Transient and Steady Pervaporation of 1-Butanol–Water Mixtures through a Poly[1-(Trimethylsilyl)-1-Propyne] (PTMSP) Membrane
Abstract
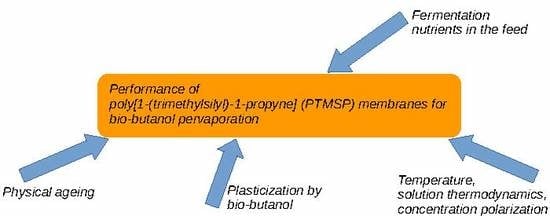
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Membrane Preparation
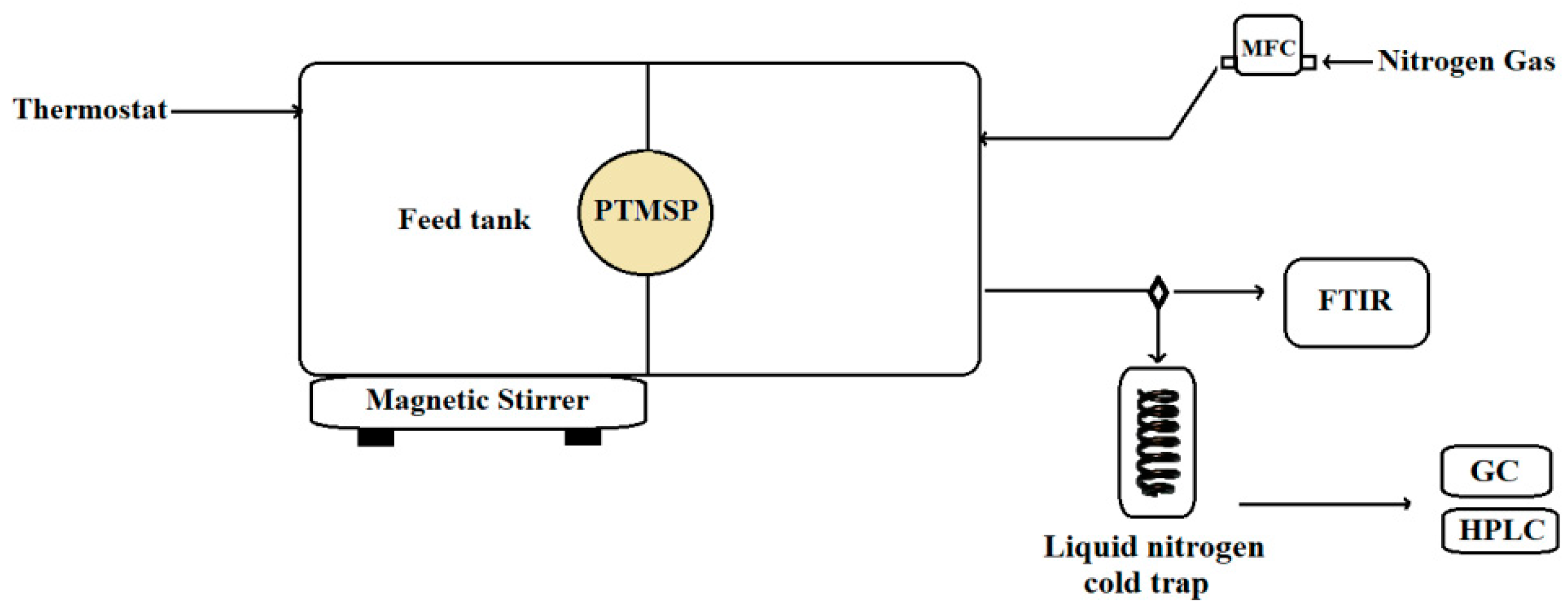
2.3. Pervaporation System
2.4. Vapor Sorption Microgravimetry
2.5. Gas Chromatography Analysis
2.6. HPLC Analysis
2.7. SEM Analysis
2.8. Contact Angle Measurement
2.9. Measurement of Transient Pervaporation
2.10. Measurement of Steady Pervaporation
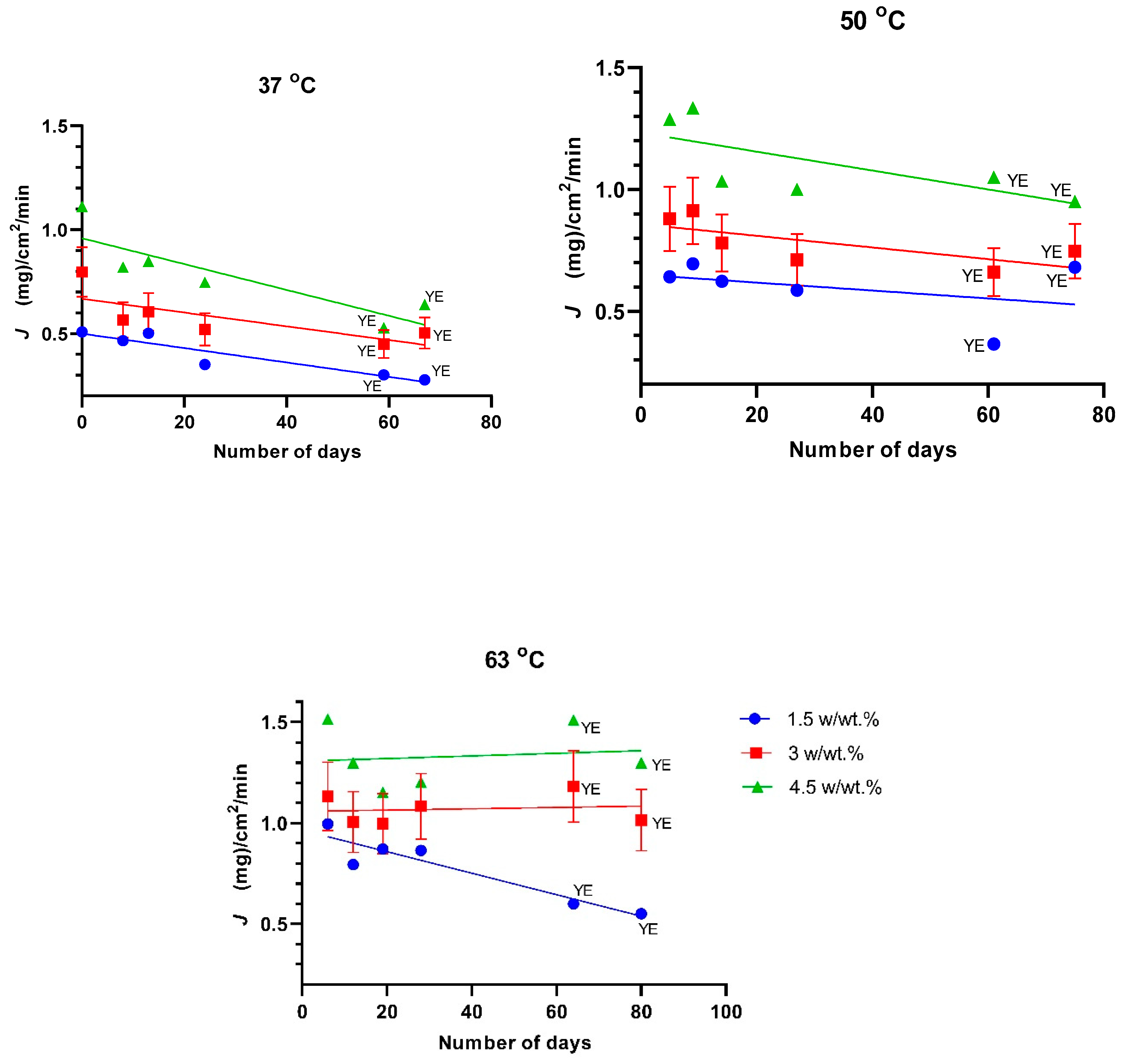
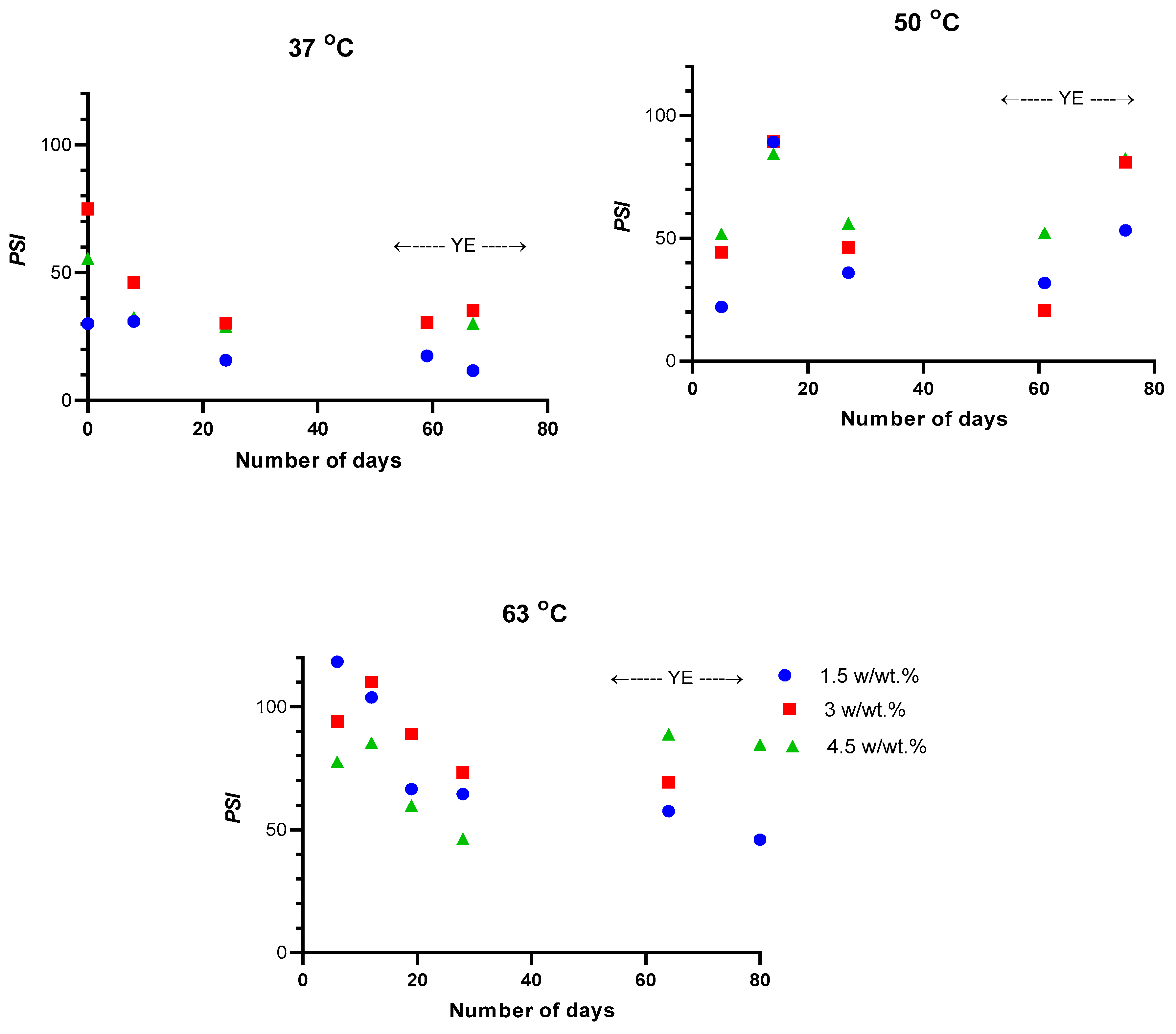
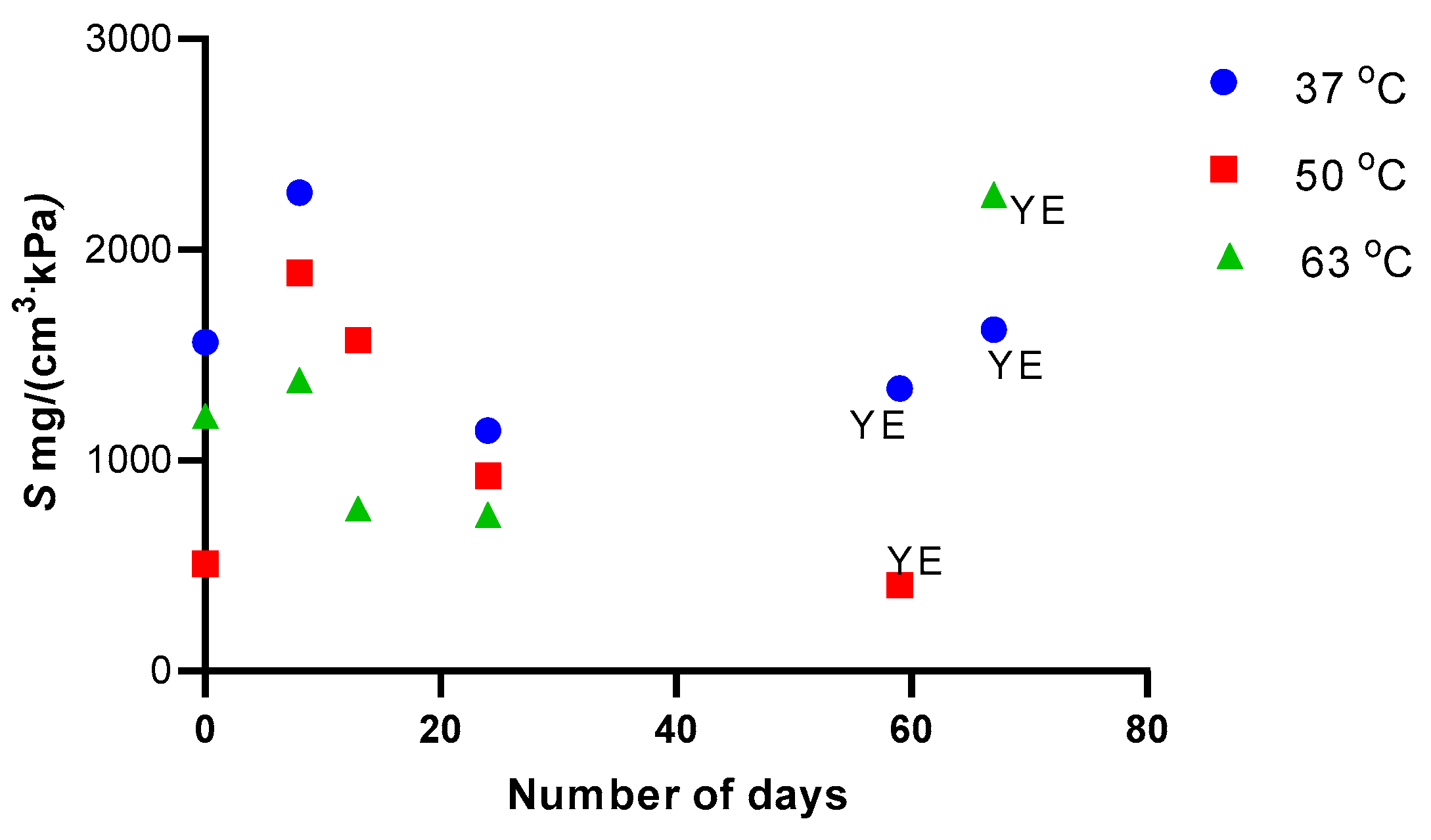
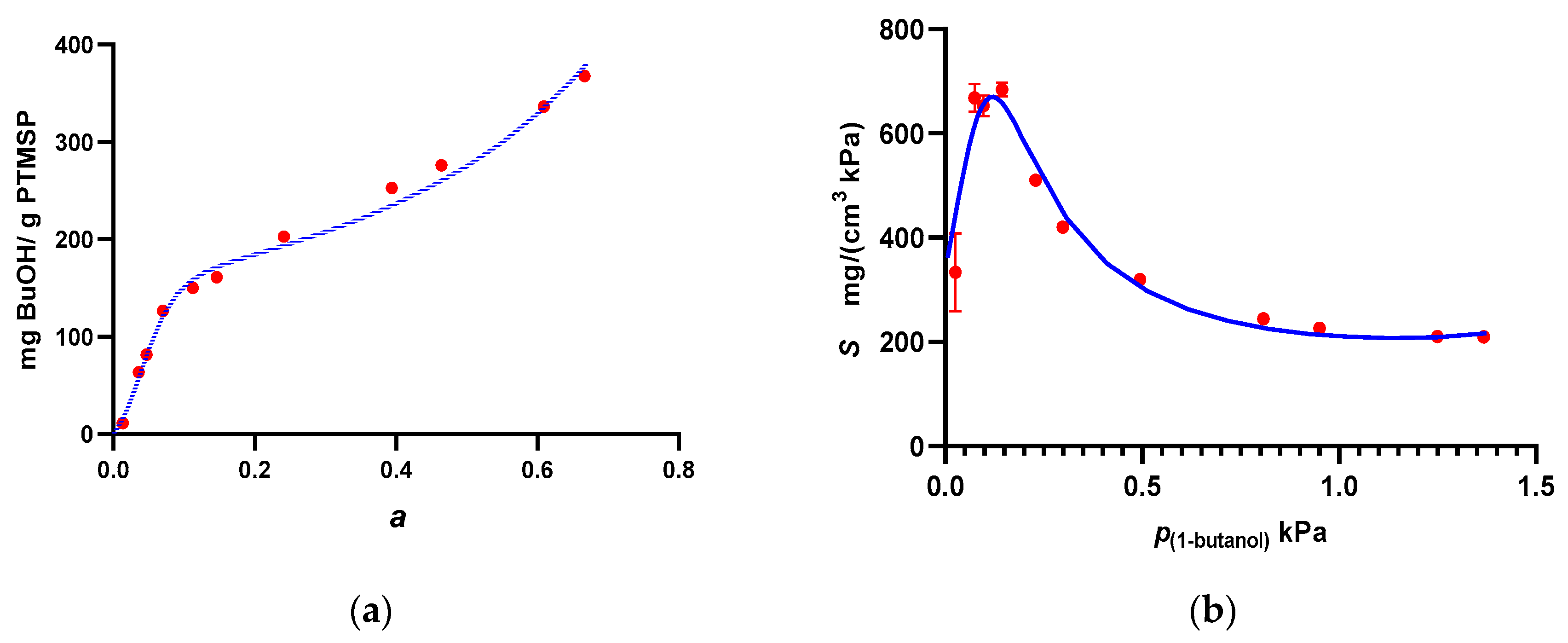
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Birgen, C.; Dürre, P.; Preisig, H.A.; Wentzel, A. Butanol production from lignocellulosic biomass: Revisiting fermentation performance indicators with exploratory data analysis. Biotechnol. Biofuels 2019, 12, 167. [Google Scholar] [CrossRef]
- Dürre, P. Biobutanol: An attractive biofuel. Biotechnol. J. 2007, 2, 1525–1534. [Google Scholar] [CrossRef]
- Bharathiraja, B.; Jayamuthunagai, J.; Sudharsanaa, T.; Bharghavi, A.; Praveenkumar, R.; Chakravarthy, M.; Yuvaraj, D. Biobutanol–An impending biofuel for future: A review on upstream and downstream processing tecniques. Renew. Sustain. Energy Rev. 2017, 68, 788–807. [Google Scholar] [CrossRef]
- Pereira, L.; Chagas, M.; Dias, M.; Cavalett, O.; Bonomi, A. Life cycle assessment of butanol production in sugarcane biorefineries in Brazil. J. Clean. Prod. 2015, 96, 557–568. [Google Scholar] [CrossRef]
- Abdehagh, N.; Tezel, F.; Thibault, J. Separation techniques in butanol production: Challenges and developments. Biomass Bioenergy 2014, 60, 222–246. [Google Scholar] [CrossRef]
- Zhu, Y.; Xin, F.; Chang, Y.; Zhao, Y.; Weichong, W. Feasibility of reed for biobutanol production hydrolyzed by crude cellulase. Biomass Bioenergy 2015, 76, 24–30. [Google Scholar] [CrossRef]
