Ultrasensitive Wearable Strain Sensors of 3D Printing Tough and Conductive Hydrogels
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials
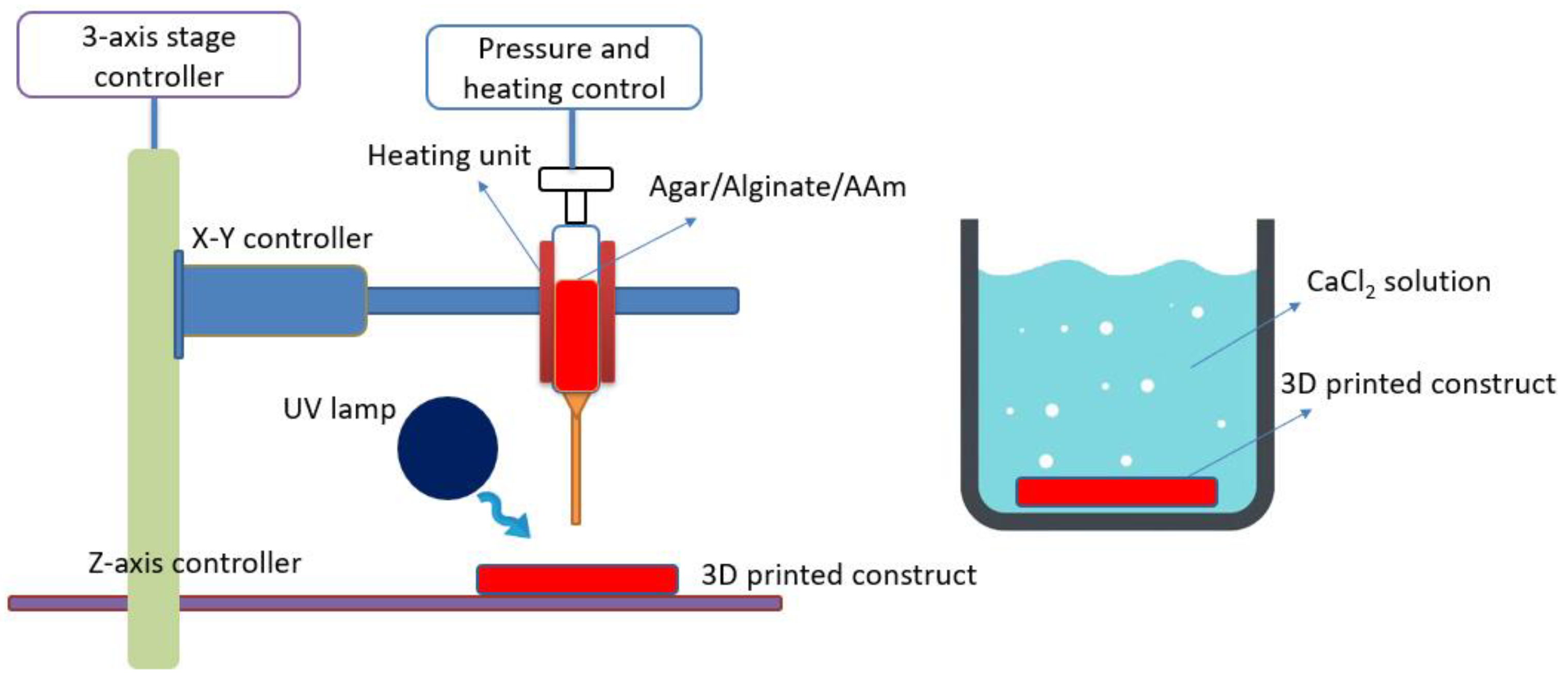
2.2. 3D Printing System
2.3. 3D Printing Hydrogels Fabrication
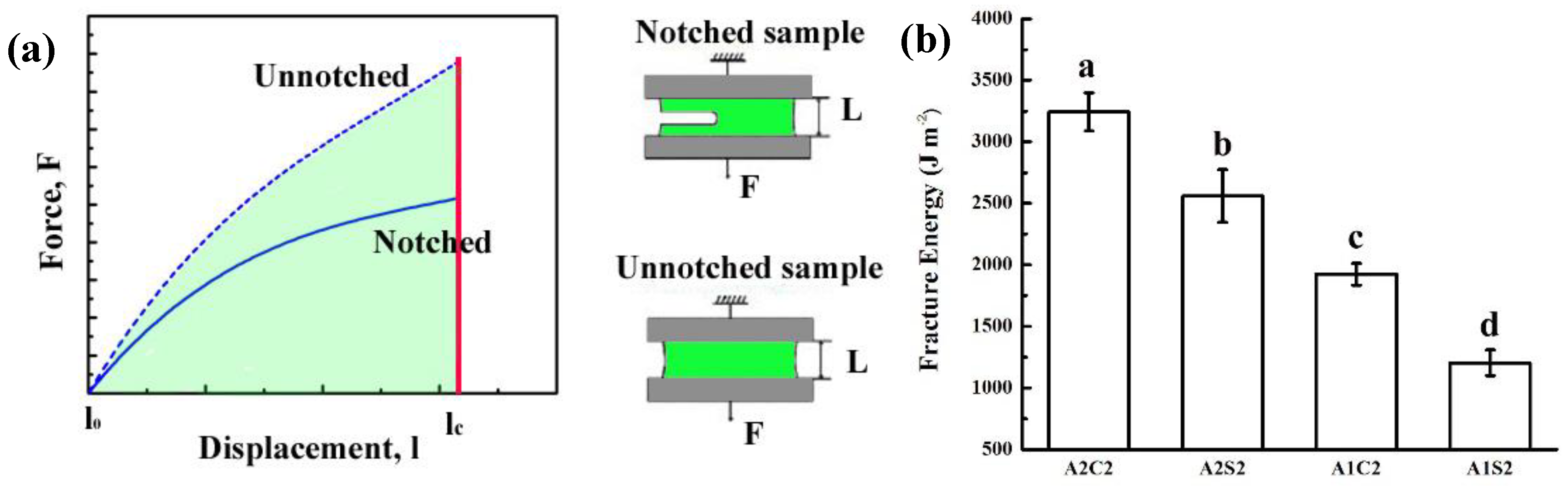
2.4. Mechanical Test
2.5. Rheological Test
2.6. Conductivity Test
2.7. Cytotoxicity Test
2.8. Swelling Test
2.9. Statistical Analysis
3. Results and Discussions
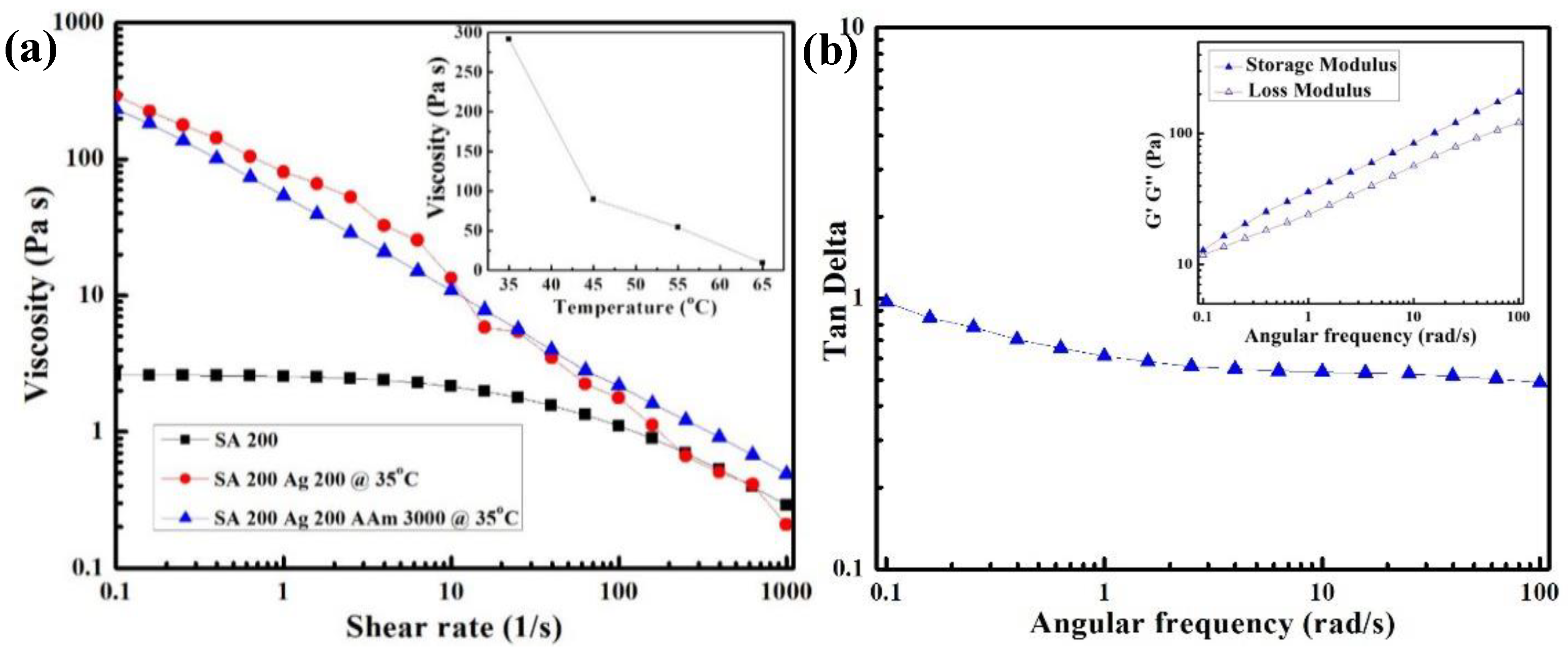
3.1. Viscosity and Printability
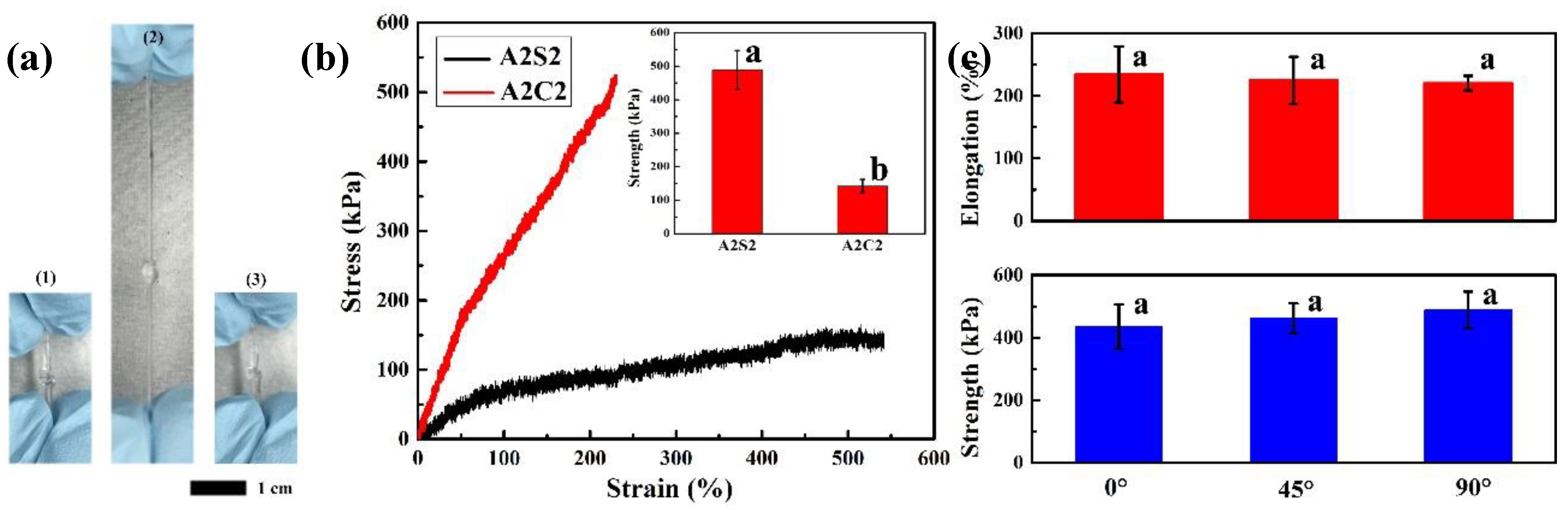
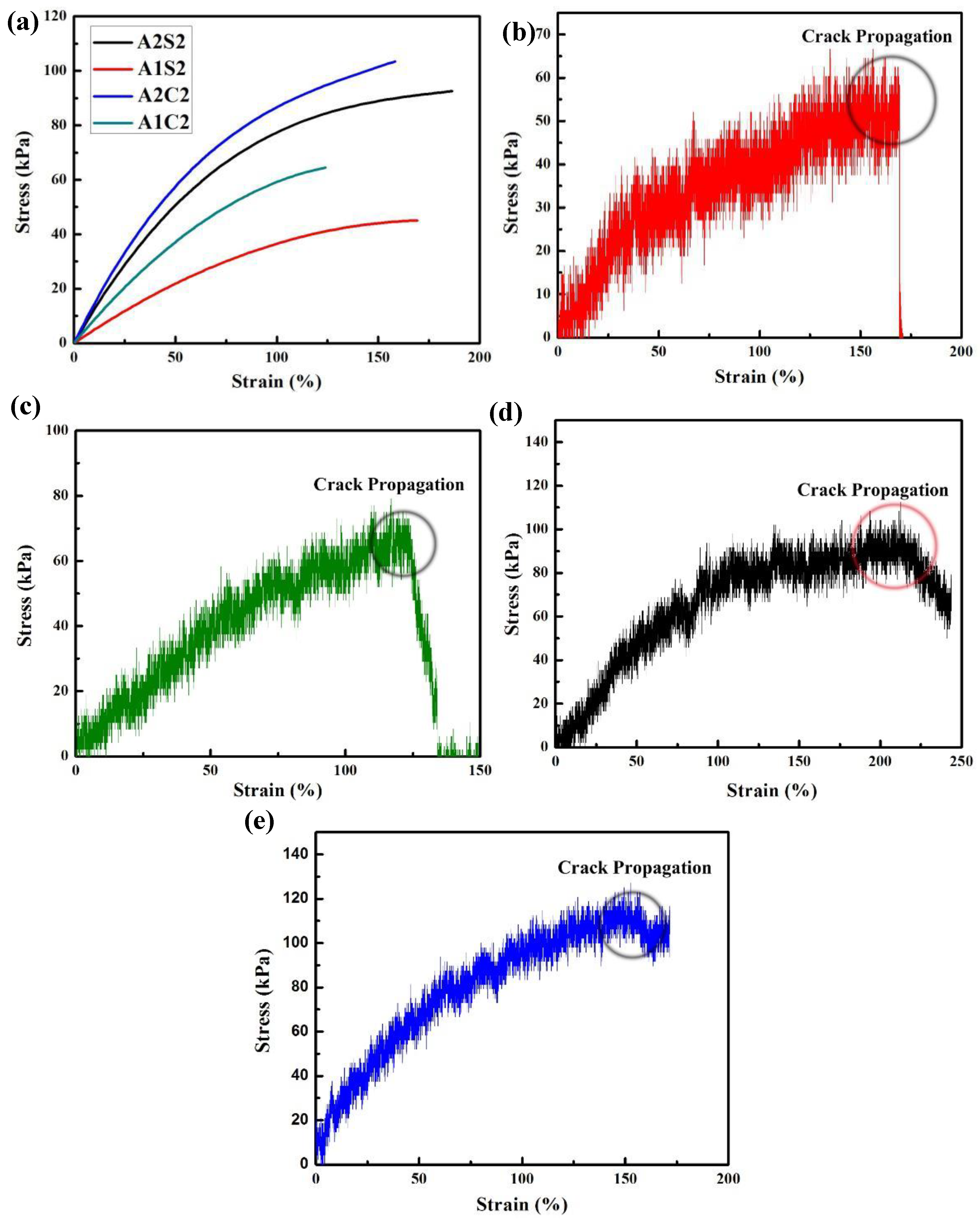
3.2. Mechanical Properties
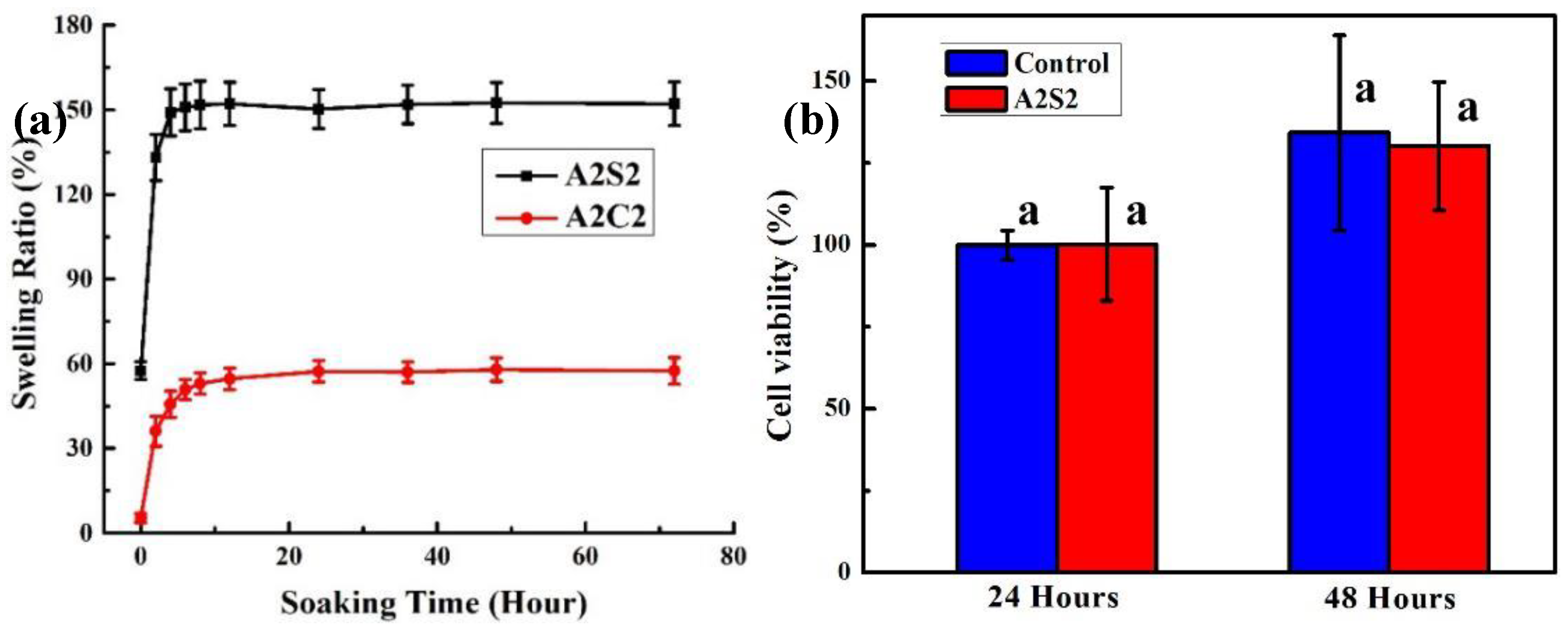
3.3. Swelling and Cytotoxicity
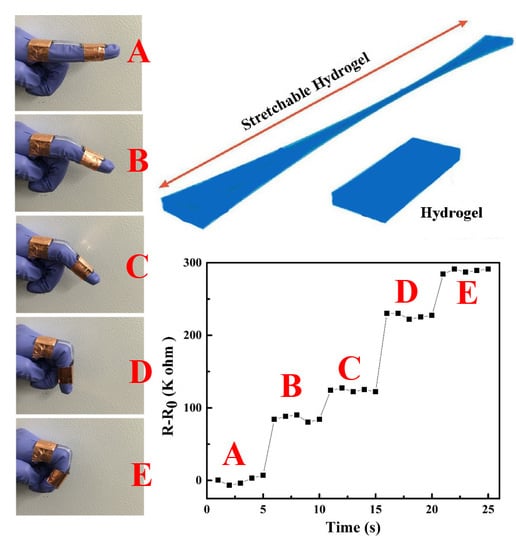
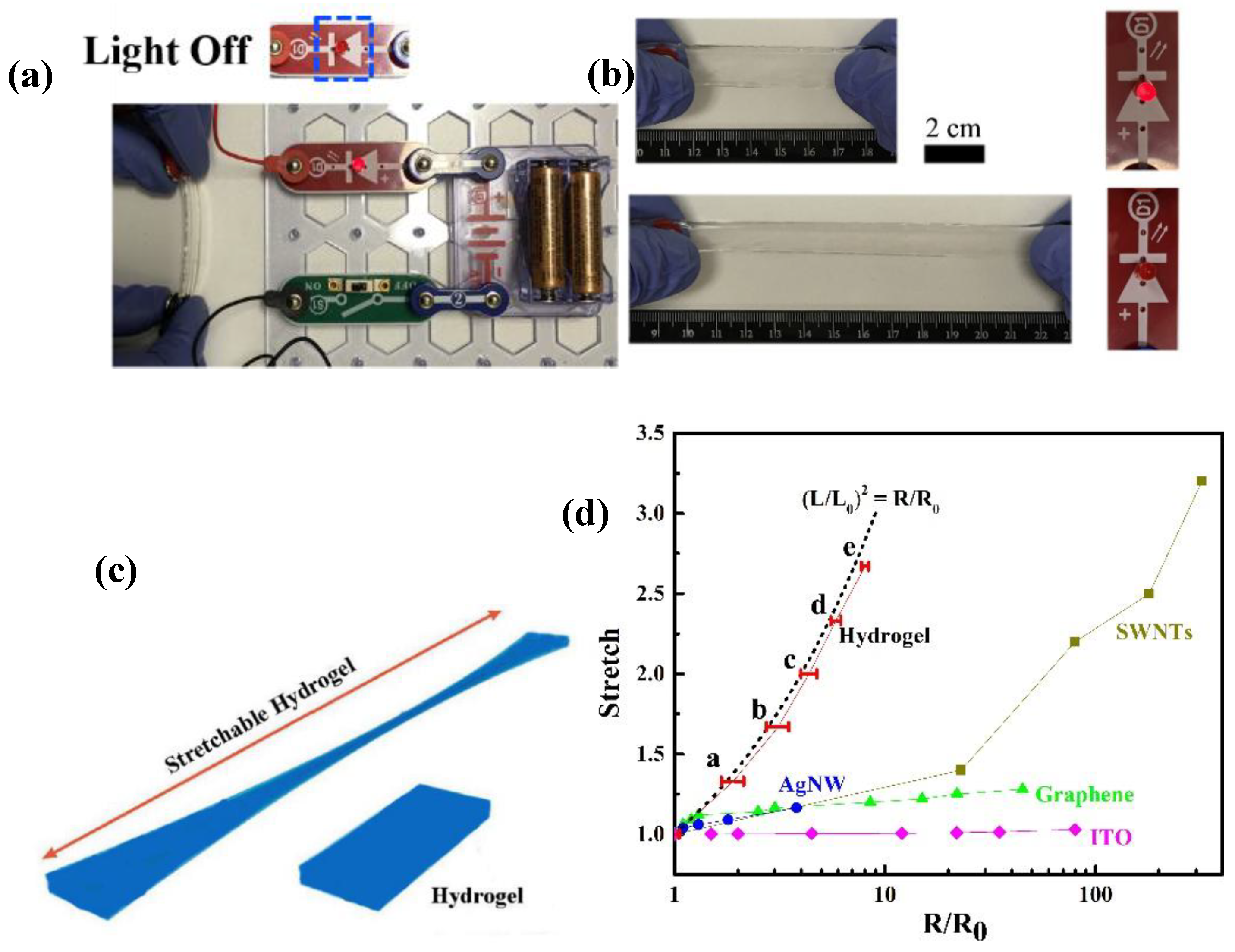
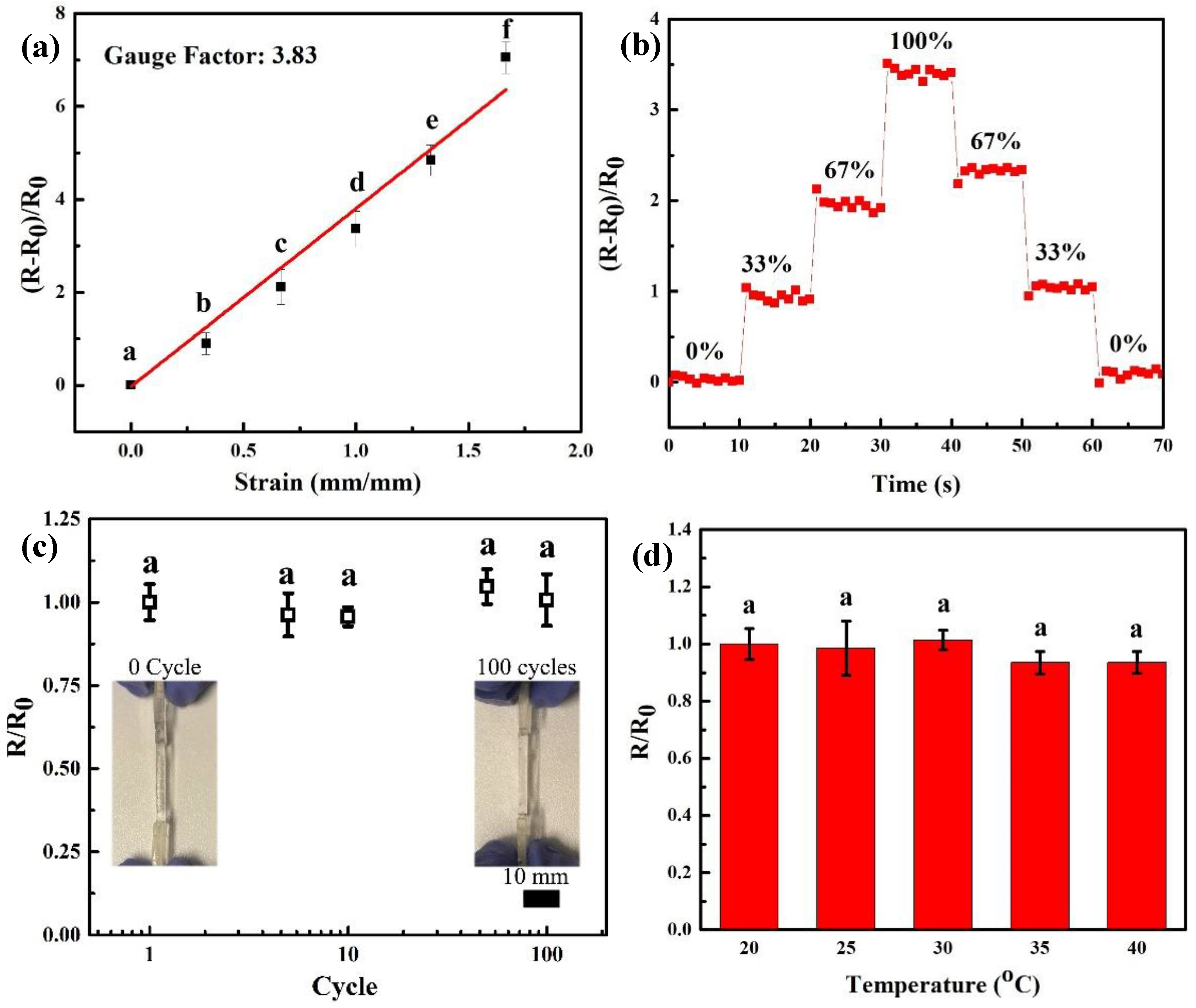
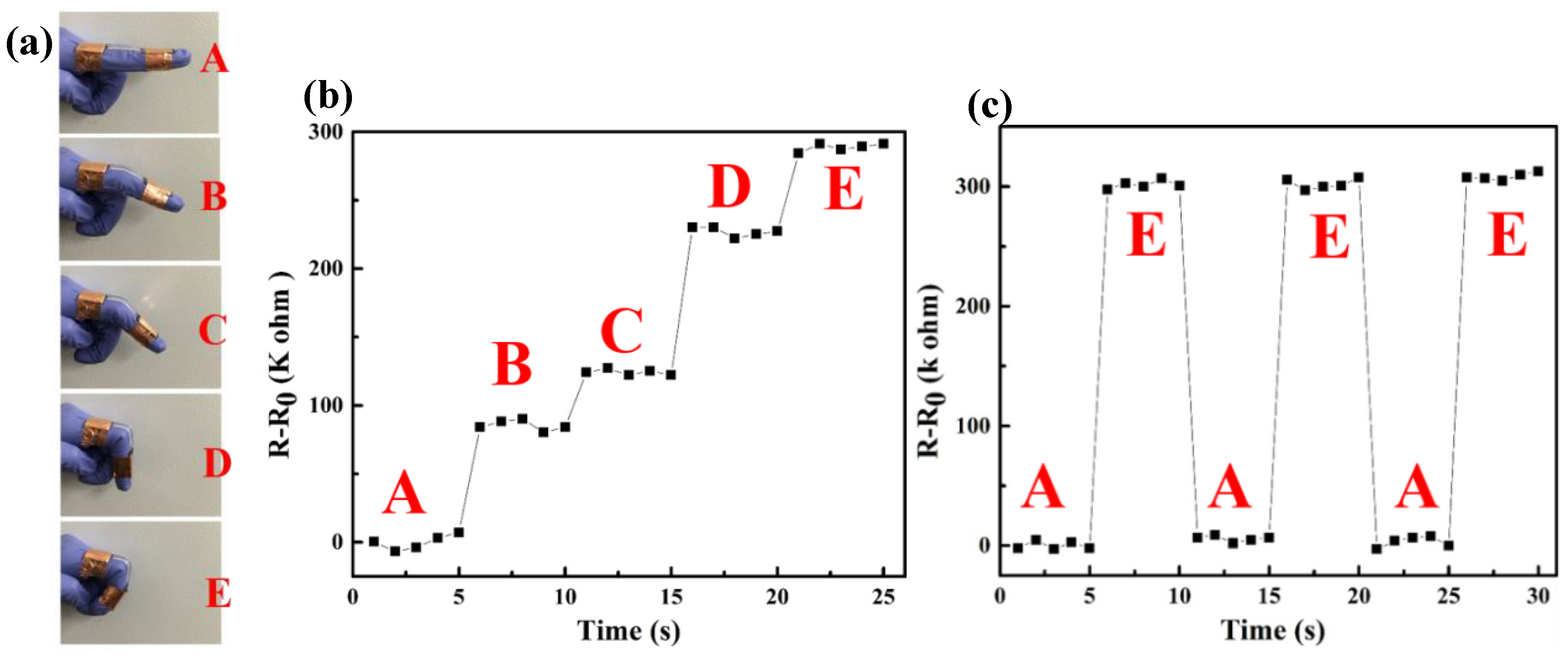
3.4. Conductivity and Sensors
4. Conclusion
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Hammock, M.L.; Chortos, A.; Tee, B.C.K.; Tok, J.B.H.; Bao, Z.A. 25th Anniversary Article: The Evolution of Electronic Skin (E-Skin): A Brief History, Design Considerations, and Recent Progress. Adv. Mater. 2013, 25, 5997–6037. [Google Scholar] [CrossRef]
- Chortos, A.; Bao, Z.N. Skin-inspired electronic devices. Mater. Today 2014, 17, 321–331. [Google Scholar] [CrossRef]
- Wang, X.D.; Dong, L.; Zhang, H.L.; Yu, R.M.; Pan, C.F.; Wang, Z.L. Recent Progress in Electronic Skin. Adv. Sci. 2015, 2, 1500169. [Google Scholar] [CrossRef]
