Abstract
The conformation of the title compounds was determined in solution by 1H-NMR spectroscopy and in solid state by single-crystal X-ray diffraction (XRD) complemented with density functional theory. The compounds were found to exist exclusively in solution and solid state as trans-2-aminochalcone epoxides with strong intramolecular hydrogen bonding interaction between the amino and carbonyl groups. These 2-aminochalcone epoxides experienced a solvent effect in DMSO-d6, which resulted in an anomalous chemical shift for the α-hydrogen signal, presumably due to complexation of solute molecules with DMSO. The solute–solvent interaction would probably fix the trans conformation of epoxyketone such that α-H is more accessible to both aryl rings, and in turn, experience their combined anisotropic effect. Intermolecular interactions in the crystal structures were confirmed and quantified using the Hirshfeld surface analysis. Moreover, the trans stereochemistry of the α-epoxyketones facilitated direct one-pot sequential sulfuric acid-mediated ring opening and aryl migration to afford the corresponding 3-arylquinolin-4(1H)-ones (azaisoflavones).
1. Introduction
Epoxides are versatile synthetic intermediates for a wide range of natural products and biologically active compounds [1,2,3]. They react with a variety of nucleophilic reagents under neutral, acidic or basic conditions via SN2 mechanism to afford 1,2-disubstituted products with trans orientation of the nucleophile and leaving oxygen atom. Ion pair (SNi), carbocationic SN1 and double inversion mechanisms have been proposed for those reactions that proceed with the retention of configuration [4]. The reactivity of the three-membered ring is due to angle strain, which makes the carbon–oxygen bond weaker and therefore more reactive than that of ethers. On the other hand, by the resonance effect, strong electron withdrawing groups (e.g., methyl ester, 4-nitrophenyl and cyano group) have been found to widen the C–O–C bond angle and to lengthen the C–C bond of the epoxide ring significantly to lead to ring-opening reactions via C–C bond breaking [5]. This effect becomes more pronounced with increasing number of electron-withdrawing substituents around the epoxide ring. The Weitz–Scheffer reaction has been used extensively to oxidize α,β-unsaturated ketones into α-epoxyketones using hydrogen peroxide under alkaline conditions [6]. The proximity of the nucleophilic centre in the case of ortho-heteroatom-substituted chalcone epoxides has been found to facilitate intramolecular ring opening to afford heterocyclic compounds. The 2-hydroxychalcones, for example, have been found to undergo epoxidation of the carbon–carbon double bond using hydrogen peroxide under alkaline conditions followed by ring opening in situ to afford the 3-flavonols [7]. This reaction, which is commonly known as the Algar–Flynn–Oyamada reaction, generally produces 3-flavonols from 2-hydroxychalcones without isolation of the incipient 2-hydroxychalcone epoxides [7]. However, the 2-hydroxychalcone epoxides have been isolated as stable products via initial epoxidation of the 2-tetrahydropyran-2-yloxychalcones and subsequent deprotection of the tetrahydropyranyl (TlHP) group [7]. The instability of the 2-hydroxychalcone epoxides under Algar–Flynn–Oyamada reaction conditions is presumably due to the increased acidity of the hydroxyl group that becomes deprotonated under strongly basic conditions to effect nucleophilic attack on the electrophilic epoxide moiety. 2-Hydroxychalcone epoxides substituted at the 6-position of ring-A with a methoxy or methyl group and no hydroxyl group at the ortho or para position of ring-B were found to undergo α-cyclization preferentially to afford aurone derivatives [8]. It is envisaged that the 6-substituent forces the carbonyl group to lie out-of-plane with the aromatic ring and thereby facilitate α-cyclization to afford aurones [9]. Under similar reaction conditions employed for the epoxidation of the 2-hydroxychalcones, the analogous 2-aminochalcones afford epoxides as stable products [10,11,12]. The 2-aminochalcone epoxides have been found to react with acetic acid to afford α-hydroxy-β-acetoxydihydrochalcones [12]. Their reaction with triflic acid [13] and indium(III) bromide (InBr3) or bismuth(III) chloride (BiCl3) [14] afforded the 2-aryl-3-hydroxy-2,3-dihydroquinolon-4(1H)-ones. Triflic-acid-promoted tandem ring closure and aryl-migration of 2-aminochalcone epoxide via 3-hydroxy-2,3-dihydroquinolin-4(1H)-one intermediate, leading to 3-aryl-4(1H)-quinolinones (azaisoflavones), has also been achieved [13]. On the other hand, the use of stereoisomers of 2-aminochalcone epoxide generated in non-chiral conditions as substrates in the reaction with triflic acid has been reported to produce mixtures of the cis- and trans-2-aryl-3-hydroxy-2,3-dihydroquinolin-4(1H)-ones [14]. Further reaction of a mixture of stereoisomers of the 2-aryl-3-hydroxy-2,3-dihydroquinolin-4(1H)-ones with triflic acid resulted in reduced yields of the 3-azaisoflavones. The observed low yields were attributed to the stereoisomer of 3-hydroxy-2,3-dihydroquinolin-4(1H)-one with the hydroxyl and the migrating aryl groups in cis-configuration, which does not undergo triflic-acid-mediated aryl migration to afford the 3-arylquinolinones [11,13]. On the other hand, the stereoisomers of 2-nitrochalcone epoxides have been found to undergo triflic-acid-mediated Meinwald rearrangement/intramolecular reductive cyclization to afford the 3-arylquinolin-4(1H)-ones in a single-pot operation [15]. The analogous 2-methoxychalcone epoxides have been found to undergo boron trifluoride etherate (BF3-Et2O)-catalysed Meerwein rearrangement and subsequent deformylation to afford the 2-methoxydeoxybenzoins in a single-pot operation [16].
