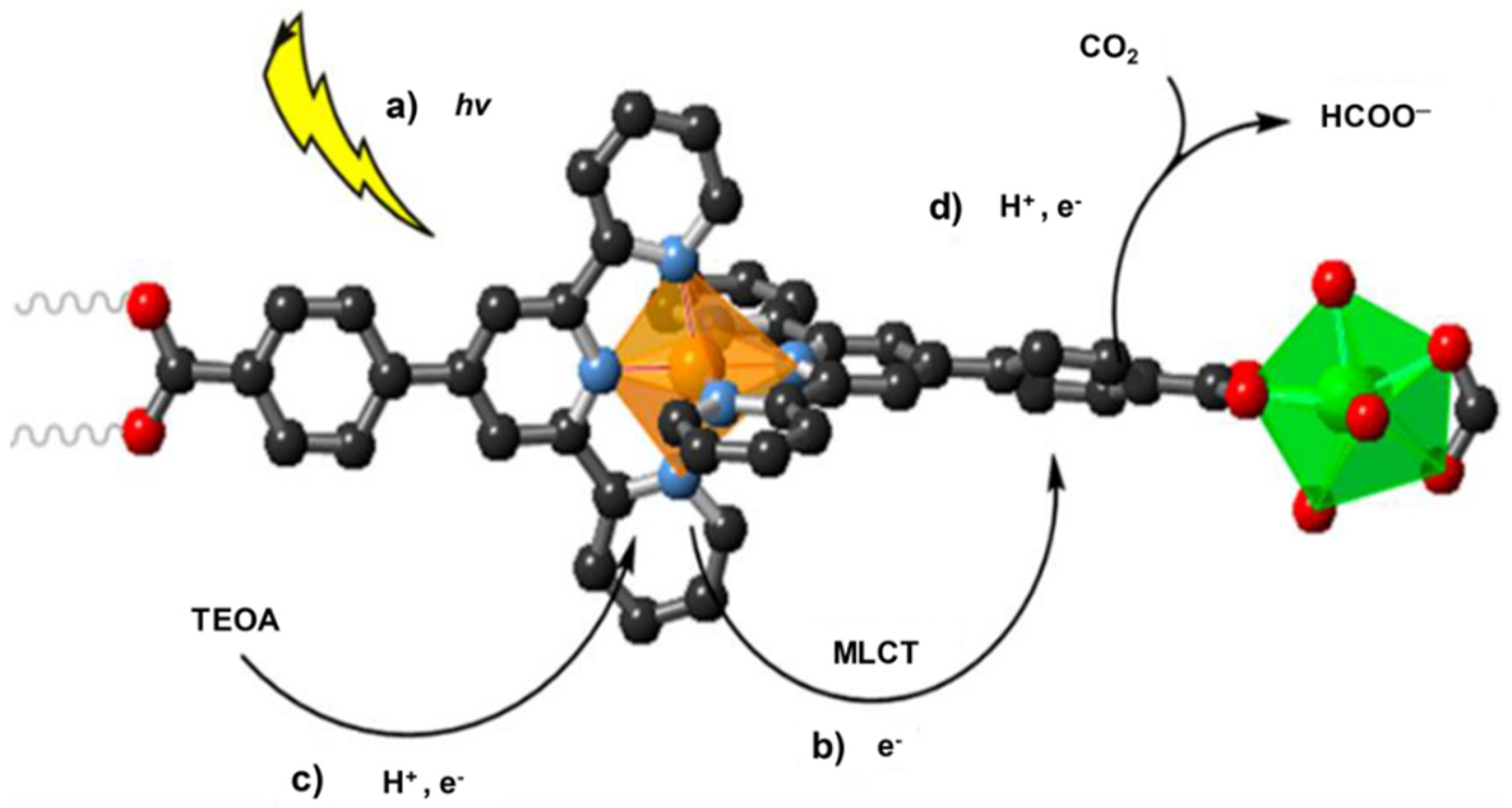
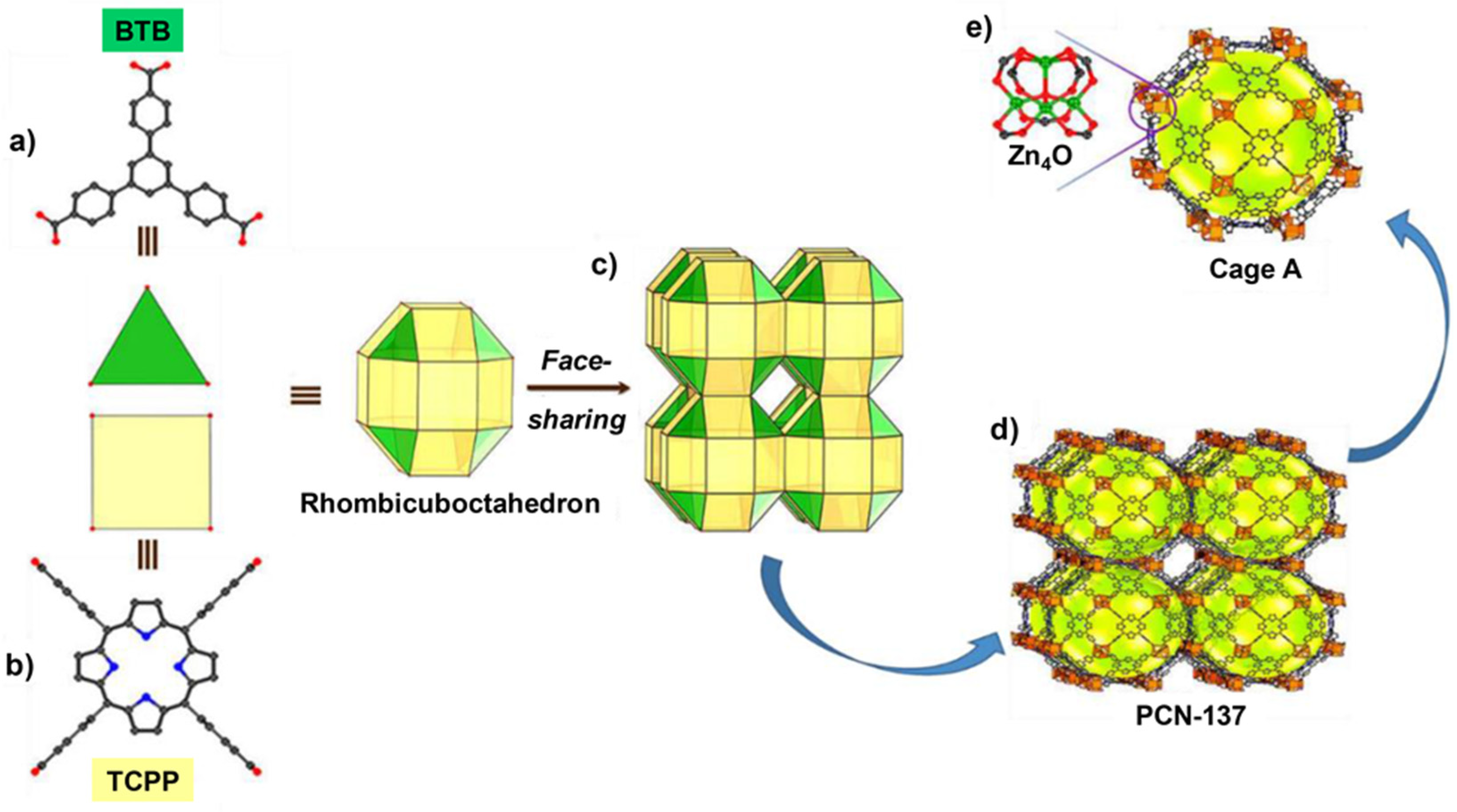
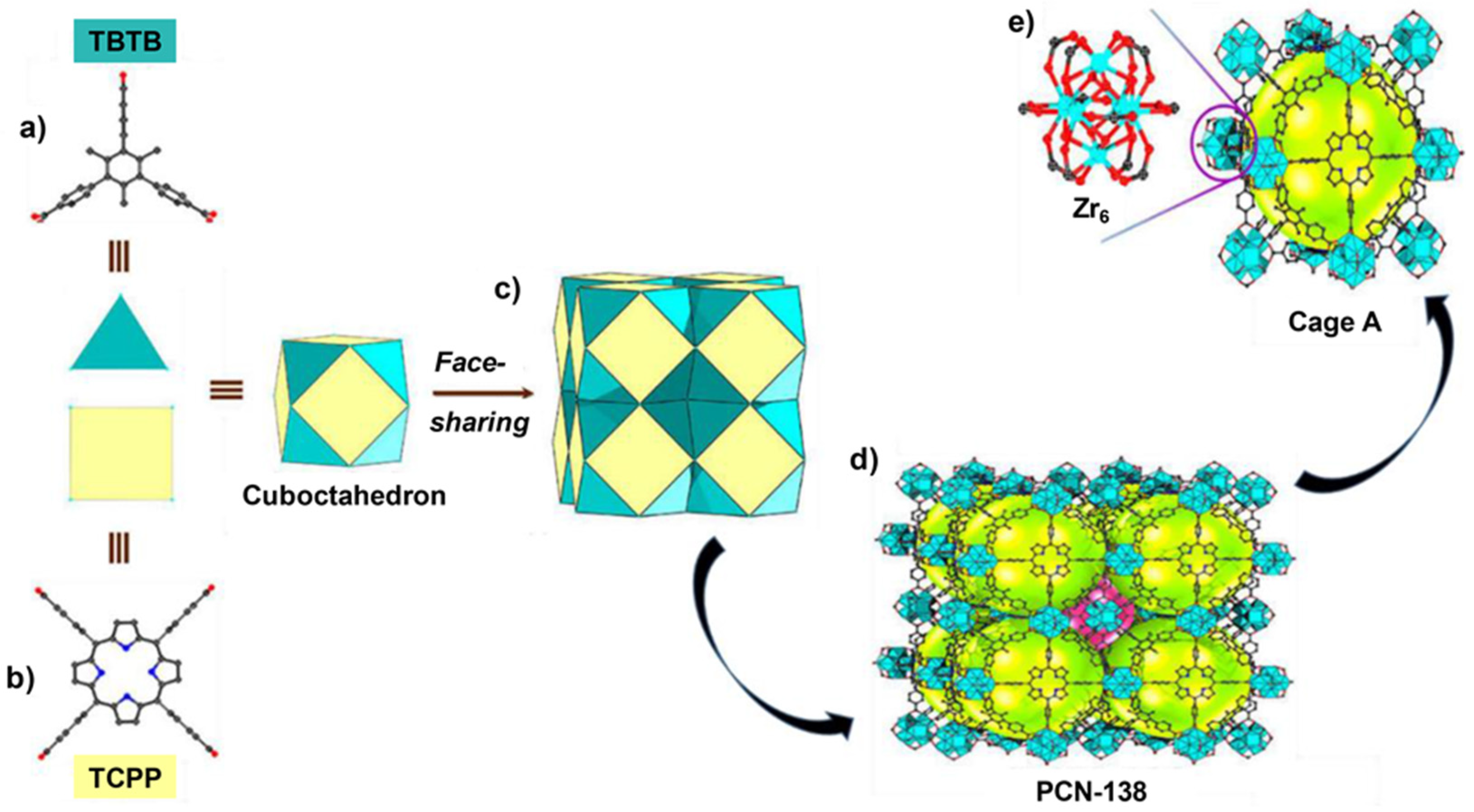
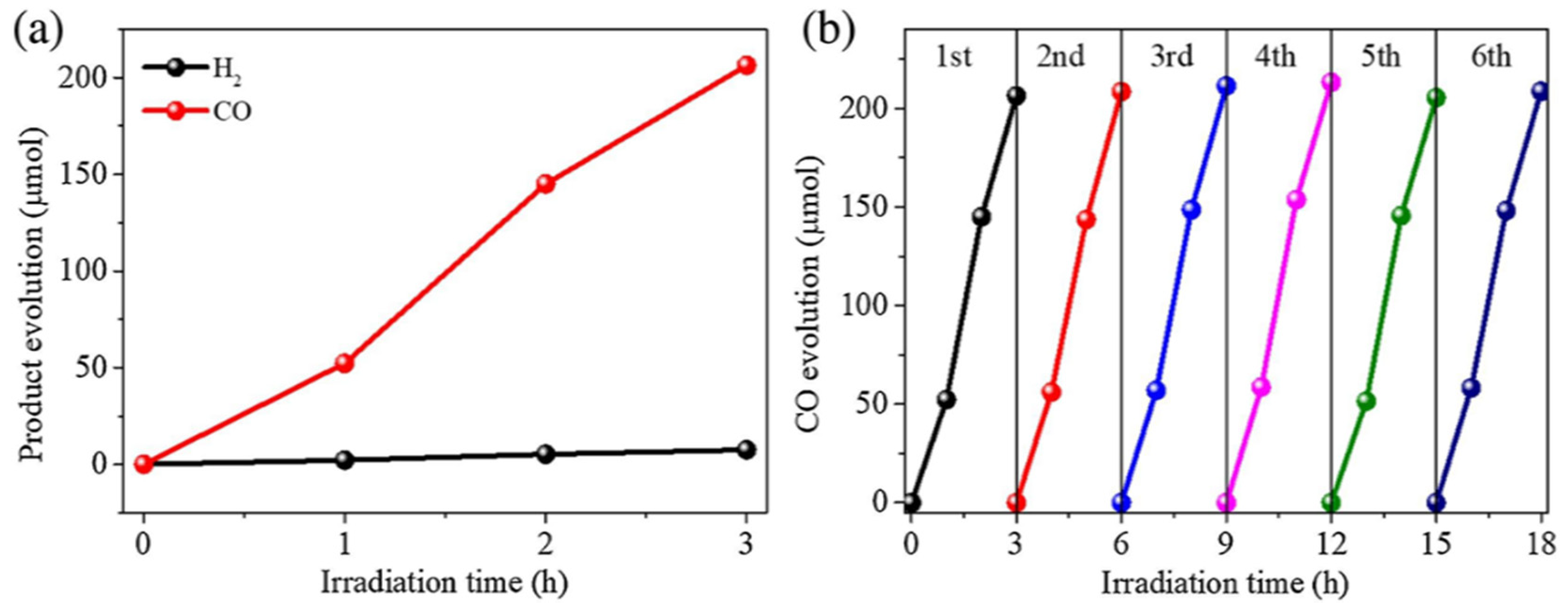
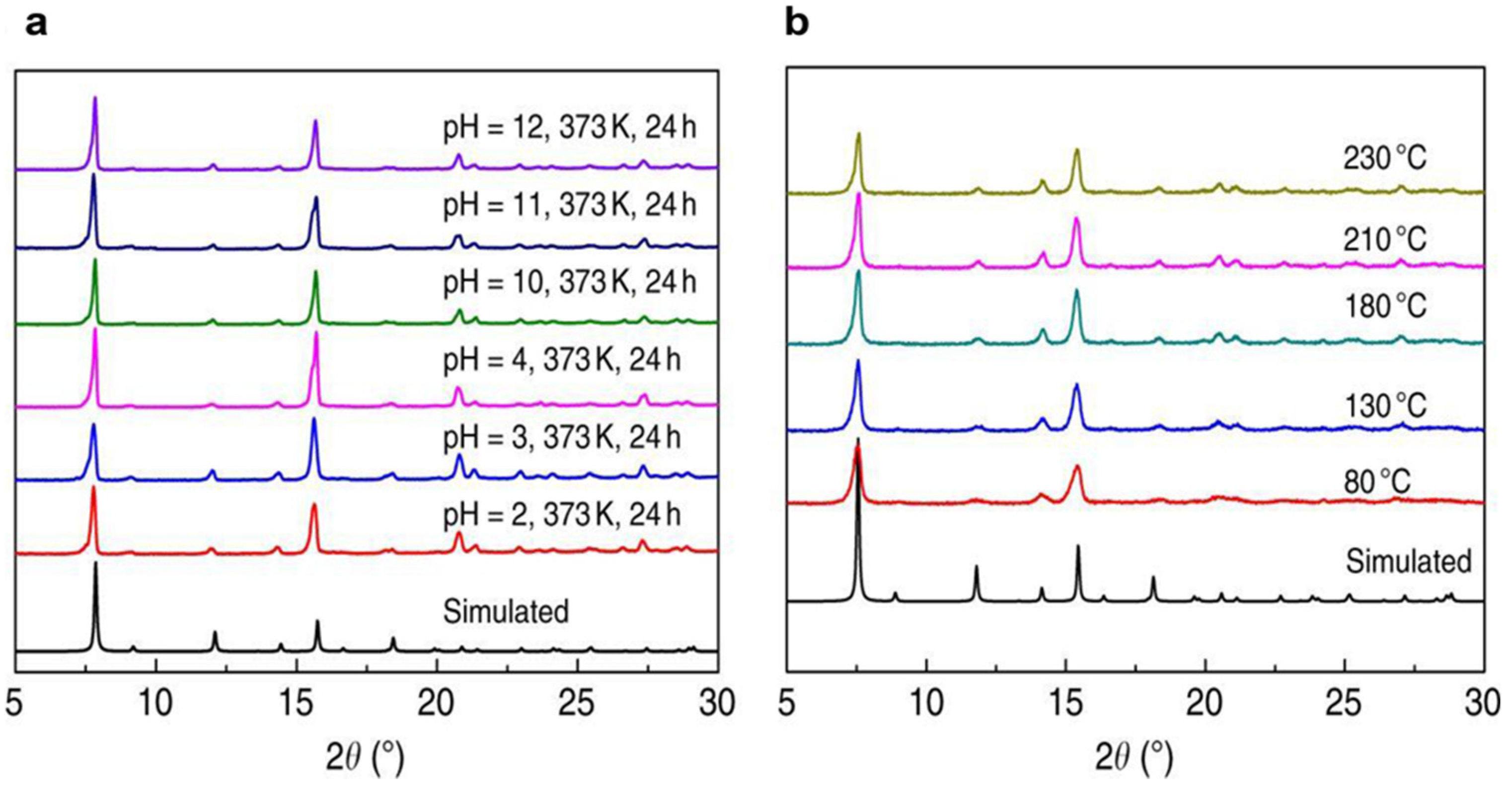
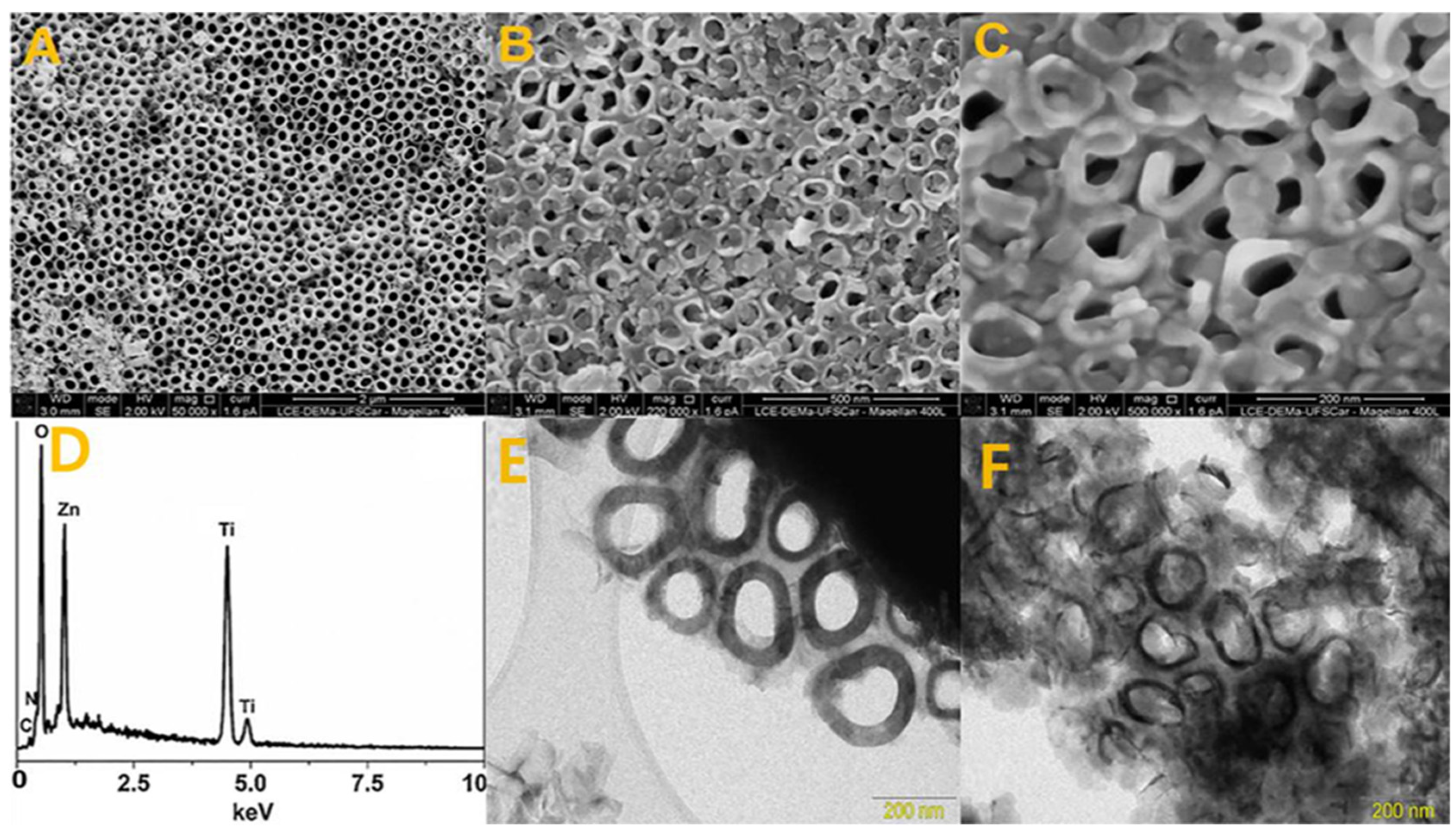
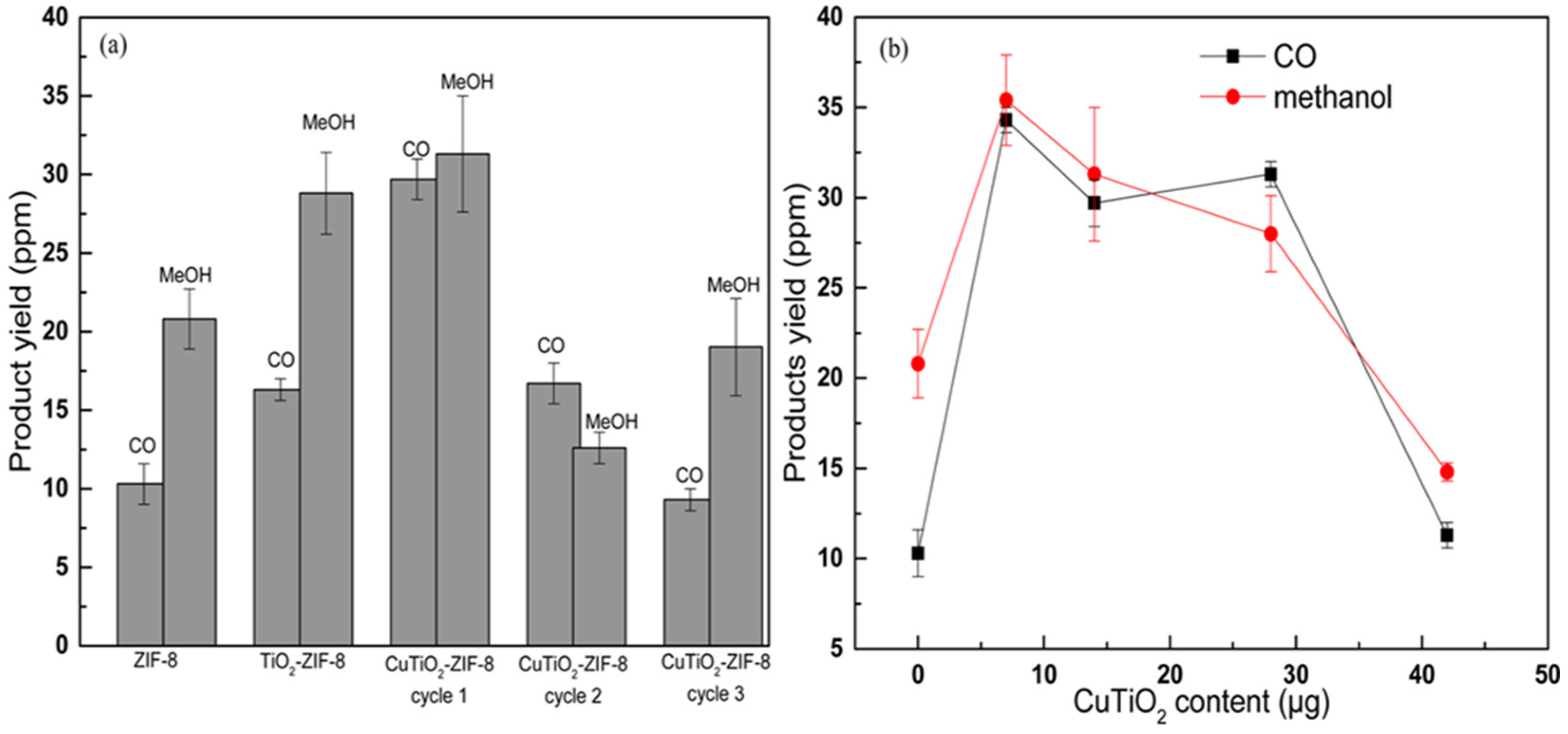
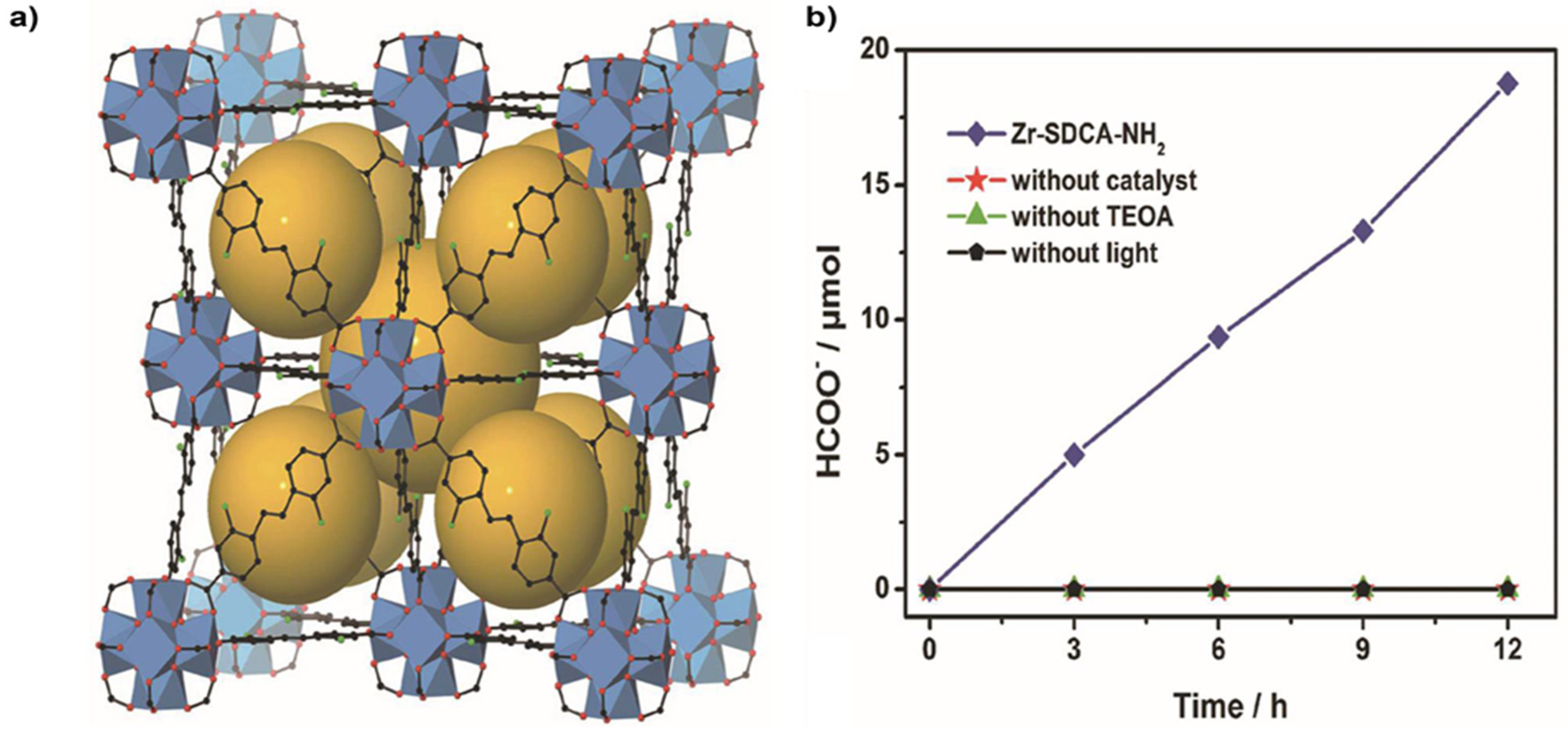
| Reactant Protonation and Electron Inoculation | Product | E (V vs. NHE, at pH = 7) |
|---|---|---|
| CO2 + 2H+ + 2e− | HCOOH | −0.61 |
| CO2 + 2H+ + 2e− | CO + H2O | −0.53 |
| CO2 + 4H+ + 4e− | HCHO + H2O | −0.48 |
| CO2 + 6H+ + 6e− | CH3OH + H2O | −0.38 |
| CO2 + 8H+ + 4e− | CH4 + 2H2O | −0.24 |
| 2H+ + 2e− | H2 | −0.41 |
| H2O + 2h+ | 1/2O2 + 2H+ | +0.41 |
| Photocatalyst | Active Metal Cluster | Organic Metal Linker | BET (m2/g) | Photocatalytic Reaction Condition [Catalyst Loading, Solvent, Power, Sacrificial Agent, Irradiation, Time (h)] | Throughput (µmol/g) | Ref. | |||
|---|---|---|---|---|---|---|---|---|---|
| CO | CH4 | H2 | HCOO− | ||||||
| ZrPP-1-Co | Zr/Co | THPP | 852.3 | (20 mg), MeCN/TEOA, 300-W xenon lamp (λ > 420 nm), TEOA, 15 h | 210 | 7.5 | [139] | ||
