The Decoloration of Anionic and Cationic Dyes Using ZnO and ZnO-Cu2O
Abstract
:1. Introduction
2. Experiments
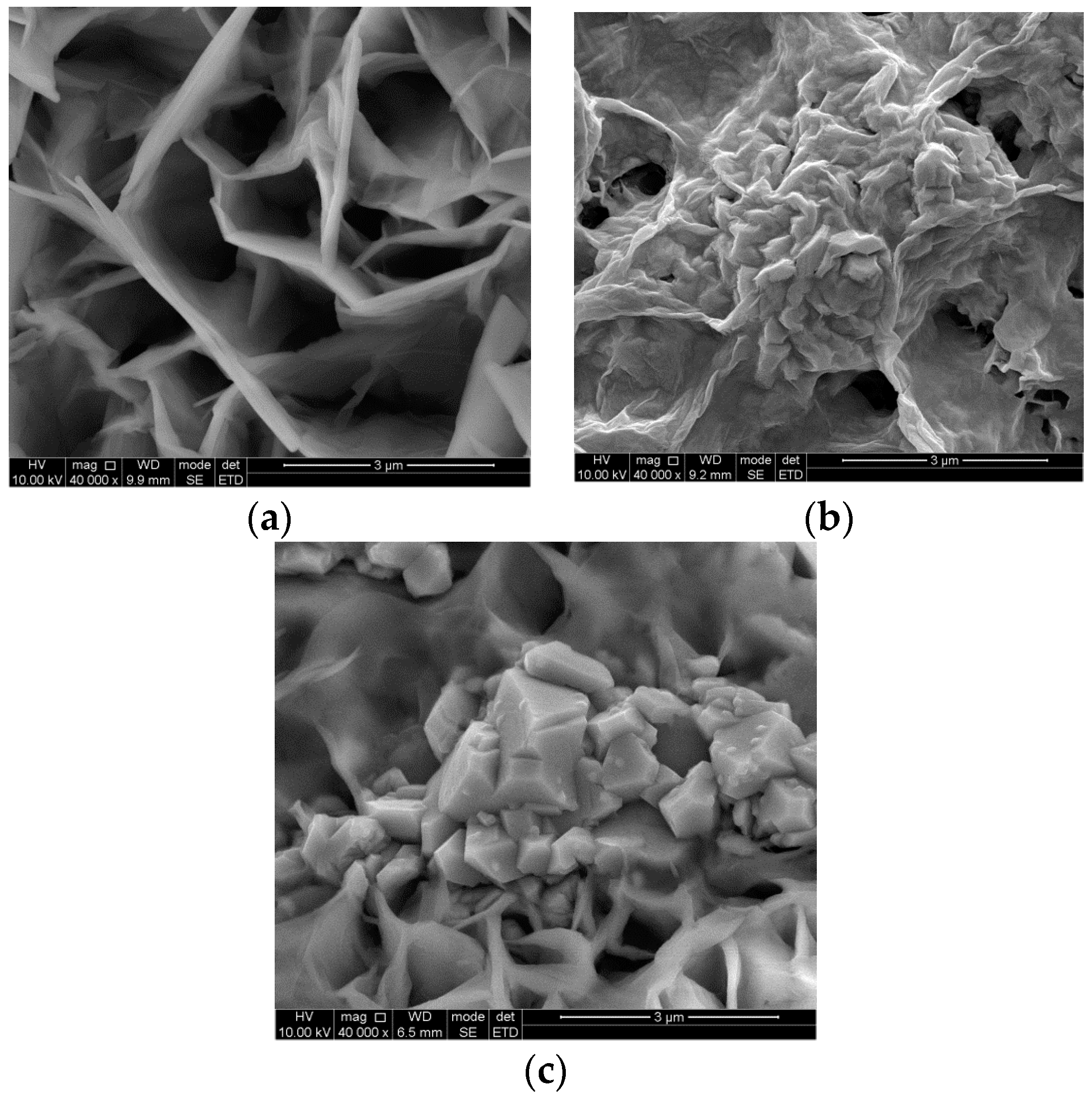
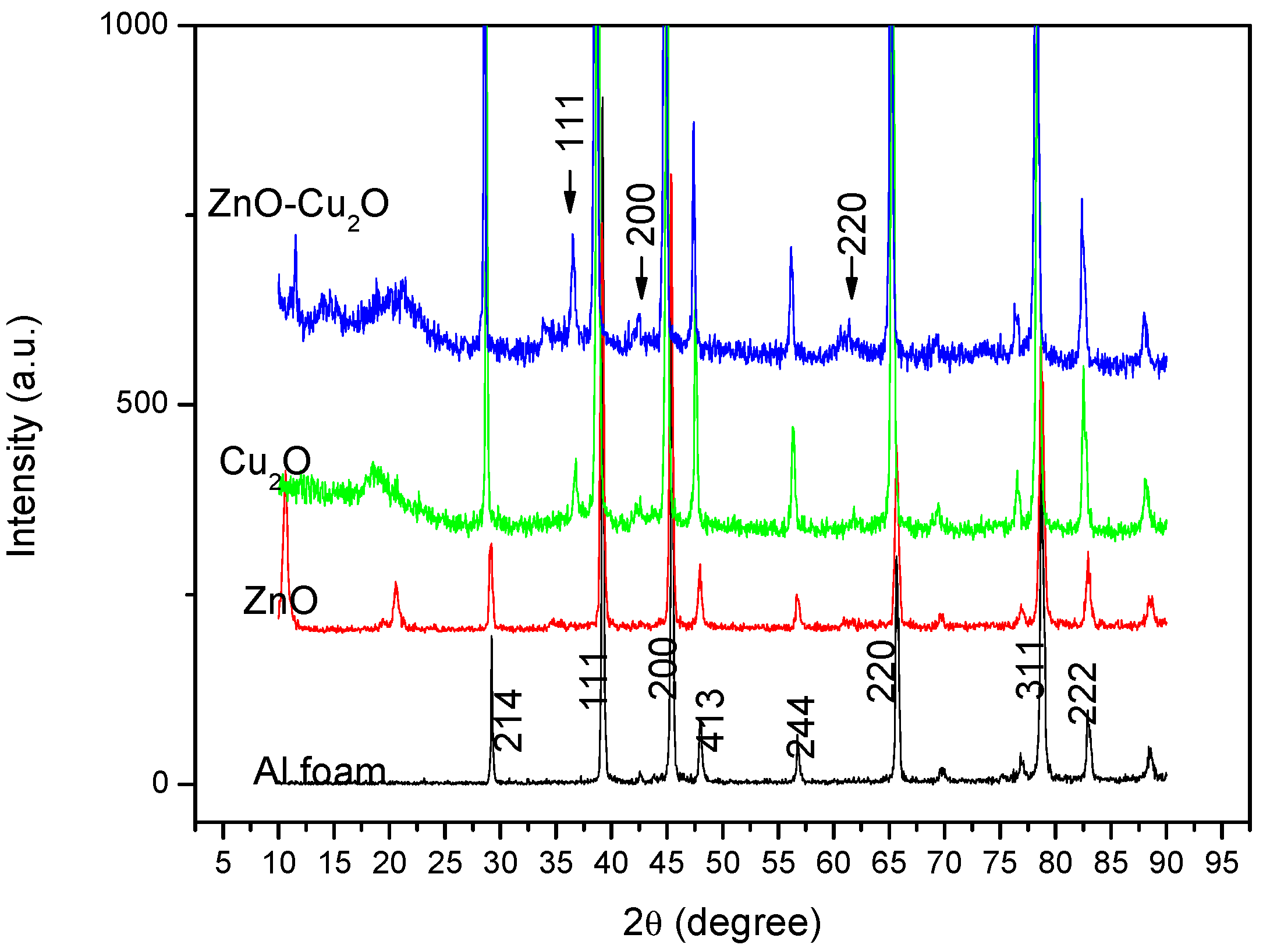
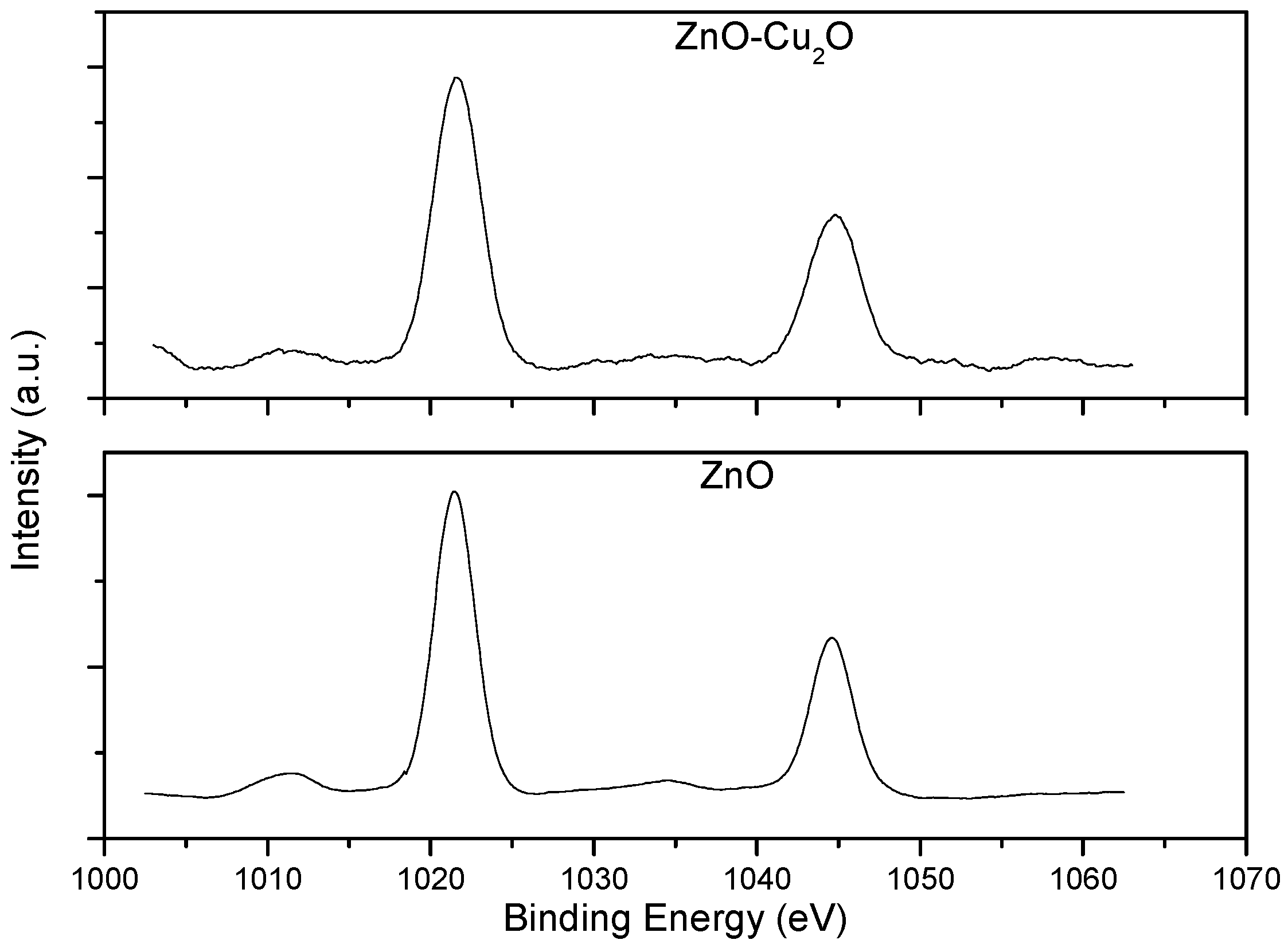
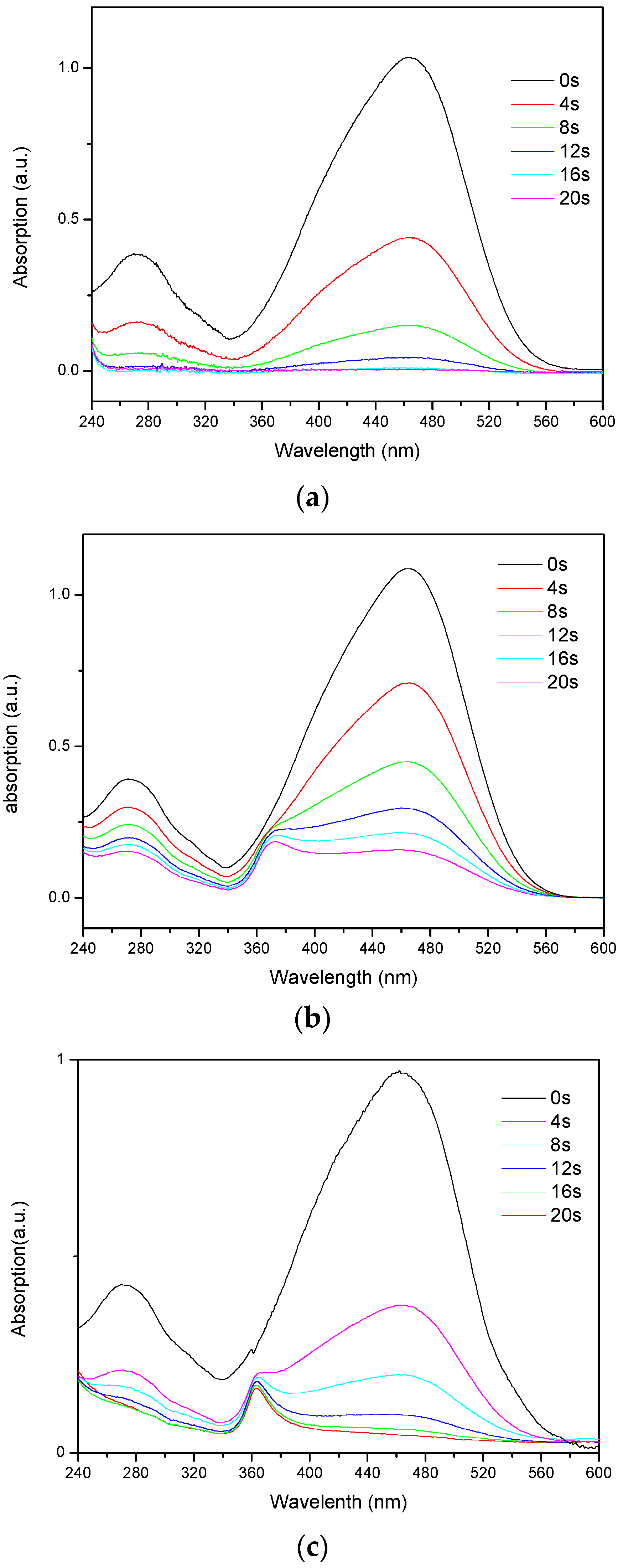
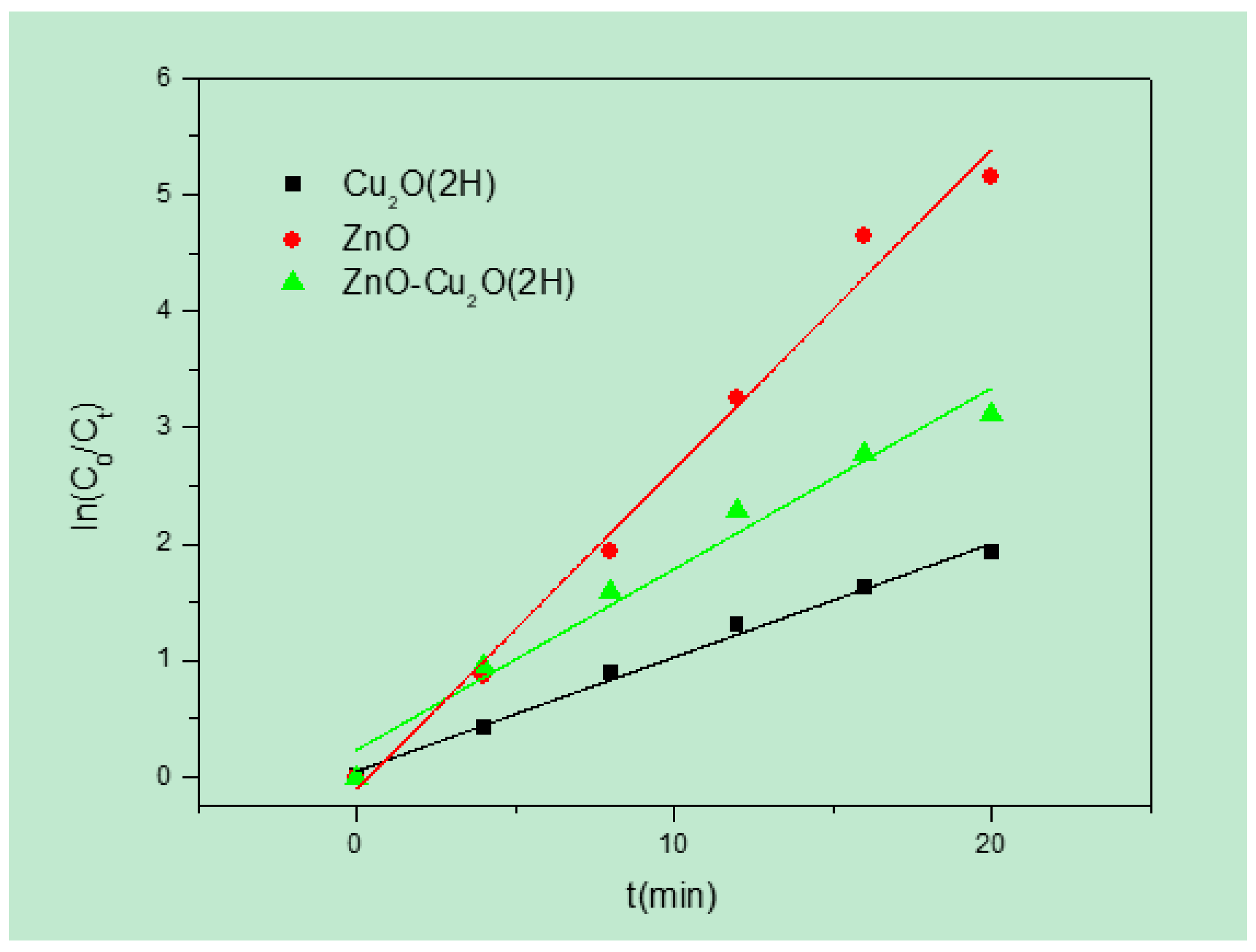
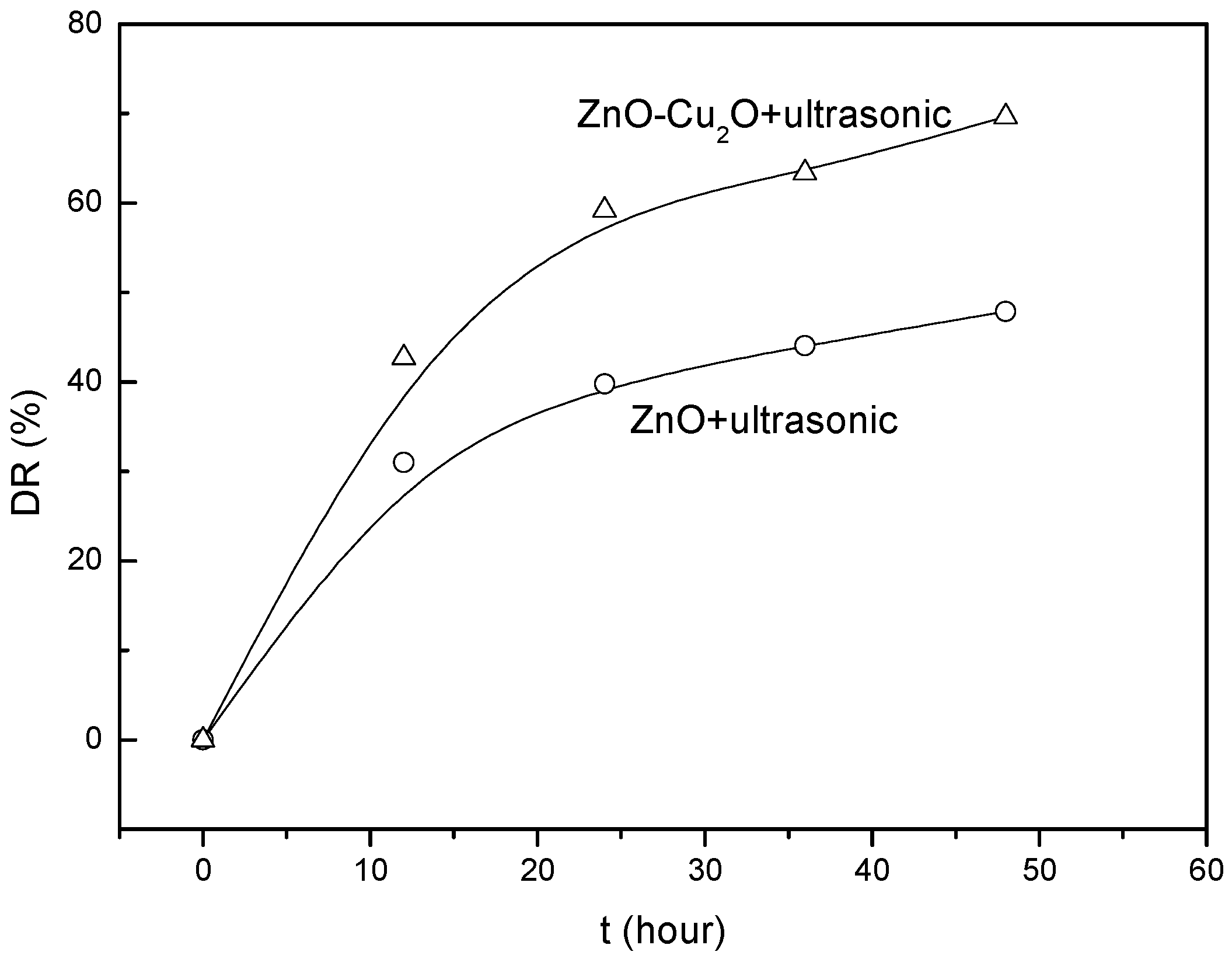
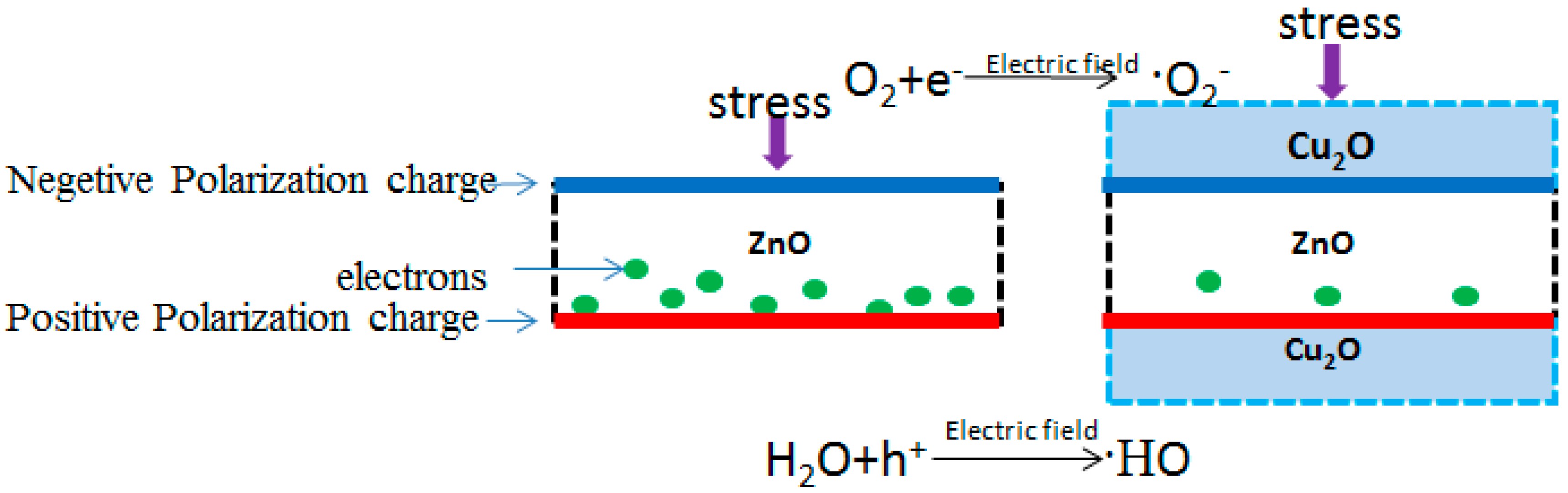
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Hosseini, A.; Faghihian, H.; Sanati, A.M. Elimination of dibenzothiophene from transportation fuel by combined photocatalytic and adsorptive method. Mater. Sci. Semicond. Process. 2018, 87, 110–118. [Google Scholar] [CrossRef]
- Singh, G.; Singh, J.; Jolly, S.S.; Rawat, R.; Kukkar, D.; Kumar, S.; Basu, S.; Rawat, M. Fructose modifed synthesis of ZnO nanoparticles and its application for removal of industrial pollutants from water. J. Mater. Sci. Mater. Electron. 2018, 29, 7364–7371. [Google Scholar] [CrossRef]
