Structural Characterization and Antioxidant Activity of Milled Wood Lignin from Xylose Residue and Corncob
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Isolation of MWLs from the Two Feedstocks
2.3. Characterization of MWLs
2.4. Antioxidant Activity against DPPH Radical
3. Results and Discussion
3.1. Composition Analysis and Associated Carbohydrates
3.2. Molecular Weight of Lignin Fractions
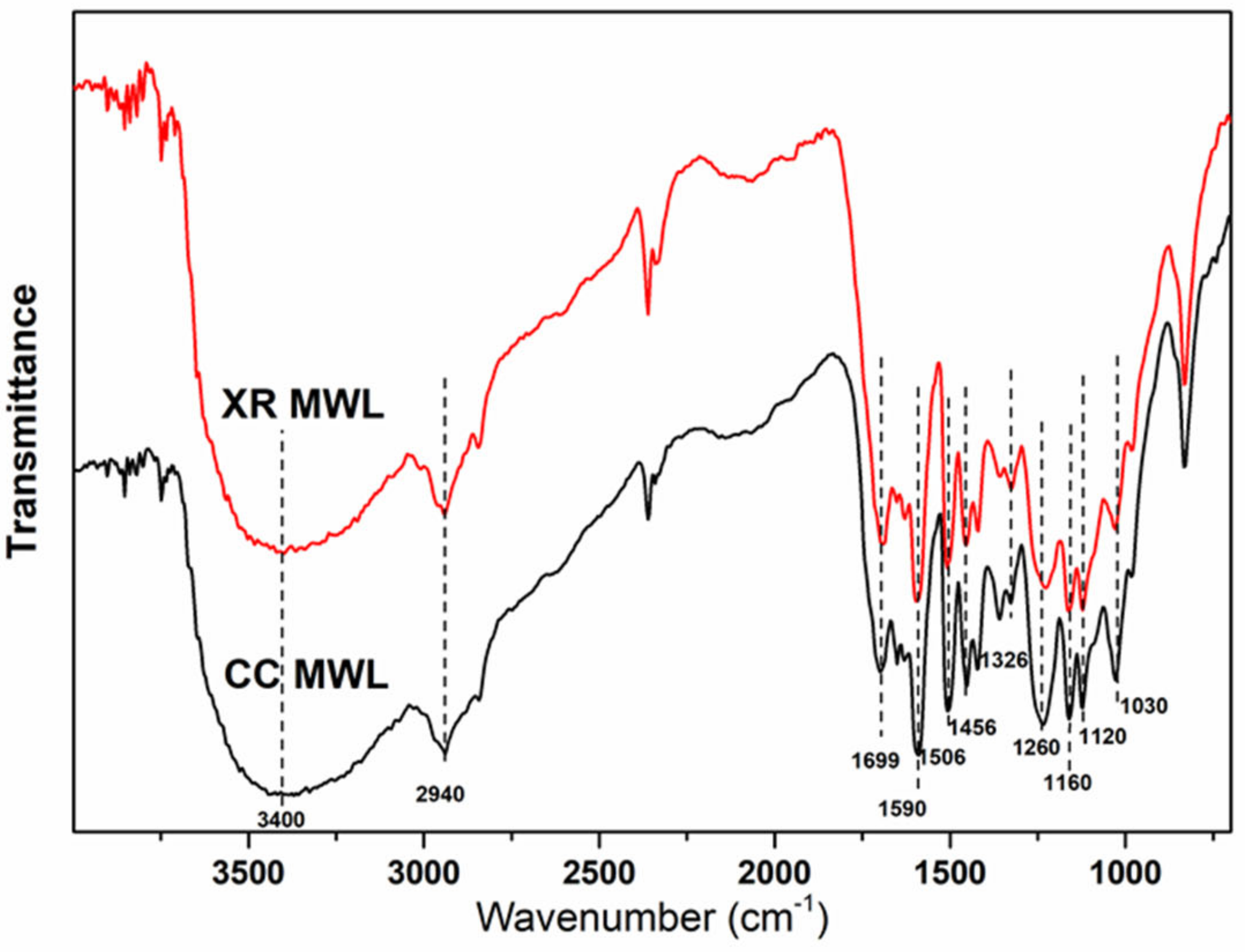
3.3. FTIR Spectra
3.4. Quantitative NMR Spectra
3.4.1. 2D-HSQC Spectra
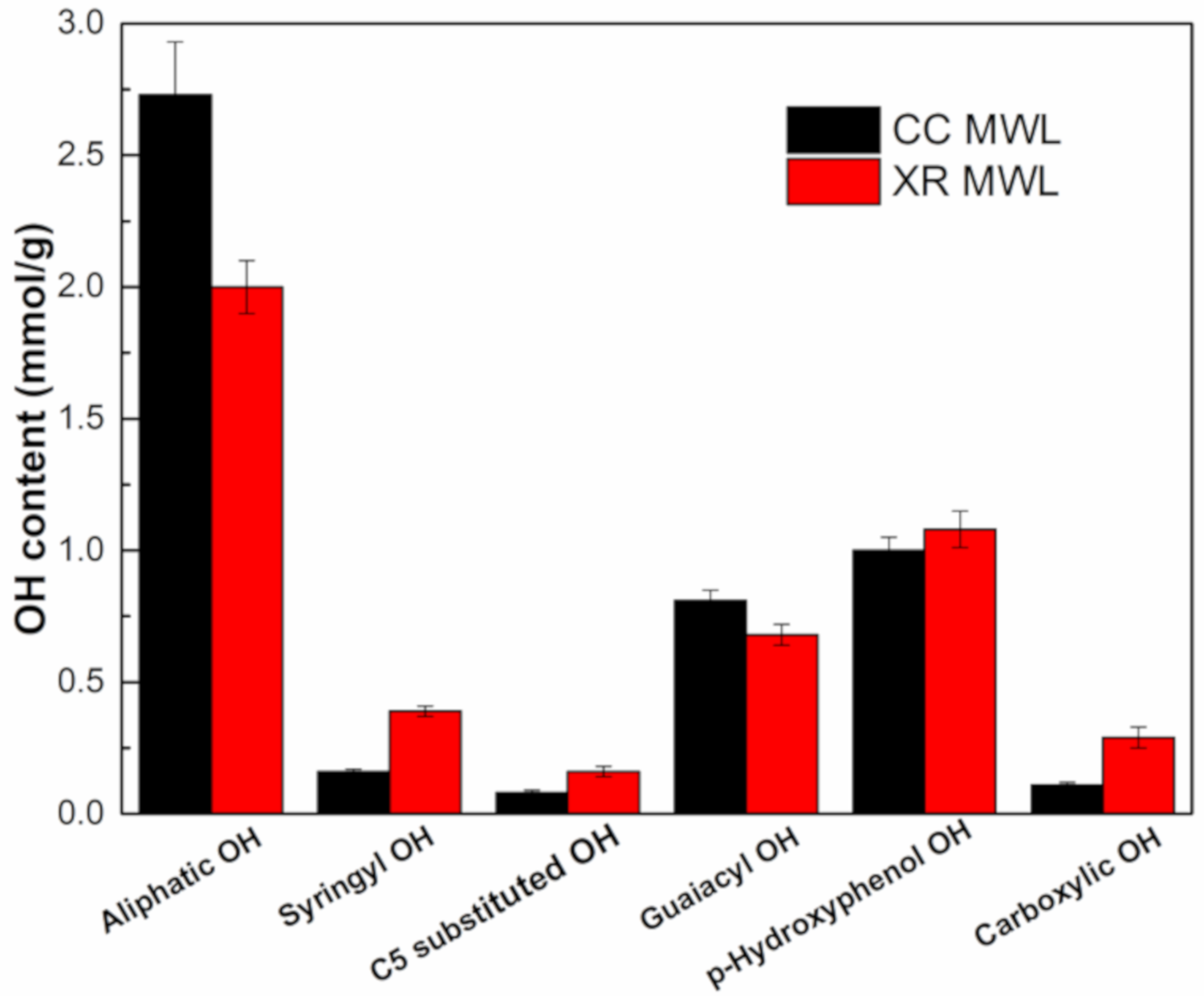
3.4.2. 31P NMR Analysis of Lignins
3.5. Thermal Analysis
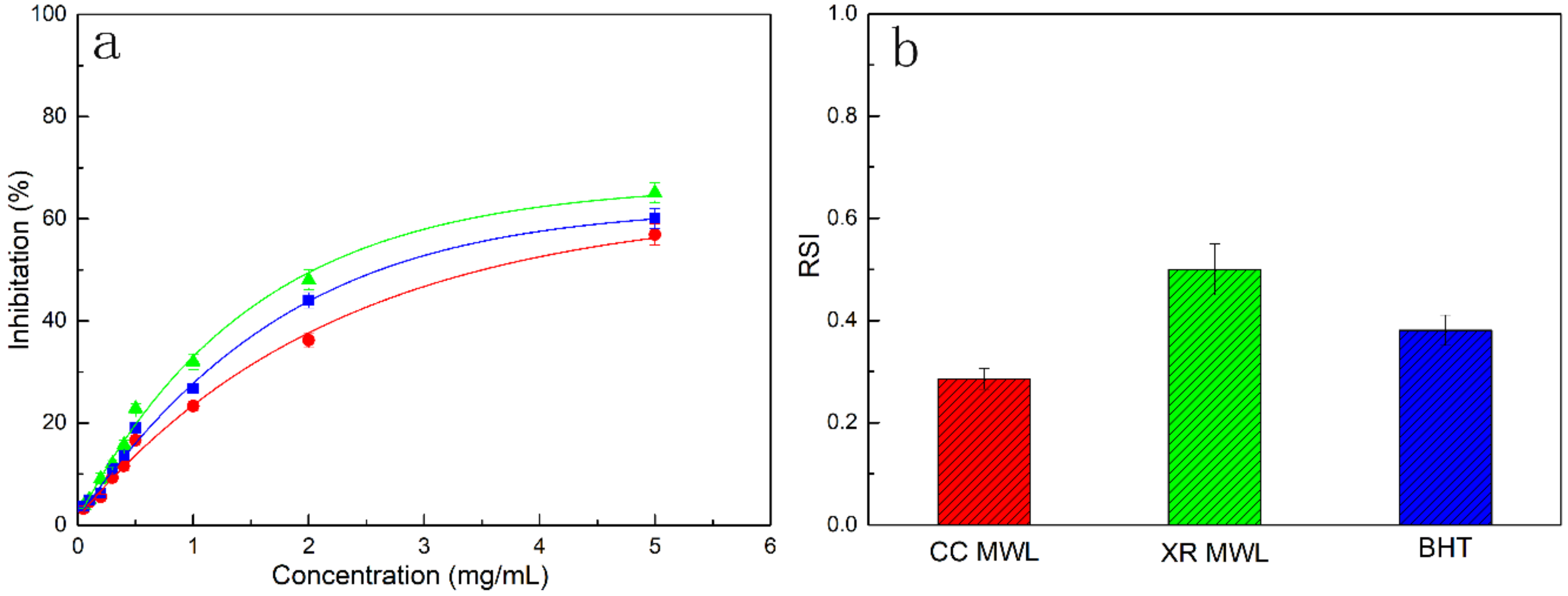
3.6. Antioxidant Activity of MWLs
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Corma, A.; Iborra, S.; Velty, A. Chemical Routes for theTransformation of Biomass into Chemicals. Chem. Rev. 2007, 107, 2411–2502. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Zhang, L.M.; Zhou, T.; Chen, J.C.; Xu, F. Synergy of Lewis and Brønsted acids on catalytic hydrothermal decomposition of carbohydrates and corncob acid hydrolysis residues to 5-hydroxymethylfurfural. Sci. Rep. 2017, 7, 40908–40917. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Zhang, Q.; Chen, Y.; Zhang, X.; Xu, F. Highly Efficient Conversion of Xylose Residues to Levulinic Acid over FeCl3 Catalyst in Green Salt Solutions. ACS Sustain. Chem. Eng. 2018, 6, 3154–3161. [Google Scholar] [CrossRef]
- Ji, Z.; Zhang, X.; Ling, Z.; Zhou, X.; Ramaswamy, S.; Xu, F. Visualization of Miscanthus x giganteus cell wall deconstruction subjected to dilute acid pretreatment for enhanced enzymatic digestibility. Biotechnol. Biofuels 2015, 8, 103–116. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Y.; Zhao, S.; Yang, S.; Ding, S.-Y. Lignin plays a negative role in the biochemical process for producing lignocellulosic biofuels. Curr. Opin. Biotechnol. 2014, 27, 38–45. [Google Scholar] [CrossRef]
