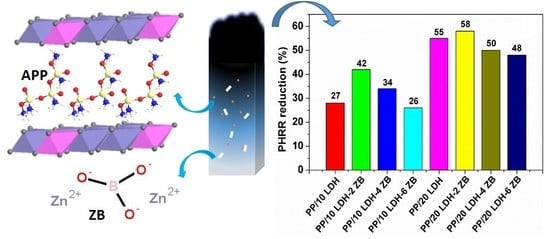
Ammonium Polyphosphate Intercalated Layered Double Hydroxide and Zinc Borate as Highly Efficient Flame Retardant Nanofillers for Polypropylene
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of Aqueous Miscible Organic (AMO)-LDH
2.3. Synthesis of Zinc Borate
2.4. Preparation of PP/APP-LDH and PP/APP-LDH/ZB Nanocomposites
2.5. Characterization of Samples
2.6. Thermal Stability and Flammability Properties
3. Results
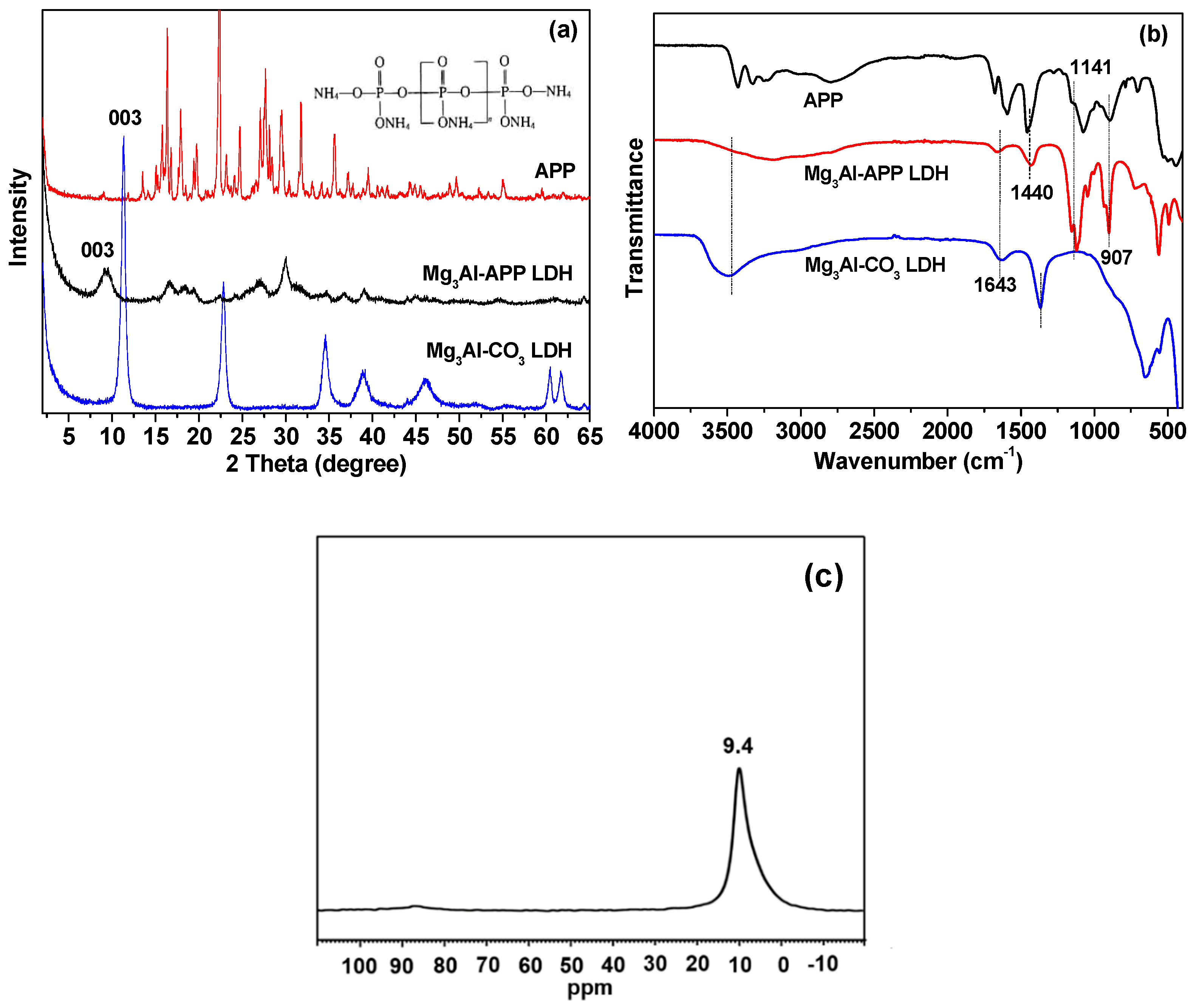
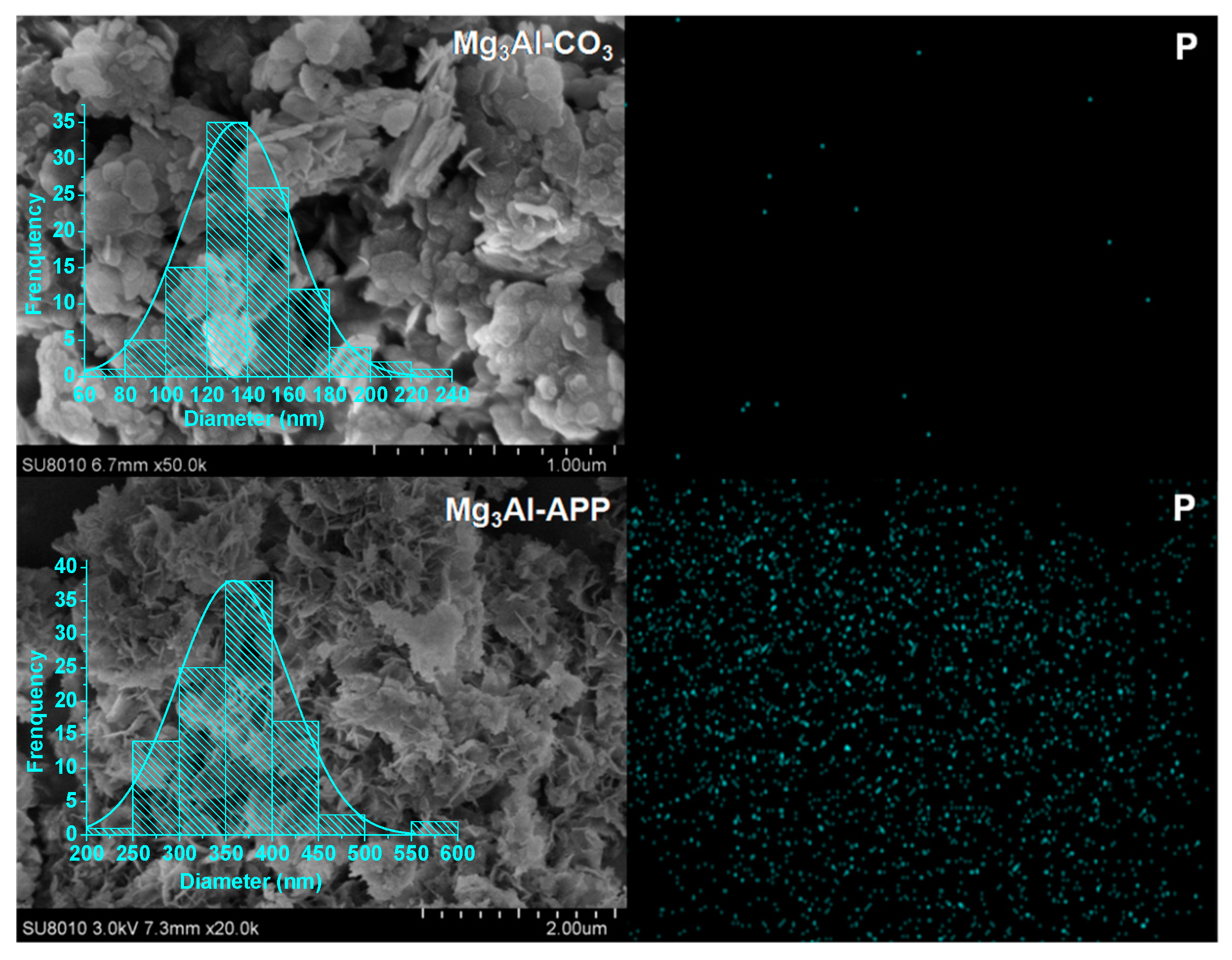
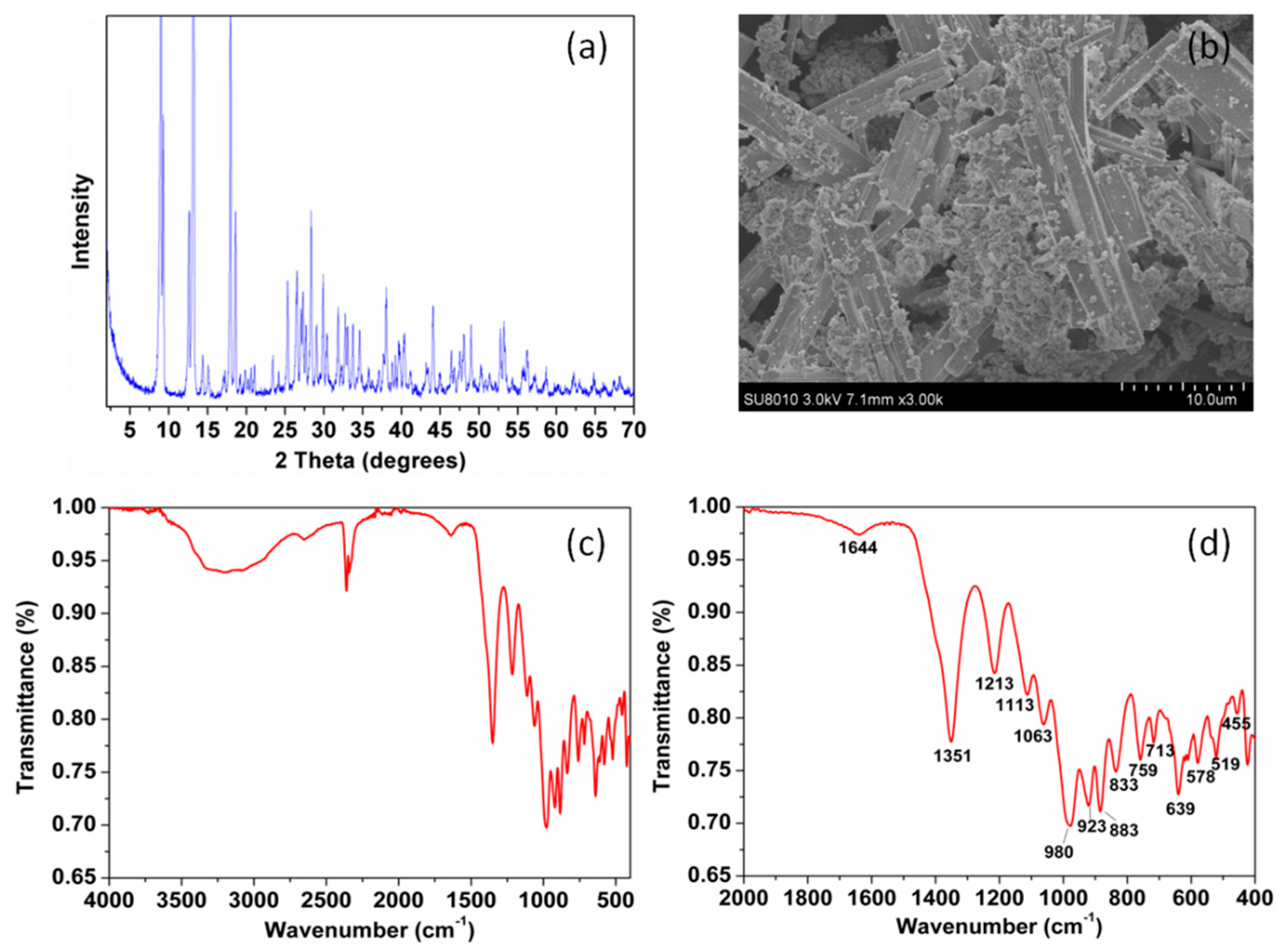
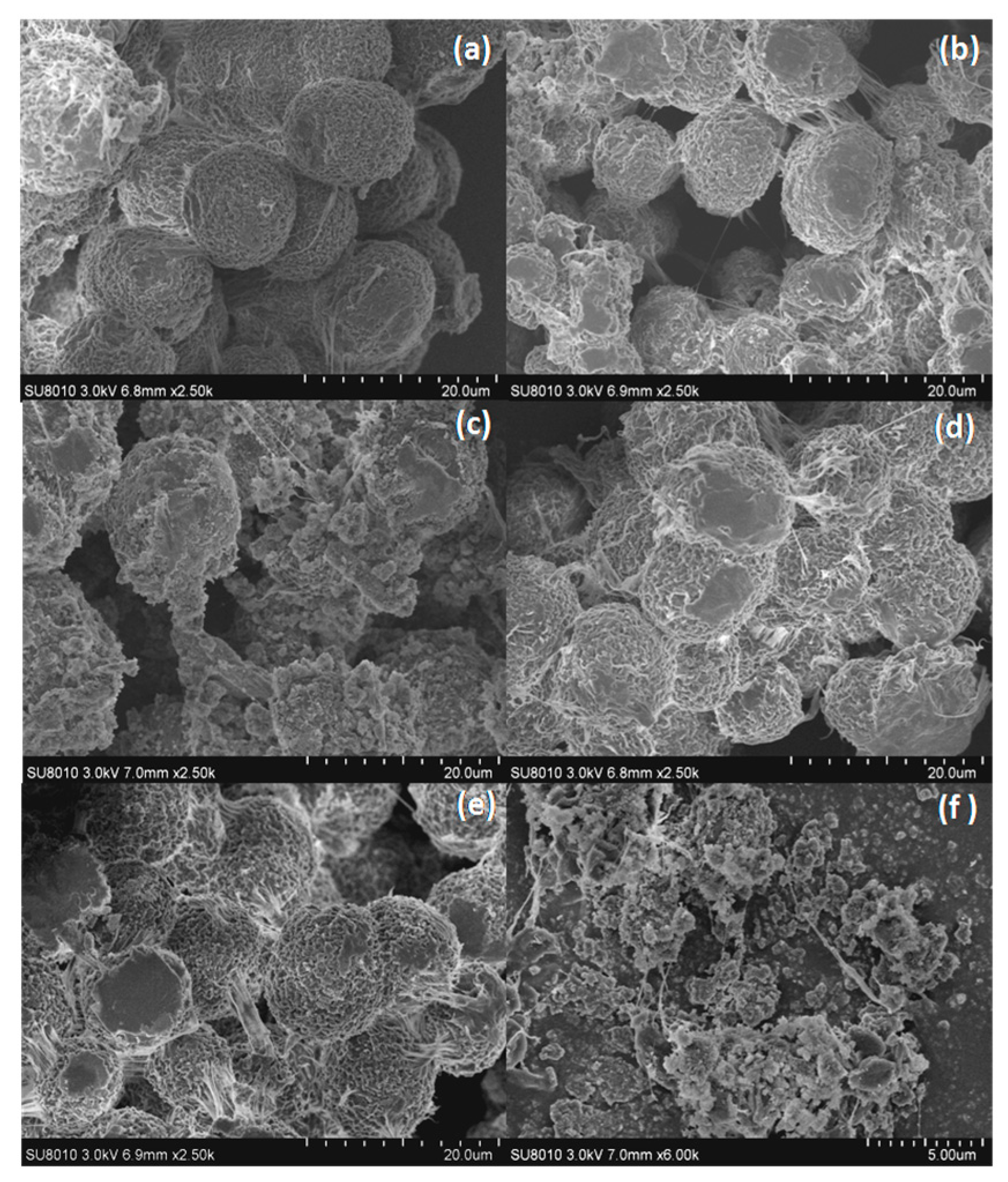
3.1. Characterization of LDHs and Zinc Borate
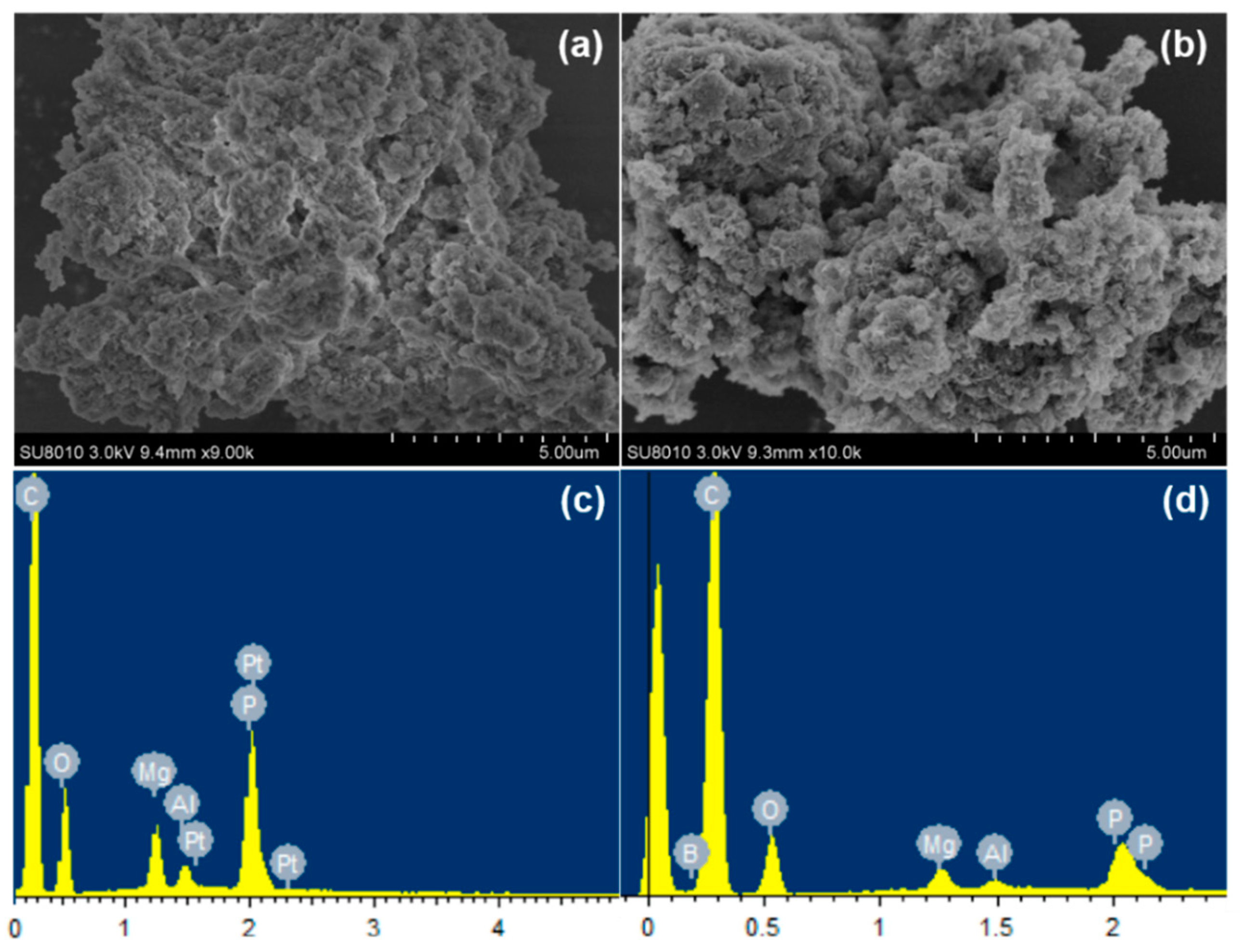
3.2. Characterization of PP/APP-LDH and PP/APP-LDH/ZB Nanocomposites
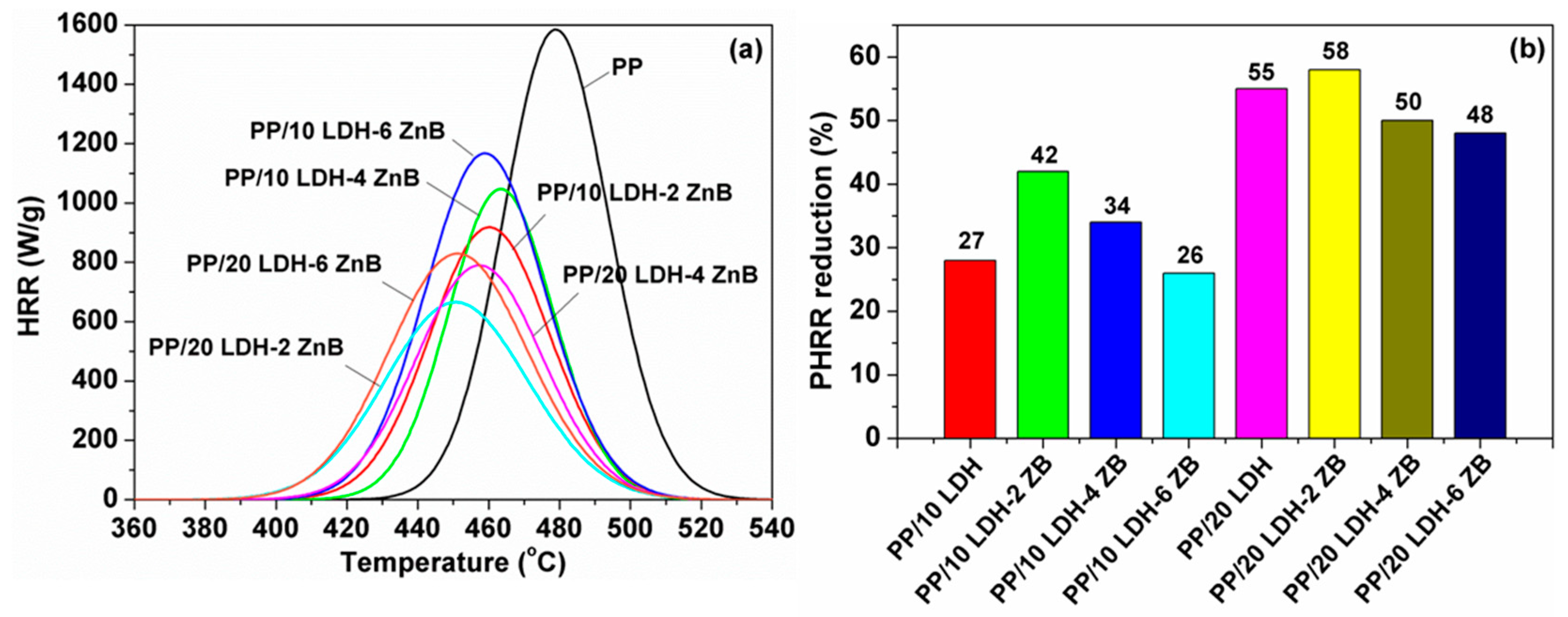
3.3. Thermal and Flammable Properties of PP-Based Nanocomposites
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Wang, L.J.; He, X.J.; Lu, H.D.; Feng, J.X.; Xie, X.L.; Su, S.P.; Wilkie, C.A. Flame retardancy of polypropylene (nano)composites containing LDH and zinc borate. Polym. Adv. Technol. 2011, 22, 1131–1138. [Google Scholar] [CrossRef]
- Lim, K.-S.; Bee, S.-T.; Sin, L.T.; Tee, T.-T.; Ratnam, C.T.; Hui, D.; Rahmat, A.R. A review of application of ammonium polyphosphate as intumescent flame retardant in thermoplastic composites. Compos. Part B Eng. 2016, 84, 155–174. [Google Scholar] [CrossRef]
- Zhou, L.; Shao, M.F.; Wei, M.; Duan, X. Advances in efficient electrocatalysts based on layered double hydroxides and their derivatives. J. Energy Chem. 2017, 26, 1094–1106. [Google Scholar] [CrossRef]