Although the 2-aminochalcone epoxides generated from 2-aminochalcones with alkaline hydrogen peroxide were transformed into various derivatives, to our knowledge, the exact stereochemistry of these α-epoxyketones has not been established. This encouraged us to subject the 2-amino-3,5-dibromochalcones to alkaline hydrogen peroxide and to establish the solution and solid-state geometry of the 2-aminochalcone epoxides generated using these conditions by means of spectroscopic and single-crystal diffraction X-ray crystallography. The experimental results were complemented with theoretical studies using density functional theory (DFT) methods. Moreover, we investigated the reactivity of these ortho-amino-substituted α-epoxyketones with sulfuric acid in tandem cyclization and aryl migration to afford the 3-arylquinolin-4(1H)-ones (azaisoflavones).
2. Materials and Methods
2.1. General Notes
The melting point (mp) values were recorded on a Thermocouple digital melting point apparatus (Mettler Toledo LLC, Columbus, OH, USA). The infrared (IR) spectra were recorded as powders using the thin-film method on a Bruker VERTEX 70 FT-IR Spectrometer (Bruker Optics, Billerica, MA, USA). Column chromatography was performed using Merck kieselgel 60 (0.063–0.200 mm) as stationary phase (Merck KGaA, Frankfurt, Germany). 1H- and 13C-NMR spectra were obtained as CDCl3 or DMSO-d6 solutions using a Bruker Ascend 400 MHz NMR spectrometer (Bruker Biospin GmhH, Karlsrushe, Germany) and an Agilent 500 MHz NMR spectrometer (Agilent Technologies, Oxford, UK), and the chemical shifts are quoted relative to tetramethylsilane (TMS) peak. The 2D NOESY spectra were acquired using a Bruker Ascend 400 MHz NMR spectrometer. The high-resolution mass spectra were recorded at the University of Stellenbosch Central Analytical Facility using a Waters Synapt G2 Quadrupole Time-of-Flight mass spectrometer (Waters Corp., Milford, MA, USA). The synthesis and analytical data for compounds 1a–c used as substrates in this investigation have been reported [17].
2.2. Typical Procedure for Epoxidation of the 2-Aminochalcones 2a–c
A stirred solution of 1a (1.00 g, 1.0 mmol) in methanol (50 mL) at 0 °C in a 100-mL round-bottomed flask was treated sequentially with 30% H2O2 (20 mL) and aqueous solution of 10% NaOH (10 mL). The mixture was stirred at room temperature for 6 h, and then poured into ice-cold water. The product was extracted into chloroform and the combined organic layers were dried over anhydrous magnesium sulphate (MgSO4). The salt was filtered off, and the solvent was evaporated under reduced pressure. The residue was recrystallized from ethanol to afford 2a as a solid.
2.2.1. (2-Amino-3,5-dibromophenyl)(3-phenyloxiran-2-yl)methanone (2a)
Solid (0.87 g, 89%); mp. 109–112 °C; νmax (ATR) 3443, 3326, 1640, 1614, 1573, 1539, 1495, 1448, 1338, 1206, 1157, 1010, 976, 737, 696, 662 cm−1; δH (500 MHz, CDCl3) 4.04 (1H, d, J = 2.0 Hz, α-H), 4.19 (1H, d, J = 2.0 Hz, β -H), 6.94 (2H, br s, NH2), 7.24–7.43 (5H. m, Ph), 7.74 (1H, d, J = 2.0 Hz, H-4), 7.86 (1H, d, J = 2.0 Hz, H-6); δC (100 MHz, CDCl3) 59.4, 60.4, 106.2, 111.9, 118.1, 125.7, 128.8, 129.1, 132.0, 135.1, 140.0, 146.6, 192.3; HRMS (ES): m/z [M + H]+ calc for C15H12NO2Br2: 395.9249; found 395.9157.
2.2.2. (2-Amino-3,5-dibromophenyl)(3-(4-fluorophenyl)oxiran-2-yl)methanone (2b)
Solid (0.78 g, 92%); mp. 121–123 °C; νmax (ATR) 3427, 3317, 1646, 1615, 1575, 1541, 1506, 1483, 1445, 1414, 1341, 1266, 1205, 1153, 1096, 1007, 978, 847, 824, 770, 739, 657 cm−1; δH (400 MHz, CDCl3) 4.03 (1H, d, J = 2.0 Hz, α-H), 4.16 (1H, d, J = 2.0 Hz, β-H), 6.91 (2H, br s, NH2), 7.06 (2H, dd, JHH = 8.5 and JHF = 9.7 Hz, H-3′,5′), 7.31 (2H, dd, JHH = 8.5 and JHF = 5.1 Hz, H-2′,6′), 7.65 (1H, d, J = 2.0 Hz, H-4), 7.77 (1H, d, J = 2.0 Hz, H-6); δC (100 MHz, CDCl3) 58.8, 60.3, 106.1, 111.8, 115.8 (d, 2JCF = 21.8 Hz), 117.9, 127.5 (d, 3JCF = 8.5 Hz), 130.8 (d, 4JCF = 3.3 Hz), 131.8, 139.9, 146.5, 163.2 (d, 1JCF = 246 Hz) 192.0; HRMS (ES): m/z [M + H]+ calc for C15H11NO2F79Br2: 413.9141; found 413.9154.
2.2.3. (2-Amino-3,5-dibromophenyl)(3-(4-chlorophenyl)oxiran-2-yl)methanone (2c)
Solid (0.90 g, 94%); mp. 125–127 °C; νmax (ATR) 3472, 3325, 3034, 1641, 1611, 1568, 1536, 1491, 1446, 1405, 1336, 1292, 1263, 1208, 1156, 1089, 1006, 981, 816, 749, 674, 640 cm−1; δH (400 MHz, CDCl3) 4.02 (1H, d, J = 2.0 Hz, α-H), 4.14 (1H, d, J = 2.0 Hz, β-H), 6.95 (2H, br s, NH2), 7.28 (2H, d, J = 8.5 Hz, H-2′,6′), 7.38 (2H, d, J = 8.5 Hz, H-3′,5′), 7.72 (1H, d, J = 2.5 Hz, H-4), 7.80 (1H, d, J = 2.0 Hz, H-6); δC (100 MHz, CDCl3) 58.7, 60.4, 106.2, 111.9, 118.0, 125.7, 128.7, 129.1, 133.6, 134.9, 140.1, 146.7, 191.9; HRMS (ES): m/z [M + H]+ calc for C15H11NO2ClBr2: 429.8859; found 429.8767.