| Pbz-MOF-1 | Zr-O | HCBB | ≈1768 | (20 mg), MeCN/H2O, 300-W xenon arc lamp (λ ≥ 420 nm), TIPA, 12 h | 176.5 | [140] | |||
| PCN-136 | Zr-O | HCHC | 1768 | 526 | |||||
| CsPbBr3 QDs (15%)/UiO-66(NH2) | Zr-O | H4L | 465.68 | (10 mg), H2O/ethyl acetate, 300-W xenon arc lamp (λ ≥ 420 nm), H2O, 12 h | 98.57 | 3.08 | [141] | ||
| MOF-253-Re-(CO)3Cl | Re-O | dcbpy | (5 mg), DMF/H2O, (400 < λ < 800 nm), TEOA, 4 h | 446 | 22 | 1900 | [142] | ||
| NH2-rGO (5 wt.%)/Al-PMOF | Zr-O | TCPP | 1180 | (50 mg), MeCN/TEOA, 125-W mercury lamp, TEOA, 6 h | 4113.6 | [143] | |||
| NH2-MIL-101(Fe) | Fe-O | H4L | (2 mg), Solvent-Free, 300-W xenon arc lamp (400 < λ < 780 nm), TEOA, 5 h | 87.6 | [144] | ||||
| NH2-MIL-53(Fe) | Fe-O | H4L | 15.7 | ||||||
| AUBM-4 | Zr-O | cptpy | 50 | (20 mg), MeCN/TEOA, 150-W (420 < λ < 800 nm), TEOA, 6 h | 2196 | [145] | |||
| PCN-222 | Zr-O | TCPP | 1728 | (20 mg), MeCN/TEOA, 300-W (420 < λ < 800 nm), TEOA, 6 h | 2197 | [146] | |||
| Eu-Ru(phen)3-MOF | Eu-O | H3L | 2205 | [147] | |||||
| NH2-MIL-53(Fe) | Fe-O | BDC | 2199 | [148] | |||||
