Preparation and Single Crystal Structure Determination of the First Biobased Furan-Polydiacetylene Using Topochemical Polymerization
Abstract
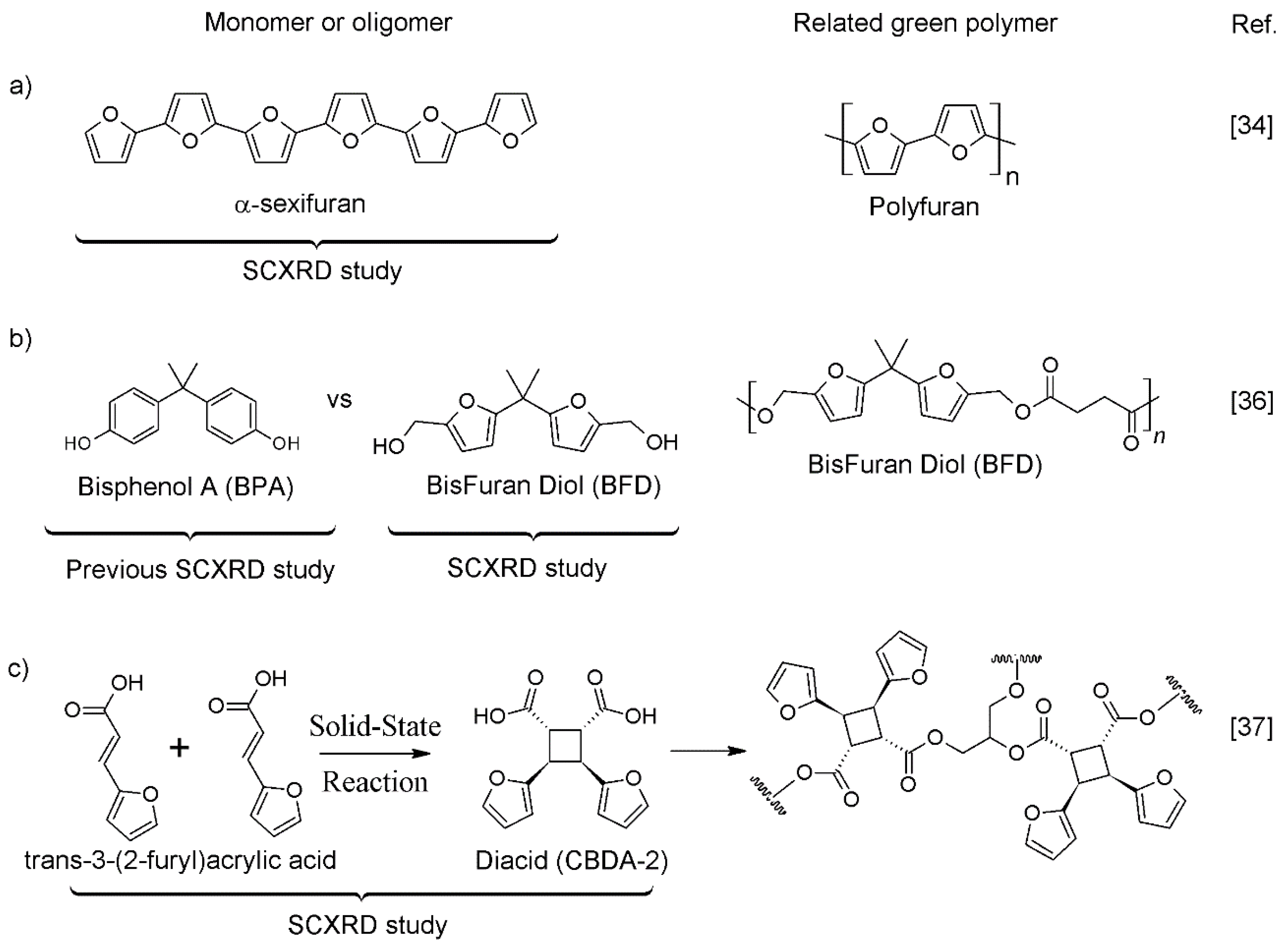
1. Introduction
2. Materials and Methods
2.1. Synthesis and Recrystallization
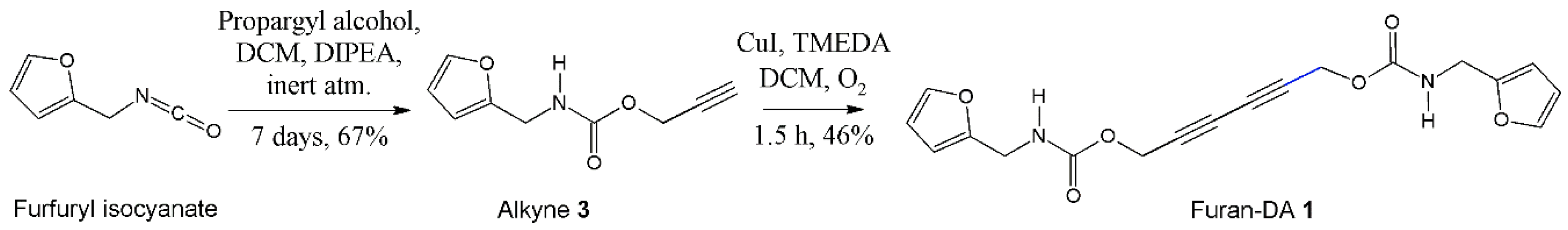
2.1.1. Synthesis of Alkyne 3
2.1.2. Synthesis and Recrystallization of Furan-DA 1
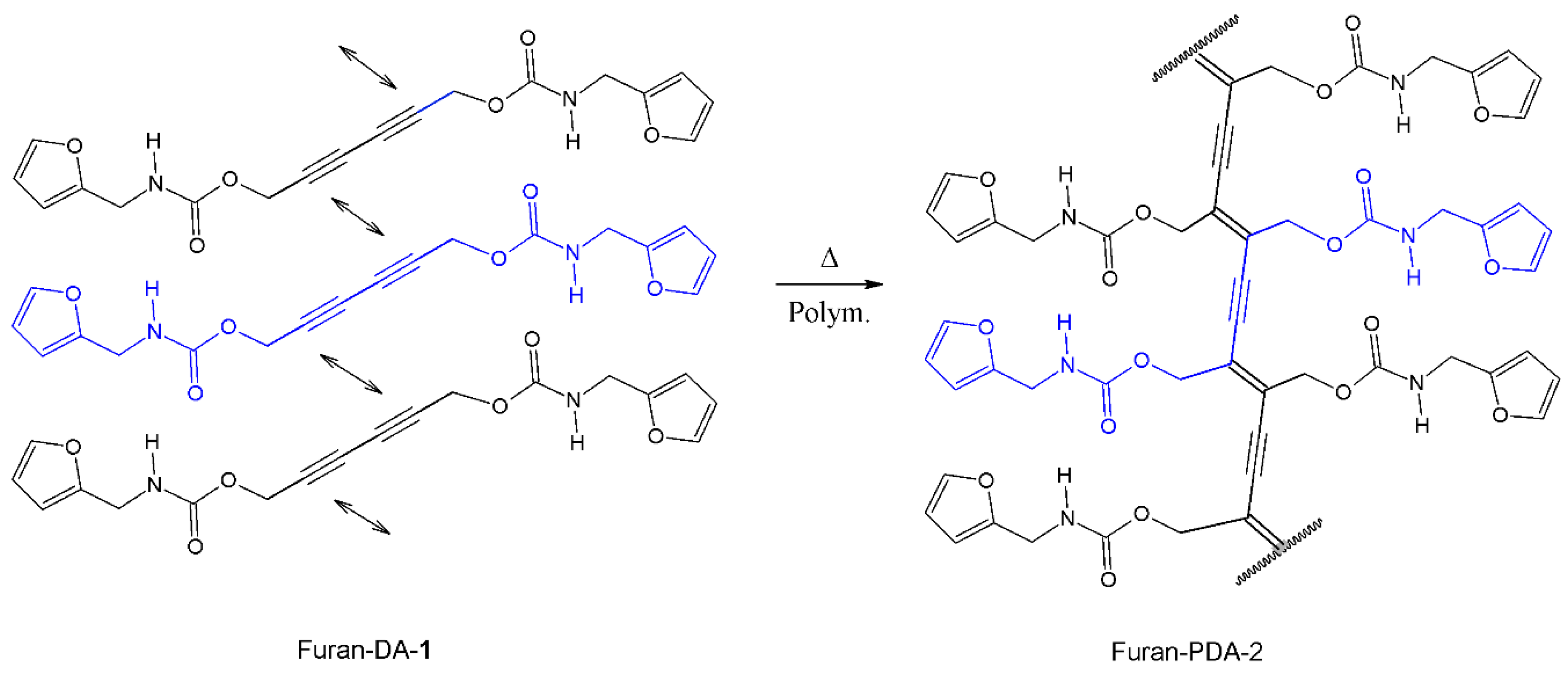
2.2. Topochemical Polymerization of Furan-DA 1 to Afford Furan-PDA 2
2.3. Single Crystal Structure Analysis of Furan-DA 1 and Furan-PDA 2
3. Results and Discussion
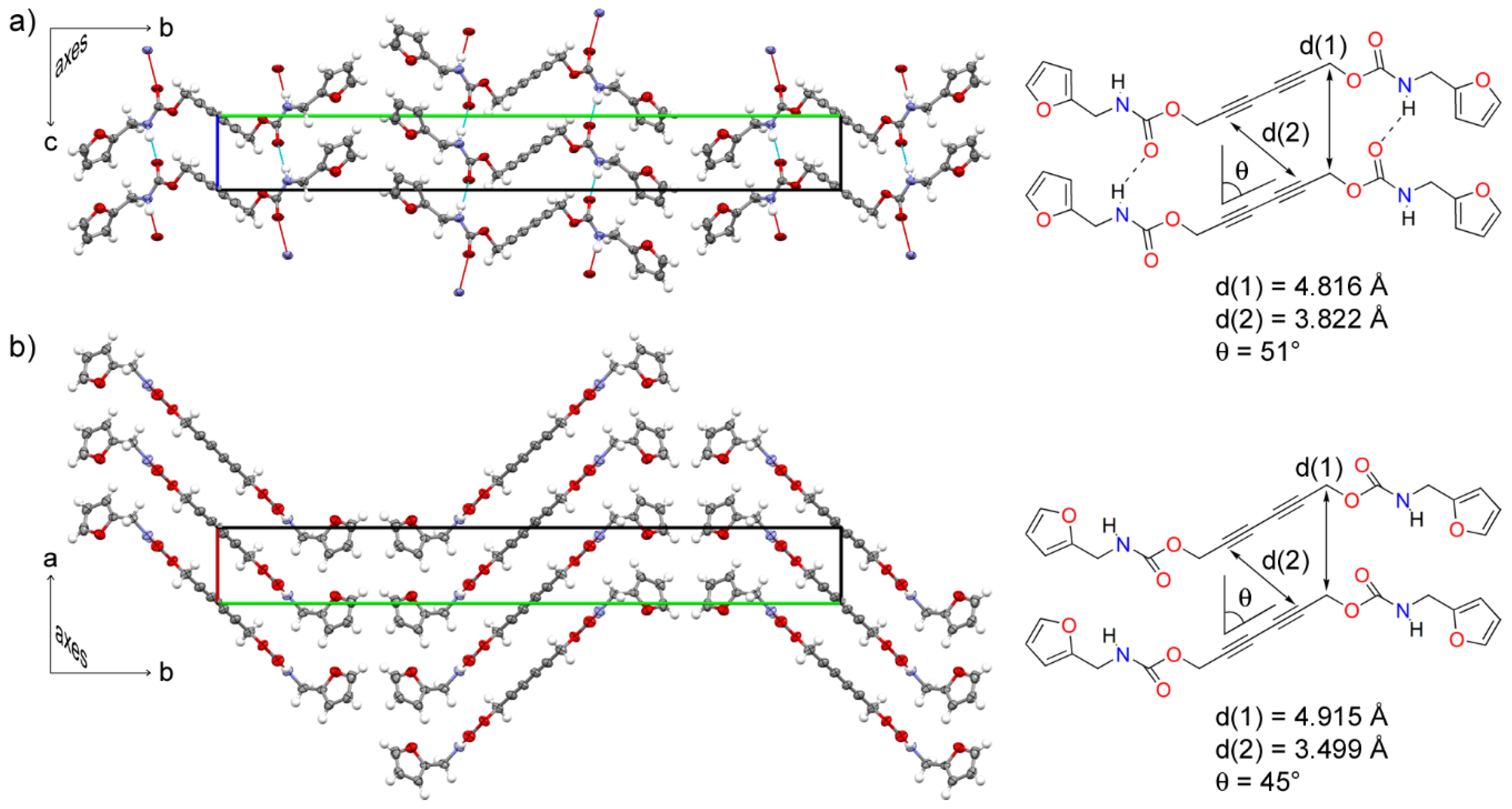
3.1. Single Crystal Structure of Furan-DA 1
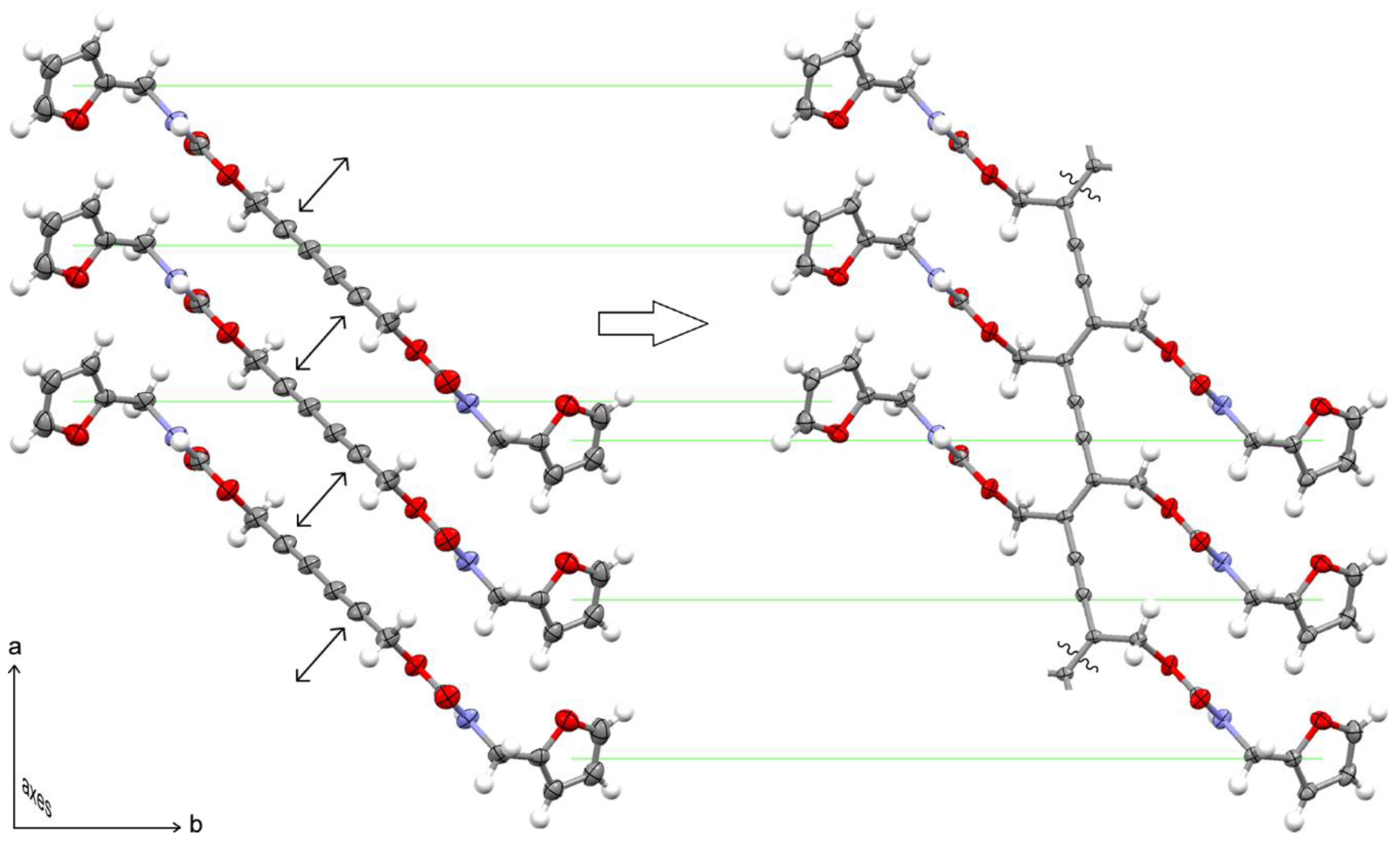
3.2. Topochemical Polymerization of Furan-DA 1 to Furan-PDA 2
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Isikgor, F.H.; Becer, C.R. Lignocellulosic Biomass: A Sustainable Platform for Production of Bio-Based Chemicals and Polymers. Polym. Chem. 2015, 6, 4497–4559. [Google Scholar] [CrossRef]
- Decostanzi, M.; Auvergne, R.; Boutevin, B.; Caillol, S. Biobased phenol and furan derivative coupling for the synthesis of functional monomers. Green Chem. 2019, 21, 724–747. [Google Scholar] [CrossRef]
- Gandini, A.; Lacerda, T.; Carvalho, A.; Trovatti, E. Progress of Polymers from Renewable Resources: Furans, Vegetable Oils, and Polysaccharides. Chem. Rev. 2016, 116, 1637–1669. [Google Scholar] [CrossRef]
- Gandini, A. Polymers from Renewable Resources: A Challenge for the Future of Macromolecular Materials. Macromolecules 2008, 41, 9491–9504. [Google Scholar] [CrossRef]
- John, G.; Nagarajan, S.; Vemula, P.K.; Silverman, J.R.; Pillai, C.K.S. Natural Monomers: A Mine for Functional and Sustainable Materials–Occurrence, Chemical Modification and Polymerization. Prog. Polym. Sci. 2019, 92, 158–209. [Google Scholar] [CrossRef]
- Yao, K.; Tang, C. Controlled Polymerization of Next-Generation Renewable Monomers and Beyond. Macromolecules 2013, 46, 1689–1712. [Google Scholar] [CrossRef]
- Sharif, A.; Hoque, M.E. Renewable Resource-Based Polymers. In Bio-based Polymers and Nanocomposites; Sanyang, M., Jawaid, M., Eds.; Springer International: Basel, Switzerland, 2019; pp. 1–28. [Google Scholar]
