Evaluation of the Properties, Gas Permeability, and Selectivity of Mixed Matrix Membrane Based on Polysulfone Polymer Matrix Incorporated with KIT-6 Silica
Abstract
1. Introduction
2. Materials and Methods
2.1. Synthesis of KIT-6 Silica
2.2. Fabrication of the Membranes
2.3. KIT-6 Characterization
2.4. Membranes Characterization
2.5. Gas Permeability and Selectivity Study
3. Results and Discussion
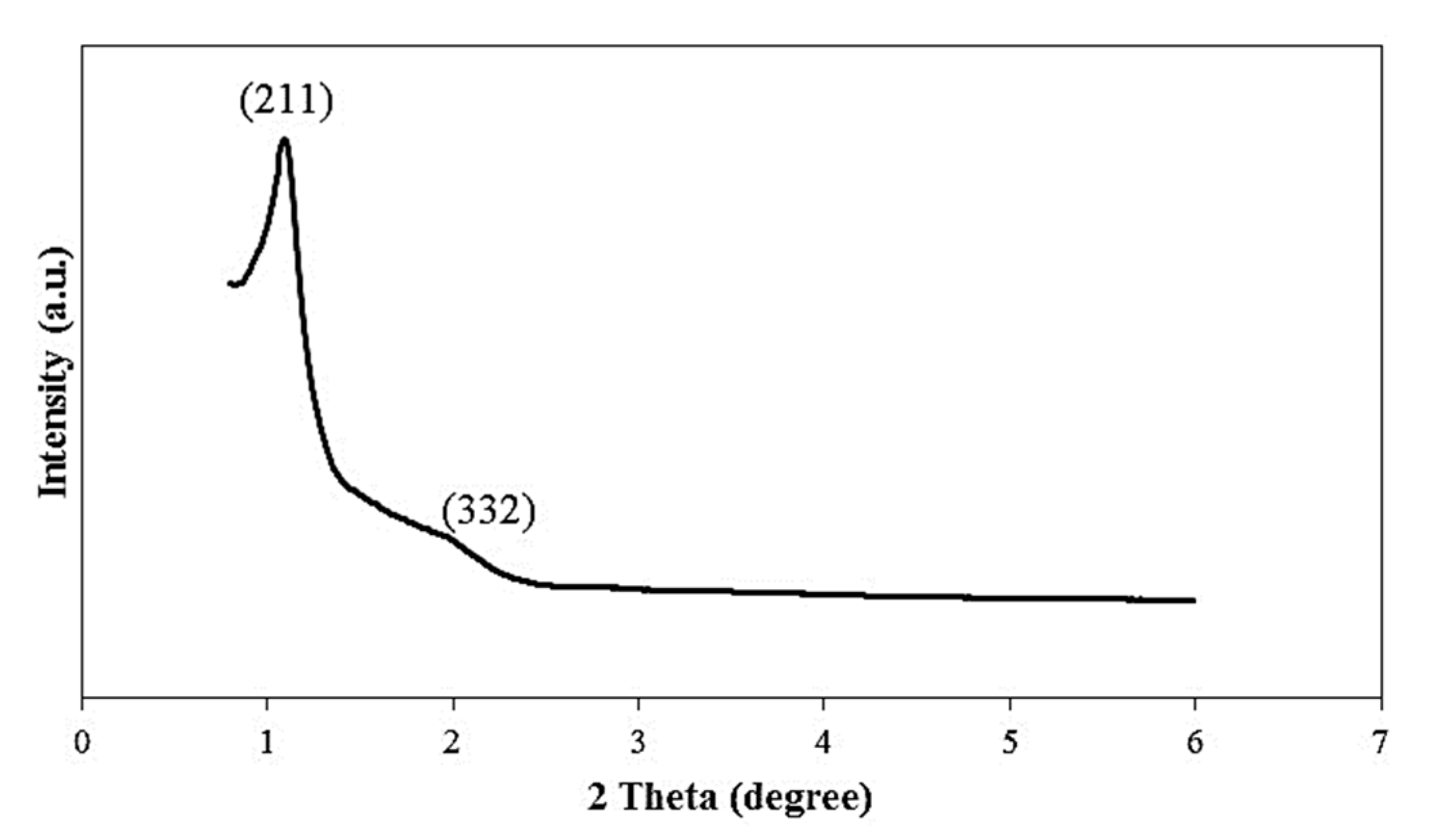
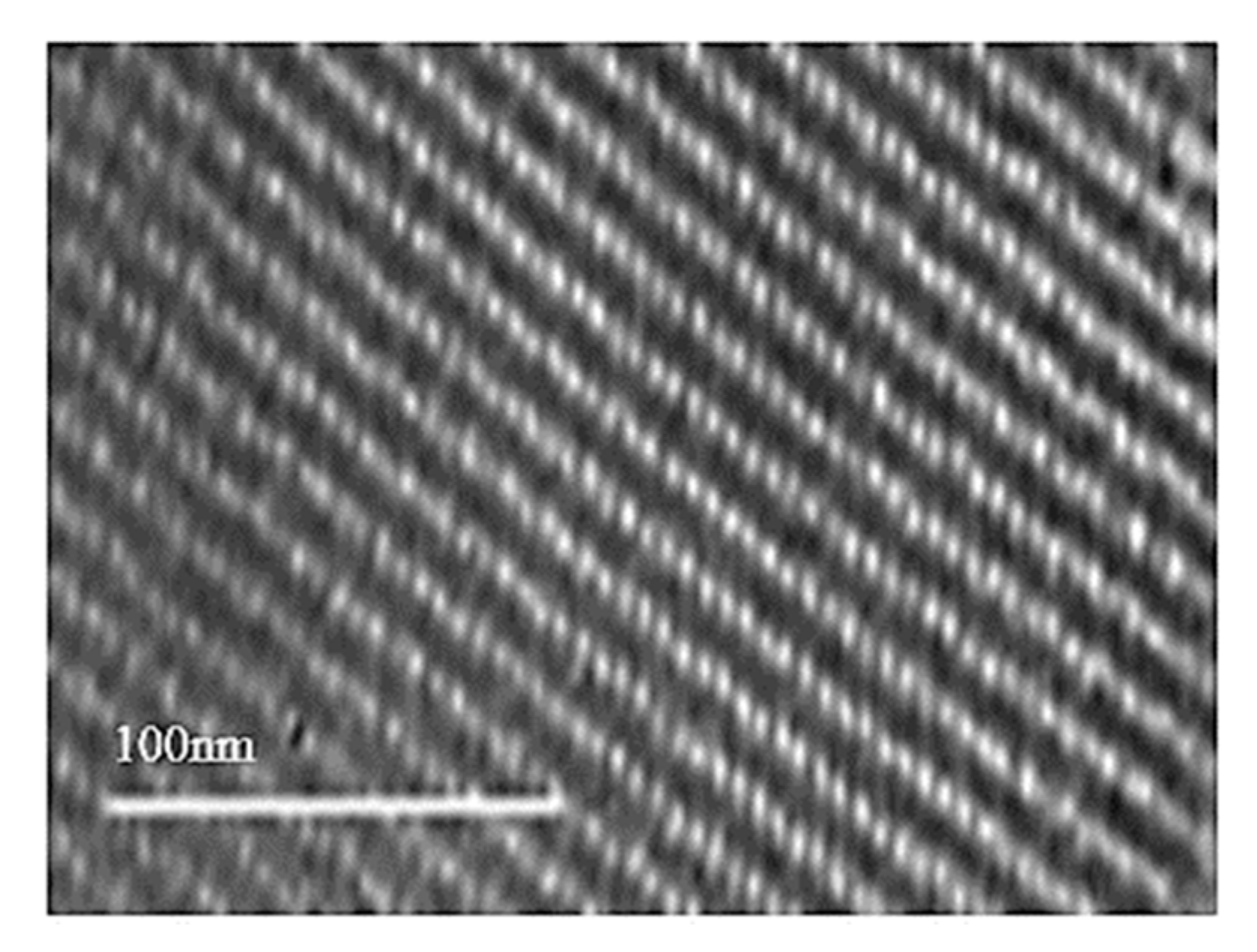
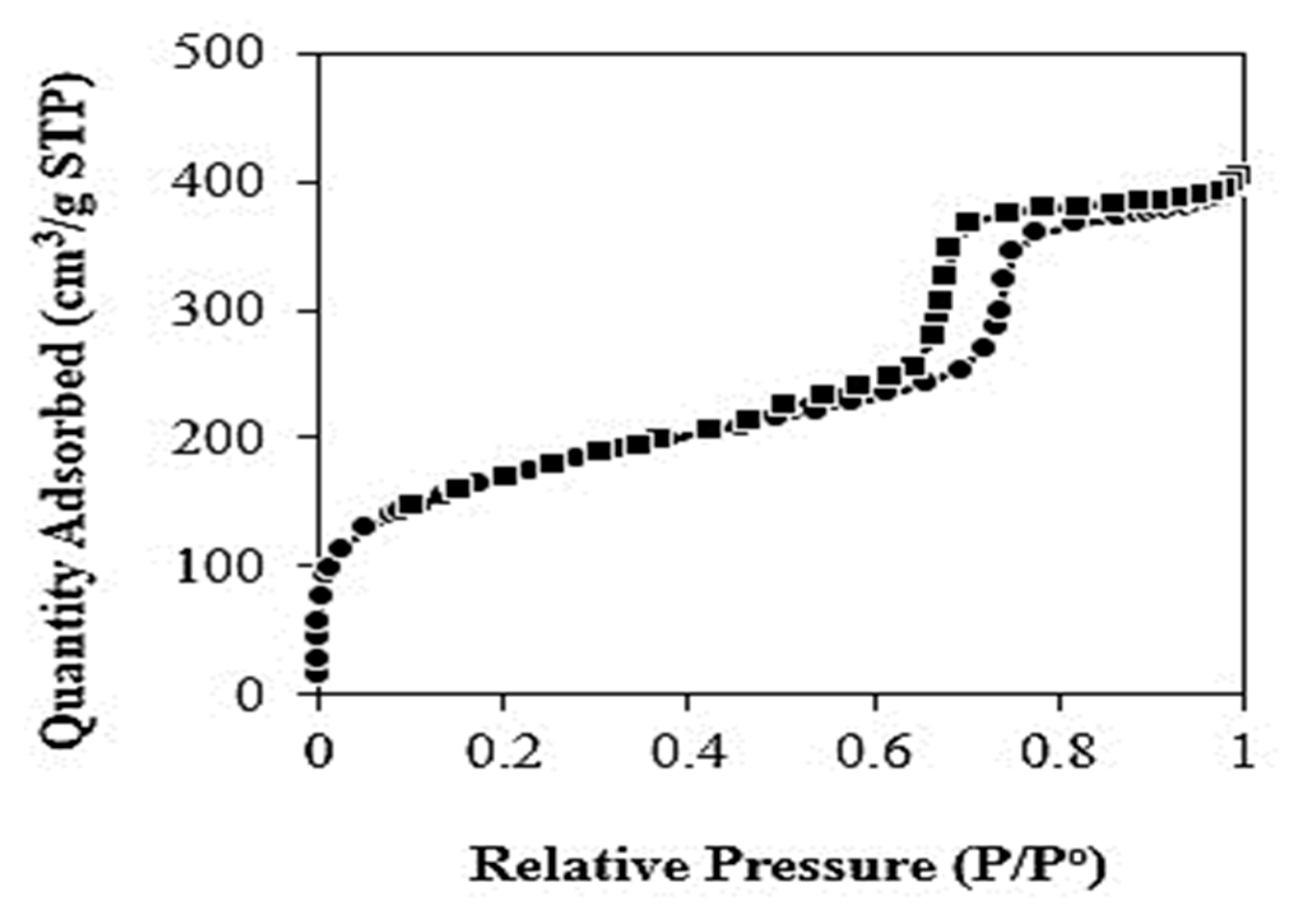
3.1. Characterization of KIT-6
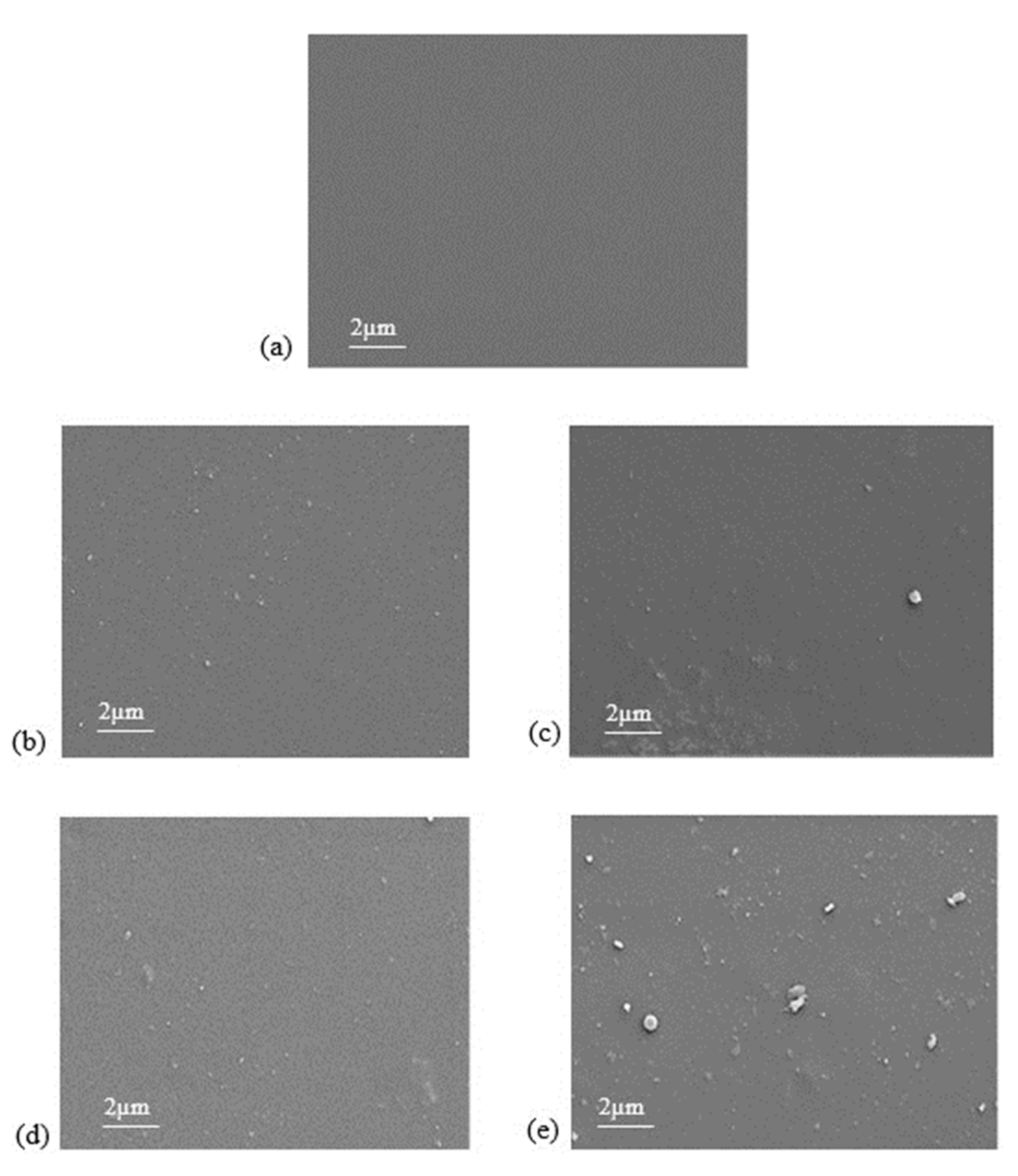
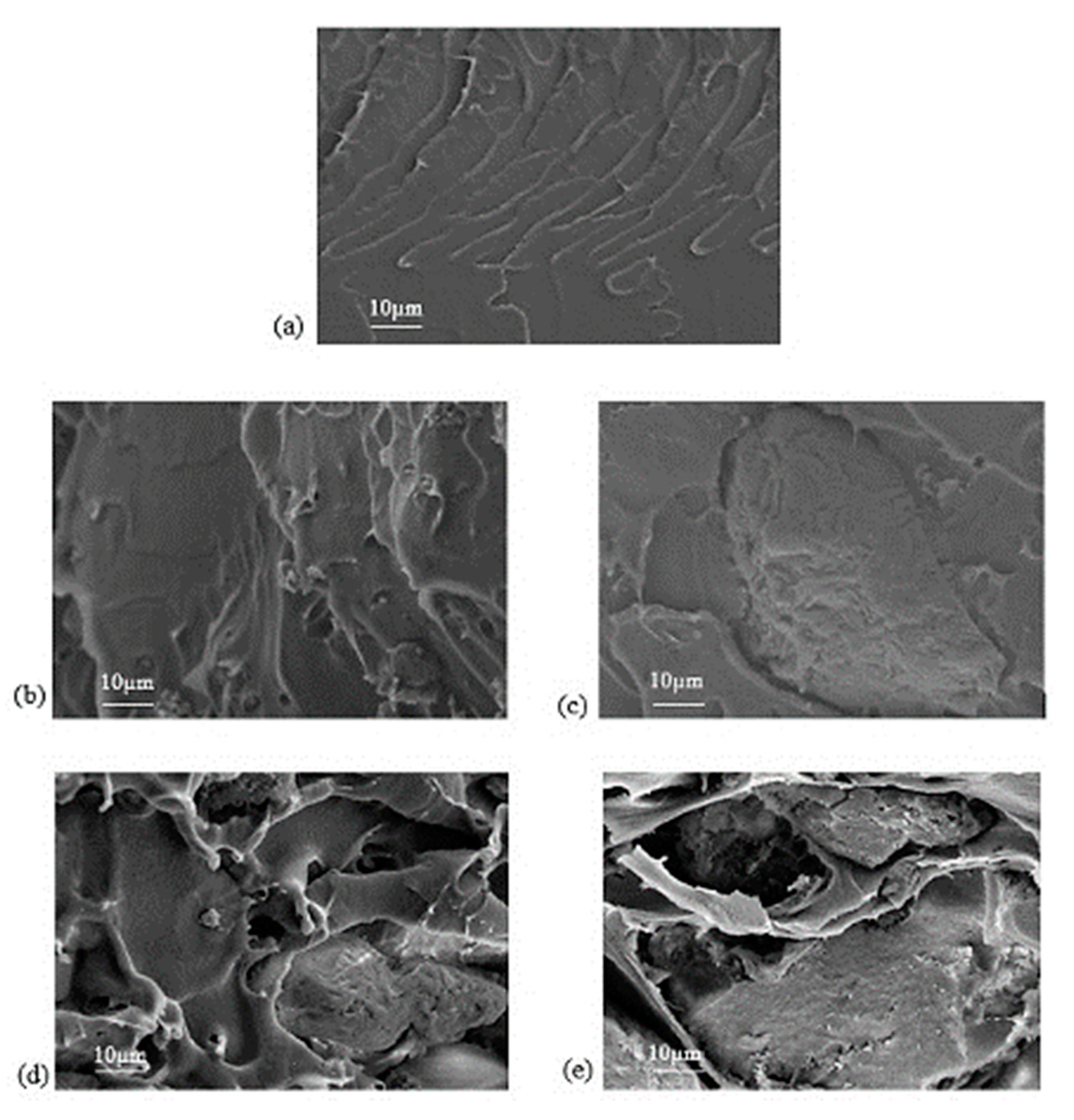
3.2. Membrane Characterization
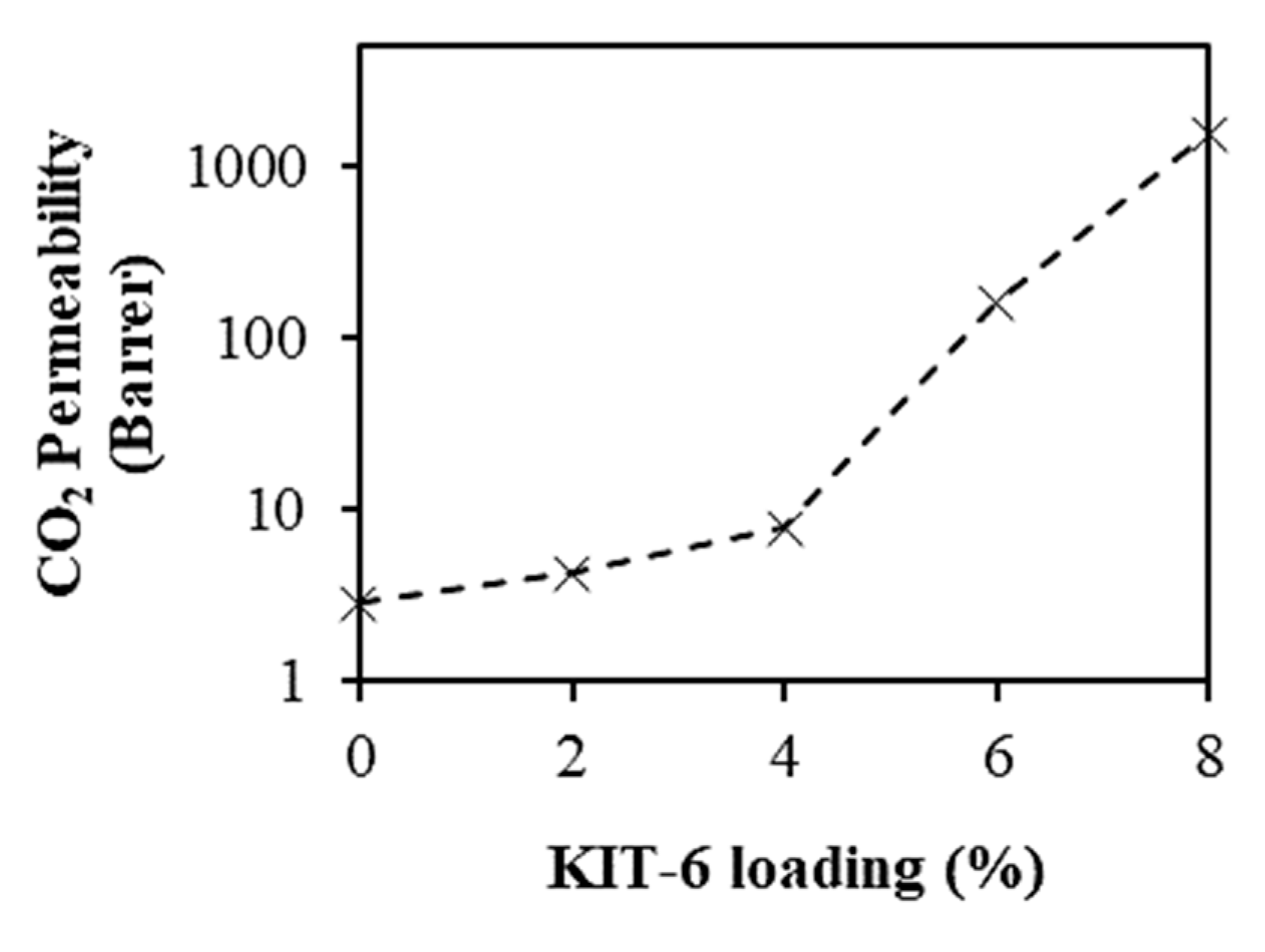
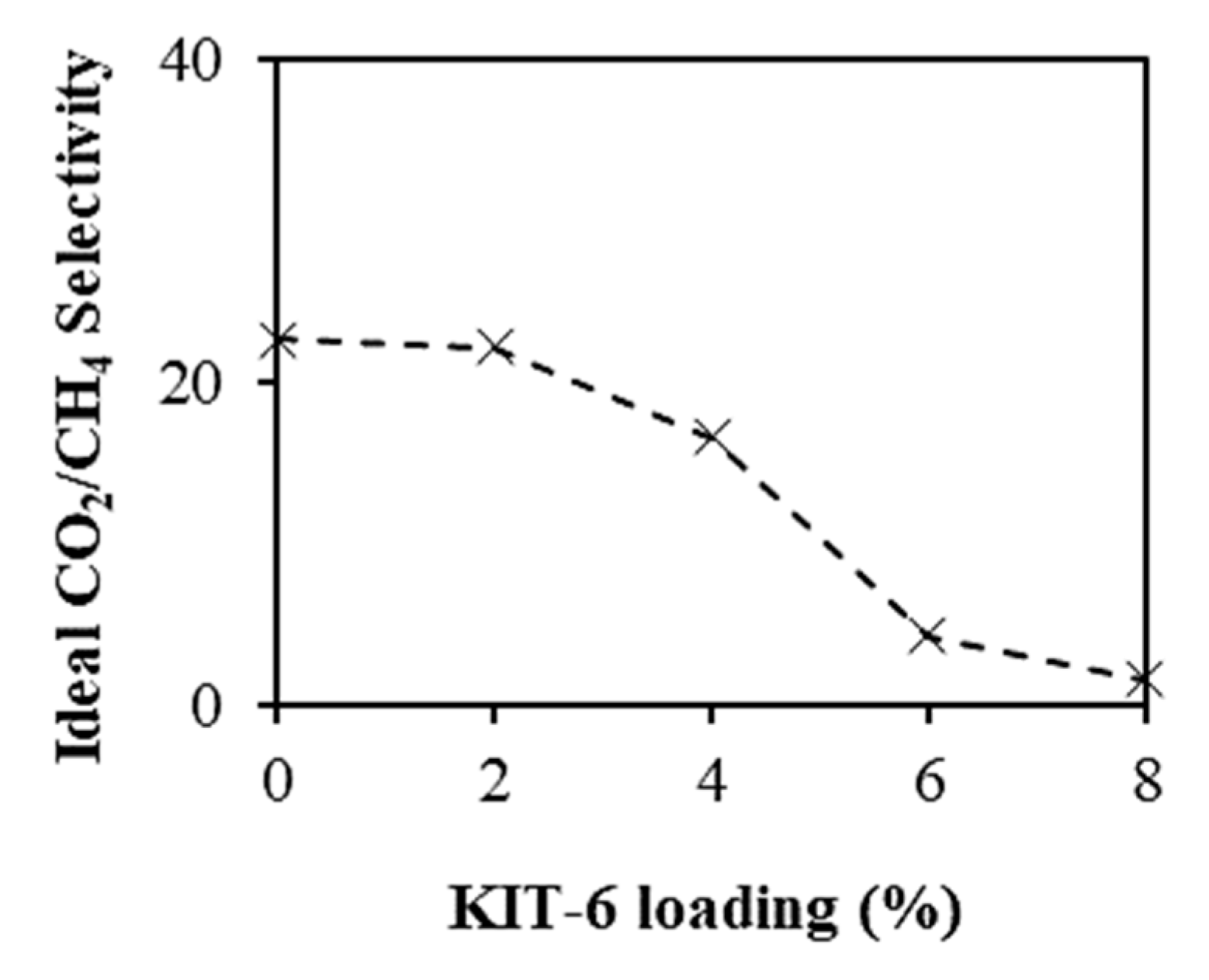
3.3. CO2 Permeability and Selectivity of the Membranes
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kinoshita, Y.; Wakimoto, K.; Gibbons, A.H.; Isfahani, A.P.; Kusuda, H.; Sivaniah, E.; Ghalei, B. Enhanced PIM-1 membrane gas separation selectivity through efficient dispersion of functionalized POSS fillers. J. Membr. Sci. 2017, 539, 178–186. [Google Scholar] [CrossRef]
- Yavari, M.; Okamoto, Y.; Lin, H. The role of halogens in polychlorotrifluoroethylene (PCTFE) in membrane gas separations. J. Membr. Sci. 2018, 548, 380–389. [Google Scholar] [CrossRef]