- Qin, Q.Q.; Wang, J.Y.; Zhou, T.T.; Zheng, Q.W.; Huang, L.; Zhang, Y.; Lu, P.; Umar, A.; Louis, B.; Wang, Q. Impact of organic interlayer anions on the CO2 adsorption performance of Mg-Al layered double hydroxides derived mixed oxides. J. Energy Chem. 2017, 26, 346–353. [Google Scholar] [CrossRef]
- Chu, X.Y.; Deng, T.; Zhang, W.; Wang, D.; Liu, X.F.; Zhang, C.; Qin, T.T.; Zhang, L.Y.; Zhang, B.S.; Chen, C.M.; et al. Architecture of Co-layered double hydroxide nanocages/graphene composite electrode with high electrochemical performance for supercapacitor. J. Energy Chem. 2018, 27, 507–512. [Google Scholar] [CrossRef]
- Qiu, L.; Gao, Y.S.; Zhang, C.; Yan, Q.H.; O’Hare, D.; Wang, Q. Synthesis of highly efficient flame retardant polypropylene nanocomposites with surfactant intercalated layered double hydroxides. Dalton Trans. 2018, 47, 2965–2975. [Google Scholar] [CrossRef] [PubMed]
- Guo, B.T.; Liu, Y.Z.; Zhang, Q.; Wang, F.Q.; Wang, Q.W.; Liu, Y.X.; Li, J.; Yu, H.P. Efficient flame-retardant and smoke-suppression properties of Mg-Al-layered double-hydroxide nanostructures on wood substrate. ACS Appl. Mater. Interfaces 2017, 9, 23039–23047. [Google Scholar] [CrossRef] [PubMed]
- Edenharter, A.; Feicht, P.; Diar-Bakerly, B.; Beyer, G.; Breu, J. Superior flame retardant by combining high aspect ratio layered double hydroxide and graphene oxide. Polymer 2016, 91, 41–49. [Google Scholar] [CrossRef]