- Chouchene, B.; Chaabane, T.B.; Mozet, K.; Girot, E.; Corbel, S.; Balan, L.; Medjahdi, G.; Schneider, R. Porous Al-doped ZnO rods with selective adsorption properties. Appl. Surf. Sci. 2017, 409, 102–110. [Google Scholar] [CrossRef]
- Hassanzadeh-Tabrizi, S.A.; Motlagh, M.M.; Salahshour, S. Synthesis of ZnO/CuO nanocomposite immobilized on -Al2O3 and application for removal of methyl orange. Appl. Surf. Sci. 2016, 384, 237–243. [Google Scholar] [CrossRef]
- Xu, X.; Jia, Y.; Xiao, L.; Wu, Z. Strong vibration-catalysis of ZnO nanorods for dye waste water decolorization via piezo-electro-chemical coupling. Chemosphere 2018, 193, 1143–1148. [Google Scholar] [CrossRef]
- Qian, W.; Wu, Z.; Jia, Y.; Hong, Y.; Xu, X.; You, H.; Zheng, Y.; Xia, Y. Thermo-electrochemical coupling for room temperature thermocatalysis in pyroelectric ZnO nanorods. Electrochem. Commun. 2017, 81, 124–127. [Google Scholar] [CrossRef]
- Hong, D.; Zang, W.; Guo, X.; Fu, Y.; He, H.; Sun, J.; Xing, L.; Liu, B.; Xue, X. High Piezo-photocatalytic Efficiency of CuS/ZnO Nanowires Using Both Solar and Mechanical Energy for Degrading Organic Dye. ACS Appl. Mater. Interfaces 2016, 8, 21302–21314. [Google Scholar] [CrossRef]
- Song, X.M.; Yuan, C.; Wang, Y.; Wang, B.; Mao, H.; Wu, S.; Zhang, Y. ZnO/CuO photoelectrode with n-p heterogeneous structure for photoelectrocatalytic oxidation of formaldehyde. Appl. Surf. Sci. 2018, 455, 181–186. [Google Scholar] [CrossRef]
- Muzakki, A.; Shabrany, H.; Saleh, R. Synthesis of ZnO/CuO and TiO2/CuO nanocomposites for light and ultrasound assisted degradation of a textile dye in aqueous solution. AIP Conf. Proc. 2015, 1725, 020051. [Google Scholar]
- Gupta, V.K.; Suhas. Application of low-cost adsorbents for dye removal–A review. J. Environ. Manag. 2009, 90, 2313–2342. [Google Scholar] [CrossRef]