- Sun, J.Y.; Keplinger, C.; Whitesides, G.M.; Suo, Z.G. Ionic Skin. Adv. Mater. 2014, 26, 7608–7614. [Google Scholar] [CrossRef] [PubMed]
- Pelrine, R.; Kornbluh, R.; Pei, Q.B.; Joseph, J. High-speed electrically actuated elastomers with strain greater than 100%. Science 2000, 287, 836–839. [Google Scholar] [CrossRef] [PubMed]
- Zang, J.F.; Ryu, S.; Pugno, N.; Wang, Q.M.; Tu, Q.; Buehler, M.J.; Zhao, X.H. Multifunctionality and control of the crumpling and unfolding of large-area graphene. Nat. Mater. 2013, 12, 321–325. [Google Scholar] [CrossRef] [PubMed]
- Lipomi, D.J.; Vosgueritchian, M.; Tee, B.C.K.; Hellstrom, S.L.; Lee, J.A.; Fox, C.H.; Bao, Z.N. Skin-like pressure and strain sensors based on transparent elastic films of carbon nanotubes. Nat. Nanotechnol. 2011, 6, 788–792. [Google Scholar] [CrossRef]
- Majidi, C.; Kramer, R.; Wood, R.J. A non-differential elastomer curvature sensor for softer-than-skin electronics. Smart Mater. Struct. 2011, 20, 105017. [Google Scholar] [CrossRef]
- Liang, J.J.; Li, L.; Niu, X.F.; Yu, Z.B.; Pei, Q.B. Elastomeric polymer light-emitting devices and displays. Nat. Photonics 2013, 7, 817–824. [Google Scholar] [CrossRef]
- Guo, C.F.; Sun, T.Y.; Liu, Q.H.; Suo, Z.G.; Ren, Z.F. Highly stretchable and transparent nanomesh electrodes made by grain boundary lithography. Nat. Commun. 2014, 5, 3121. [Google Scholar] [CrossRef]
- Wang, J.L.; Wei, J.H.; Su, S.H.; Qiu, J.J.; Wang, S.R. Ion-linked double-network hydrogel with high toughness and stiffness. J. Mater. Sci. 2015, 50, 5458–5465. [Google Scholar] [CrossRef]
