Viscosity Behavior of P(DAC-AM) with Serial Cationicity and Intrinsic Viscosity in Inorganic Salt Solutions
Abstract
1. Introduction
2. Experimental
2.1. Samples
2.2. Measurement of Apparent Viscosity
2.3. Methods
- (1)
- Determination of viscosity property of polymer in salt solutions
- (2)
- Assessment of the influencing factors on the viscosity property.
- (3)
- The interaction mechanism model of salt ions and polyelectrolyte molecules.
3. Results and Discussion
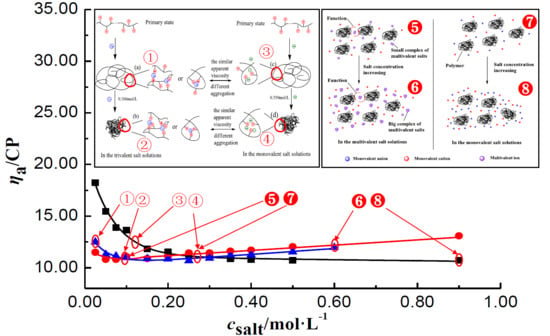
3.1. The General Regulations of ηa of P(DAC-AM) Samples in the Salt Solutions
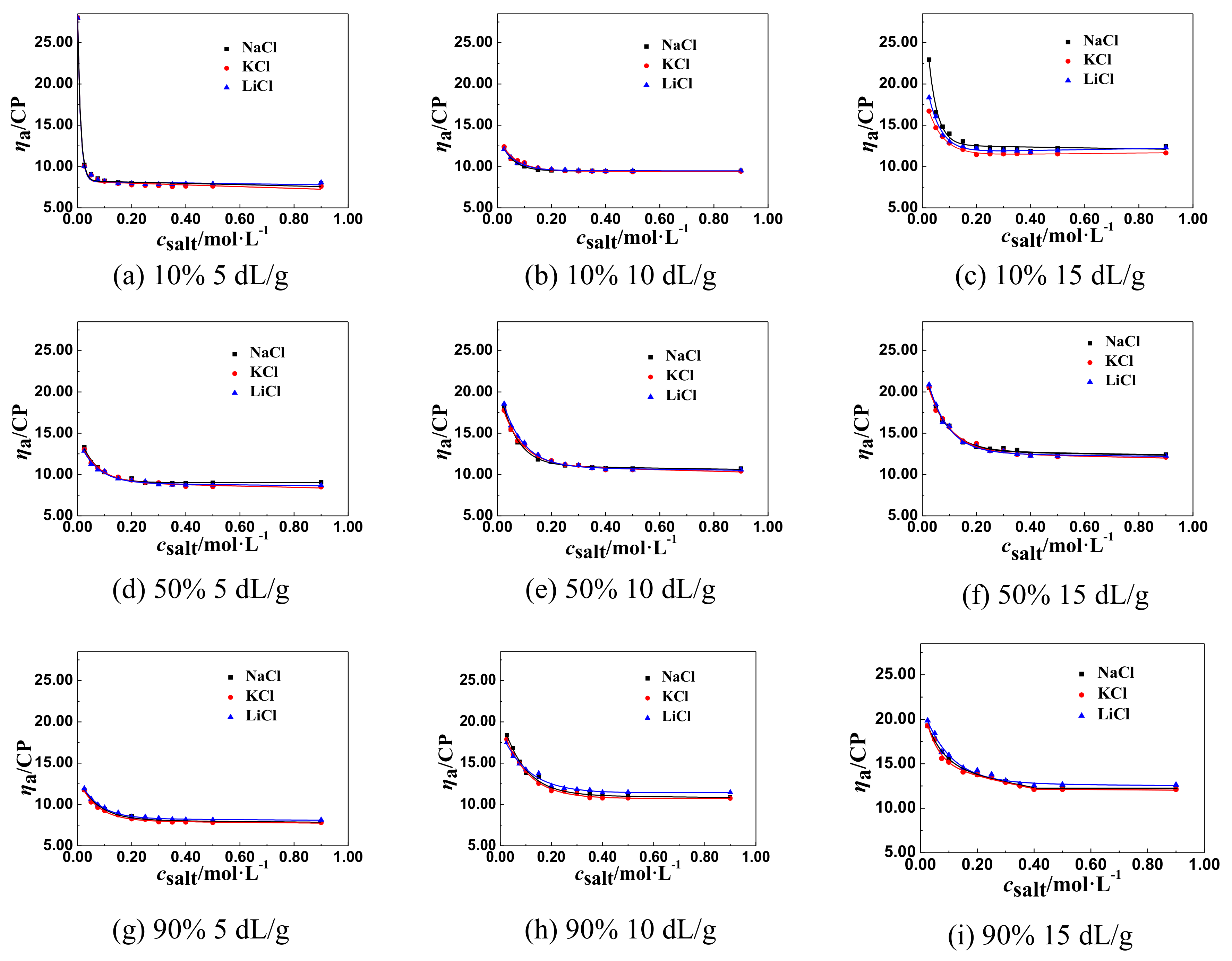
3.1.1. The Change Tendency of ηa of P(DAC-AM) Samples with Salt Concentration in the Monovalent Salt Solutions
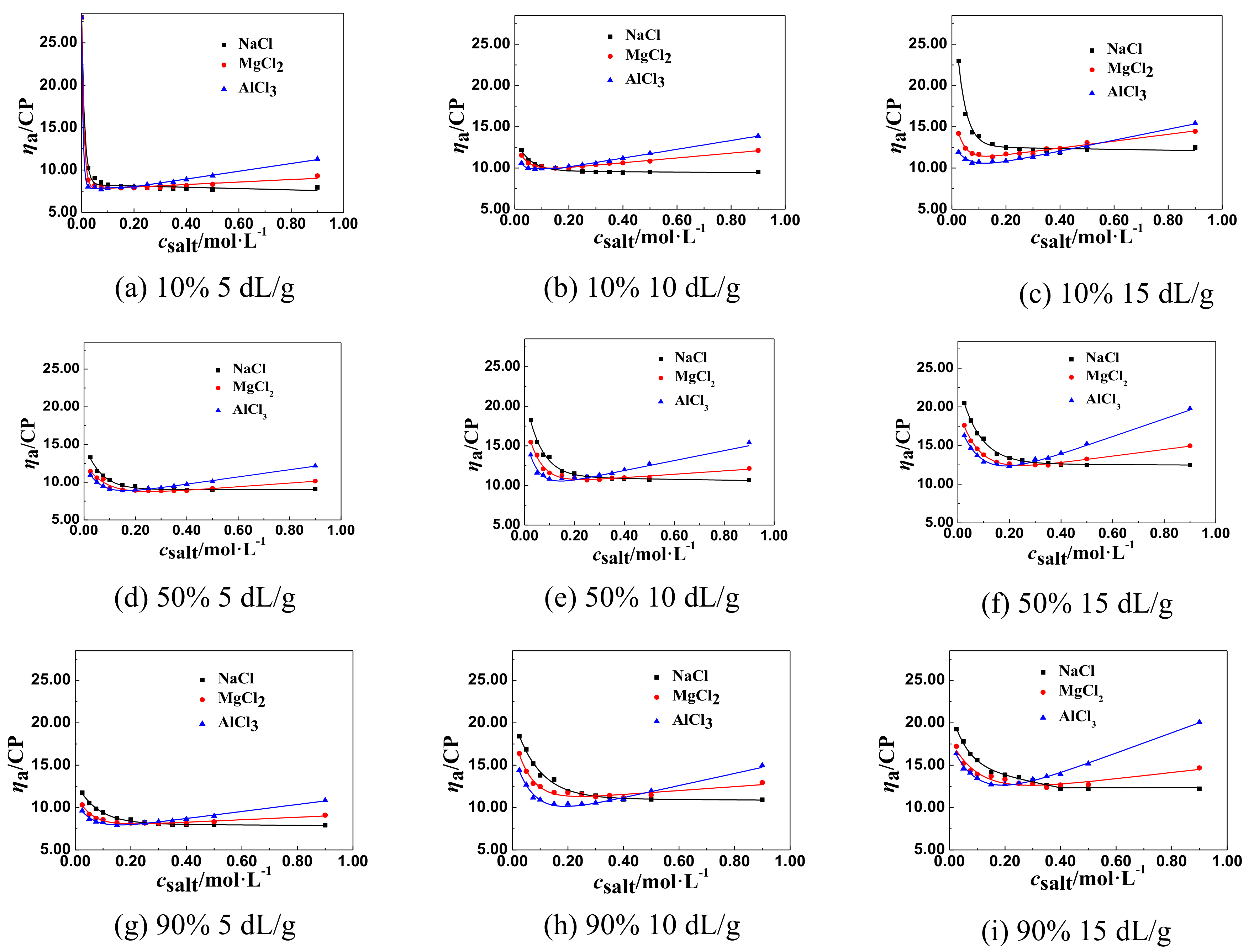
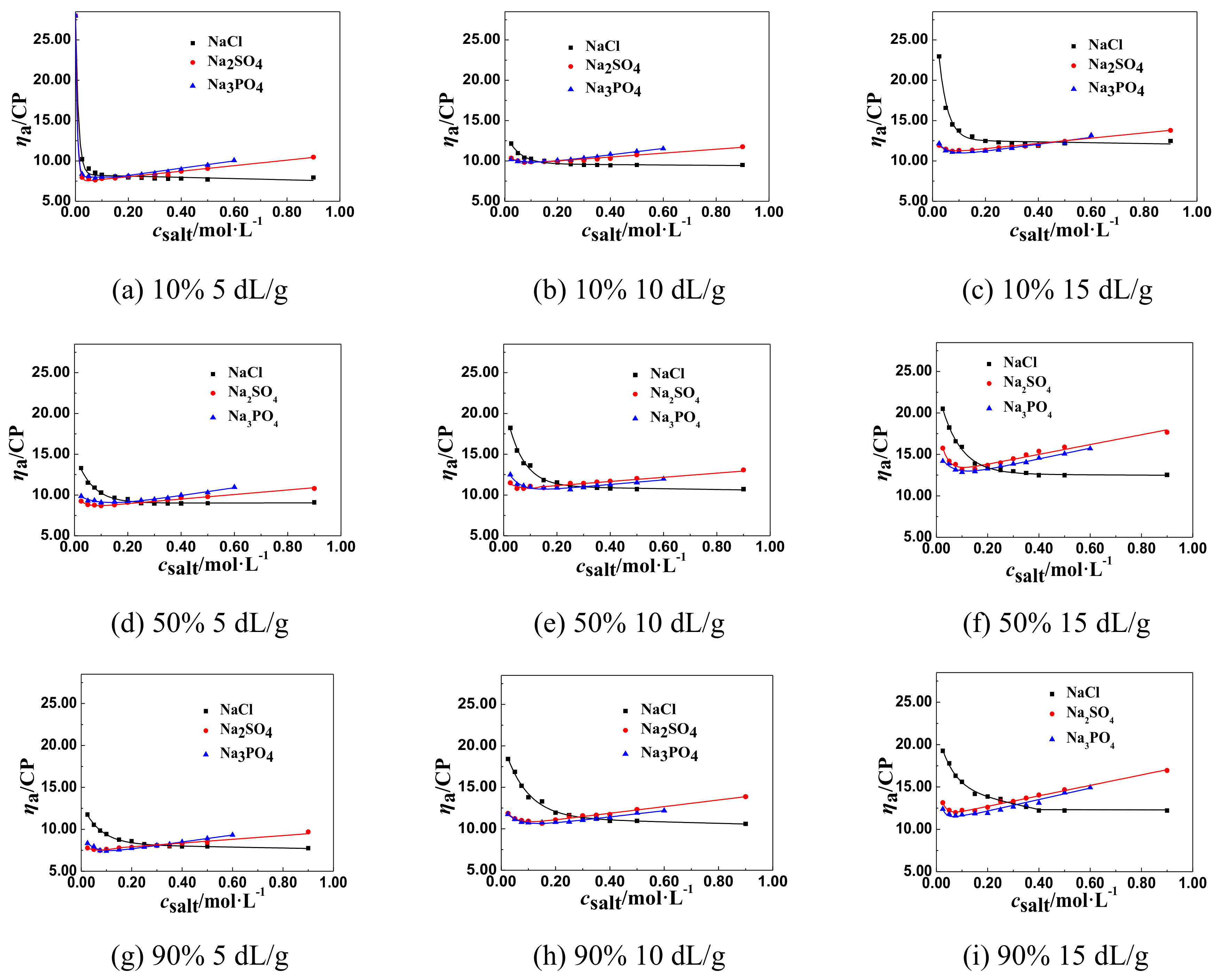
3.1.2. The Change Tendency of ηa of P(DAC-AM) Samples with Salt Concentration in the Multivalent Salt Solutions
The ηa of P(DAC-AM) Samples in the Multivalent Salt Solutions of Different Cations
The ηa of P(DAC-AM) Samples in the Multivalent Salt Solutions of Different Anions
The General Regulations of ηa of P(DAC-AM) Samples in the Multivalent Salt Solutions
3.2. The Inflection Point of the ηa of P(DAC-AM) Samples in Salt Solutions and Its Influencing Factors
3.2.1. The Inflection Point of the ηa of P(DAC-AM) Samples and Its Influencing Factors
3.2.2. The Effect of [η] on csalt and ηaL of P(DAC-AM) Samples
The Effect of [η] on ηaL
The Effect of [η] on csalt
3.2.3. The Effect of Cationicity on csalt and ηaL of P(DAC-AM) Samples
3.2.4. The Effect of Salt Ion Valence on csalt and ηaL of the P(DAC-AM) Samples
- (1)
- The effect of the cation radius. For the monovalent salts, like LiCl, NaCl, and KCl in their solutions, with the same anion and different cations, their csalt decreased a little with the increase of the cation radius of salts from LiCl to KCl which were in the same group and different period, comparing all corresponding csalt at the ηaL of the P(DAC-AM), as shown for example in Table 3. This might be due to the electronegativity of cations decreasing with their ionic radius increasing, and their ability of ionizing or releasing Cl− increasing [35], which resulted in the increase of the charge shielding effect of free Cl− on the P(DAC-AM) molecules, i.e., the csalt at the ηaL of the P(DAC-AM) exampled decreased.