| 253-Ru(5,5′-dcbpy)(CO)2Cl2 | Ru-O | dcbpy | 2200 | [149] | |||||
| MIL-101(Fe) | Fe-O | ATA | 2201 | [148] | |||||
| Zr-SDCA-NH2 | Zr-O | H2L1 | 2546 | 2202 | [150] | ||||
| NNU-28 | Zr-O | H2L | 1490 | 2203 | [151] | ||||
| Ir-CP | Ir-ligand | dcbpy | (20 mg), MeCN/TEOA, 500-W (420 < λ < 800 nm), TEOA, 6 h | 2204 | [152] | ||||
| NH2-UiO-66(Zr) | Zr-O | BPDC | 778 | 2198 | [153] | ||||
| Zr-SDCA-NH2 | Zr-O | H2L1 | 2546 | (40 mg), MeCN/TEOA, 300-W xenon lamp (420 < λ < 800 nm), TEOA, 12 h | 470 | [150] | |||
| co-cat. ZIF-67 | Zn-O | TCPP | 445 | (10 mg), MeCN/MeOH/TEOA, 300-W xenon lamp (420 < λ < 800 nm), TEOA, 6 h | 2120 | 209 | [154] | ||
| ZnMn2O4, nanoparticle | Zn-O | 2.7 | (100 mg), H2O, 500-W Xenon arc lamp (λ = 400 nm), TEOA, 8 h | 94.7 | [155] | ||||
| ZMO-350 | Zn-O | PTCDA | 24.7 | 159.9 | |||||
| ZMO-450 | Zn-O | PTCDA | 45.8 | 191.9 | |||||
| ZMO-550 | Zn-O | PTCDA | 109.1 | 126.6 | |||||
| ZMO-650 | Zn-O | PTCDA | 8.4 | 106.9 | |||||
| Co6-MOF | Co-O | NTB | 1957.5 | (3 mg), MeCN/H2O, 150-W xenon lamp (420 ≤ λ ≤ 780), TEOA,3 h, PS | 13.120 | 9376.7 | [156] | ||
