Integrated Chromogenic Analysis of Freshwater Pearls: Revealing the Internal Factors Driving Color Variation
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Description
2.2. Methods
2.2.1. Spectroscopy Analysis
2.2.2. Scanning Electron Microscope
2.2.3. Chemical Composition Analysis
3. Results
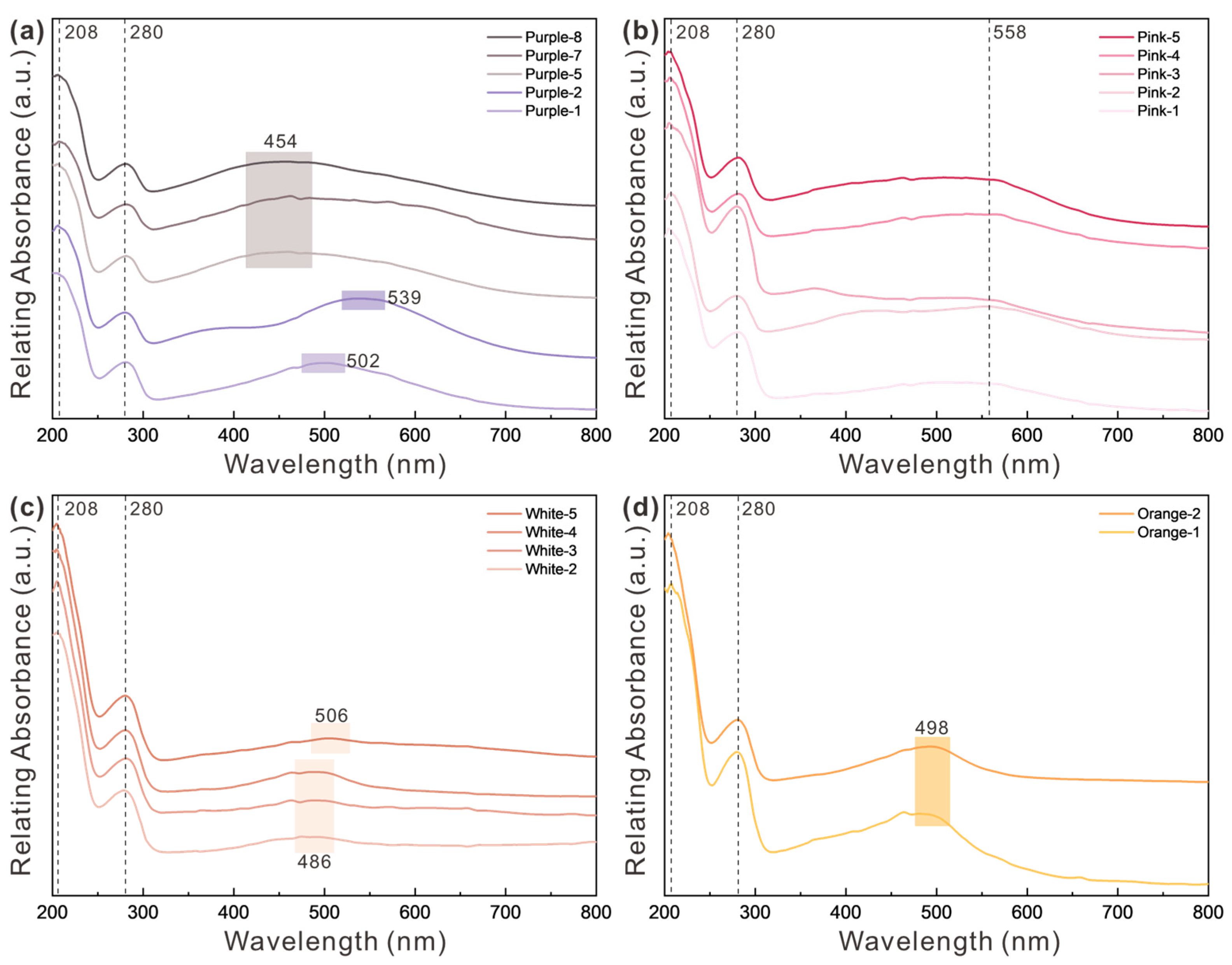