- Heitmann, S.; Krüger, V.; Welz, D.; Lutze, P. Experimental investigation of pervaporation membranes for biobutanol separation. J. Membr. Sep. Technol. 2013, 2, 245–262. [Google Scholar]
- Kumar, M.; Gayen, K. Developments in biobutanol production: New insight. Appl. Energy 2011, 88, 1999–2012. [Google Scholar] [CrossRef]
- Jadhav, S.; Harde, S.; Bankar, S.; Granström, T.; Ojamo, H.; Singhal, R.; Survase, S. A green process for the production of butanol from butyraldehyde using alcohol dehydrogenase: Process details. Rsc Adv. 2014, 4, 14597–14602. [Google Scholar] [CrossRef]
- Dong, H.; Tao, W.; Dai, Z.; Yang, L.; Gong, F.; Zhang, Y.; Li, Y. Biobutanol. In Advances in Biochemical Engineering/Biotechnology; Springer: Berlin/Heidelberg, Germany, 2011; Volume 128, pp. 85–100. [Google Scholar]
- Qureshi, N.; Liu, S.; Ezeji, T. Cellulosic butanol production from agricultural biomass and residues: Recent advances in technology. In Advanced Biofuels and Bioproducts; James, W.L., Ed.; Springer: New York, NY, USA, 2013; Volume 15, pp. 247–265. [Google Scholar]
- Li, X.; Li, Z.; Zheng, J.; Shi, Z.; Li, L. Yeast extract promotes phase shift of bio-butanol fermentation by Clostridium acetobutylicum ATCC824 using cassava as substrate. Bioresour. Technol. 2012, 125, 43–51. [Google Scholar] [CrossRef]
- Liu, X.; Gu, Q.; Liao, C.; Yu, X. Enhancing butanol tolerance and preventing degeneration in Clostridium acetobutylicum by 1-butanol–glycerol storage during long-term preservation. Biomass Bioenergy 2014, 69, 192–197. [Google Scholar] [CrossRef]
- García, V.; Päkkilä, J.; Ojamo, H.; Muurinen, E.; Keiski, R. Challenges in biobutanol production: How to improve the efficiency. Renew. Sustain. Energy Rev. 2011, 15, 964–980. [Google Scholar] [CrossRef]
- Mariano, A.; Qureshi, N.; Filho, R.; Ezeji, T. Bioproduction of butanol in bioreactors: New insights from simultaneous in situ butanol recovery to eliminate product toxicity. Biotechnol. Bioeng. 2011, 108, 1757–1765. [Google Scholar] [CrossRef] [PubMed]