- Mohamad, M.B.; Fong, Y.Y.; Shariff, A. Gas separation of carbon dioxide from methane using polysulfone membrane incorporated with Zeolite-T. Procedia Eng. 2016, 148, 621–629. [Google Scholar] [CrossRef]
- Aroon, M.; Ismail, A.; Montazer-Rahmati, M.; Matsuura, T. Morphology and permeation properties of polysulfone membranes for gas separation: Effects of non-solvent additives and co-solvent. Sep. Purif. Technol. 2010, 72, 194–202. [Google Scholar] [CrossRef]
- Bastani, D.; Esmaeili, N.; Asadollahi, M. Polymeric mixed matrix membranes containing zeolites as a filler for gas separation applications: A review. J. Ind. Eng. Chem. 2013, 19, 375–393. [Google Scholar] [CrossRef]
- Brunetti, A.; Scura, F.; Barbieri, G.; Drioli, E. Membrane technologies for CO2 separation. J. Membr. Sci. 2010, 359, 115–125. [Google Scholar] [CrossRef]
- Wang, X.; Chen, H.; Zhang, L.; Yu, R.; Qu, R.; Yang, L. Effects of coexistent gaseous components and fine particles in the flue gas on CO2 separation by flat-sheet polysulfone membranes. J. Membr. Sci. 2014, 470, 237–245. [Google Scholar] [CrossRef]
- Shahid, S.; Nijmeijer, K. Matrimid®/polysulfone blend mixed matrix membranes containing ZIF-8 nanoparticles for high pressure stability in natural gas separation. Sep. Purif. Technol. 2017, 189, 90–100. [Google Scholar] [CrossRef]