- (2)
- The effect of cation valence. For the salt, as NaCl, MgCl2, and AlCl3 in their solutions, with the same anion and different valent cations, their csalt decreased with the increase of the cation valence, comparing all corresponding csalt at ηaL of the P(DAC-AM) as shown in Table 3. However, according to the anion and cation concentration, the corresponding cation concentration at the inflection point decreased from 0.350 to 0.162 mol/L with the valence of cation, Na+, Mg2+, and Al3+. However, the corresponding concentration of anion Cl− was 0.350, 0.452, and 0.486 mol/L, which indicated that the concentration of the counter ion, Cl− required to shrink the P(DAC-AM) molecule to the minimum volume in the MgCl2 and AlCl3 solutions was larger than that in the NaCl solution. Via a comparison with the effect of cation radius in the above (1), the reason might be easily understood that the ability of ionizing or releasing Cl− decreased with both the increase of the nuclear electronic number and the decrease of their ion radius of the cations in the same period, like Na+, Mg2+, and Al3+, which resulted in the decrease of the charge shielding effect of free Cl− on the positive charges in P(DAC-AM) molecules.
- (3)
- The effect of anion valence. For the salt, as NaCl, Na2SO4, and Na3PO4 in solutions, with the same cation and different valent anions, their csalt decreased clearly with the increase of the anion valence, comparing all the corresponding csalt at the ηaL of the P(DAC-AM) as shown in Table 3. According to the anion and cation concentration, it was clear to see that the csalt, equal to the anion concentration here as well, decreased sharply from 0.350 to 0.106 mol/L with the valence increase of anions, like Cl−, SO42−, and PO43−, which meant the increase of the charge number of the anions. In this time, however, the corresponding concentration of Na+ was 0.350, 0.212, and 0.318 mol/L. This illustrated that the salt cation, Na+, had no notable effect on the P(DAC-AM) molecules shrinking, and meanwhile indicated the charge number of the salt anion had a greater effect on the P(DAC-AM) molecules shrinking. The larger the charge number of the anion was, the P(DAC-AM) more easily the molecules shrank. This might be because the multivalent anion could shield more hanging cation groups in P(DAC-AM) molecules than the monovalent anion to make the polymer molecules shrink strongly [19,36].
3.3. Mechanism Analysis of Effect of Salt Concentration on the ηa of P(DAC-AM) Samples After Inflection Point
3.3.1. Speculation and Hypothesis of the Mechanism
3.3.2. Verifying Experiments and Results
- (1)
- The ηa of pure salt solutions of different concentrations.
- (2)
- The changes of the residual values of ηa of P(DAC-AM) after deducting the ηa of pure salt solutions.
- (3)
- Mechanism analysis and the numerical simulation of the interaction regulation.
3.4. Model of the Interaction Mechanism between Salt Ions and Polymer P(DAC-AM)
3.4.1. Model of the Interaction Mechanism Before and at the Inflection Point of ηa
3.4.2. Model of the Interaction Mechanism After the Inflection Point of the ηa
4. Conclusions
- (1)
- The values of apparent viscosity (ηa) of P(DAC-AM) with cationicity of 10%, 50%, and 90%, and intrinsic viscosity ([η]) of 5, 10, and 15 dL/g in the inorganic salt, such as NaCl, LiCl, KCl, MgCl2, AlCl3, Na2SO4, and Na3PO4, solutions firstly decreased sharply to their inflection point with the salt concentration, then tended to maintain their values with the increase of the monovalent salt concentration, or increased with the increase of the multivalent salt concentration after the inflection point.
- (2)
- It was discovered that the [η], cationicity, the kinds of salts, and the salt valence shared in the effect on the values of apparent viscosity (ηaL) at the inflection point of ηa of P(DAC-AM) samples, and corresponding salt concentrations (csalt). For the polymer P(DAC-AM), samples of the same cationicity, the csalt and ηaL increased with the [η] of P(DAC-AM) increasing. For the polymer P(DAC-AM) samples of the same [η], the csalt increased with the cationicity increasing, however, the cationicity of the polymer P(DAC-AM) samples hardly impacted the ηaL. For the same P(DAC-AM) sample, the csalt required to arrive at the inflection point of the ηa of P(DAC-AM) decreased with the increase of the cation or anion valence in the salt solution, and here, it decreased lower with the anion valence in the salt solutions.
- (3)
- From the mechanism hypothesis and verifying experiments, we discovered that the reasons for the increase of the ηa of P(DAC-AM) samples in the multivalent salt solutions after passing the inflection point were caused by not only the increase of ηa of the pure salt solutions resulting from the formation of the salt ion complexes, but also the interaction between the polymers and salt ion complexes. The linear quantitative relationship of the latter was developed and obtained. Meanwhile, we recognized that an increase in both the multivalent salt ion valences (meaning the volume and charge of the complexes) and the cationicity and [η] of the P(DAC-AM) samples (meaning the charge and the molecular size) were all enhanced in these interactions, in their individual form, and to different degrees.