- Yuanchun, S. Biomass: To Win the Future; Lexington Books: Lanham, Maryland, 2013; pp. 173–174. [Google Scholar]
- Mekonnen, T.; Mussone, P.; Khalil, H.; Bressler, D. Progress in bio-based plastics and plasticizing modifications. J. Mater. Chem. A 2013, 1, 13379–13398. [Google Scholar] [CrossRef]
- Ten, E.; Vermerris, W. Functionalized Polymers from Lignocellulosic Biomass: State of the Art. Polymers 2013, 5, 600–642. [Google Scholar] [CrossRef]
- Motagamwala, A.H.; Won, W.; Sener, C.; Alonso, D.M.; Maravelias, C.T.; Dumesic, J.A. Toward biomass-derived renewable plastics: Production of 2,5-furandicarboxylic acid from fructose. Sci. Adv. 2018, 4, eaap9722. [Google Scholar] [CrossRef]
- Storz, H.; Vorlop, K.D. Bio-based plastics: Status, challenges and trends. Appl. Agric. For. Res. 2013, 63, 321–332. [Google Scholar] [CrossRef]
- Luzi, F.; Torre, L.; Kenny, J.M.; Puglia, D. Bio- and Fossil-Based Polymeric Blends and Nanocomposites for Packaging: Structure–Property Relationship. Materials 2019, 12, 471. [Google Scholar] [CrossRef]
- Poulopoulou, N.; Kasmi, N.; Bikiaris, D.N.; Papageorgiou, D.G.; Floudas, G.; Papageorgiou, G.Z. Sustainable Polymers from Renewable Resources: Polymer Blends of Furan-Based Polyesters. Macromol. Mater. Eng. 2018, 303, 1800153. [Google Scholar] [CrossRef]
- Mariscal, R.; Maireles-Torres, P.; Ojeda, M.; Sábada, I.; López Granados, M. Furfural: A renewable and versatile platform molecule for the synthesis of chemicals and fuels. Energy Environ. Sci. 2016, 9, 1144–1189. [Google Scholar] [CrossRef]
- Lee, Y.; Kwon, E.E.; Lee, J. Polymers derived from hemicellulosic parts of lignocellulosic biomass. Rev. Environ. Sci. Biotechnol. 2019, 18, 317–334. [Google Scholar] [CrossRef]
- Sousa, A.; Vilela, C.; Fonseca, A.; Matos, M.; Freire, C.; Gruter, G.; Coelho, J.; Silvestre, A. Biobased polyesters and other polymers from 2,5-furandicarboxylic acid: a tribute to furan excellency. Polym. Chem. 2015, 6, 5961–5983. [Google Scholar] [CrossRef]
- Gandini, A. Furan Monomers and their Polymers: Synthesis, Properties and Applications. In Biopolymers–New Materials for Sustainable Films and Coatings; Plackett, D., Ed.; Wiley: New York, NY, USA, 2011; pp. 179–209. [Google Scholar]