- Guo, Z.; Ling, Z.; Wang, C.; Zhang, X.; Xu, F. Integration of facile deep eutectic solvents pretreatment for enhanced enzymatic hydrolysis and lignin valorization from industrial xylose residue. Bioresour. Technol. 2018, 265, 334–339. [Google Scholar] [CrossRef]
- Wang, C.; Lyu, G.; Yang, G.; Chen, J.; Jiang, W. Characterization and hydrothermal conversion of lignin produced from corncob acid hydrolysis residue. BioResources 2014, 9, 4596–4607. [Google Scholar] [CrossRef]
- Sluiter, A.; Hames, B.; Ruiz, R.; Scarlata, C.; Sluiter, J.; Templeton, D.; Crocker, D. Determination of Structural Carbohydrates and Lignin in Biomass. NREL 2008, 1617, 1–16. [Google Scholar]
- Del Rio, J.C.; Rencoret, J.; Gutierrez, A.; Nieto, L.; Jimenez-Barbero, J.; Martinez, A.T. Structural characterization of guaiacyl-rich lignins in flax (Linum usitatissimum) fibers and shives. J. Agric. Food Chem. 2011, 59, 11088–11099. [Google Scholar] [CrossRef]
- Huang, F.; Singh, P.M.; Ragauskas, A.J. Characterization of milled wood lignin (MWL) in loblolly pine stem wood, residue, and bark. J. Agric. Food Chem. 2011, 59, 12910–12916. [Google Scholar] [CrossRef]
- You, T.T.; Mao, J.Z.; Yuan, T.Q.; Wen, J.L.; Xu, F. Structural elucidation of the lignins from stems and foliage of Arundo donax Linn. J. Agric. Food Chem. 2013, 61, 5361–5370. [Google Scholar] [CrossRef] [PubMed]
- Granata, A.; Argyropoulos, D.S. 2-Chloro-4,4,5,5-tetramethyl-1,3,2-dioxaphospholane, a Reagent for the Accurate Determination of the Uncondensed and Condensed Phenolic Moieties in Lignins. J. Agric. Food Chem. 1995, 43, 1538–1544. [Google Scholar] [CrossRef]