- (4)
- Taking the monovalent ion and trivalent ion as examples, a mechanism model of the interaction between the P(DAC-AM) molecules and inorganic salt ions or their complexes was set, in order to illustrate the entire progress from the decrease of the ηa of P(DAC-AM) samples to their inflection point and after passing through the inflection point in the salt solutions. On the basis of the model, the dependence of the ηa of P(DAC-AM) samples on both the cationicity and [η] themselves, and the different kinds and valence of salt solutions were described and explained.
Author Contributions
Funding
Conflicts of Interest
References
- Jaeger, W.; Bohrisch, J.; Laschewsky, A. Synthetic polymers with quaternary nitrogen atoms - Synthesis and structure of the most used type of cationic polyelectrolytes. Prog. Polym. Sci. 2010, 35, 511–577. [Google Scholar] [CrossRef]
- Siyam, T. Development of acrylamide polymers for the treatment of waste water. Des. Monomers Polym. 2001, 4, 107–168. [Google Scholar] [CrossRef]
- Jia, X. Researches on the Preparation, Polymerization Mechanism and Relationships Between Structure, Properties and Performances of Poly (Dimethyldiallylammonium Chloride); Nanjing University of Science and Technology: Nanjing, China, 2010. [Google Scholar]
- Zhang, B.H.; Zhu, C.Y.; Guo, T.Y. Modern Polymer Science; Chemical Industry Press: Beijing, China, 2006; pp. 318–342. [Google Scholar]
- Ghimici, L.; Bercea, M.; Dragan, E.S. Rheological Behavior of Some Cationic Polyelectrolytes. J. Macromol. Sci. 2009, 48, 1011–1024. [Google Scholar] [CrossRef]
- Velazquezgarcia, A.I.; Cadenaspliego, G.; Riveravallejo, C.C. Influence of Surfactant and Salt Concentration on the Rheological Properties of Three Different Microstructures of Associative Polyelectrolytes Obtained by Solution Polymerization. J. Mod. Phys. 2014, 5, 1387–1396. [Google Scholar] [CrossRef][Green Version]
- Chimamkpam, T.O.; Rasteriro, M.G.; Garia, F.A.P.; Antunes, E.; Ferreira, P.; Hunkeler, D.; Wandrey, C. Solution viscosity and flocculation characteristics of linear polymeric flocculants in various media. Chem. Eng. Res. Des. 2011, 89, 1037–1044. [Google Scholar] [CrossRef]
- Hu, Z.Q.; Ma, X.P.; Wang, N.S. Study on the Properties of Bicopolymer P (DMC-AM) solution. J. Yangtze Univ. Nat. Sci. Ed. Sci. Eng. 2007, 4, 51–54. [Google Scholar]
- Delgado, J.D.; Schlenoff, J.B. Static and dynamic solution behavior of a polyzwitterion using a Hofmeister salt series. Macromolecules 2017, 50, 4454–4464. [Google Scholar] [CrossRef]