- Venturi, D.; Grupkovic, D.; Sisti, L.; Baschetti, M.G. Effect of humidity and nanocellulose content on Polyvinylamine-nanocellulose hybrid membranes for CO2 capture. J. Membr. Sci. 2018, 548, 263–274. [Google Scholar] [CrossRef]
- Bera, B.; Das, J.K.; Das, N. Mesoporous silica based composite membrane formation by in-situ cross-linking of phenol and formaldehyde at room temperature for enhanced CO2 separation. Microporous Mesoporous Mater. 2018, 256, 177–189. [Google Scholar] [CrossRef]
- Shete, M.; Kumar, P.; Bachman, J.E.; Ma, X.; Smith, Z.P.; Xu, W.; Mkhoyan, A.; Long, J.R.; Tsapatsis, M. On the direct synthesis of Cu (BDC) MOF nanosheets and their performance in mixed matrix membranes. J. Membr. Sci. 2018, 549, 312–320. [Google Scholar] [CrossRef]
- Benavides, M.E.; David, O.; Johnson, T.; Łozińska, M.M.; Orsi, A.; Wright, P.A.; Mastel, S.; Hillenbrand, R.; Kapteijn, F.; Gascon, J. High performance mixed matrix membranes (MMMs) composed of ZIF-94 filler and 6FDA-DAM polymer. J. Membr. Sci. 2018, 550, 198–207. [Google Scholar] [CrossRef]
- Sakaguchi, T.; Nakao, S.; Irie, S.; Hashimoto, T. Gas permeability of mixed matrix membranes composed of poly(diphenylacetylene)s and dispersed metal chloride particles. Polymer 2018, 140, 208–214. [Google Scholar] [CrossRef]