| QS-Co3O4HoMSs (ZIF-67) | Co-O | 2-mIM | (5 mg), H2O, 200-W xenon lamp (AM 1.5 filter), 5 h | 231.5 | [157] | ||||
| BIF-101 | Co-O | NBDC | 328.1 | (10 mg), MeCN/H2O, (λ > 420 nm), 10 h, PS | 58.300 | 11.000 | [158] | ||
| MOF-74 | Zn-O | DHBDC | (30 mg), H2O, 500-W xenon lamp, 5 h | 7.42 | [159] | ||||
| Pt/MOF-74 | Zn-O | DHBDC | 8.85 | 9.04 | |||||
| Au@Pd@MOF-74 | Zn-O | DHBDC | 12.31 | ||||||
| Pt/Au@Pd@MOF-74 | Zn-O | DHBDC | 2.42 | 12.35 | |||||
| Co1.11Te2⊂C | Co-/Te-O | 2-mIM | 107 | (1 mg), MeCN/TEOA/H2O, 200-W white LEDs lamp, TEOA, 3 h, PS | 34.200 | 73.4 | 12.394 | [160] | |
| Ni MOLs (Pure CO2) | Ni-O | BDC | 48.9 | (1 mg), MeCN/TEOA, 5-W white LED light (400 nm ≤ λ ≤ 800 nm), TEOA, 2 h, PS | 12.500 | 280 | [161] | ||
| Ni MOLs (10% dilute CO2) | Ni-O | BDC | 12.5 | 0.38 | |||||
| Co MOLs (Pure CO2) | Co-O | BDC | 44.6 | 4.61 | 2.34 | ||||
| Co MOLs (10% dilute CO2) | Co-O | BDC | 0.44 | 4.15 | |||||
| Zn2GeO4 | 56.4 | (20 mg), MeCN/H2O, 300-W xenon arc lamp (200 < λ < 1100 nm), 9 h | 1.8 | [162] | |||||