- Gao, Y.S.; Wang, Q.; Wang, J.Y.; Huang, L.; Yan, X.R.; Zhang, X.; He, Q.L.; Xing, Z.P.; Guo, Z.H. Synthesis of highly efficient flame retardant HDPE nanocomposites with inorgano-LDH as nanofiller using solvent mixing method. ACS Appl. Mater. Interfaces 2014, 6, 5094–5104. [Google Scholar] [CrossRef] [PubMed]
- Ling, S.; Li, D.Q.; Li, S.F.; Wang, J.R.; Evans, D.G.; Duan, X. Structure, flame retarding and smoke suppressing properties of Zn-Mg-Al-CO3 layered double hydroxides. Chin. Sci. Bull. 2005, 50, 1101–1104. [Google Scholar]
- Shao, Z.B.; Deng, C.; Tan, Y.; Chen, M.J.; Chen, L.; Wang, Y.Z. An efficient mono-component polymeric intumescent flame retardant for polypropylene: preparation and application. ACS Appl. Mater. Interfaces 2014, 6, 7363–7370. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.B.; Ding, P.; Zhang, M.; Qu, B.J. Synergistic effects of layered double hydroxide with hyperfine magnesium hydroxide in halogen-free flame retardant EVA/HFMH/LDH nanocomposites. Polym. Degrad. Stab. 2007, 92, 1715–1720. [Google Scholar] [CrossRef]
- Levchik, S.V.; Weil, E.D. A review of recent progress in phosphorus-based flame retardant. J. Fire Sci. 2006, 24, 345–364. [Google Scholar] [CrossRef]
- Zhao, C.X.; Liu, Y.; Wang, D.Y.; Wang, D.L.; Wang, Y.Z. Synergistic effect of ammonium poluphosphate and layered double hydroxide on flame retardant properties of poly(vinyl alcohol). Polym. Degrad. Stab. 2008, 93, 1323–1331. [Google Scholar] [CrossRef]
- Wang, X.; Zhou, S.; Xing, W.Y.; Yu, B.; Feng, X.M.; Song, L.; Hu, Y. Self-assembly of Ni-Fe layered double hydroxide/graphene hybrids for reducing fire hazard in epoxy composites. J. Mater. Chem. A 2013, 1, 4383–4390. [Google Scholar] [CrossRef]