- De Jong, E.; Dam, M.A.; Sipos, L.; Gruter, G.-J.M. Furandicarboxylic Acid (FDCA), A Versatile Building Block for a Very Interesting Class of Polyesters. In Biobased Monomers, Polymers, and Materials; Smith, P.B., Gross, R.A., Eds.; ACS Symposium Series 1105; American Chemical Society: Washington, DC, USA, 2012; pp. 1–13. [Google Scholar]
- Amarasekara, A.S. 5-Hydroxymethylfurfural Based Polymers. In Renewable Polymers: Synthesis, Processing, and Technology; Mittal, V., Ed.; Wiley: Hoboken, NJ, USA, 2011; pp. 381–428. [Google Scholar]
- Gandini, A. Furans as offspring of sugars and polysaccharides and progenitors of a family of remarkable polymers: A review of recent progress. Polym. Chem. 2010, 1, 245–251. [Google Scholar] [CrossRef]
- González-Tejera, M.J.; Sánchez de la Blanca, E.; Carrillo, I. Polyfuran conducting polymers: Synthesis, properties, and applications. Synth. Met. 2008, 158, 165–189. [Google Scholar] [CrossRef]
- Zhang, J.; Liang, Q.; Xie, W.; Peng, L.; He, L.; He, Z.; Chowdhury, S.P.; Christensen, R.; Ni, Y. An Eco-Friendly Method to Get a Bio-Based Dicarboxylic Acid Monomer 2,5-Furandicarboxylic Acid and Its Application in the Synthesis of Poly(hexylene 2,5-furandicarboxylate) (PHF). Polymers 2019, 11, 197. [Google Scholar] [CrossRef]
- Zeng, C.; Seino, H.; Ren, J.; Hatanaka, K.; Yoshie, N. Bio-Based Furan Polymers with Self-Healing Ability. Macromolecules 2013, 46, 1794–1802. [Google Scholar] [CrossRef]
- Feng, Z.; Hu, J.; Yu, B.; Tian, H.; Zuo, H.; Ning, N.; Tian, M.; Zhang, L. Environmentally Friendly Method to Prepare Thermo-Reversible, Self-Healable Biobased Elastomers by One-Step Melt Processing. ACS Appl. Polym. Mater. 2019, 1, 169–177. [Google Scholar] [CrossRef]
- Gronau, G.; Krishnaji, S.T.; Kinahan, M.E.; Giesa, T.; Wong, J.Y.; Kaplan, D.L.; Buehler, M.J. A review of combined experimental and computational procedures for assessing biopolymer structure–process–property relationships. Biomaterials 2012, 33, 8240–8255. [Google Scholar] [CrossRef]
- Murthy, N.S. Recent developments in polymer characterization using x-ray diffraction. Rigaku J. 2004, 21, 15–24. [Google Scholar]
- Favirov, S. Methods for the Characterization and Investigation of Polymers. In Fundamentals of Polymer Science for Engineers; Wiley-VCH: Weinheim, Germany, 2017; pp. 189–218. [Google Scholar]