- Kim, C.C.; Lee, H.H.; Oh, K.H.; Sun, J.Y. Highly stretchable, transparent ionic touch panel. Science 2016, 353, 682–687. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.L.; Su, S.H.; Qiu, J.J. Biocompatible swelling graphene oxide reinforced double network hydrogels with high toughness and stiffness. New J. Chem. 2017, 41, 3781–3789. [Google Scholar] [CrossRef]
- Wang, J.; Wei, J.; Qiu, J. Facile Synthesis of Tough Double Network Hydrogel. MRS Adv. 2016, 1, 1953–1958. [Google Scholar] [CrossRef]
- Wei, J.; Wang, J.; Su, S.; Wang, S.; Qiu, J. Tough and fully recoverable hydrogels. J. Mater. Chem. B 2015, 3, 5284–5290. [Google Scholar] [CrossRef]
- Wei, J.H.; Su, S.H.; Wang, J.L.; Qiu, J.J. Imitation proteoglycans improve toughness of double network hydrogels. Mater. Chem. Phys. 2015, 166, 66–72. [Google Scholar] [CrossRef]
- Wei, J.H.; Wang, J.L.; Su, S.H.; Wang, S.R.; Qiu, J.J.; Zhang, Z.H.; Christopher, G.; Ning, F.D.; Cong, W.L. 3D printing of an extremely tough hydrogel. RSC Adv. 2015, 5, 81324–81329. [Google Scholar] [CrossRef]
- Markstedt, K.; Mantas, A.; Tournier, I.; Avila, H.M.; Hagg, D.; Gatenholm, P. 3D Bioprinting Human Chondrocytes with Nanocellulose-Alginate Bioink for Cartilage Tissue Engineering Applications. Biomacromolecules 2015, 16, 1489–1496. [Google Scholar] [CrossRef]
- Hull, C.W. Apparatus for Production of Three-Dimensional Objects by Stereolithography. U.S. Patents US4575330A, 11 March 1986. [Google Scholar]