2.3. Typical Procedure for the Reaction of 2a–c with Sulfuric Acid to Afford 3a–c
A stirred solution of 2a (1.00 g, 1.0 mmol) in dichloromethane (10 mL) at 0 °C was treated dropwise with concentrated sulfuric acid (0.75 g, 3.0 mmol). The mixture was stirred at 0 °C for 2 hours and then quenched with ice-cold water. The product was extracted into dichloromethane, and the combined organic phases were washed with water. The organic phase was dried over anhydrous MgSO4, and the salt was filtered off. The solvent was evaporated under reduced pressure to afford 3a as a solid.
2.3.1. 6,8-Dibromo-3-phenylquinolin-4(1H)-one (3a)
Solid (0.81 g, 67%), mp 147–149 °C, νmax (ATR) 3332, 1655, 1604, 1480, 1332, 1303, 1261, 1215, 1154, 1115, 1076, 1024, 999, 915, 765, 699, 617 cm−1; δH (500 MHz, DMSO-d6) 7.30–7.33 (1H, m, Ph), 7.42 (2H, t, J = 8.5 Hz, Ph), 7.66 (2H, dd, J = 1.5 and 8.5 Hz, Ph), 8.00 (1H, s, H-2), 8.24 (1H, d, J = 2.5 Hz, H-7), 8.30 (1H, d, J = 2.5 Hz, H-5), 11.57 (1H, s, NH); δC (125 MHz, DMSO-d6) 113.3, 116.1, 121.2, 127.5, 128.2, 128.3, 128.5, 128.8, 135.4, 136.6, 137.3, 139.4, 173.6; HRMS (ES): m/z [M + H]+ calc for C15H10NO79Br2: 377.9129; found 377.9119.
2.3.2. 6,8-Dibromo-3-(4-fluorophenyl)quinolin-4(1H)-one (3b)
Solid (0.39 g, 70%), mp 118–120 °C, νmax (ATR) 3299, 1645, 1603, 1505, 1479, 1436, 1355, 1309, 1223, 1154, 1120, 1001, 913, 860, 836, 796, 755, 639 cm−1; δH (500 MHz, DMSO-d6) 7.23 (2H, dd, JHH = 8.7 and JHF = 9.7 Hz, H-3′,5′), 7.71 (2H, dd, JHH = 8.7 Hz and JHF = 5.4 Hz, H-2′,6′), 8.00 (1H, s, H-2), 8.24 (1H, d, J = 2.5 Hz, H-7), 8.29 (1H, d, J = 2.5 Hz, H-5), 11.59 (1H, s, NH); δC (125 MHz, DMSO-d6) 113.4, 115.4 (d, 2JCF = 21.8 Hz), 116.2, 120.2, 128.3 (d, 3JCF = 8.5 Hz), 130.8 (d, 4JCF = 3.3 Hz), 131.7, 131.8, 136.6, 137.4, 139.4, 160.8 (d, 1JCF = 246 Hz) 173.5; HRMS (ES): m/z [M + H]+ calc for C15H9NOF79Br2: 395.9035; found 395.9022.
2.3.3. 6,8-Dibromo-3-(4-chlorophenyl)quinolin-4(1H)-one (3c)
Solid (0.65 g, 63%), mp 142–143 °C, νmax (ATR) 3306, 1651, 1604, 1508, 1480, 1410, 1326, 1250, 1211, 1151, 1118, 1089, 1015, 916, 825, 764, 685, 647 cm−1; δH (500 MHz, DMSO-d6) 7.59 (2H, d, J = 8.5 Hz, H-2′,6′), 7.65 (2H, d, J = 8.5 Hz, H-3′,5′), 8.04 (1H, s, H-2), 8.24 (1H, d, J = 2.0 Hz, H-7), 8.29 (1H, d, J = 2.0 Hz, H-5), 11.60 (1H, s, NH); δC (125 MHz, DMSO-d6) 113.3, 116.3, 119.8, 120.5, 128.2, 128.3, 130.8, 131.4, 134.7, 136.6, 137.4, 139.6, 173.4; HRMS (ES): m/z [M + H]+ calc for C15H10NO35Cl79Br2: 411.8739; found 411.8732.
2.4. Data Collection and Refinement of 2a
Intensity data were determined on a Bruker Venture D8 Photon CMOS diffractometer (Bruker AXS, Madison, WI, USA) with graphite-monochromated MoKα1 (λ = 0.71073 Å) radiation at 173 K using an Oxford Cryostream 600 cooler. Data reduction was performed using the program SAINT+, version 6.02 [18]. Face-indexed absorption corrections were performed with SADABS [19]. The space group assignments were made using XPREP [19]. The structure was solved in the WinGX [19] Suite of programs using direct methods through using SHELXS-97 [20], and refined using full-matrix least-squares/difference Fourier techniques on F2 using SHELXL-2017 [20]. All hydrogen atoms were placed at idealized positions and refined as riding atoms with isotropic parameters 1.2 times those of their parent atoms. The disorder around epoxide group C8–O1–C9 was resolved by finding alternate positions in the difference Fourier map and by refining their occupancies to final values of 0.578(8) and 0.422(8). The disorder around the phenyl ring of atoms C11, C12, C14 and C15 was resolved by finding alternative positions in the difference Fourier map and refining their occupancies to final values of 0.536(7) and 0.464(7). Diagrams and publication material were generated using ORTEP-3 [19] and PLATON [21].
2.5. Computational Methods
The density functional theory computations were carried out using the hybrid functional Becke’s three-parameter nonlocal exchange functional with the Lee–Yang–Parr correlation function (B3LYP) [22], together with the 6-311++G(d,p) [23,24] basis set for all atoms. The computations were performed using the Gaussian 09 software suite [25].
2.6. Hirshfeld Surface Analyses
The Hirshfeld (HF) surface analyses were obtained with the support of Crystal Explorer software [26] in order to explain the intermolecular interactions between molecules in the crystal as well as between atoms in the molecule. The normalized contact distance (dnorm) is based on de (distance from the point to the nearest nucleus external to the surface) and di (distance to the nearest nucleus internal to the surface) [27].