- Sun, S.L.; Wen, J.L.; Ma, M.G.; Sun, R.C. Structural elucidation of sorghum lignins from an integrated biorefinery process based on hydrothermal and alkaline treatments. J. Agric. Food Chem. 2014, 62, 8120–8128. [Google Scholar] [CrossRef] [PubMed]
- Li, M.F.; Sun, S.N.; Xu, F.; Sun, R.C. Microwave-assisted organic acid extraction of lignin from bamboo: structure and antioxidant activity investigation. Food Chem. 2012, 134, 1392–1398. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Yang, G.; Zhang, X.; Shao, L.; Lyu, G.; Mao, J.; Liu, S.; Xu, F. A kinetic study on the hydrolysis of corncob residues to levulinic acid in the FeCl3–NaCl system. Cellulose 2019, 26, 8313–8323. [Google Scholar] [CrossRef]
- Sun, S.-N.; Li, M.-F.; Yuan, T.-Q.; Xu, F.; Sun, R.-C. Effect of ionic liquid/organic solvent pretreatment on the enzymatic hydrolysis of corncob for bioethanol production. Part 1: Structural characterization of the lignins. Ind. Crops Prod. 2013, 43, 570–577. [Google Scholar] [CrossRef]
- Tana, T.; Zhang, Z.; Moghaddam, L.; Rackemann, D.W.; Rencoret, J.; Gutiérrez, A.; del Río, J.C.; Doherty, W.O.S. Structural Changes of Sugar Cane Bagasse Lignin during Cellulosic Ethanol Production Process. ACS Sustain. Chem. Eng. 2016, 4, 5483–5494. [Google Scholar] [CrossRef]
- Wen, J.L.; Sun, S.L.; Xue, B.L.; Sun, R.C. Quantitative structures and thermal properties of birch lignins after ionic liquid pretreatment. J. Agric. Food Chem. 2013, 61, 635–645. [Google Scholar] [CrossRef]