- Tencé-Girault, S.; Lebreton, S.; Bunau, O.; Dang, P.; Bargain, F. Simultaneous SAXS-WAXS Experiments on Semi-Crystalline Polymers: Example of PA11 and Its Brill Transition. Crystals 2019, 9, 271. [Google Scholar] [CrossRef]
- Maini, L.; Gigli, M.; Gazzano, M.; Lotti, N.; Bikiaris, D.N.; Papageorgiou, G.Z. Structural Investigation of Poly(ethylene furanoate) Polymorphs. Polymers 2018, 10, 296. [Google Scholar] [CrossRef]
- Mao, Y.; Kriegel, R.; Bucknall, D. The crystal structure of Poly(ethylene furanoate). Polymer 2016, 102, 308–314. [Google Scholar] [CrossRef]
- Zhu, J.; Cai, J.; Xie, W.; Chen, P.-H.; Gazzano, M.; Scandola, M.; Gross, R.A. Poly(butylene 2,5-furan dicarboxylate), a Biobased Alternative to PBT: Synthesis, Physical Properties, and Crystal Structure. Macromolecules 2013, 46, 796–804. [Google Scholar] [CrossRef]
- Hong, S.; Min, K.-D.; Nam, B.-U.; Park, O.O. High molecular weight bio furan-based co-polyesters for food packaging applications: Synthesis, characterization and solid-state polymerization. Green Chem. 2016, 18, 5142–5150. [Google Scholar] [CrossRef]
- Gidron, O.; Diskin-Posner, Y.; Bendikov, M. α-Oligofurans. J. Am. Chem. Soc. 2010, 132, 2148–2150. [Google Scholar] [CrossRef]
- Gidron, O.; Bendikov, M. α-Oligofurans: An Emerging Class of Conjugated Oligomers for Organic Electronics. Angew. Chem. Int. Ed. 2014, 53, 2546–2555. [Google Scholar] [CrossRef]
- Gaitonde, V.; Lee, K.; Kirschmaum, K.; Sucheck, S.J. Bio-Based Bisfuran: Synthesis, Crystal Structure and Low Molecular Weight Amorphous Polyester. Tetrahedron Lett. 2014, 55, 4141–4145. [Google Scholar] [CrossRef]
- Wang, Z.D.; Elliott, Q.; Wang, Z.; Setien, R.A.; Puttkammer, J.; Ugrinov, A.; Lee, J.; Webster, D.C.; Chu, Q.R. Furfural-Derived Diacid Prepared by Photoreaction for Sustainable Materials Synthesis. ACS Sustain. Chem. Eng. 2018, 6, 8136–8141. [Google Scholar] [CrossRef]
- Barancelli, D.A.; Mantovani, A.C.; Jesse, C.; Nogueira, C.W.; Zeni, G. Synthesis of Natural Polyacetylenes Bearing Furan Rings. J. Nat. Prod. 2009, 72, 857–860. [Google Scholar] [CrossRef]
- Fiandanese, V.; Bottalico, D.; Marchese, G.; Punzi, A. Synthesis of naturally occurring polyacetylenes via a bis-silylated diyne. Tetrahedron 2006, 62, 5126–5132. [Google Scholar] [CrossRef]