- Qian, Y.; Zhou, S.J.; Chen, X.L. Flammability and thermal degradation behavior of ethylene-vinyl acetate/layered double hydroxides/zinc borate composites. Polym. Adv. Technol. 2017, 28, 353–361. [Google Scholar] [CrossRef]
- Li, Z.; Zhang, J.H.; Dufosse, F.; Wang, D.Y. Ultrafine nickel nanocatalyst-engineering of an organic layered double hydroxide towards a super-efficient fire-safe epoxy resin via interfacial catalysis. J. Mater. Chem. A 2018, 6, 8488–8498. [Google Scholar] [CrossRef]
- Wang, X.; Spörer, Y.; Leuteritz, A.; Kuehnert, I.; Wagenknecht, U.; Heinrich, G.; Wang, D.Y. Comparative study of the synergistic effect of binary and ternary LDH with intumescent flame retardant on the properties of polypropylene composites. RSC Adv. 2015, 5, 78979–78985. [Google Scholar] [CrossRef]
- Zhao, C.X.; Peng, G.; Liu, B.L.; Jiang, Z.W. Synergistic effect of organically modified layered double hydroxide on thermal and flame-retardant properties of poly(butyl acrylate-vinyl acetate). J. Polym. Res. 2011, 18, 1971–1981. [Google Scholar] [CrossRef]
- Lewin, M. Synergistic and catalytic effects in flame retardancy of polymeric materials—An overview. J. Fire Sci. 1999, 17, 3–19. [Google Scholar] [CrossRef]
- Li, L.; Qian, Y.; Jiao, C.M. Synergistic flame retardant effects of ammonium polyphosphate in ethylene-vinyl acetate/layered double hydroxides composites. Polym. Eng. Sci. 2014, 54, 766–776. [Google Scholar] [CrossRef]