- Ikeda, S.; Takata, T.; Komoda, M.; Hara, M.; Kondo, J.N.; Domen, K.; Tanaka, A.; Hosono, H.; Kawazoe, H. Mechano-catalysis a novel method for overall water splitting. Phys. Chem. Chem. Phys. 1999, 1, 4485–4491. [Google Scholar] [CrossRef]
- Guo, B.; Liu, G.; Zeng, Y.; Dong, G.; Wang, C. Rapid mineralization of methyl orange by nanocrystalline-assembled mesoporous Cu2O microspheres. Nanotechnology 2018, 29, 445701. [Google Scholar] [CrossRef]
- Wang, Y.; Gao, J.; Wang, X.; Jin, L.; Fang, L.; Zhang, M.; He, G.; Sun, Z. Facile synthesis of core-shell ZnO/Cu2O heterojunction with enhanced visible light-driven photocatalytic performance. J. Sol-Gel Sci. Technol. 2018, 88, 172–180. [Google Scholar] [CrossRef]
- Ghamgosar, P.; Rigoni, F.; You, S.; Dobryden, I.; Kohan, M.G.; Pellegrino, A.L.; Concina, I.; Almqvist, N.; Malandrino, G.; Vomiero, A. ZnO-Cu2O core-shell nanowires as stable and fast response photodetectors. Nano Energy 2018, 51, 308–316. [Google Scholar] [CrossRef]