- Patakova, P.; Kolek, J.; Sedlar, K.; Koscova, P.; Branska, B.; Kupkova, K.; Paulova, L.; Provaznik, I. Comparative analysis of high butanol tolerance and production in clostridia. Biotechnol. Adv. 2018, 36, 721–738. [Google Scholar] [CrossRef] [PubMed]
- Sharif, A.R.; Mehrani, P.; Thibault, J. Comparison of in-situ recovery methods of gas stripping, pervaporation, and vacuum separation by multiobjective optimization for producing biobutanol via fermentation process. Can. J. Chem. Eng. 2015, 93, 986–997. [Google Scholar] [CrossRef]
- Liu, C.; Wei, W.; Wu, H.; Dong, X.; Jiang, M.; Jin, W. Pervaporation performance of PDMS/ceramic composite membrane in acetone butanol ethanol (ABE) fermentation-PV coupled process. J. Membr. Sci. 2011, 373, 121–129. [Google Scholar] [CrossRef]
- Peng, P.; Shi, B.; Lan, Y. A review of membrane materials for ethanol recovery by pervaporation. Sep. Sci. Technol. 2011, 46, 234–246. [Google Scholar] [CrossRef]
- Liu, F.; Liu, L.; Feng, X. Separation of acetone-butanol-ethanol (ABE) from dilute aqueous solutions by pervaporation. Sep. Purification Technol. 2005, 42, 273–282. [Google Scholar] [CrossRef]
- Li, S.; Srivastava, R.; Parnas, R. Separation of 1-butanol by pervaporation using a novel tri-layer PDMS composite membrane. J. Membr. Sci. 2010, 363, 287–294. [Google Scholar] [CrossRef]
- Azimi, H.; Tezel, F.; Thibault, J. Effect of embedded activated carbon nanoparticles on the performance of polydimethylsiloxane (PDMS) membrane for pervaporation separation of butanol. J. Chem. Technol. Biotechnol. 2017, 92, 2901–2911. [Google Scholar] [CrossRef]
- Hickey, P.J.; Juricic, F.P.; Slater, C.S. The effect of process parameters on the pervaporation of alcohols through organophilic membranes. Sep. Sci. Technol. 1992, 27, 843–861. [Google Scholar] [CrossRef]
- Schmidt, C.L.; Myers, M.D.; Kelley, S.S.; McMillan, J.D.; Paducone, N. Evaluation of PTMSP membranes in achieving enhanced ethanol removal from fermentations by pervaporation. Appl. Biochem. Biotechnol. 1997, 63, 469–482. [Google Scholar] [CrossRef] [PubMed]