- Shusteff, M.; Browar, A.E.M.; Kelly, B.E.; Henriksson, J.; Weisgraber, T.H.; Panas, R.M.; Fang, N.X.; Spadaccini, C.M. One-step volumetric additive manufacturing of complex polymer structures. Sci. Adv. 2017, 3, 5496. [Google Scholar] [CrossRef]
- de Beer, M.P.; van der Laan, H.L.; Cole, M.A.; Whelan, R.J.; Burns, M.A.; Scott, T.F. Rapid, continuous additive manufacturing by volumetric polymerization inhibition patterning. Sci. Adv. 2019, 5, 8723. [Google Scholar] [CrossRef]
- Kelly, B.E.; Bhattacharya, I.; Heidari, H.; Shusteff, M.; Spadaccini, C.M.; Taylor, H.K. Volumetric additive manufacturing via tomographic reconstruction. Science 2019, 363, 1075–1079. [Google Scholar] [CrossRef] [PubMed]
- Murphy, S.V.; Atala, A. 3D bioprinting of tissues and organs. Nat. Biotechnol. 2014, 32, 773–785. [Google Scholar] [CrossRef] [PubMed]
- Lee, V.; Singh, G.; Trasatti, J.P.; Bjornsson, C.; Xu, X.W.; Tran, T.N.; Yoo, S.S.; Dai, G.H.; Karande, P. Design and Fabrication of Human Skin by Three-Dimensional Bioprinting. Tissue Eng. Part C Methods 2014, 20, 473–484. [Google Scholar] [CrossRef] [PubMed]
- Song, S.J.; Choi, J.; Park, Y.D.; Lee, J.J.; Hong, S.Y.; Sun, K. A Three-Dimensional Bioprinting System for Use with a Hydrogel-Based Biomaterial and Printing Parameter Characterization. Artif. Organs 2010, 34, 1044–1048. [Google Scholar] [CrossRef]