- Knechtle, P.; Diefenbacher, M.; Greve, K.B. The natural diyne-furan fatty acid EV-086 is an inhibitor of fungal delta-9 fatty acid desaturation with efficacy in a model of skin dermatophytosis. Antimicrob. Agents Chemother. 2014, 58, 455–466. [Google Scholar] [CrossRef]
- Wen, J.T.; Roper, J.M.; Tsutsui, H. Polydiacetylene Supramolecules: Synthesis, Characterization, and Emerging Applications. Ind. Eng. Chem. Res. 2018, 57, 9037–9053. [Google Scholar] [CrossRef]
- Jelinek, R.; Ritenberg, M. Polydiacetylenes—Recent molecular advances and applications. RSC Adv. 2013, 3, 21192–21201. [Google Scholar] [CrossRef]
- Qian, X.; Städler, B. Recent Developments in Polydiacetylene-Based Sensors. Chem. Mater. 2019, 31, 1196–1222. [Google Scholar] [CrossRef]
- Lee, S.; Kim, J.Y.; Chen, X.; Yoon, J. Recent progress in stimuli-induced polydiacetylenes for sensing temperature, chemical and biological targets. Chem. Commun. 2016, 52, 9178–9196. [Google Scholar] [CrossRef]
- Jelinek, R. Polydiacetylene Bio- and Chemo-Sensors. In Encyclopedia of Analytical Chemistry; Meyers, R.A., Ed.; Wiley: New York, NY, USA, 2019; pp. 1–24. [Google Scholar]
- Ahn, D.J.; Lee, S.; Kim, J.-M. Rational Design of Conjugated Polymer Supramolecules with Tunable Colorimetric Responses. Adv. Funct. Mater. 2009, 19, 1483–1496. [Google Scholar] [CrossRef]
- Yoon, B.; Lee, S.; Kim, J.-M. Recent conceptual and technological advances in polydiacetylene-based supramolecular chemosensors. Chem. Soc. Rev. 2009, 38, 1958–1968. [Google Scholar] [CrossRef]
- Sun, X.; Chen, T.; Huang, S.; Li, L.; Peng, H. Chromatic polydiacetylene with novel sensitivity. Chem. Soc. Rev. 2010, 39, 4244–4257. [Google Scholar] [CrossRef]
- Chen, X.; Zhou, G.; Peng, X.; Yoon, J. Biosensors and chemosensors based on the optical responses of polydiacetylenes. Chem. Soc. Rev. 2012, 41, 4610–4630. [Google Scholar] [CrossRef]
- Huo, J.; Deng, Q.; Fan, T.; He, G.; Hu, X.; Hong, X.; Chen, H.; Luo, S.; Wang, Z.; Chen, D. Advances in polydiacetylene development for the design of side chain groups in smart material applications—A mini review. Polym. Chem. 2017, 8, 7438. [Google Scholar] [CrossRef]