- Shi, S.; Xu, J.; Li, L. Preparation and photocatalytic activity of ZnO nanorods and ZnO/Cu2O nanocomposites. Main Group Chem. 2017, 16, 47–55. [Google Scholar] [CrossRef]
- Wang, Q.; Qiu, Y.; Yang, D.; Li, B.; Zhang, X.; Tang, Y.; Hu, L. Improvement in piezoelectric performance of a ZnO nanogenerator by modulating interface engineering of CuO-ZnO heterojunction. Appl. Phys. Lett. 2018, 113, 053901. [Google Scholar] [CrossRef]
- Pang, Y.L.; Abdullah, A.Z.; Bhatia, S. Review on sonochemical methods in the presence of catalysts and chemical additives for treatment of organic pollutants in wastewater. Desalination 2011, 277, 1–14. [Google Scholar] [CrossRef]
- Ye, F.; Li, H.; Yu, H.; Chen, S.; Quan, X. Hydrothermal fabrication of few-layer MoS2 nanosheets within nanopores on TiO2 derived from MIL-125(Ti) for efficient photocatalytic H2 evolution. Appl. Surf. Sci. 2017, 426, 177–184. [Google Scholar] [CrossRef]
- Chen, Y.P.; Liu, S.Y.; Yu, H.Q.; Yin, H.; Li, Q.R. Radiation-induced degradation of methyl orange in aqueous solutions. Chemosphere 2008, 72, 532–536. [Google Scholar] [CrossRef]
- Jing, H.Y.; Wen, T.; Fan, C.M.; Gao, G.Q.; Zhong, S.L.; Xu, A.W. Efficient adsorption/photodegradation of organic pollutants from aqueous systems using Cu2O nanocrystals as a novel integrated photocatalytic adsorbent. J. Mater. Chem. A 2014, 2, 14563. [Google Scholar] [CrossRef]
- Chen, D.S.; Yu, W.B.; Deng, Z.; Liu, J.; Jin, J.; Li, Y.; Wu, M.; Chen, L.H.; Su, B.L. Hollow Cu2O microspheres with two active {111}and {110} facets for highly selective adsorption and photo degradation of anionic dye. RSC Adv. 2015, 5, 55520. [Google Scholar] [CrossRef]
- Liu, C.M.; Huang, X.Y.; Zhang, H.Y.; Dai, J.D.; Ning, C.C. The decoloration of methyl orange using aluminum foam, ultrasound and direct electric current. Mater. Res. Express 2018, 5, 015501. [Google Scholar] [CrossRef]