- Khan, A.L.; Klaysom, C.; Gahlaut, A.; Vankelecom, I.F. Polysulfone acrylate membranes containing functionalized mesoporous MCM-41 for CO2 separation. J. Membr. Sci. 2013, 436, 145–153. [Google Scholar] [CrossRef]
- Tzi, E.C.N.; Ching, O.P. Surface modification of AMH-3 for development of mixed matrix membranes. Procedia Eng. 2016, 148, 86–92. [Google Scholar] [CrossRef][Green Version]
- Wang, J.; Li, Y.; Zhang, Z.; Hao, Z. Mesoporous KIT-6 silica–polydimethylsiloxane (PDMS) mixed matrix membranes for gas separation. J. Mater. Chem. A 2015, 3, 8650–8658. [Google Scholar] [CrossRef]
- Wu, H.; Li, X.Q.; Li, Y.F.; Wang, S.F.; Guo, R.L.; Jiang, Z.Y.; Wu, C.; Xin, Q.P.; Lu, X. Facilitated transport mixed matrix membranes incorporated with amine functionalized MCM-41 for enhanced gas separation properties. J. Membr. Sci. 2014, 465, 78–90. [Google Scholar] [CrossRef]
- Kim, S.; Marand, E. High permeability nano-composite membranes based on mesoporous MCM-41 nanoparticles in a polysulfone matrix. Microporous Mesoporous Mater. 2008, 114, 129–136. [Google Scholar] [CrossRef]
- Jomekian, A.; Pakizeh, M.; Mansoori, S.; Poorafshari, M.; Hemmati, M.; Ataee Dil, P. Gas transport behavior of novel modified MCM-48/polysulfone mixed matrix membrane coated by PDMS. J. Membr. Sci. Technol. 2011, 1, 1–6. [Google Scholar]