- Nyambo, C.; Kandare, E.; Wang, D.Y.; Wilkie, C.A. Flame-retarded polystyrene: Investigating chemical interactions between ammonium polyphosphate and MgAl layered double hydroxide. Polym. Degrad. Stab. 2008, 93, 1656–1663. [Google Scholar] [CrossRef]
- Kalali, E.N.; Montes, A.; Wang, X.; Zhang, L.; Shabestari, M.J.; Li, Z.; Wang, D.Y. Effect of phytic acid–modified layered double hydroxide on flammability and mechanical properties of intumescent flame retardant polypropylene system. Fire Mater. 2018, 42, 213–220. [Google Scholar] [CrossRef]
- Schubert, D.M.; Alam, F.; Visi, M.Z.; Knobler, C.B. Structural characterization and chemistry of the industrially important zinc borate Zn[B3O4(OH)3]. Chem. Mater. 2003, 15, 866–871. [Google Scholar] [CrossRef]
- Wu, X.F.; Wang, L.C.; Wu, C.; Wang, G.L.; Jiang, P.K. Flammability of EVA/IFR (APP/PER/ZB system) and EVA/IFR/synergist (CaCO3, NG, and EG) composites. J. Appl. Polym. Sci. 2012, 126, 1917–1928. [Google Scholar] [CrossRef]
- Bourbigot, S.; Bras, M.L.; Leeuwendal, R.; Shen, K.K.; Schubert, D. Recent advances in the use of zinc borates in flame retardancy of EVA. Polym. Degrad. Stab. 1999, 64, 419–425. [Google Scholar] [CrossRef]
- Shi, X.X.; Xiao, Y.; Li, M.; Yuan, L.J.; Sun, J.T. Synthesis of an industrially important zinc borate 2ZnO·3B2O3·3H2O by a rheological phase reaction method. Powder Technol. 2008, 186, 263–266. [Google Scholar] [CrossRef]
- Gao, Y.S.; Zhang, Y.; Williams, G.R.; O’are, D.; Wang, Q. Layered double hydroxide-oxidized carbon nanotube hybrids as highly efficient flame retardant nanofillers for polypropylene. Sci. Rep. 2016, 6, 35502. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, Z.C.; Xue, Z.H.; Yang, B.J.; Wang, B.N.; Peng, X.H. One-pot In Situ Synthesis of hollow layered double hydroxide ammonium polyphosphate nanoshells toward flame retardant. Chem. Lett. 2014, 43, 1879–1881. [Google Scholar] [CrossRef]
- Occelli, M.L.; Olivier, J.P.; Auroux, A.; Kalwei, M.; Eckert, H. Basicity and Porosity of a Calcined Hydrotalcite-Type Material from Nitrogen Porosimetry and Adsorption Microcalorimetry Methods. Chem. Mater. 2003, 15, 4231–4238. [Google Scholar] [CrossRef]
- Gao, Y.S.; Wang, Q.; Qiu, L.; Wu, J.W.; Yan, X.R.; Umar, A.; Guo, J.; Zhang, X.; Wang, J.Y.; Guo, Z.H. Ethylene-vinyl acetate/LDH nanocomposites with enhanced thermal stability, flame retardancy, and rheological property. Polym. Compos. 2016, 37, 3449–3459. [Google Scholar] [CrossRef]
- Gao, Y.H.; Liu, Z.H. Synthesis and thermochemistry of two zinc borates, Zn2B6O11·7H2O and Zn3B10O18·14H2O. Thermochim. Acta 2009, 484, 27–31. [Google Scholar] [CrossRef]
- Li, J.; Xia, S.P.; Gao, S.Y. FT-IR and Raman spectroscopic study of hydrated borates. Spectrochim. Acta 1995, 51, 519–532. [Google Scholar] [CrossRef]
- Guo, Y.W.; Mao, L.; Rong, F.; Liu, Z.H. Preparation of Zn3B10O18·14H2O nanomaterials and their thermochemical properties. Thermochim. Acta 2012, 539, 56–61. [Google Scholar] [CrossRef]
- Patra, N.; Salerno, M.; Cozzoli, P.D.; Athanassiou, A. Surfactant-induced thermomechanical and morphological changes in TiO2-polystyrene nanocomposites. J. Colloid Interface Sci. 2013, 405, 103–108. [Google Scholar] [CrossRef] [PubMed]