- Khan, M.B. Rheological behavior of polyacrylamide solution in the presence of cationic Gemini surfactants/conventional surfactants. Asia Pac. J. Chem. Eng. 2017, 12, 671–678. [Google Scholar] [CrossRef]
- Carrillo, J.Y.; Dobrynin, A.V. Polyelectrolytes in Salt Solutions: Molecular Dynamics Simulations. Macromolecule 2011, 44, 5798–5816. [Google Scholar] [CrossRef]
- McAfee, A.S.; Zhang, H.X.; Annunziata, O. Amplification of Salt-Induced Polymer Diffusiophoresis by Increasing Salting-Out Strength. Langmuir 2014, 30, 12210–12219. [Google Scholar] [CrossRef]
- Ye, T.; Song, Y.H.; Zheng, Q. Salt response and rheological behavior of acrylamide-sulfobetaine copolymer. Colloid Polym. Sci. 2016, 294, 389–397. [Google Scholar] [CrossRef]
- Lundberg, R.D.; Bailey, F.E.; Callard, R.W. Interactions of Inorganic Salts with Poly (ethylene Oxide). J. Polym. Sci. 1966, 4, 1563–1577. [Google Scholar] [CrossRef]
- Podlas, T.J.; Ander, P. Interactions of Sodium and Potassium Ions with Sodium and Potassium Alginate in Aqueous Solution with and without Added Salt. Macromolecules 1970, 3, 154–157. [Google Scholar] [CrossRef]
- Gemma, G.G.; Tomasz, K.; Alina, M.A. Monitoring the synthesis and properties of copolymeric polycations. J. Phys. Chem. B 2008, 112, 14597–14608. [Google Scholar]
- Francois, J.; Truong, N.D.; Medjahdi, G.; Mestdagh, M.M. Aqueous solutions of acrylamide-acrylic acid copolymers: Stability in the presence of alkalinoearth cations. Polymer 1997, 38, 6115–6127. [Google Scholar] [CrossRef]
- Wen-Hong, L.; LeonYu, T.; Hsiu-Li, L. Shear thickening behavior of dilute poly (diallyl dimethyl ammonium chloride) aqueous solutions. Polymer 2007, 48, 4152–4165. [Google Scholar]
- Liu, X.L.; Wang, Y.; Lu, Z.Y.; Chen, Q.; Feng, Y. Effect of inorganic salts on viscosifying behavior of a thermoassociative water-soluble terpolymer based on 2-acrylamido-methylpropane sulfonic acid. J. Appl. Polym. Sci. 2012, 125, 4041–4048. [Google Scholar] [CrossRef]
- Liu, L.H.; Cao, J.; Ling, Y.L. Synthesis and Solution Properties of Poly (diallylethylbenzyl ammonium chloride). J. Funct. Polym. 2011, 24, 191–197. [Google Scholar]
- Liu, Y.Y. The Synthesis of New Polymer Suitable for High Temperature and High Salt of Oilreservoir and Its Development; Southwest Oilfield University: ChengDu, China, 2011. [Google Scholar]
- Wei, J.J.; Hoagland, D.A.; Zhang, G.Y.; Su, Z. Effect of divalent counterions on polyelectrolyte multilayer properties. Macromolecules 2016, 49, 1790–1797. [Google Scholar] [CrossRef]