- Fadeev, A.G.; Selinskaya, Y.A.; Kelley, S.S.; Meagher, M.M.; Litvinova, E.G.; Khotimsky, V.S.; Volkov, V.V. Extraction of butanol from aqueous solutions by pervaporation through poly(1-trimethylsilyl-1-propyne). J. Membr. Sci. 2001, 186, 205–217. [Google Scholar] [CrossRef]
- Fadeev, A.G.; Kelley, S.; McMillan, J.; Selinskaya, Y.; Khotimsky, V.; Volkov, V.V. Effect of yeast fermentation by-products on poly[1-(trimethylsilyl)-1-propyne] pervaporative performance. J. Membr. Sci. 2003, 214, 229–238. [Google Scholar] [CrossRef]
- Pinnau, I.; Casillas, C.G.; Morisato, A.; Freeman, B.D. Long-term permeation properties of poly(1-trimethylsilyl-1-propyne) membranes in hydrocarbon—Vapor environment. J. Polym. Sci. Part B: Polym. Phys. 1997, 33, 1483–1490. [Google Scholar] [CrossRef]
- Fadeev, A.G.; Meagher, M.M.; Kelley, S.S.; Volkov, V.V. Fouling of poly[-1-(trimethylsilyl)-1-propyne] membranes in pervaporative recovery of butanol from aqueous solutions and ABE fermentation broth. J. Membr. Sci. 2000, 173, 133–144. [Google Scholar] [CrossRef]
- Zhang, G.; Cheng, H.; Pengcheng, S.; Zhang, X.; Zheng, J.; Yinghua, L.; Qinglin, L. PIM-1/PDMS hybrid pervaporation membrane for high-efficiency separation of n-butanol-water mixture under low concentration. Sep. Purif. Technol. 2019, 216, 83–91. [Google Scholar] [CrossRef]
- Žák, M.; Klepic, M.; Štastná, L.Č.; Sedláková, Z.; Vychodilová, H.; Hovorka, Š.; Friess, K.; Randová, A.; Brožová, L.; Jansen, J.C.; et al. Selective removal of butanol from aqueous solution by pervaporation with a PIM-1 membrane and membrane aging. Sep. Purif. Technol. 2015, 151, 108–114. [Google Scholar] [CrossRef]
- Zhou, H.; Jin, W. Membranes with Intrinsic Micro-Porosity: Structure, Solubility, and Applications. Membranes 2019, 9, 3. [Google Scholar] [CrossRef]
- Srinivasan, R.; Auvil, S.R.; Burban, P.M. Elucidating the mechanism(s) of gas transport in poly[1-(trimethylsilyl)-1-propyne] (PTMSP) membranes. J. Membr. Sci. 1994, 86, 67–86. [Google Scholar] [CrossRef]