- Almeida, C.R.; Serra, T.; Oliveira, M.I.; Planell, J.A.; Barbosa, M.A.; Navarro, M. Impact of 3-D printed PLA- and chitosan-based scaffolds on human monocyte/macrophage responses: Unraveling the effect of 3-D structures on inflammation. Acta Biomater. 2014, 10, 613–622. [Google Scholar] [CrossRef]
- Sun, J.Y.; Zhao, X.H.; Illeperuma, W.R.K.; Chaudhuri, O.; Oh, K.H.; Mooney, D.J.; Vlassak, J.J.; Suo, Z.G. Highly stretchable and tough hydrogels. Nature 2012, 489, 133–136. [Google Scholar] [CrossRef]
- Kamata, H.; Akagi, Y.; Kayasuga-Kariya, Y.; Chung, U.; Sakai, T. “Nonswellable” Hydrogel without Mechanical Hysteresis. Science 2014, 343, 873–875. [Google Scholar] [CrossRef]
- Liu, Z.Y.; Qi, D.P.; Guo, P.Z.; Liu, Y.; Zhu, B.W.; Yang, H.; Liu, Y.Q.; Li, B.; Zhang, C.G.; Yu, J.C.; et al. Thickness-Gradient Films for High Gauge Factor Stretchable Strain Sensors. Adv. Mater. 2015, 27, 6230–6237. [Google Scholar] [CrossRef]
- Kong, H.J.; Lee, K.Y.; Mooney, D.J. Decoupling the dependence of rheological/mechanical properties of hydrogels from solids concentration. Polymer 2002, 43, 6239–6246. [Google Scholar] [CrossRef]
- Chung, J.H.Y.; Naficy, S.; Yue, Z.L.; Kapsa, R.; Quigley, A.; Moulton, S.E.; Wallace, G.G. Bio-ink properties and printability for extrusion printing living cells. Biomater. Sci. 2013, 1, 763–773. [Google Scholar] [CrossRef]
- Lee, J.S.; Hong, J.M.; Jung, J.W.; Shim, J.H.; Oh, J.H.; Cho, D.W. 3D printing of composite tissue with complex shape applied to ear regeneration. Biofabrication 2014, 6, 024103. [Google Scholar] [CrossRef] [PubMed]