- Dong, D.P.; Hooper, J.B.; Bedrov, D. Structural and Dynamical Properties of Tetraalkylammonium Bromide Aqueous Solutions: A Molecular Dynamics Simulation Study Using a Polarizable Force Field. J. Phys. Chem. B 2017, 121, 4853–4863. [Google Scholar] [CrossRef]
- Guo-liang, M.; Qing-xiang, L.; Ke-hui, L.; Xue-song, C.; Gang, L.I. Interaction of sodium dodecylsulfate with aqueous soluten of copolymer of acrylamide and acryloyloxyethy trimethyl ammonium chloride. J. Daqing Pet. Ist. 2004, 28, 38–43. [Google Scholar]
- Huang, P.; Ye, L. In situ polymerization of cationic polyacrylamide/montmorillonite composites and its flocculation characteristics. J. Thermoplast. Compos. Mater. 2016, 29, 58–73. [Google Scholar] [CrossRef]
- Rattanakawin, C.; Hogg, R. Viscosity behavior of polymeric flocculant solutions. Miner. Eng. 2007, 20, 1033–1038. [Google Scholar] [CrossRef]
- Zhang, S.Y.; Xia, H.L. Physical Chemistry; Medical Science and Technology Press: Beijing, China, 2014; pp. 289–303. [Google Scholar]
- Zhang, Y.J.; Chen, T.T. The synthesis of P(DAC-AM) with high intrinsic viscosity and serial cationicity. CN Patent 201910073959.0, 25 January 2019. [Google Scholar]
- Li, X.X.; Zhang, Y.J.; Zhao, X.L. The Characteristics of Sludge from Enhanced Coagulation Processes usingPAC/PDMDAAC Composite Coagulants in treatment of Micro-Polluted Raw Water. Sep. Purif. Technol. 2015, 147, 125–131. [Google Scholar] [CrossRef]
- Ghimici, L.; Dragan, S. Behaviour of cationic polyelectrolytes upon binging of electrolytes: Effects of polycation structure, counterions and nature of the solvent. Colloid Polym. Sci. 2002, 280, 130–134. [Google Scholar] [CrossRef]
- Santo, K.P.; Vishnyakov, A.; Kumar, R.; Neimark, A.V. Elucidating the Effects of Metal Complexesation on Morphological and Rheological Properties of Polymer Solutions by a Dissipative Particle Dynamics Model. Macromolecules 2018, 51, 4987–5000. [Google Scholar] [CrossRef]
- He, J.J. An Introduction to Polymer Solution Theories; Lanzhou University Press: Lanzhou, China, 1987. [Google Scholar]
- Dong, Y.M.; Zhu, P.P.; Xu, S.A. Polymer Structure and Properties; East China University of Science and Technology Press: Shanghai, China, 2010. [Google Scholar]
- Wang, Y. Water Soluble Polymer; Chemical Industry Press: Beijing, China, 2017. [Google Scholar]
- Sun, T.; Zhang, X. Inorganic Chemistry; Metallurgical Industry Press: Beijing, China, 2011; pp. 219–234. [Google Scholar]