- Ortiz-Cervantes, C.; Román-Román, P.I.; Vazquez-Chavez, J.; Hernández-Rodríguez, M.; Solis-Ibarra, D. Thousand-fold Conductivity Increase in 2D Perovskites by Polydiacetylene Incorporation and Doping. Angew. Chem. Int. Ed. 2018, 57, 13882–13886. [Google Scholar] [CrossRef]
- Marikhin, V.A.; Guk, E.G.; Myasnikova, L.P. New approach to achieving the potentially high conductivity of polydiacetylene. Phys. Solid State 1997, 39, 686–689. [Google Scholar] [CrossRef]
- Tabata, H.; Tokoyama, H.; Yamakado, H.; Okuno, T. Preparation and properties of two-legged ladder polymers based on polydiacetylenes. J. Mater. Chem. 2012, 22, 115–122. [Google Scholar] [CrossRef]
- Xu, X.-Q.; He, Y.; Liu, H.; Wang, Y. Polydiacetylene–Polyurethane Crisscross Elastomer as an Intrinsic Shape Memory Conductive Polymer. ACS Macro Lett. 2019, 8, 409–413. [Google Scholar] [CrossRef]
- Zhang, X.; Dong, H.; Hu, W. Organic Semiconductor Single Crystals for Electronics and Photonics. Adv. Mater. 2018, 30, 1801048. [Google Scholar] [CrossRef]
- Ulaganathan, M.; Varghese Hansen, R.; Drayton, N.; Hingorani, H.; Kutty, R.G.; Joshi, H.; Sreejith, S.; Liu, Z.; Yang, J.; Zhao, Y. Photopolymerization of Diacetylene on Aligned Multiwall Carbon Nanotube Microfibers for High-Performance Energy Devices. ACS Appl. Mater. Interfaces 2016, 8, 32643–32648. [Google Scholar] [CrossRef]
- Takami, K.; Kuwahara, Y.; Ishii, T.; Akai-Kasaya, M.; Saito, A.; Aono, M. Significant increase in conductivity of polydiacetylene thin film induced by iodine doping. Surface Sci. Lett. 2005, 591, 273–279. [Google Scholar] [CrossRef]
- Yao, Y.; Dong, H.; Liu, F.; Russel, T.P.; Hu, W. Approaching Intra- and Interchain Charge Transport of Conjugated Polymers Facilely by Topochemical Polymerized Single Crystals. Adv. Mater. 2017, 29, 1701251. [Google Scholar] [CrossRef] [PubMed]
- Matsuda, H.; Nakanishi, H.; Kato, S.-I.; Kato, M. Conductivity of polydiacetylene with π-conjugation between polymer backbone and substituents. J. Polym. Sci. Part A Polym. Chem. 1987, 25, 1663–1669. [Google Scholar] [CrossRef]
- Tang, Z.; Li, M.; Song, M.; Jiang, L.; Li, J.; He, Y.; Zhou, L. Good conductivity of a single component polydiacetylene film. Org. Electron. 2017, 49, 174–178. [Google Scholar] [CrossRef]