- Lakshmanan, L.W.; Hopfenberg, H.; Chern, R. Sorption and transport of organic vapors in poly[1-(trimethylsilyl)-1-propyne]. J. Membr. Sci. 1990, 48, 321–331. [Google Scholar] [CrossRef]
- Masuda, T.; Isobe, E.; Higashimura, T. Polymerization of 1-(trimethylsilyl)-1-propyne by halides of niobium(V) and tantalum(V) and polymer properties. Macromolecules 1985, 18, 841–845. [Google Scholar] [CrossRef]
- Hasegawa, Y.; Kimura, K.; Nemoto, Y.; Nagase, T.; Kiyozumi, Y.; Nishide, T.; Mizukami, F. Real-time monitoring of permeation properties through polycrystalline MFI-type zeolite membranes during pervaporation using mass-spectrometry. Sep. Purif. Technol. 2008, 58, 386–392. [Google Scholar] [CrossRef]
- Bowen, T.; Wyss, J.; Noble, R.; Falconer, J. Measurement of diffusion through a zeolite membrane using isotopic-transient pervaporation. Microporous Mesoporous Mater. 2004, 71, 199–210. [Google Scholar] [CrossRef]
- Dudek, G.; Borys, P. A Simple Methodology to Estimate the Diffusion Coefficient in Pervaporation-Based Purification Experiments. Polymers 2019, 11, 343. [Google Scholar] [CrossRef] [Green Version]
- Gotō, A.; Takahashi, M.; Kuwahara, N.; Kubota, K. Numerical simulation of solvent diffusion for binary alcohol mixtures in polyacrylonitrile membrane. J. Membr. Sci. 1995, 99, 107–115. [Google Scholar] [CrossRef]
- Schafer, T.; Vital, J.; Crespo, J. Coupled pervaporation/mass spectrometry for investigating membrane mass transport phenomena. J. Membr. Sci. 2004, 241, 197–205. [Google Scholar] [CrossRef]
- Oh, K.; Koo, Y.; Jung, K. Characterization of a sheet membrane interface for sample introduction into a time-of-flight mass spectromete. Int. J. Mass Spectrom. 2006, 253, 65–70. [Google Scholar] [CrossRef]
- Johnson, R.; Cooks, R.; Allen, T.; Cisper, M.; Hemberger, P.H. Membrane introduction mass spectrometry: Trends and applications. Mass Spectrom. Rev 2000, 19, 1–37. [Google Scholar] [CrossRef]