- Qian, L.; Ren, Y.; Liu, T.; Pan, D.; Wang, H.; Chen, G. Influence of KIT-6′s pore structure on its surface properties evaluated by inverse gas chromatography. Chem. Eng. J. 2012, 213, 186–194. [Google Scholar] [CrossRef]
- Ayad, M.M.; Salahuddin, N.A.; El-Nasr, A.A.; Torad, N.L. Amine-functionalized mesoporous silica KIT-6 as a controlled release drug delivery carrier. Microporous Mesoporous Mater. 2016, 229, 166–177. [Google Scholar] [CrossRef]
- Kishor, R.; Ghoshal, A.K. APTES grafted ordered mesoporous silica KIT-6 for CO2 adsorption. Chem. Eng. J. 2015, 262, 882–890. [Google Scholar] [CrossRef]
- Hafizi, H.; Chermahini, A.N.; Saraji, M.; Mohammadnezhad, G. The catalytic conversion of fructose into 5-hydroxymethylfurfural over acid-functionalized KIT-6, an ordered mesoporous silica. Chem. Eng. J. 2016, 294, 380–388. [Google Scholar] [CrossRef]
- Loganathan, S.; Ghoshal, A.K. Amine tethered pore-expanded MCM-41: A promising adsorbent for CO2 capture. Chem. Eng. J. 2017, 308, 827–839. [Google Scholar] [CrossRef]
- Ahn, J.; Chung, W.-J.; Pinnau, I.; Guiver, M.D. Polysulfone/silica nanoparticle mixed-matrix membranes for gas separation. J. Membr. Sci. 2008, 314, 123–133. [Google Scholar] [CrossRef]