| Zn2GeO4/Mg-MOF-74 | Zn-O | H4DOBDC | 406.7 | 12.94 | |||||
| PCN-138 | Zr-O | TCPP | 1261 | (10 mg), MeCN/H2O, 300-W xenon lamp (λ ≥ 420 nm), TIPA, 12 h | 2021 | [163] | |||
| Co-cat. Ni3(HITP)2 | Ni-N4/Ni2+ | HATP | 630 | (2 mg), MeCN/H2O/TEOA, 100-W LED light (λ = 420 nm), TEOA, 3 h, PS | 103.50 | 3745 | [164] | ||
| O-ZnO/UiO-66-NH2 | Zr/Zn-O | ATA | (100 mg), NaHCO3 aq., 300-W xenon lamp (λ > 420 nm), 6 h | 29.6 | [165] | ||||
| O-ZnO/rGO/UiO-66-NH2 | Zr/Zn-O | ATA | 877.3 | 38.5 | |||||
| TiO2 | 42 | (3 mg), 150-W xenon lamp (λ > 325 nm), H2, 6 h | 17.1 | [166] | |||||
| NH2-UiO-66 | Zr-O | BPDC | 871 | 9 | |||||
| 1-TiMOF | Zr/Ti-O | BPDC | 173 | 22.44 | |||||
| 2-TiMOF | Zr/Ti-O | BPDC | 202 | 25.44 | |||||
| 3-TiMOF | Zr/Ti-O | BPDC | 268 | 20.22 | |||||
| 4-TiMOF | Zr/Ti-O | BPDC | 284 | 17.1 | |||||
| MOF-525 | Zr-O | TCPP | 2127.7 | (2 mg), MeCN/TEOA, 300-W xenon arc lamp (400 nm < λ < 800 nm), TEOA, 6 h | 384.12 | 37.2 | [167] | ||
| MOF-525-Co | Zr-O | TCPP | 1317.8 | 1203.6 | 220.56 | ||||
| MOF-525-Zn | Zr-O | TCPP | 1264.2 | 670.2 | 69.81 | ||||