3. Results and Discussion
3.1. Synthesis and Solution Phase Structural Analysis
The 2-amino-3,5-dibromochalcones 1a–c were reacted with hydrogen peroxide in the presence of sodium hydroxide in methanol at room temperature (RT) for 6 h to afford the corresponding 2-aminochalcone epoxides 2a–c (Scheme 1). Structural information on the α-epoxyketones 2 in solution were obtained from 1D- and 2D-NMR studies. Their 1H-NMR spectra were recorded in CDCl3 and DMSO-d6 to investigate solvent effects (Figure 1). The 1H-NMR spectra of these epoxides obtained as CDCl3 solutions revealed the presence of a group of signals in the aromatic region and a broad singlet (br s) with chemical shift (δ) value around 7.00 ppm for the amino group (Figure 1, bottom spectrum in blue). The signal for -NH2 resonated as a relatively intense singlet slightly downfield in the region δ = 7.45–7.49 ppm in DMSO-d6 (Figure 1, top spectrum in red). The aliphatic region of their spectra in CDCl3 revealed the presence of a set of doublets around δ 4.06 ppm and δ 4.20 ppm with coupling constant (3Jvic) value of about 2.0 Hz. This small 3JHH value is typical for the trans-stereochemistry of relatively rigid 3-membered ring systems (Jcis > Jtrans) [28] and compares favourably with the literature value for the trans-2-benzylaminochalcone epoxides [9] and the analogous 2-aminochalcone epoxides [14]. The set of doublet of doublets corresponded to α-hydrogen and β-hydrogen atoms, and their corresponding carbon atom signals resonated at δ 57.9 ppm (β-C) and δ 59.0 ppm (α-C), respectively. The doublet for the α-hydrogen resonated slightly upfield around δ 4.06 ppm in CDCl3, but showed an anomalous downfield shift to around δ 4.86–4.89 ppm in DMSO-d6. This is presumably due to dipole–dipole interaction of DMSO with the carbonyl group oxygen and the β-carbon. A similar effect by DMSO on the α-hydrogen has previously been proposed for the phenyl vinyl ketones [29]. Such interaction in the case of the title compounds would probably fix the trans conformation of the α-epoxyketone and, in turn, make the α-hydrogen more accessible to both aryl groups to experience their combined magnetic anisotropy. In our view, solute–solvent interaction together with the combined magnetic anisotropic effect of the two phenyl groups contributed significantly to the anomalous solvent effect on the chemical shift of the α-hydrogen. On the other hand, solute–solvent interaction showed no effect on the chemical shift of the β-hydrogen in DMSO-d6.
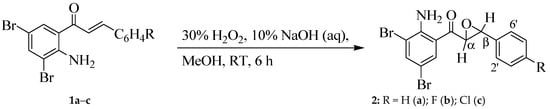
Scheme 1.
Synthesis of 2-aminochalcone epoxides 2a–c.
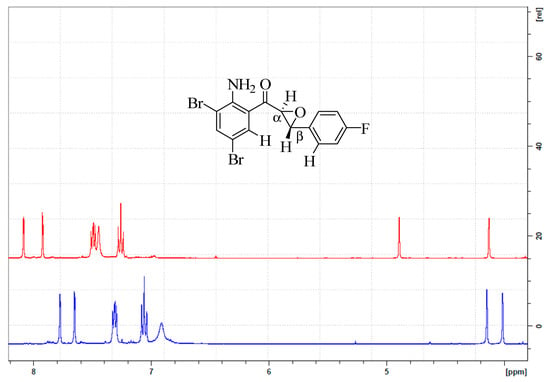
Figure 1.
1H-NMR spectra of 2b in CDCl3 (blue) and DMSO-d6 (red) at 400 MHz.
The trans orientation of the α- and β-hydrogens about the epoxide ring was further confirmed by lack of correlation of their corresponding signals in the 2D NOESY spectra of compounds 2a–c, represented in Figure 2 by the spectrum of 2c (the NOESY spectra of 2a and 2b have been included as Figure S2 in the Supplementary Materials). The 2D NOESY experiments revealed a strong NOE correlation peak between a doublet around δ 4.20 ppm with a doublet for H-6 on ring-A and the hydrogen atom on ring-B (either H-2′ or H-6′) ortho to the β-carbon (see structure insert in Figure 2). The other doublet of the epoxide ring, which was assigned to the α-hydrogen atom, resonated slightly upfield around δ = 4.06 ppm and showed no interaction with any of ring-A or ring-B protons. The NOE correlation between the signal around δ 4.20 ppm with H-6 and an ortho hydrogen atom on ring-B (ortho relative to the β-carbon) presumably suggests these protons to be on the same face of the epoxide ring. Moreover, this orientation further confirms the trans stereochemistry of the α-epoxyketone moiety. Based on the observed vicinal diaxial coupling constant value (Jvic) of 2.0 Hz and lack of correlation between signals of the α-H and β-H in the NOESY spectrum (refer to Figure 2 below and/or Figure S2 in the Supplementary Materials), we concluded that these α-epoxyketones existed exclusively in solution as the trans-conformers. This conformation would probably enable intramolecular hydrogen bond interaction between the carbonyl oxygen and hydrogen atoms of the amino group. In our view, the involvement of NH function in a six-membered intramolecular hydrogen bonding was confirmed by the lack of H/D exchange in DMSO-d6 for all the compounds and by the slight increase in chemical shift (δ) of about 0.45 ppm in this solvent from around δ 7.00 ppm in CDCl3. This intramolecular hydrogen bonding interaction appeared to be stable in both solvents without disruption by H/D exchange or rotation about the Ar–NH2 bond.