- Tahir, M.N.; Nyayachavadi, A.; Morin, J.-F.; Rondeau-Gagné, S. Recent progress in the stabilization of supramolecular assemblies with functional polydiacetylenes. Polym. Chem. 2018, 9, 3019–3028. [Google Scholar] [CrossRef]
- Halasz, I. Single-Crystal-to-Single-Crystal Reactivity: Gray, Rather than Black or White. Cryst. Growth Des. 2010, 10, 2817–2823. [Google Scholar] [CrossRef]
- Lauher, J.W.; Fowler, F.W. Single-Crystal-to-Single-Crystal Topochemical Polymerizations by Design. Acc. Chem. Res. 2008, 41, 1215–1229. [Google Scholar] [CrossRef] [PubMed]
- Curtis, S.M.; Le, N.; Nguyen, T.; Ouyang, X.; Tran, T.; Fowler, F.W.; Lauher, J.W. What have We Learned about Topochemical Diacetylene Polymerizations? Supramol. Chem. 2005, 17, 31–36. [Google Scholar] [CrossRef]
- Biradha, K.; Santra, R. Crystal engineering of topochemical solid state reactions. Chem. Soc. Rev. 2013, 42, 950. [Google Scholar] [CrossRef]
- Chaudhary, A.; Mohammad, A.; Mobin, S.M. Recent Advances in Single-Crystal-to-Single-Crystal Transformation at the Discrete Molecular Level. Cryst. Growth Des. 2017, 17, 2893–2910. [Google Scholar] [CrossRef]
- Hay, A.S. Oxidative Coupling of Acetylenes II. J. Org. Chem. 1962, 27, 3320–3321. [Google Scholar] [CrossRef]
- Baughman, R.H. Solid-State Synthesis of Large Polymer Single-Crystals. J. Polym. Sci. Polym. Phys. Ed. 1974, 12, 1511–1535. [Google Scholar] [CrossRef]
- Turowska-Tyrk, I.; Grześniak, K.; Trzop, E.; Zych, T. Monitoring structural transformations in crystals. Part 4. Monitoring structural changes in crystals of pyridine analogs of chalcone during [2+2]-photodimerization and possibilities of the reaction in hydroxy derivatives. J. Solid State Chem. 2003, 174, 459–465. [Google Scholar] [CrossRef]
- Bertault, M.; Canceill, J.; Collet, A.; Toupet, L. Synthesis and Solid-state Polymerization Properties of Symmetrical and Unsymmetrical Diacetylene Derivatives containing a ‘Polymerogenic’ Side Group. J. Chem. Soc. Chem. Commun. 1988, 163–166. [Google Scholar] [CrossRef]
- Rigas, G.-P.; Payne, M.P.; Anthony, J.E.; Horton, P.N.; Castro, A.F. Spray printing of organic semiconducting single crystals. Nat. Commun. 2016, 7, 13531. [Google Scholar] [CrossRef] [PubMed]
- Cao, X.; Zhao, K.; Chen, L.; Liu, J.; Han, Y. Conjugated polymer single crystals and nanowires. Polym. Cryst. 2019, e10064. [Google Scholar] [CrossRef]