- Zornoza, B.; Irusta, S.; Téllez, C.; Coronas, J. Mesoporous silica sphere−polysulfone mixed matrix membranes for gas separation. Langmuir 2009, 25, 5903–5909. [Google Scholar] [CrossRef]
- Waheed, N.; Mushtaq, A.; Tabassum, S.; Gilani, M.A.; Ilyas, A.; Ashraf, F.; Jamal, Y.; Bilad, M.R.; Khan, A.U.; Khan, L.A. Mixed matrix membranes based on polysulfone and rice husk extracted silica for CO2 separation. Sep. Purif. Technol. 2016, 170, 122–129. [Google Scholar] [CrossRef]
- Khan, A.L.; Klaysom, C.; Gahlaut, A.; Khan, A.U.; Vankelecom, I.F. Mixed matrix membranes comprising of Matrimid and–SO3H functionalized mesoporous MCM-41 for gas separation. J. Membr. Sci. 2013, 447, 73–79. [Google Scholar] [CrossRef]
- Rezakazemi, M.; Amooghin, A.E.; Montazer-Rahmati, M.M.; Ismail, A.F.; Matsuura, T. State-of-the-art membrane based CO2 separation using mixed matrix membranes (MMMs): An overview on current status and future directions. Prog. Polym. Sci. 2014, 39, 817–861. [Google Scholar] [CrossRef]
- Kim, S.; Marand, E.; Ida, J.; Guliants, V.V. Polysulfone and mesoporous molecular sieve MCM-48 mixed matrix membranes for gas separation. Chem. Mater. 2006, 18, 1149–1155. [Google Scholar] [CrossRef]