- Shah, M.; Noble, R.; Clough, D. Measurement of sorption and diffusion in nonporous membranes by transient permeation experiments. J. Membr. Sci. 2007, 287, 111–118. [Google Scholar] [CrossRef]
- Vopicka, O.; Hynek, V.; Rabová, V. Measuring the transient diffusion of vapor mixtures through dense membranes. J. Membr. Sci. 2010, 350, 217–225. [Google Scholar] [CrossRef]
- Vopicka, O.; Radotínský, D.; Friess, K. Sorption of vapor mixtures of methanol and dimethyl carbonate in PDMS: Experimental study. Eur. Polym. J. 2015, 73, 480–486. [Google Scholar] [CrossRef]
- Vopicka, O.; Hynek, V.; Zgažar, M.; Friess, K.; Šípek, M. A new sorption model with a dynamic correction for the determination of diffusion coefficients. J. Membr. Sci. 2009, 330, 51–56. [Google Scholar] [CrossRef]
- Design Institute for Physical Properties, Sponsored by AICHE (2005; 2008; 2009; 2010). Dippr Project 801—Full Version. Design Institute for Physical Property Research/AICHE. Available online: http://knovel.com/web/portal/browse/display?_ext_knovel_display_bookid=1187&verticalid=0 (accessed on 12 August 2019).
- Nagai, K.; Higuchi, A.; Nakagawa, T. Gas permeability and stability of poly(1-trimethylsilyl-1-propyne-co-1-phenyl-1-propyne) membranes. J. Polym. Sci. Part B Polym. Phys. 1995, 33, 289–298. [Google Scholar] [CrossRef]
- Barker, J.J.; Treybal, R.E. Mass transfer coefficients for solids suspended in agitated liquids. AIChE J. 1960, 6, 289–295. [Google Scholar] [CrossRef]
- Vopicka, O.; Pilnácek, K.; Cíhal, P.; Friess, K. Sorption of methanol, dimethyl carbonate, methyl acetate, and acetone vapors in CTA and PTMSP: General findings from the GAB Analysis. J. Polym. Sci. Part B Polym. Phys. 2015, 54, 561–569. [Google Scholar] [CrossRef]
- Radotínský, D.; Vopicka, O.; Hynek, V.; Izák, P.; Friess, K. Aparatura Pro Stanovení Sorpce a Permeace Organických Par V Polymerech Pomocí InfraNoervené Spektroskopie. Chem. Listy 2015, 109, 619–624. (In Czech) [Google Scholar]