- Yang, X.; Li, N.; Lin, X.; Pan, X.; Zhou, Y. Selective Cleavage of the Aryl Ether Bonds in Lignin for Depolymerization by Acidic Lithium Bromide Molten Salt Hydrate under Mild Conditions. J. Agric. Food Chem. 2016, 64, 8379–8387. [Google Scholar] [CrossRef]
- Rencoret, J.; Marques, G.; Gutierrez, A.; Ibarra, D.; Li, J.; Gellerstedt, G.; Santos, J.; Santos, I.; Jimenez-Barbero, J.; Martinez, A.; et al. Structural characterization of milled wood lignins from different eucalypt species. Holzforschung 2008, 62, 514–526. [Google Scholar] [CrossRef]
- Del Rio, J.C.; Rencoret, J.; Prinsen, P.; Martinez, A.T.; Ralph, J.; Gutierrez, A. Structural characterization of wheat straw lignin as revealed by analytical pyrolysis, 2D-NMR, and reductive cleavage methods. J. Agric. Food Chem. 2012, 60, 5922–5935. [Google Scholar] [CrossRef] [PubMed]
- Kuwabara, H.; Mouri, K.M.; Otsuka, H.; Kasai, R.; Yamasaki, K. Tricin from a Malagasy Connar aceous Plant with Potent Antihistaminic Activity. J. Nat. Prod. 2003, 66, 1273–1275. [Google Scholar] [CrossRef] [PubMed]
- Ashour, A.; Amer, M.; Marzouk, A.; Shimizu, K.; Kondo, R.; El-Sharkawy, S. Corncobs as a potential source of functional chemicals. Molecules 2013, 18, 13823–13830. [Google Scholar] [CrossRef] [PubMed]
- Sun, R.; Tomkinson, J.; Jones, G.L. Fractional characterization of ash-AQ lignin by successive extraction with organic solvents from oil palm EFB fibre. Polym. Degrad. Stab. 2000, 68, 111–119. [Google Scholar] [CrossRef]
- Bu, L.; Tang, Y.; Gao, Y. Comparative characterization of milled wood lignin from furfural residues and corncob. Chem. Eng. J. 2011, 175, 176–184. [Google Scholar] [CrossRef]
- Faustino, H.; Gil, N.; Baptista, C.; Duarte, A.P. Antioxidant activity of lignin phenolic compounds extracted from kraft and sulphite black liquors. Molecules 2010, 15, 9308–9322. [Google Scholar] [CrossRef]