- Khan, A.L.; Sree, S.P.; Martens, J.A.; Raza, M.T.; Vankelecom, I.F. Mixed matrix membranes comprising of matrimid and mesoporous COK-12: Preparation and gas separation properties. J. Membr. Sci. 2015, 495, 471–478. [Google Scholar] [CrossRef]
- Pakizeh, M.; Hokmabadi, S. Experimental study of the effect of zeolite 4A treated with magnesium hydroxide on the characteristics and gas-permeation properties of polysulfone-based mixed-matrix membranes. J. Appl. Polymer Sci. 2017, 134, 1–7. [Google Scholar] [CrossRef]
- Feijani, E.A.; Mahdavi, H.; Tavasoli, A. Poly(vinylidene fluoride) based mixed matrix membranes comprising metal organic frameworks for gas separation applications. Chem. Eng. Res. Des. 2015, 96, 87–102. [Google Scholar] [CrossRef]
- Perez, E.V.; Balkus, K.J., Jr.; Ferraris, J.P.; Musselman, I.H. Metal-organic polyhedra 18 mixed-matrix membranes for gas separation. J. Membr. Sci. 2014, 463, 82–93. [Google Scholar] [CrossRef]

| Membrane Samples | Weight Percentage Loss (%) | |||
|---|---|---|---|---|
| Desorption of Absorbed Water at 30–500 °C | Main Thermal Degradation at 500–580 °C | Decomposition into Ash at 580–800 °C | Total Weight Loss | |
| Pristine PSF | 2.5 | 54.5 | 15.8 | 72.8 |
| 2%-KIT-6/PSF | 4.4 | 64.2 | 13.5 | 82.1 |
| 4%-KIT-6/PSF | 4.0 | 55.2 | 12.2 | 71.4 |
| 6%-KIT-6/PSF | 1.7 | 54.3 | 10.0 | 66.0 |