- Lei, J.; Yin, B.; Qiu, Y.; Zhang, H.; Chang, Y.; Luo, Y.; Zhao, Y.; Ji, J.; Hu, L. Flexible piezoelectric nanogenerator based on Cu2O–ZnO p–n junction for energy harvesting. RSC Adv. 2015, 5, 59458. [Google Scholar] [CrossRef]
- Starr, M.B.; Wang, X. Fundamental Analysis of Piezocatalysis Process on the Surfaces of Strained Piezoelectric Materials. Sci. Rep. 2013, 3, 2160. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.; Kumar, Y.B.; Seo, J.S.; Kim, K.H.; Sohn, J.I.; Cha, S.N.; Choi, D.; Wang, Z.L.; Kim, S.W. p-type polymer-hybridized highperformance piezoelectric nanogenerators. Nano Lett. 2012, 12, 1959. [Google Scholar] [CrossRef] [PubMed]
- Shin, S.H.; Lee, M.; Jung, J.Y.; Seol, J.; Nah, J. Piezoelectric performance enhancement of ZnO flexible nanogenerator by a CuO-ZnO p-n junction formation. J. Mater. Chem. C 2013, 1, 8103–8107. [Google Scholar] [CrossRef]
- Wu, J.M.; Chang, W.E.; Chang, Y.T.; Chang, C.K. Piezo-Catalytic Effect on the Enhancement of the Ultra-High Degradation Activity in the Dark by Singleand Few-Layers MoS2 Nanoflowers. Adv. Mater. 2016, 28, 3718–3725. [Google Scholar] [CrossRef] [PubMed]

© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dai, J.D.; Gan, L.; Zhang, H.Y.; Liu, C.M. The Decoloration of Anionic and Cationic Dyes Using ZnO and ZnO-Cu2O. Crystals 2019, 9, 229. https://doi.org/10.3390/cryst9050229
Dai JD, Gan L, Zhang HY, Liu CM. The Decoloration of Anionic and Cationic Dyes Using ZnO and ZnO-Cu2O. Crystals. 2019; 9(5):229. https://doi.org/10.3390/cryst9050229
Chicago/Turabian StyleDai, Jiang Dong, Luo Gan, Hai Yan Zhang, and Chun Ming Liu. 2019. "The Decoloration of Anionic and Cationic Dyes Using ZnO and ZnO-Cu2O" Crystals 9, no. 5: 229. https://doi.org/10.3390/cryst9050229
APA StyleDai, J. D., Gan, L., Zhang, H. Y., & Liu, C. M. (2019). The Decoloration of Anionic and Cationic Dyes Using ZnO and ZnO-Cu2O. Crystals, 9(5), 229. https://doi.org/10.3390/cryst9050229