- Huang, P.L.; Tian, H.Z. Basic Elemental Chemistry; Beijing Normal University Publication Hose: Beijing, China, 1994; pp. 230–248. [Google Scholar]
- Gadkari, P.V.; Tu, S.; Chiyarda, K.; Reaney, M.J.T.; Ghosh, S. Rheological characterization of fenugreek gum and comparison with other galactomannans. Int. J. Biol. Macromol. 2018, 119, 486–495. [Google Scholar] [CrossRef]
- Tang, H.X. Inorganic Polymer Flocculation Theory and Flocculants; China Building Industry Press: Beijing, China, 2006; pp. 58–59. [Google Scholar]
- Chen, J.F.; Tan, G.X. Production and Application of Phosphate; Chengdu University of Science and Technology Press: ChengDu, China, 1989; pp. 36–39. [Google Scholar]
- Francis, P.S. Solution properties of water-soluble polymers. I. Control of aggregation of sodium carboxymethylcellulose (CMC) by choice of solvent and/or electrolyte. J. Appl. Polym. Sci. 1961, 5, 261–270. [Google Scholar] [CrossRef]
- Lopez, C.G.; Richtering, W. Influence of divalent counterions on the solution rheology and supramolecular aggregation of carboxymethyl cellulose. Cellulose 2019, 26, 1517–1534. [Google Scholar] [CrossRef]
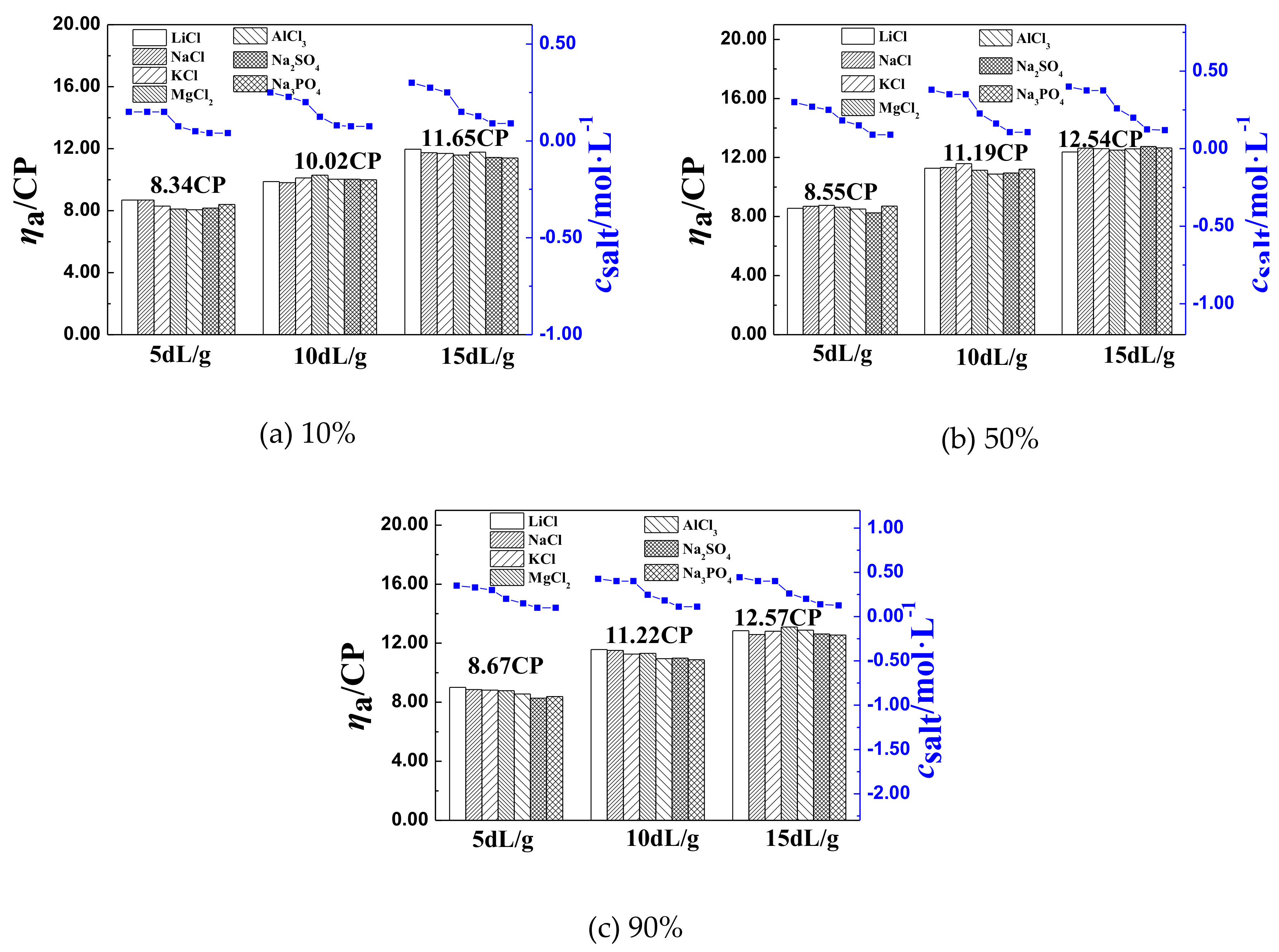
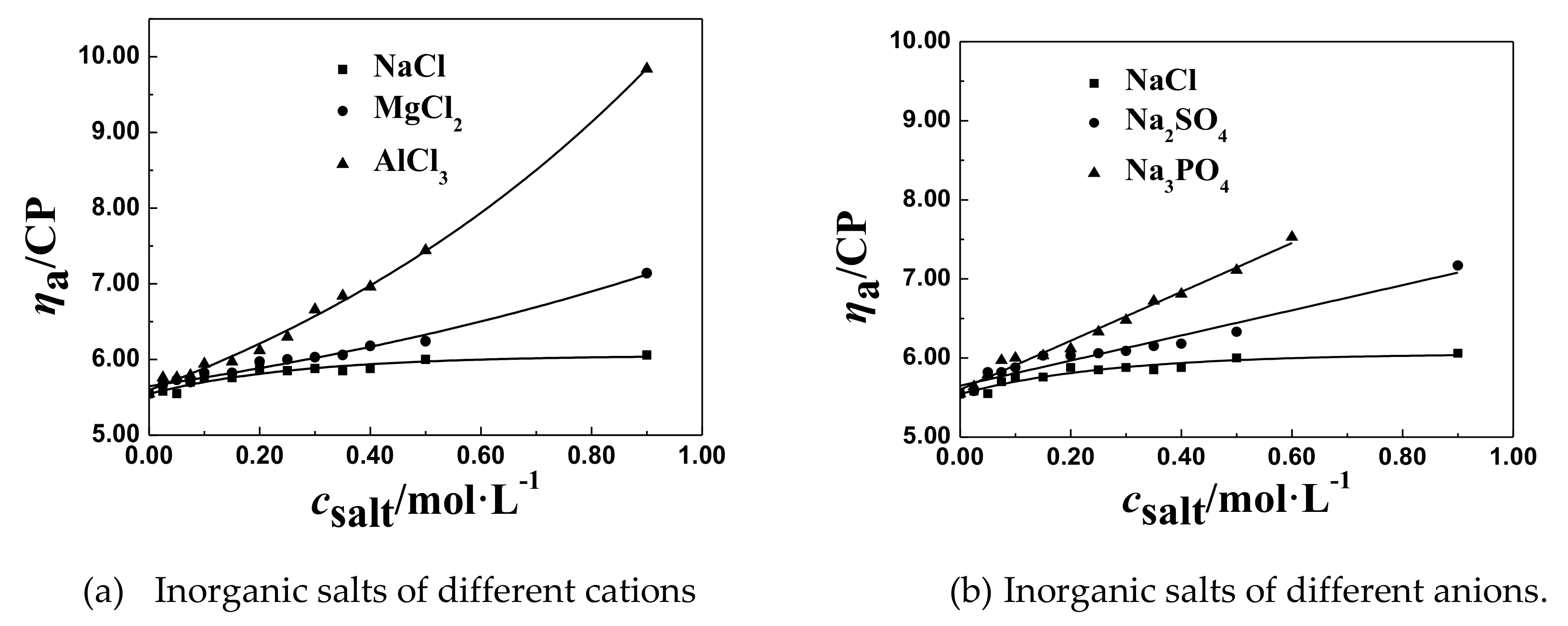
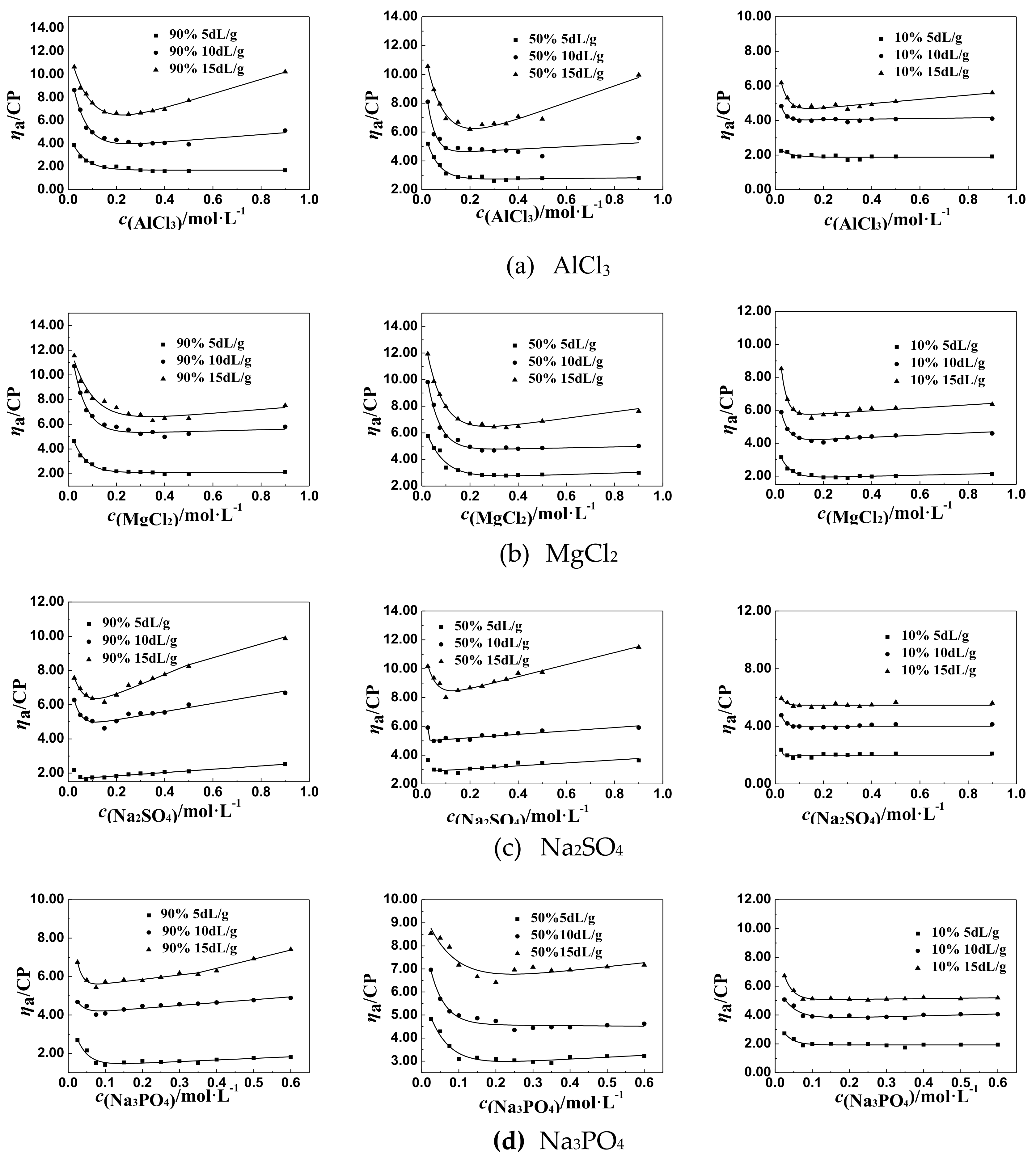
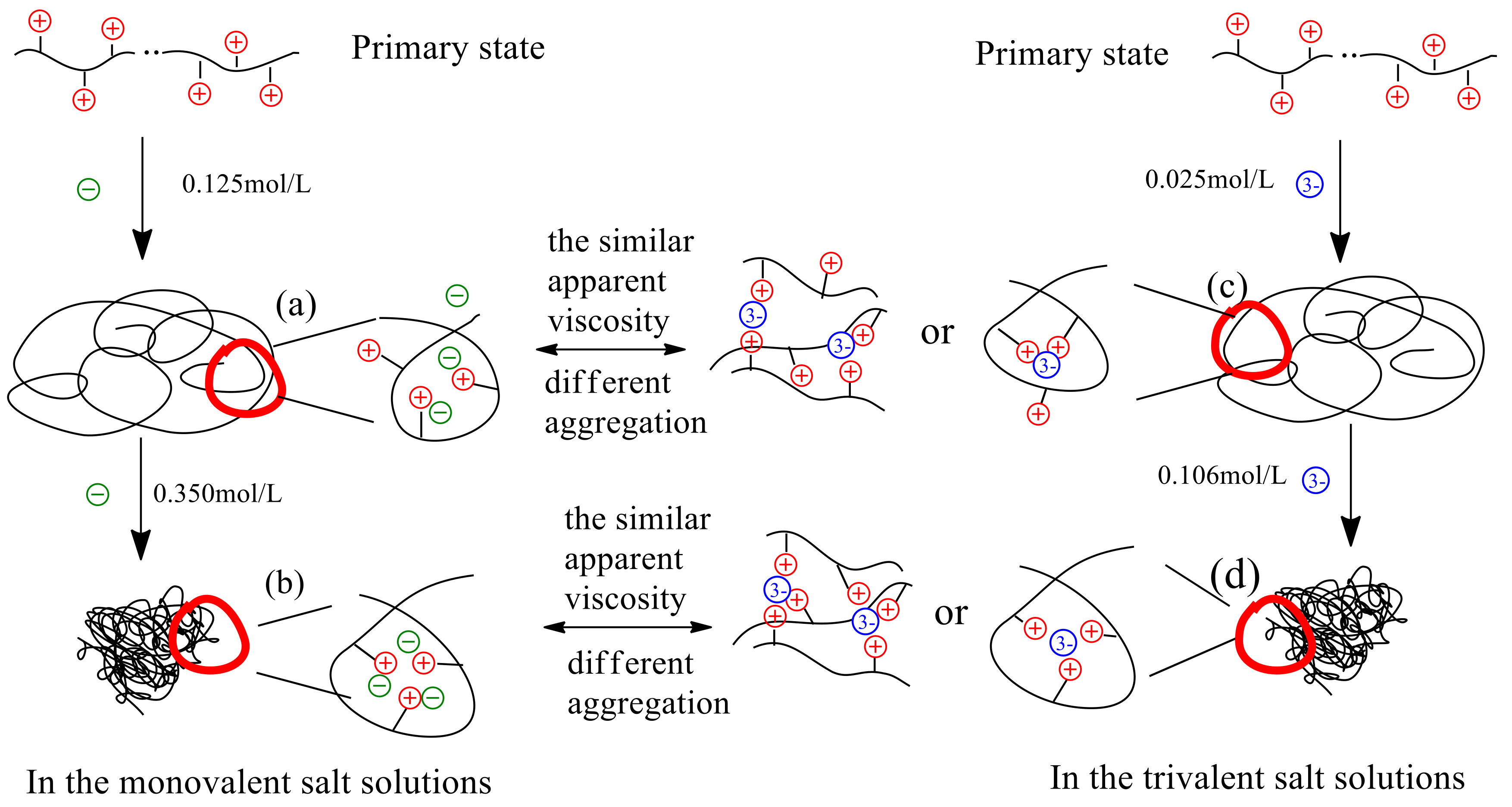

| No. | [η]/dL·g−1 | ηaL/CP | Relative Average Deviation/% | |||
|---|---|---|---|---|---|---|
| 10% | 50% | 90% | Average ηa | |||
| 1 | 5 | 8.34 | 8.55 | 8.67 | 8.52 | 1.41 |
| 2 | 10 | 10.02 | 11.19 | 11.22 | 10.81 | 4.87 |
| 3 | 15 | 11.65 | 12.54 | 12.57 | 12.25 | 3.29 |
| [η]/ dL·g−1 | Cationicity/ % | csalt/ (mol•L−1) | ||||||
|---|---|---|---|---|---|---|---|---|
| LiCl | NaCl | KCl | MgCl2 | AlCl3 | Na2SO4 | Na3PO4 | ||
| 5 | 10 | 0.150 | 0.150 | 0.150 | 0.075 | 0.050 | 0.040 | 0.040 |
| 50 | 0.300 | 0.270 | 0.250 | 0.182 | 0.150 | 0.090 | 0.090 | |
| 90 | 0.350 | 0.328 | 0.300 | 0.200 | 0.150 | 0.100 | 0.100 | |
| 10 | 10 | 0.250 | 0.227 | 0.200 | 0.124 | 0.080 | 0.075 | 0.075 |
| 50 | 0.380 | 0.350 | 0.350 | 0.226 | 0.162 | 0.106 | 0.106 | |
| 90 | 0.425 | 0.400 | 0.400 | 0.247 | 0.182 | 0.112 | 0.112 | |
| 15 | 10 | 0.300 | 0.274 | 0.250 | 0.150 | 0.127 | 0.090 | 0.090 |
| 50 | 0.400 | 0.375 | 0.375 | 0.260 | 0.200 | 0.124 | 0.119 | |
| 90 | 0.445 | 0.400 | 0.400 | 0.260 | 0.200 | 0.139 | 0.127 | |
| Salt Names | ηaL = 11.19 CP | ||
|---|---|---|---|
| csalt/(mol·L−1) | c(cation)/(mol·L−1) | c(anion)/(mol·L−1) | |
| LiCl | 0.380 | 0.380 | 0.380 |