- Tabriz, A.G.; Hermida, M.A.; Leslie, N.R.; Shu, W.M. Three-dimensional bioprinting of complex cell laden alginate hydrogel structures. Biofabrication 2015, 7, 045012. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, D.; Bharath, S. Design of lab model mechanical strength test instrument for tensile strength determination of film formulations. J. Dent. Orofac. Res. 2018, 14, 18–22. [Google Scholar]
- Hong, S.; Sycks, D.; Chan, H.F.; Lin, S.T.; Lopez, G.P.; Guilak, F.; Leong, K.W.; Zhao, X.H. 3D Printing of Highly Stretchable and Tough Hydrogels into Complex, Cellularized Structures. Adv. Mater. 2015, 27, 4035–4040. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, J.; Lou, L.; Qiu, J. Super-tough hydrogels using ionically crosslinked networks. J. Appl. Polym. Sci. 2019, 136, 48182. [Google Scholar] [CrossRef]
- Chen, Q.; Zhu, L.; Huang, L.N.; Chen, H.; Xu, K.; Tan, Y.; Wang, P.X.; Zheng, J. Fracture of the Physically Cross-Linked First Network in Hybrid Double Network Hydrogels. Macromolecules 2014, 47, 2140–2148. [Google Scholar] [CrossRef]
- Liu, Y.J.; Cao, W.T.; Ma, M.G.; Wan, P.B. Ultrasensitive Wearable Soft Strain Sensors of Conductive, Selfhealing, and Elastic Hydrogels with Synergistic “Soft and Hard” Hybrid Networks. ACS Appl. Mater. Interfaces 2017, 9, 25559–25570. [Google Scholar] [CrossRef]
- Keplinger, C.; Sun, J.Y.; Foo, C.C.; Whitesides, G.M.; Suo, Z.G. Stretchable, Transparent, Ionic Conductors. Science 2013, 341, 984–987. [Google Scholar] [CrossRef] [Green Version]