- Patra, N.; Salerno, M.; Cozzoli, P.D.; Barone, A.C.; Ceseracciu, L.; Pignatelli, F.; Carzino, R.; Marini, L.; Athanassiou, A. Thermal and mechanical characterization of poly(methyl methacrylate) nanocomposites filled with TiO2 nanorods. Compos. Part B Eng. 2012, 43, 3114–3119. [Google Scholar] [CrossRef]
- Giraud, S.; Bourbigot, S.; Rochery, M.; Vroman, I.; Tighzert, L.; Delobel, R. Microencapsulation of phosphate: application to flame retarded coated cotton. Polym. Degrad. Stab. 2002, 77, 285–297. [Google Scholar] [CrossRef]
- Li, Y.M.; Deng, C.; Long, J.W.; Huang, S.C.; Zhao, Z.Y.; Wang, Y.Z. Improving fire retardancy of ceramifiable polyolefin system via a hybrid of zinc borate@melamine cyanurate. Polym. Degrad. Stab. 2018, 153, 325–332. [Google Scholar] [CrossRef]

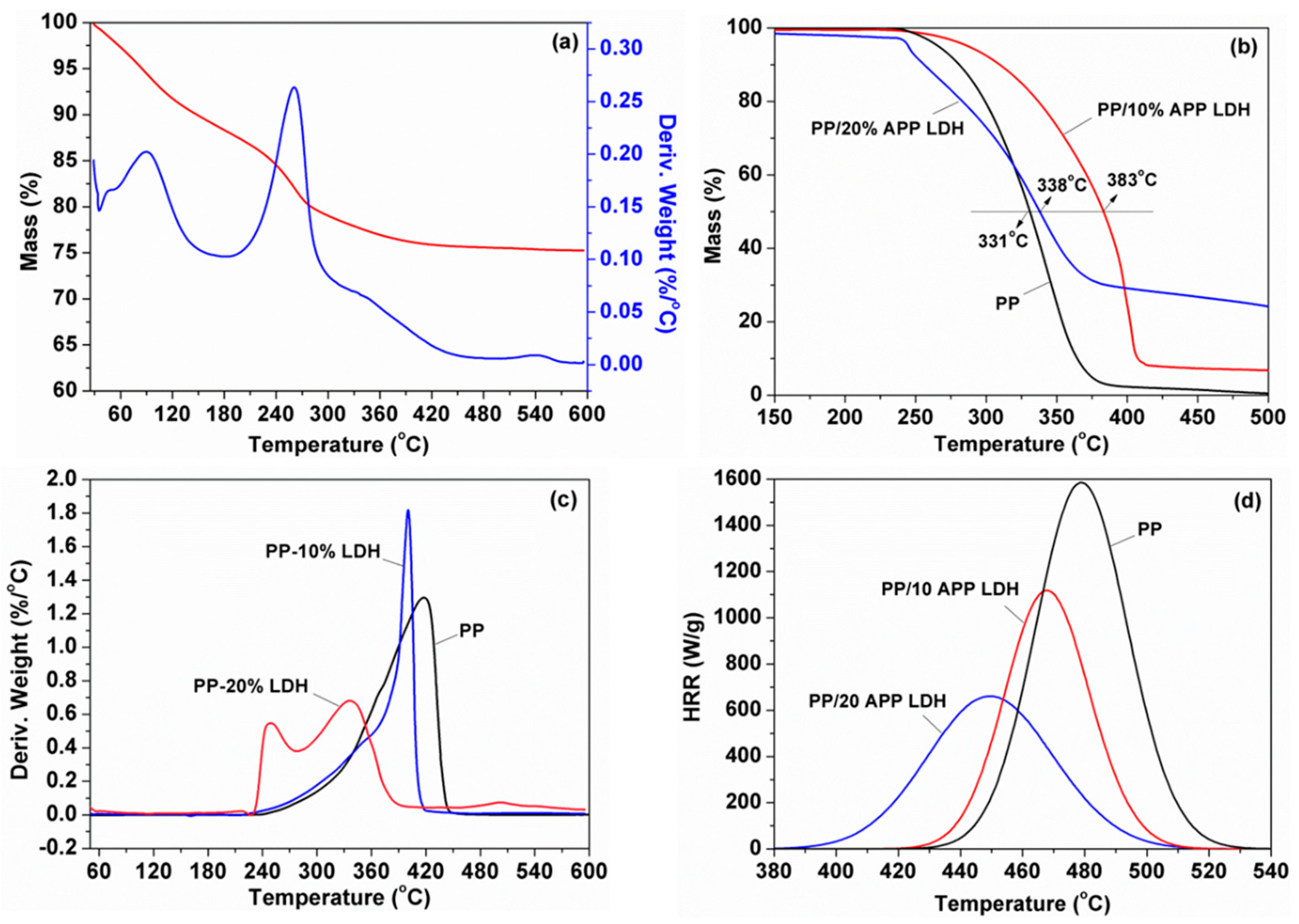


| Samples | T10% (oC) | ΔT10% (°C) | T50% (°C) | ΔT50% (°C) | Char(E) b (wt %) | Char(T) b (wt %) |
|---|---|---|---|---|---|---|
| PP | 280 | NA | 331 | NA | 0.7 | - |
| PP/10% APP-LDH | 310 | 30 | 383 | 52 | 7.0 | 6.8 |
| PP/20% APP-LDH | 257 | −23 | 336 | 7 | 24.1 | 15.6 |
| PP/10% APP LDH-2% ZB | 308 | 28 | 367 | 36 | 12.0 | 9.6 |
| PP/10% APP LDH-4% ZB | 288 | 8 | 367 | 36 | 15.4 | 11.1 |
| PP/10% APP LDH-6% ZB | 281 | 1 | 362 | 31 | 17.6 | 12.6 |
| PP/20% APP LDH-2% ZB | 255 | −25 | 341 | 10 | 26.3 | 17.1 |
| PP/20% APP LDH-4% ZB | 267 | −13 | 358 | 27 | 28.3 | 18.5 |
| PP/20% APP LDH-6% ZB | 259 | −21 | 360 | 29 | 27.4 | 20.0 |
| Samples | PHRR (Wg−1) | Reduction (%) | THR (kJg−1) | Tmax (°C) | HRC (Jg−1K−1) |
|---|---|---|---|---|---|
| PP | 1585 | NA | 47.6 | 479 | 1163 |