| C-Cu2−xS@g-C3N4 | Cu-O | TAA | 95.1 | (1 mg), H2O, (300 nm < λ < 800 nm), H2O, 12 h | 1062.6 | 26.42 | [168] | ||
| QS-Co3O4HoMSs(ZIF-67) | Co-O | 2-mIM | (1 mg), H2O, 200-W xenon lamp, H2O, 5 h | 231.5 | [157] | ||||
| g-C3N4 | 34.55 | (20 mg), MeCN/TEOA, 300-W xenon arc lamp, TEOA, 6 h | 32.442 | 9.294 | [169] | ||||
| BIF-20@g-C3N4, (10% g-C3N4) | Zn-O | BH-(mim)3− | 378.83 | 170.85 | 56.1 | ||||
| BIF-20@g-C3N4, (15% g-C3N4) | Zn-O | BH-(mim)3- | 283.67 | 242.75 | 72.7 | ||||
| BIF-20@g-C3N4, (20% g-C3N4) | Zn-O | BH-(mim)3- | 1276.01 | 305.85 | 88.15 | ||||
| BIF-20@g-C3N4, (25% g-C3N4) | Zn-O | BH-(mim)3- | 1177.77 | 257 | 75.35 | ||||
| AD-MOF-1 | Co-O | HAD/BA | (5 mg), MeCN/H2O, 300-W xenon lamp (420 < λ < 800 nm), TIPA, 4h | 716 | [170] | ||||
| AD-MOF-2 | Co-O | HAD/IA | (5 mg), MeCN/H2O, 300-W xenon lamp (420 < λ < 800 nm), TIPA, 5 h | 1773 | |||||
| MIL-101-EN | Cr-O | TPA | 839.7 | (5 mg), H2O/TEOA, light-intensity-controlled xenon lamp, TEOA, 10 h | 472 | 17.12 | [171] | ||
| MIL-101-SO3H | Cr-O | TPA | 1936.9 | 217 | 13 | ||||