- Daynes, H.A. The Process of Diffusion through a Rubber Membrane. Proc. R. Soc. 1920, 97, 286–307. [Google Scholar] [CrossRef]
- Crank, J. The Mathematics of Diffusion, 2nd ed.; Oxford University Press: London, UK, 1975; pp. 69–88. [Google Scholar]
- Kappert, E.J.; Raaijmakers, M.J.; Tempelman, K.; Cuperus, F.P.; Ogieglo, W.; Benes, N.E. Swelling of 9 polymers commonly employed for solvent-resistant nanofiltration membranes: A comprehensive dataset. J. Membr. Sci. 2019, 569, 177–199. [Google Scholar] [CrossRef] [Green Version]
- Cihal, P.; Vopicka, O.; Pilnacek, K.; Poustka, J.; Friess, K.; Hajšlová, J.; Dobiáš, J.; Dole, P. Aroma scalping characteristics of polybutylene succinate based films. Polym. Test. 2015, 46, 108–115. [Google Scholar] [CrossRef]
- Torra, V.; Tachoire, H. Conduction Calorimeters Heat Transmission Systems with Uncertainties. J. Therm. Anal. Calorim. 1998, 52, 663–681. [Google Scholar] [CrossRef]
- LePage, W. Complex Variables and the Laplace Transform for Engineers; Dover Publications, Inc.: New York, NY, USA, 1961. [Google Scholar]
- Oberhettinger, F.; Badii, L. Tables of Laplace Transforms; Springer: Berlin, Germany, 1973. [Google Scholar]
- Baker, R.; Wijmans, J.; Huang, Y. Permeability, permeance and selectivity: A preferred way of reporting pervaporation performance data. J. Membr. Sci. 2010, 348, 346–352. [Google Scholar] [CrossRef]
- Renon, H.; Prausnitz, J. Local compositions in thermodynamic excess functions for liquid mixtures. AIChE J. 1968, 14, 135–144. [Google Scholar] [CrossRef]
- KoichiI, W.; Hitoshi, K. A correlation method for isobaric vapor–liquid and vapor–liquid–liquid equilibria data of binary systems. Fluid Phase Equilibria 2008, 266, 202–210. [Google Scholar]
- Nakanishi, K.; Odani, H.; Kurata, M.; Masuda, T.; Higashimura, T. Sorption of Alcohol Vapors in a Disubstituted Polyacetylene. Polym. J. 1987, 19, 293–296. [Google Scholar] [CrossRef] [Green Version]