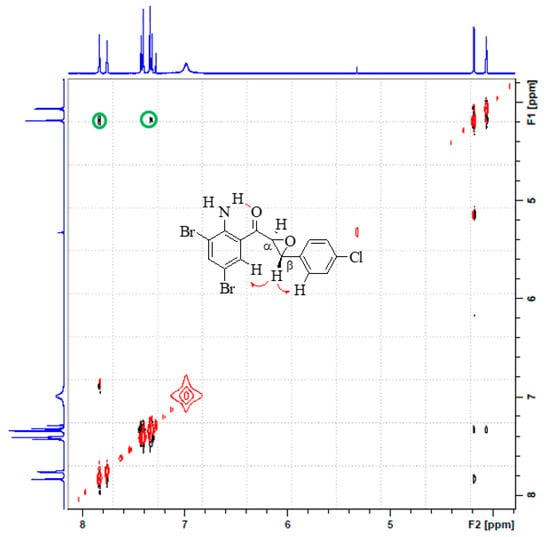
Figure 2.
NOESY Spectrum of 2c showing dipole–dipole interaction between β-H and the ring-A and B protons.
Although DMSO-d6 caused an unusual solvent effect on the chemical shifts of α-H, it showed no chemical shift changes for the corresponding α-carbon and β-carbon atoms, or for the carbonyl carbon for all compounds (Figure 3). The presence in each case of the carbonyl carbon resonance around δ 192.0 ppm in their 13C-NMR spectra obtained as CDCl3 or DMSO-d6 solutions in our view confirmed the existence of the title compounds in solution as single conformers.
Figure 3.
13C-NMR spectra of 2c in CDCl3 (red) and DMSO-d6 (blue) at 100 MHz.
3.2. Solid-State Analysis
α-Epoxyketones have been found to generally exist exclusively in the form of trans isomers in the solid phase [30,31], although the analogous 1,3-diphenyl-2,3-epoxy-1-propanone has been reported to exist in cis conformation [32]. The quinolyl framework and the p-tolyl ring in the analogous 3-(2-chloro-6-methylquinolin-3-yl)oxiran-2-yl-(p-tolyl)methanone have also been reported to exist in cis configuration with a dihedral angle of 65.80(7)° [33]. The possibility of the existence of the α-epoxyketones in either trans or cis configuration encouraged us to study the solid-state geometry of the 2-aminochalcone epoxides 2. The infrared (IR) carbonyl band of the α-epoxyketones 2a–c obtained as powders was found to absorb in the region υmax 1640–1645 cm−1, which may suggest existence as single conformers in the solid state. Single crystals suitable for X-ray diffraction analysis were obtained by recrystallization of compound 2a from ethanol. The solid-state geometry of α-epoxyketones 2a–c was established by means of single-crystal X-ray diffraction (XRD). Compound 2a was found to crystallize in the monoclinic system with P21/n space group, and one molecule was found in the asymmetric unit. The crystallographic numbering shown in Figure 4 was used in the context of the X-ray analysis in place of the systematic numbering. The crystal data and structure refinement for compound 2a are shown in Table 1 below. The trans conformation suggested in the solution phase is in fact evident in the X-ray diffraction structure of 2-aminochalcone epoxide 2a. The core of the ortho-aminoketoaryl group is co-planar, and this rotation brings the NH2 fragment in relatively close proximity to the carbonyl oxygen. Intramolecular N–H···O hydrogen bond interaction exists between the carbonyl oxygen atom (O2) and the amino group with a hydrogen bond distance of 2.35 Å between hydrogen and oxygen. Both the exocyclic bonds of the ketoaryl and aryl groups are in trans orientation relative to each other about the oxirane ring with torsion angle C7–C8a–C9a–C10 of 154.8°. This orientation may support the lack of NOE correlation between the α- and β-hydrogens observed in the solution-phase geometry.
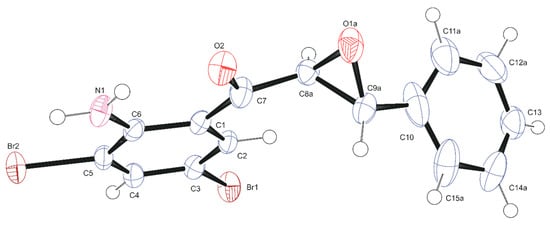
Figure 4.
Oak Ridge Thermal Ellipsoid Plot (ORTEP) diagram (50% probability level) of 2a.

Table 1.
Crystal data and structure refinement for compound 2a.
Examining the short contacts in the crystal structure of 2a suggests the existence of weak C–H···Br and N–H···Br bond interactions. The molecules were aligned in parallel planes and held together via multiple weak C−H···O and C−H···N interactions and further stabilized by π···π stacking (Figure 5).
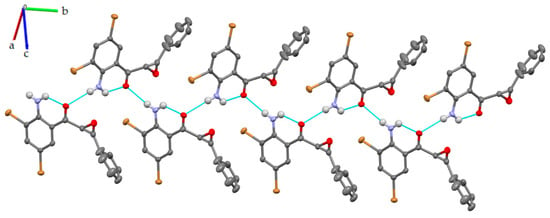
Figure 5.
Intra- and intermolecular hydrogen bonding interactions for compound 2a.
3.3. Optimized Structure from Density Functional Theory (DFT) Analysis
The geometry-optimized structure of 2-aminochalcone epoxide 2a is shown in Figure 6 below, and was obtained in the gas phase using B3LYP function and the 6-311G basis set. The optimal structure confirmed the trans orientation of the ortho-aminoketoaryl group and the aryl substituent about the epoxide ring (Figure 6). Some of the DFT optimized parameters, namely, the bond lengths, bond angles and torsion angles are compared with the XRD values in Table 2 below. Assignment of the simulated values is in accordance with atomic numbering scheme for the XRD analysis given in Figure 3 above. The gas phase calculations have been reported to frequently compare favourably with crystal structures [34]. The deviation observed and calculated values are probably due to the fact that the DFT simulations deal with the isolated molecule in the gaseous phase. The experimental results, on the other hand, deal on the molecule in the solid state. The experimental (1.468 Å) and the calculated (1.471 Å) C(8a)–C(9a) bond lengths of 2a are comparable to those of ethylene oxide/oxirane (1.46–1.47 Å) [5], and in our view this observation rules out the possibility of a conjugative effect between the epoxide ring and the ketoaryl group.