| MIL-101-Cr | Cr-O | TPA | 3126.5 | 83 | 17 | ||||
| NNU-29 | Zn-O | L | (10 mg), H2O/TEOA, 300-W xenon arc lamp (420 < λ < 800 nm), TEOA, 16 h, PS | 18 | 58 | 3520 | [172] | ||
| MOF-Cu | Cu-O | TCA | (5 mg), MeCN/H2O, 300-W xenon lamp (λ ≥ 420 nm), TIPA, 12 h, PS | 344 | 1162 | [173] | |||
| MOF-Co | Co-O | TCA | 4564 | 5062 | |||||
| MOF-Ni | Ni-O | TCA | 4472 | 104 | |||||
| TiO2 | 138.07 | (10 mg), H2O, 300-W xenon lamp (λ > 300 nm), 8 h | 6.56 | [174] | |||||
| PCN-224(Cu) | Zr-O | TCPP | 2285 | 29.76 | 10.86 | ||||
| 15% PCN-224(Cu)/TiO2 | Zr-O | TCPP | 178.05 | 297.68 | 1.69 | ||||
| (Co/Ru)2.4-UiO-67(bpydc) | Co-O | bpydc | 103.3 | (1 mg), MeCN/H2O, 450 nm LED light, TEOA, 16 h, PS | 4520.4 | 9121.5 | [175] | ||
| 36% CdS/MIL-101(Cr) | Cr-O | TPA | 0.1324 | (10 mg), H2SO4 + NaHCO3, (λ ≥ 400 nm), 3.33 h | 54.5 | [176] | |||
| CdS | (10 mg), MeCN/TEOA, 300-W xenon lamp (λ ≥ 420 nm), TEOA, 10 h | 230 | 2480 | ||||||
| CdS/UiO-bpy | Zr-O | bpydc | 52.6 | (10 mg), MeCN/TEOA, 300-W xenon lamp (λ ≥ 420 nm), TEOA, 10 h | 2750 | [177] | |||
| CdS/UiO-bpy/Co | Zr-O | bpydc | 25.5 | 2350 | 410 | ||||