- Hu, W.; Niu, X.; Li, L.; Yun, S.; Yu, Z.; Pei, Q. Intrinsically stretchable transparent electrodes based on silver-nanowire–crosslinked-polyacrylate composites. Nanotechnology 2012, 23, 344002. [Google Scholar] [CrossRef]
- Yu, Z.B.; Zhang, Q.W.; Li, L.; Chen, Q.; Niu, X.F.; Liu, J.; Pei, Q.B. Highly flexible silver nanowire electrodes for shape-memory polymer lightemitting diodes. Adv. Mater. 2011, 23, 664–668. [Google Scholar] [CrossRef]
- Kim, K.S.; Zhao, Y.; Jang, H.; Lee, S.Y.; Kim, J.M.; Kim, K.S.; Ahn, J.H.; Kim, P.; Choi, J.Y.; Hong, B.H. Large-scale pattern growth of graphene films for stretchable transparent electrodes. Nature 2009, 457, 706–710. [Google Scholar] [CrossRef] [PubMed]
- Hu, L.; Yuan, W.; Brochu, P.; Gruner, G.; Pei, Q. Highly stretchable, conductive, and transparent nanotube thin films. Appl. Phys. Lett. 2009, 94, 161108. [Google Scholar] [CrossRef]
| Sample | Agar (mg) | Sodium Alginate (mg) | Irgacure 2959 (mg) | Acrylamide (mg) | MBAA (mg) | Concentration of CaCl2 (mM) |
|---|---|---|---|---|---|---|
| A1C2 | 100 | 200 | 90 | 3000 | 3 | 100 |
| A2C2 | 200 | 200 | 90 | 3000 | 3 | 100 |
| A3C2 | 300 | 200 | 90 | 3000 | 3 | 100 |
| A2C1 | 200 | 100 | 90 | 3000 | 3 | 100 |
| A2C3 | 200 | 300 | 90 | 3000 | 3 | 100 |
| A1S2 | 100 | 200 | 90 | 3000 | 3 | N/A |
| A2S2 | 200 | 200 | 90 | 3000 | 3 | N/A |
| Sample | Young’s Modulus (kPa) | Strength (kPa) | Elongation (%) | Toughness (kJ m−3) |
|---|---|---|---|---|
| A1C2 | 26.74 ± 4.95 | 385.56 ± 31.10 | 223.63 ± 62.96 | 479.71 ± 150.97 |