| Samples | Ara a | Glu a | Xyl a | Gal a | Lignin | Others |
|---|---|---|---|---|---|---|
| CC | 5.05 | 39.14 | 32.43 | 2.39 | 13.31 | 7.68 |
| XR | 0.20 | 60.22 | 5.04 | - | 23.12 | 11.42 |
| Sample | Yield a | Ara b | Glu b | Xyl b | Total Sugar b |
|---|---|---|---|---|---|
| CC MWL | 5.25 | 0.21 | 0.85 | 1.58 | 2.64 |
| XR MWL | 8.78 | 0.14 | 0.50 | 0.47 | 1.11 |
| Sample | Mw | Mn | PI (Mw/Mn) |
|---|---|---|---|
| CC MWL | 2847 | 2283 | 1.25 |
| XR MWL | 6121 | 4104 | 1.49 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, M.; Wang, C.; Lyu, G.; Zhong, L.; Yang, L.; Wang, Z.; Qin, C.; Ji, X.; Yang, G.; Chen, J.; et al. Structural Characterization and Antioxidant Activity of Milled Wood Lignin from Xylose Residue and Corncob. Polymers 2019, 11, 2092. https://doi.org/10.3390/polym11122092
Xu M, Wang C, Lyu G, Zhong L, Yang L, Wang Z, Qin C, Ji X, Yang G, Chen J, et al. Structural Characterization and Antioxidant Activity of Milled Wood Lignin from Xylose Residue and Corncob. Polymers. 2019; 11(12):2092. https://doi.org/10.3390/polym11122092
Chicago/Turabian StyleXu, Miaomiao, Chao Wang, Gaojin Lyu, Lei Zhong, Liyuan Yang, Zhiwei Wang, Chengrong Qin, Xingxiang Ji, Guihua Yang, Jiachuan Chen, and et al. 2019. "Structural Characterization and Antioxidant Activity of Milled Wood Lignin from Xylose Residue and Corncob" Polymers 11, no. 12: 2092. https://doi.org/10.3390/polym11122092
APA StyleXu, M., Wang, C., Lyu, G., Zhong, L., Yang, L., Wang, Z., Qin, C., Ji, X., Yang, G., Chen, J., & Xu, F. (2019). Structural Characterization and Antioxidant Activity of Milled Wood Lignin from Xylose Residue and Corncob. Polymers, 11(12), 2092. https://doi.org/10.3390/polym11122092