| PP/10% CO3-LDH | 1422 | 10 | 43 | 481.8 | 1071 |
| PP/20% CO3-LDH | 1099 | 31 | 37.7 | 484.5 | 822 |
| PP/10% APP LDH | 1154 | 27 | 42.5 | 468 | 1097 |
| PP/20% APP LDH | 707 | 55 | 34.0 | 455 | 661 |
| PP/10% APP LDH-2% ZB | 918 | 42 | 40.3 | 460 | 869 |
| PP/10% APP LDH-4% ZB | 1047 | 34 | 40.0 | 463 | 990 |
| PP/10% APP LDH-6% ZB | 1167 | 26 | 37.3 | 459 | 880 |
| PP/20% APP LDH-2% ZB | 668 | 58 | 34.5 | 451 | 636 |
| PP/20% APP LDH-4% ZB | 790 | 50 | 35.0 | 458 | 750 |
| PP/20% APP LDH-6% ZB | 830 | 48 | 29.7 | 452 | 619 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gao, Y.; Wang, Q.; Lin, W. Ammonium Polyphosphate Intercalated Layered Double Hydroxide and Zinc Borate as Highly Efficient Flame Retardant Nanofillers for Polypropylene. Polymers 2018, 10, 1114. https://doi.org/10.3390/polym10101114
Gao Y, Wang Q, Lin W. Ammonium Polyphosphate Intercalated Layered Double Hydroxide and Zinc Borate as Highly Efficient Flame Retardant Nanofillers for Polypropylene. Polymers. 2018; 10(10):1114. https://doi.org/10.3390/polym10101114
Chicago/Turabian StyleGao, Yanshan, Qiang Wang, and Weiran Lin. 2018. "Ammonium Polyphosphate Intercalated Layered Double Hydroxide and Zinc Borate as Highly Efficient Flame Retardant Nanofillers for Polypropylene" Polymers 10, no. 10: 1114. https://doi.org/10.3390/polym10101114
APA StyleGao, Y., Wang, Q., & Lin, W. (2018). Ammonium Polyphosphate Intercalated Layered Double Hydroxide and Zinc Borate as Highly Efficient Flame Retardant Nanofillers for Polypropylene. Polymers, 10(10), 1114. https://doi.org/10.3390/polym10101114