| A2C2 | 38.14 ± 2.99 | 488.75 ± 58.31 | 220.30 ± 11.74 | 603.22 ± 61.78 |
| A3C2 | 55.45 ± 9.33 | 744.57 ± 144.17 | 235.78 ± 31.95 | 1049.77 ± 241.21 |
| A2C1 | 17.14 ± 4.63 | 283.33 ± 31.07 | 215.44 ± 12.10 | 362.01 ± 31.26 |
| A2C3 | 41.20 ± 3.61 | 596.84 ± 28.43 | 218.54 ± 24.81 | 762.02 ± 43.76 |
| A2S2 | 16.29 ± 0.54 | 142.67 ± 19.60 | 566.38 ± 23.47 | 493.27 ± 42.00 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, J.; Liu, Y.; Su, S.; Wei, J.; Rahman, S.E.; Ning, F.; Christopher, G.; Cong, W.; Qiu, J. Ultrasensitive Wearable Strain Sensors of 3D Printing Tough and Conductive Hydrogels. Polymers 2019, 11, 1873. https://doi.org/10.3390/polym11111873
Wang J, Liu Y, Su S, Wei J, Rahman SE, Ning F, Christopher G, Cong W, Qiu J. Ultrasensitive Wearable Strain Sensors of 3D Printing Tough and Conductive Hydrogels. Polymers. 2019; 11(11):1873. https://doi.org/10.3390/polym11111873
Chicago/Turabian StyleWang, Jilong, Yan Liu, Siheng Su, Junhua Wei, Syed Ehsanur Rahman, Fuda Ning, Gordon Christopher, Weilong Cong, and Jingjing Qiu. 2019. "Ultrasensitive Wearable Strain Sensors of 3D Printing Tough and Conductive Hydrogels" Polymers 11, no. 11: 1873. https://doi.org/10.3390/polym11111873
APA StyleWang, J., Liu, Y., Su, S., Wei, J., Rahman, S. E., Ning, F., Christopher, G., Cong, W., & Qiu, J. (2019). Ultrasensitive Wearable Strain Sensors of 3D Printing Tough and Conductive Hydrogels. Polymers, 11(11), 1873. https://doi.org/10.3390/polym11111873