| NaCl | 0.350 | 0.350 | 0.350 |
| KCl | 0.350 | 0.350 | 0.350 |
| MgCl2 | 0.226 | 0.226 (<0.350) | 0.452 (>0.350) |
| AlCl3 | 0.162 | 0.162 (<0.350) | 0.486 (>0.350) |
| Na2SO4 | 0.106 | 0.212 (<0.350) | 0.106 (<0.350) |
| Na3PO4 | 0.106 | 0.318 (<0.350) | 0.106 (<0.350) |
| Salts | [η]/ (dL·g−1) | The Fitted Equations | |||||
|---|---|---|---|---|---|---|---|
| 90% | R2 | 50% | R2 | 10% | R2 | ||
| AlCl3 | 5 | y = 0.17x + 1.53 | 0.9547 | y = 0.06x + 2.76 | 0.9901 | y = 0.06x + 1.90 | 0.9592 |
| 10 | y = 2.19x + 3.11 | 0.9618 | y = 1.95x + 3.82 | 0.9960 | y = 0.06x + 4.05 | 0.9286 | |
| 15 | y = 6.45x + 4.44 | 0.9968 | y = 6.02x + 4.50 | 0.9691 | y = 1.35x + 4.40 | 0.9918 | |
| MgCl2 | 5 | y = 0.41x + 1.78 | 0.9949 | y = 0.36x + 2.67 | 0.9695 | y = 0.30x + 1.86 | 1.0000 |
| 10 | y = 1.56x + 4.39 | 0.9791 | y = 0.41x + 4.65 | 0.9835 | y = 0.34x + 4.28 | 0.9592 | |
| 15 | y = 2.25x + 5.48 | 0.9286 | y = 2.17x + 5.69 | 0.9495 | y = 0.49x + 5.91 | 0.9887 | |
| Na2SO4 | 5 | y = 0.94x + 1.66 | 0.9662 | y = 0.47x + 3.21 | 0.9945 | y = 0.08x + 2.04 | 0.9945 |
| 10 | y = 2.12x + 4.81 | 0.9110 | y = 0.75x + 5.24 | 0.9737 | y = 0.06x + 4.09 | 0.9592 | |
| 15 | y = 4.18x + 6.12 | 0.9984 | y = 3.81x + 8.02 | 0.9488 | y = 0.39x + 5.35 | 0.9235 | |
| Na3PO4 | 5 | y = 0.60x + 1.45 | 0.9286 | y = 0.25x + 3.08 | 0.9737 | y = 0.10x + 1.90 | 1.0000 |
| 10 | y = 1.20x + 4.17 | 1.0000 | y = 0.75x + 4.18 | 0.9737 | y = 0.15x + 3.96 | 0.9286 | |
| 15 | y = 5.55x + 4.11 | 0.9879 | y = 1.05x + 6.55 | 0.9629 | y = 0.30x + 5.01 | 0.9286 | |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, T.; Fu, X.; Zhang, L.; Zhang, Y. Viscosity Behavior of P(DAC-AM) with Serial Cationicity and Intrinsic Viscosity in Inorganic Salt Solutions. Polymers 2019, 11, 1944. https://doi.org/10.3390/polym11121944
Chen T, Fu X, Zhang L, Zhang Y. Viscosity Behavior of P(DAC-AM) with Serial Cationicity and Intrinsic Viscosity in Inorganic Salt Solutions. Polymers. 2019; 11(12):1944. https://doi.org/10.3390/polym11121944
Chicago/Turabian StyleChen, Tingting, Xingqin Fu, Luzi Zhang, and Yuejun Zhang. 2019. "Viscosity Behavior of P(DAC-AM) with Serial Cationicity and Intrinsic Viscosity in Inorganic Salt Solutions" Polymers 11, no. 12: 1944. https://doi.org/10.3390/polym11121944
APA StyleChen, T., Fu, X., Zhang, L., & Zhang, Y. (2019). Viscosity Behavior of P(DAC-AM) with Serial Cationicity and Intrinsic Viscosity in Inorganic Salt Solutions. Polymers, 11(12), 1944. https://doi.org/10.3390/polym11121944