| 8%-KIT-6/PSF | 1.9 | 48.9 | 11.8 | 62.6 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ding, S.H.; Ng, T.Y.S.; Chew, T.L.; Oh, P.C.; Ahmad, A.L.; Ho, C.-D. Evaluation of the Properties, Gas Permeability, and Selectivity of Mixed Matrix Membrane Based on Polysulfone Polymer Matrix Incorporated with KIT-6 Silica. Polymers 2019, 11, 1732. https://doi.org/10.3390/polym11111732
Ding SH, Ng TYS, Chew TL, Oh PC, Ahmad AL, Ho C-D. Evaluation of the Properties, Gas Permeability, and Selectivity of Mixed Matrix Membrane Based on Polysulfone Polymer Matrix Incorporated with KIT-6 Silica. Polymers. 2019; 11(11):1732. https://doi.org/10.3390/polym11111732
Chicago/Turabian StyleDing, Sie Hao, Tiffany Yit Siew Ng, Thiam Leng Chew, Pei Ching Oh, Abdul Latif Ahmad, and Chii-Dong Ho. 2019. "Evaluation of the Properties, Gas Permeability, and Selectivity of Mixed Matrix Membrane Based on Polysulfone Polymer Matrix Incorporated with KIT-6 Silica" Polymers 11, no. 11: 1732. https://doi.org/10.3390/polym11111732
APA StyleDing, S. H., Ng, T. Y. S., Chew, T. L., Oh, P. C., Ahmad, A. L., & Ho, C.-D. (2019). Evaluation of the Properties, Gas Permeability, and Selectivity of Mixed Matrix Membrane Based on Polysulfone Polymer Matrix Incorporated with KIT-6 Silica. Polymers, 11(11), 1732. https://doi.org/10.3390/polym11111732