- Tsarkov, S.; Khotimsky, V.; Budd, P.; Volkov, V.; Kukushkina, J.; Volkov, A. Solvent nanofiltration through high permeability glassy polymers: Effect of polymer and solute nature. J. Membr. Sci. 2012, 423, 65–72. [Google Scholar] [CrossRef]
- Claes, S.; Vandezande, P.; Mullens, S.; van Bael, M.K.; Maurer, F.H.J. Free volume expansion of Poly[1-(trimethylsilyl)-1-propyne] treated in supercritical carbon dioxide as revealed by positron annihilation lifetime spectroscopy. Macromolecules 2011, 44, 2766–2772. [Google Scholar] [CrossRef]
- Masuda, T.; Takatsuka, M.; Tang, B.-Z.; Higashimura, T. Pervaporation of organic liquid—Water mixtures through substituted polyacetylene membrane. J. Membr. Sci. 1990, 49, 69–83. [Google Scholar] [CrossRef] [Green Version]
- Dubreuil, M.; Vandezande, P.; van Hecke, W.; Porto-Carrero, W.; Dotremont, C. Study on aging/fouling phenomena and the effect of upstream nanofiltration on in-situ product recovery of n-butanol through poly[1-(trimethylsilyl)-1-propyne] pervaporation membranes. J. Membr. Sci. 2013, 447, 134–143. [Google Scholar] [CrossRef]
- Tuliakova, T.; Paschin, A.; Sedov, V. Yeast extracts as safety sources of vitamins, mineral substances and amino acids. Food Ind. 2004, 6, 2–4. (In Russian) [Google Scholar]




© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Talluri, V.P.; Patakova, P.; Moucha, T.; Vopicka, O. Transient and Steady Pervaporation of 1-Butanol–Water Mixtures through a Poly[1-(Trimethylsilyl)-1-Propyne] (PTMSP) Membrane. Polymers 2019, 11, 1943. https://doi.org/10.3390/polym11121943
Talluri VP, Patakova P, Moucha T, Vopicka O. Transient and Steady Pervaporation of 1-Butanol–Water Mixtures through a Poly[1-(Trimethylsilyl)-1-Propyne] (PTMSP) Membrane. Polymers. 2019; 11(12):1943. https://doi.org/10.3390/polym11121943
Chicago/Turabian StyleTalluri, VSSL Prasad, Petra Patakova, Tomas Moucha, and Ondrej Vopicka. 2019. "Transient and Steady Pervaporation of 1-Butanol–Water Mixtures through a Poly[1-(Trimethylsilyl)-1-Propyne] (PTMSP) Membrane" Polymers 11, no. 12: 1943. https://doi.org/10.3390/polym11121943