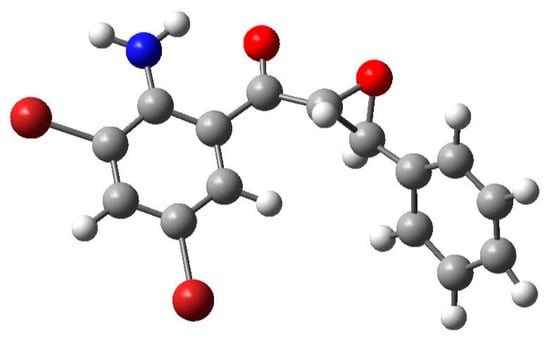
Figure 6.
A view of the optimized structure of compound 2a.

Table 2.
Comparison of selected XRD and DFT bond lengths (Å) and angles (°) of 2a.
3.4. Hirshfeld Surface Analysis of Compound 2a
The Hirshfeld surfaces and their associated two-dimensional (2D) fingerprint plots for compound 2a were computed based on the single-crystal X-ray structure as input. The quantifying and decoding of the inter-contact in the crystal packing were visualized using dnorm (normalized contact distance) and 2D fingerprint plots, respectively. The red spots over the HF surface indicate the interactions that involve hydrogen bonding (Figure 7) and analysis of the surface confirmed H-bonds and π–π stack interactions obtained by XRD packing analyses. The major contributions on the HF surface were from H···H (32.0%), Br···H (16.7%), C···H (10.6%), C···C (10.9%), O···H (5.3%), C···O (1.8%) and N···H (0.2%), as represented in Figure 8 below.
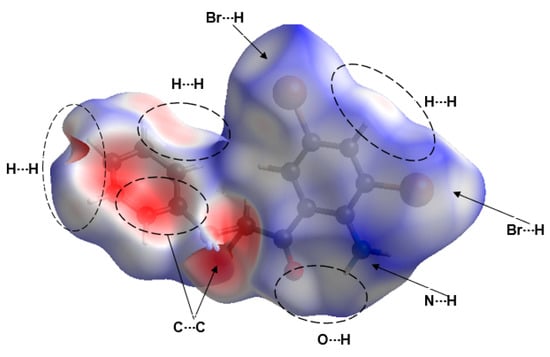
Figure 7.
Hirshfeld surface of 2a mapped with the dnorm function, and the regions of the most important intermolecular interactions are indicated by the dashed ovals and/or arrows.
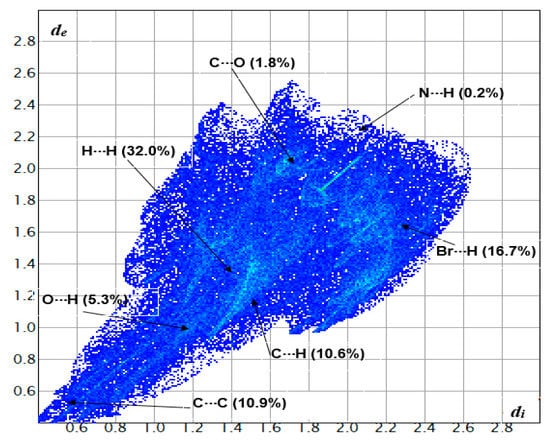
Figure 8.
Two-dimensional fingerprint plots showing the highest interactions of 2a. di corresponds to the closest internal distance from a given point of the HF surface, and de to the closest external contacts (i.e., external distance).
The difference in energy between the HOMO (highest occupied molecular orbital) and LUMO (lowest unoccupied molecular orbital) is an important quantitative structure–activity relationship (QSAR) descriptor and has also been found to relate to molecular reactivity [35]. The electron distribution of 2a was scattered in the HOMO over the epoxy ortho-aminoketoaryl moiety excluding the bromine atoms, whereas the LUMO was mainly disseminated over the entire ortho-aminoketoaryl group (Figure 9). The frontier molecular orbital energy gap between the HOMO (−0.229 eV) and the LUMO (−0.089 eV) of 2a was found to be 0.14 eV. This high energy gap value may suggest high chemical stability and high excitation energy for this molecule.
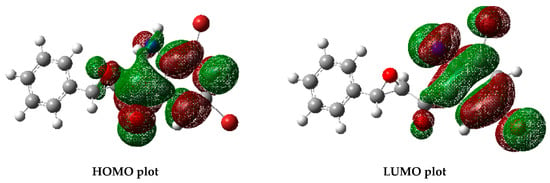
Figure 9.
HOMO–LUMO distribution of 2a.
We envisaged that the trans-2-aminochalcone epoxides 2 would undergo direct one-pot sequential acid-mediated cyclization and aryl migration of the intermediate trans-2-aryl-3-hydroxyquinolinones to yield the corresponding 3-azaisoflavones, exclusively. In our view, efficient solvation by the strongly polar protic acid and intermolecular hydrogen bonding would probably stabilize the trans conformer of the incipient 2-aryl-3-hydroxy-2,3-dihydroquinolin-4(1H)-one intermediate and, in turn, facilitate aryl migration to afford the 3-azaisoflavones as sole products. In order to prove this assumption, we subjected epoxides 2a–c to an excess of sulfuric acid (3 mol equivalent) in dichloromethane at 0 °C for 2 h with thin-layer chromatography (TLC) monitoring (Scheme 2). Thin-layer chromatography analysis of the reaction mixtures revealed in each case the presence of a single spot, which upon aqueous work-up and recrystallization were characterized using a combination of spectroscopic techniques as the azaisoflavones 3a–c. The NH-4-oxo nature of the potentially tautomeric 3a–c in solution was confirmed by the presence in the 13C-NMR spectra of a singlet around δ 173.5 ppm, which is typical for the carbonyl carbon for the quinolin-4(1H)-one framework [36]. Their ketone nature in the solid state, on the other hand, was confirmed by the intense infrared carbonyl absorption band in the region νmax 1645–1655 cm−1.
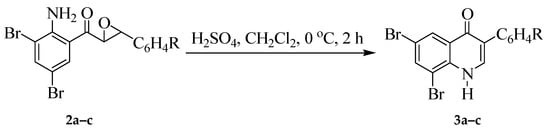
Scheme 2.