| CsPbBr3@ZIF-8 | Zn-O | 2-mIM | (4.5 mg), H2O, 100-W xenon lamp AM 1.5G filter, H2O, 3 h | 1.515 | 5.434 | [178] | |||
| CsPbBr3@ZIF-67 | Co-O | 2-Mim | 2.301 | 10.537 | |||||
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kidanemariam, A.; Lee, J.; Park, J. Recent Innovation of Metal-Organic Frameworks for Carbon Dioxide Photocatalytic Reduction. Polymers 2019, 11, 2090. https://doi.org/10.3390/polym11122090
Kidanemariam A, Lee J, Park J. Recent Innovation of Metal-Organic Frameworks for Carbon Dioxide Photocatalytic Reduction. Polymers. 2019; 11(12):2090. https://doi.org/10.3390/polym11122090
Chicago/Turabian StyleKidanemariam, Alemayehu, Jiwon Lee, and Juhyun Park. 2019. "Recent Innovation of Metal-Organic Frameworks for Carbon Dioxide Photocatalytic Reduction" Polymers 11, no. 12: 2090. https://doi.org/10.3390/polym11122090
APA StyleKidanemariam, A., Lee, J., & Park, J. (2019). Recent Innovation of Metal-Organic Frameworks for Carbon Dioxide Photocatalytic Reduction. Polymers, 11(12), 2090. https://doi.org/10.3390/polym11122090