| Furan-DA 1 | Furan-PDA 2 | |
| formula | C18H16N2O6 | (C18H16N2O6)n |
| CCDC Number | 1922071 | 1922076 |
| MW/g mol−1 | 356.33 | 356.33 |
| crystal color | clear light pink | lustrous dark red |
| crystal system | monoclinic | monoclinic |
| space group | P21/c | P21/c |
| a/Å | 4.9153(3) | 4.8908(1) |
| b/Å | 38.0403(19) | 37.6027(9) |
| c/Å | 4.8163(2) | 4.8297(1) |
| β/deg | 109.884(4) | 112.000(1) |
| V/Å3 | 846.86(8) | 823.54(3) |
| Z | 2 | 2 |
| density (calculated)/g cm−3 | 1.397 | 1.437 |
| total number of reflections | 8755 | 10411 |
| independent reflections | 1548 | 1544 |
| Rint | 0.0842 | 0.0717 |
| R1 [I >2σ(I)] | 0.0863 | 0.0524 |
| wR2 [I >2σ(I)] | 0.1696 | 0.1295 |
| GoF | 1.100 | 1.077 |
| Significant Geometric Parameters for Polymerization of DA | Optimal Values | Furan-DA 1 (Direction Parallel to the c-axis) | Furan-DA 1 (Direction Parallel to the a-axis) |
|---|---|---|---|
| d(1) | 4.7–5.2Å | 4.816 Å | 4.915 Å |
| d(2) | ≤3.8Å | 3.822 Å | 3.499 Å |
| θ | ≈45° | 51° | 45° |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dory, Y.L.; Caron, M.; Duguay, V.O.; Chicoine-Ouellet, L.; Fortin, D.; Baillargeon, P. Preparation and Single Crystal Structure Determination of the First Biobased Furan-Polydiacetylene Using Topochemical Polymerization. Crystals 2019, 9, 448. https://doi.org/10.3390/cryst9090448
Dory YL, Caron M, Duguay VO, Chicoine-Ouellet L, Fortin D, Baillargeon P. Preparation and Single Crystal Structure Determination of the First Biobased Furan-Polydiacetylene Using Topochemical Polymerization. Crystals. 2019; 9(9):448. https://doi.org/10.3390/cryst9090448
Chicago/Turabian StyleDory, Yves L., Mia Caron, Vincent Olivier Duguay, Lucas Chicoine-Ouellet, Daniel Fortin, and Pierre Baillargeon. 2019. "Preparation and Single Crystal Structure Determination of the First Biobased Furan-Polydiacetylene Using Topochemical Polymerization" Crystals 9, no. 9: 448. https://doi.org/10.3390/cryst9090448
APA StyleDory, Y. L., Caron, M., Duguay, V. O., Chicoine-Ouellet, L., Fortin, D., & Baillargeon, P. (2019). Preparation and Single Crystal Structure Determination of the First Biobased Furan-Polydiacetylene Using Topochemical Polymerization. Crystals, 9(9), 448. https://doi.org/10.3390/cryst9090448