Transformation of trans-α-epoxyketones 2a–c into 3-arylquinolin-4(1H)-ones 3a–c.
4. Conclusions
The reaction of the 2-aminochalcone derivatives with hydrogen peroxide in alkaline medium is stereospecific and occurs via syn-addition with retention of the trans-stereochemistry of the parent alkene. The 2-aminochalcone epoxides were confirmed by 1H-NMR spectroscopy and XRD analysis to exist exclusively in trans geometry in solution and in the solid state, respectively, and this geometry was corroborated by DFT method. An anomalous chemical shift effect for the α-hydrogen signal was observed in the strongly dipolar aprotic DMSO, presumably due to possible complexation of solute molecules with the solvent. The complexation would probably fix the trans conformation of the α-epoxyketone such that the β-hydrogen is kept away from the phenyl substituent while the α-hydrogen experienced the combined magnetic anisotropic effect of both phenyl rings. On the other hand, analysis of the Hirshfeld surface revealed the occurrence of weak intermolecular interactions in the crystalline state of these α-epoxyketones. Their trans conformation facilitated direct one-pot sulfuric-acid-mediated ring opening and aryl ring migration to afford the 3-arylquinolin-4(1H)-ones, exclusively. This observation suggests retention of the trans-conformation by the incipient 2-aryl-3-hydroxy-2,3-dihydroquinolin-4(1H)-ones in solution to afford azaisoflavones, exclusively. Ring opening via C–O bond cleavage, on the other hand, ruled out the possibility of conjugative effect between the epoxide ring and the ketoaryl group of α-epoxyketones, which has been found to favour epoxide ring opening via C–C bond cleavage. The proximity of 8-Br to the nucleophilic NH in the azaisoflavones 3 would probably facilitate site-selective tandem-palladium-catalysed Sonogashira cross-coupling and heteroannulation with terminal acetylenes to afford the 6-oxo-pyrrolo[3,2,1-ij]quinoline derivatives.
Supplementary Materials
The following are available online at https://www.mdpi.com/2073-4352/9/6/277/s1, Figure S1: Copies of 1H- and 13C-NMR spectra of compounds 2a–c and 3a–c, Figure S1: NOESY spectra of 2a and 2c, as well as the CIF file for 2a.
Author Contributions
M.J.M. reviewed the literature and wrote the manuscript. The synthesis and the DFT calculations were carried out by M.M.M., who also assisted in the discussion of the corresponding results. R.M.M. acquired and interpreted the 2D-NMR spectra.
Funding
The project was funded by the University of South Africa (UNISA) and the University of Limpopo as well as the National Research Foundation (GUN: 118554).
Acknowledgments
The authors are grateful to the University of Stellenbosch Central Analytical Facility and the University of the Witwatersrand for high-resolution mass spectrometric analysis and X-ray data, respectively.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Lattanzi, A.; Russo, A. Diaryl-2-pyrrolidinemethanols catalyzed enantioselective epoxidation of α,β-enones: New insight into the effect of structural modification of the catalyst on reaction efficiency. Tetrahedron 2006, 62, 12264–12269. [Google Scholar] [CrossRef]
- Adger, B.M.; Barkley, J.V.; Bergeron, S.; Cappi, M.W.; Flowerdew, B.E.; Jackson, M.P.; McCague, R.; Nugent, T.C.; Roberts, S.M. Improved procedure for Juliá–Colonna asymmetric epoxidation of α,β-unsaturated ketones: Total synthesis of diltiazem and TaxolTM side-chain. J. Chem. Soc. Perkin Trans. 1 1997, 3501–3507. [Google Scholar] [CrossRef]
- Border, Z.-M.; Marais, C.; Bezuidenhoudt, B.C.B.; Steenkamp, J.A. Studies towards the stereoselective α-hydroxylation of flavanones. Biosynthetic significance. Aus. J. Chem. 2008, 61, 122–130. [Google Scholar] [CrossRef]
- Bonollo, S.; Lanari, D.; Caccaro, L. Ring-Opening of Epoxides in Water. Eur. J. Org. Chem. 2011, 2587–2598. [Google Scholar] [CrossRef]
- Grabowsky, S.; Schirmeister, T.; Paulmann, C.; Pfeuffer, T.; Luger, P. Effect of electron-withdrawing substituents on the epoxide ring: An experimental and theoretical electron density analysis of a series of epoxide derivatives. J. Org. Chem. 2011, 76, 1305–1318. [Google Scholar] [CrossRef]
- Pillai, U.R.; Sahle-Demessie, E.; Varma, R.S. Microwave-expedited olefin epoxidation over hydrotalcites using hydrogen peroxide and acetonitrile. Tetrahedron Lett. 2002, 43, 2909–2911. [Google Scholar] [CrossRef]
- Adams, C.A.; Main, L. Synthesis of 2′-hydroxychalcone epoxides. Tetrahedron 1991, 47, 4959–4978. [Google Scholar] [CrossRef]
- Gormley, T.R.; O′Sullivan, W.I. Flavanoid epoxides-XIII: Acid and base catalysed reactions of 2′-tosyloxychalcone epoxides. Mechanism of the Algar-Flynn-Oyamada reaction. Tetrahedron 1972, 29, 369–373. [Google Scholar] [CrossRef]
- Adams, C.J.; Main, L. Cyclisation and subsequent reactions of 2′-hydroxy-6′-methoxychalcone epoxide and related compounds. Tetrahedron 1991, 47, 4979–4990. [Google Scholar] [CrossRef]
- Tðkés, A.L.; Janzsó, G. Reactions of 2′-aminochalcones. Synthetic Commun. 1989, 19, 3159–3168. [Google Scholar]
- Donnelly, J.A.; Farrell, D.F. Chalcone derivatives as precursors of 1,2,3,4-tetrahydro-4-quinolones. Tetrahedron 1990, 46, 885–894. [Google Scholar] [CrossRef]
- Litkei, G.; Tðkes, A.L. Oxidation of the 2′-NHR analogues of 2′-OR-chalcones; conversion of 2′-NHR-chalcone epoxides. Synthetic Commun. 1991, 21, 1597–1609. [Google Scholar] [CrossRef]
- Praveen, C.; Parthasarathy, K.; Perumal, P.T. Triflic acid promoted tandem ring-closure-aryl-migration of 2′-amino chalcone epoxide: A new synthetic route to azaisoflavones. SynLett 2010, 1635–1640. [Google Scholar] [CrossRef]
- Ahmed, N.; Kumar, H.; Babu, B.V. Intramolecular aminolysis of 2′-aminochalcone epoxides using InBr3 or BiCl3 as efficient catalysts. Synthetic Commun. 2013, 43, 567–581. [Google Scholar] [CrossRef]
- Wang, S.; Zhao, C.; Liu, T.; Yu, L.; Yang, F.; Tang, J. Efficient construction of 3-arylquinolin-4(1H)-ones via in situ Meinwald rearrangement/intramolecular reductive cyclization of 2′-nitrochalcone epoxides. Tetrahedron 2016, 72, 7025–7031. [Google Scholar] [CrossRef]
- Ruan, L.; Shi, M.; Mao, S.; Yu, L.; Yang, F.; Tang, J. An efficient approach to construct 2-arylbenzo[b]furans from 2-methoxychalcone epoxides. Tetrahedron 2014, 70, 1065–1070. [Google Scholar] [CrossRef]
- Khoza, T.A.; Maluleka, M.M.; Mama, N.; Mphahlele, M.J. Synthesis and photophysical properties of the 2-aryl-6,8-bis(arylethenyl)-4-methoxyquinolines. Molecules 2012, 17, 14186–14204. [Google Scholar] [CrossRef]
- APEX-3, SAINT+, version 6.02 (Includes XPREP and SADABS); Bruker AXS Inc.: Madison, WI, USA, 2016.
- Farrugia, L.J. ORTEP-3 for Windows-a version of ORTEP-III with a graphical user interface (GUI). J. Appl. Crystallogr. 2012, 45, 849–854. [Google Scholar] [CrossRef]
- Sheldrick, G.M. Short history of SHELX. Acta Cryst. C 2015, 71, 3–8. [Google Scholar] [CrossRef]
- Spek, A.L. Structure validation in chemical crystallography. Acta Cryst. D 2009, 65, 148–155. [Google Scholar] [CrossRef]
- Becke, A.D. Density-functional thermochemistry III. The role of exact exchange. J. Chem. Phys. 1993, 98, 5648–5652. [Google Scholar] [CrossRef]
- Lee, C.; Yang, W.; Parr, R.G. Development of the Colle-Salvetti correlation-energy formula into a functional of the electron density. Phys. Rev. B 1988, 37, 785–789. [Google Scholar] [CrossRef]
- Krishnan, R.; Binkley, J.S.; Seeger, R.; Pople, J.A. Self-consistent molecular orbital methods. XX. A basis set for correlated wave functions. J. Chem. Phys. 1980, 72, 650–654. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.A.; et al. Gaussian 09, revision A.01; Gaussian Inc.: Wallingford, CT, USA, 2009.
- Wolff, S.K.; Grimwood, D.J.; McKinnon, J.J.; Jayatilaka, D.; Spackman, M.A. CrystalExplorer 3.0, University of Western Australia: Perth, Australia, 2001.
- Spackman, M.A.; McKinnon, J.J. Fingerprinting intermolecular interactions in molecular crystals. Cryst. Eng. Comm. 2002, 4, 378–392. [Google Scholar] [CrossRef]
- Ohtsuru, M.; Tori, K.; Fukuyama, M. Proton magnetic resonance spectra of ethylene episulfoxides. Anomaly in magnitudes of spin-coupling constants between ring protons. Tetrahedron Lett. 1970, 11, 2877–2879. [Google Scholar] [CrossRef]
- Lien, J.-C.; Chen, S.-C.; Huang, L.-J.; Kuo, S.-C. Solvent effect of dimethyl sulfoxide on the chemical shifts of phenyl vinyl ketones. J. Chin. Chem. Soc. 2004, 51, 847–852. [Google Scholar] [CrossRef]
- Orlov, V.D.; Korotkov, S.A.; Sukach, Y.A.; Lavrushin, V.F. Investigation of the conformation of epoxychalcones by IR spectroscopy. Chem. Heterocyclic Comp. 1975, 11, 264–266. [Google Scholar] [CrossRef]
- Obregón-Mendoza, M.A.; Escobedo-Martínez, C.; Lozada, M.C.; Gnecco, D.; Soriano-García, M.; Enríquez, R.G. Investigation of three diasteromeric chalcone epoxides derivatives by NMR spectroscopy and X-ray crystallography. J. Chem. Crystallogr. 2014, 44, 512–519. [Google Scholar] [CrossRef]
- Kumar, A.; Bhakuni, V. Enantioselective epoxidation using liposomised m-chloroperbenzoic acid (LIP MCPBA). Tetrahedron Lett. 1996, 37, 4751–4754. [Google Scholar] [CrossRef]
- Praveena, N.; Hosamani, A.A.; Praveen, M.; Nagendrappa, G.; Row, T.N.G. cis-[3-(2-Chloro-6-methylquinolin-3-yl)oxiran-2-yl]-(p-tolyl)methanone. IUCrData 2017, 2, x170434. [Google Scholar] [CrossRef]
- Fleming, I. Frontier Orbitals and Organic Chemical Reactions; Wiley: Hoboken, NJ, USA, 1977. [Google Scholar]
- Honarparvar, B.; Govender, T.; Maguire, G.E.; Soliman, M.E.; Kruger, H.G. Integrated approach to structure-based enzymatic drug design: Molecular modeling, spectroscopy, and experimental bioactivity. Chem. Rev. 2013, 114, 493–537. [Google Scholar] [CrossRef] [PubMed]
- Mphahlele, M.J.; El-Nahas, A.M. Tautomeric 2-arylquinolin-4(1H)-one derivatives- spectroscopic, X-ray and quantum chemical structural property studies. J. Mol. Struct. 2004, 688, 129–136. [Google Scholar] [CrossRef]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).