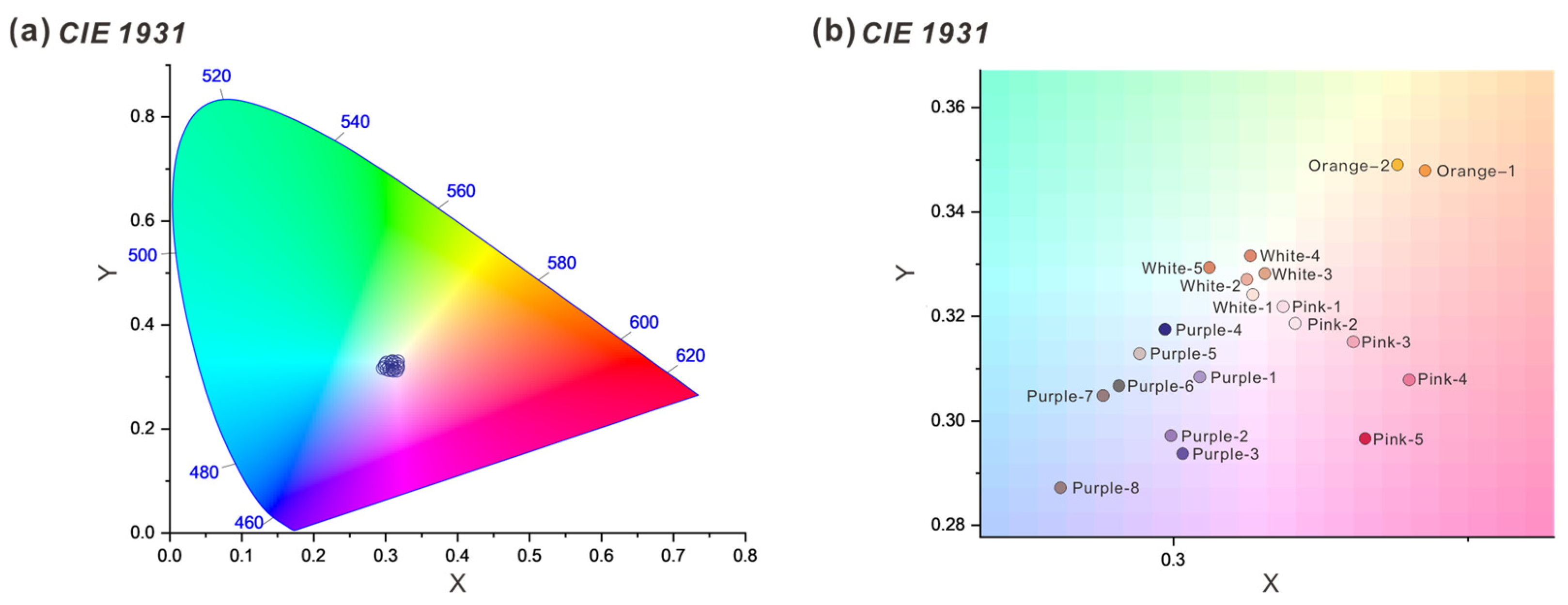
3.1. Absorption Spectra
3.1.1. UV–Vis Spectra
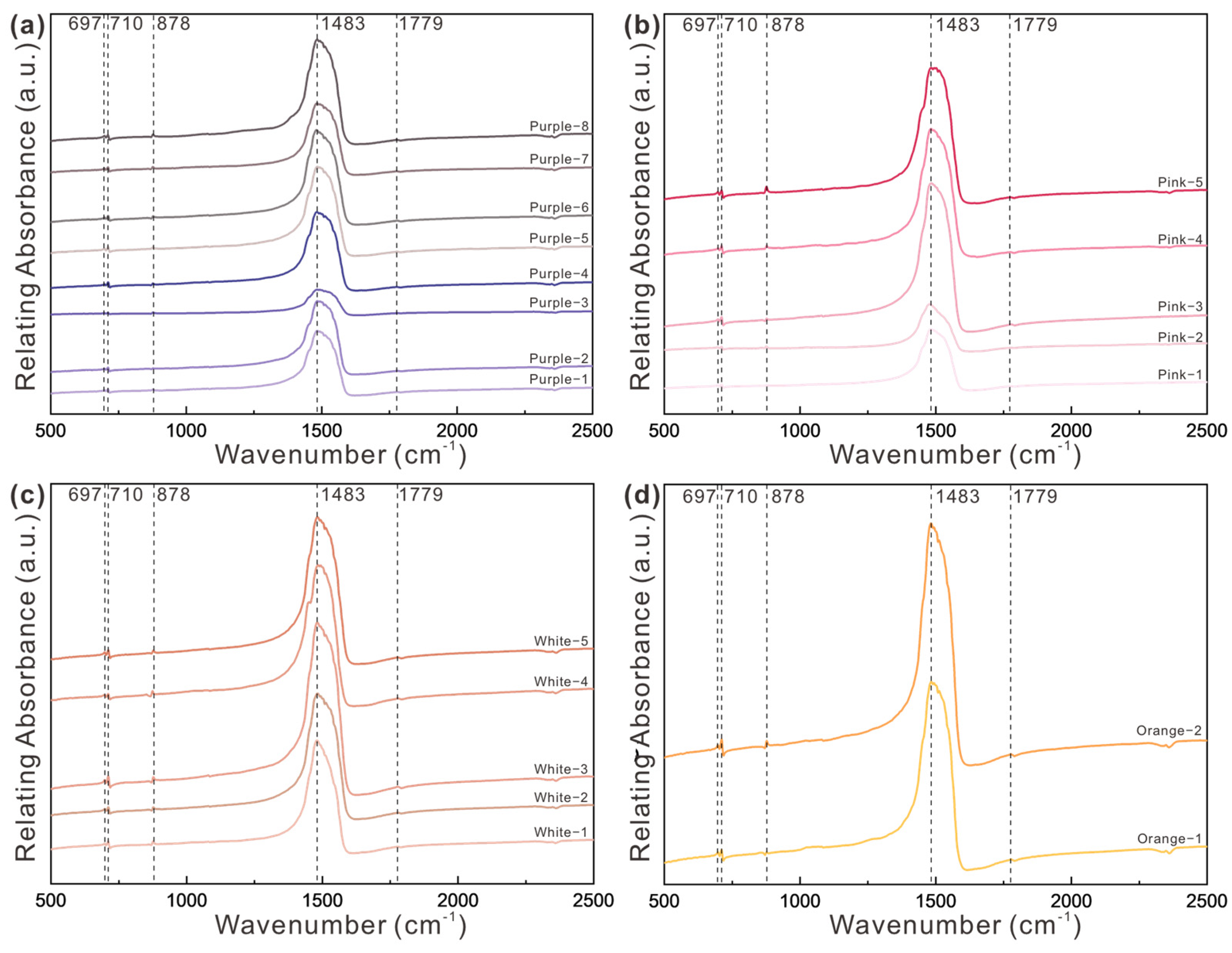
3.1.2. FTIR Spectra
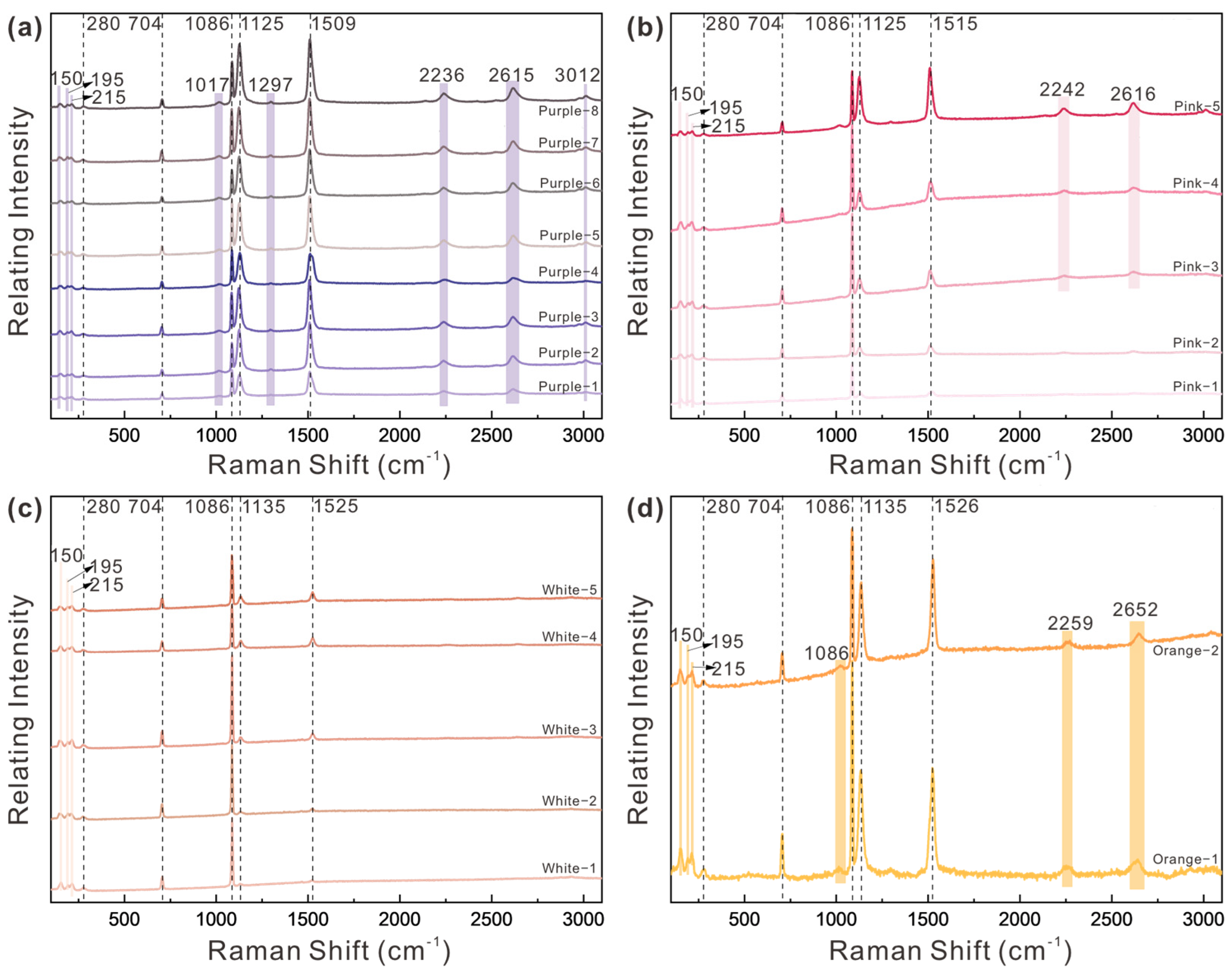
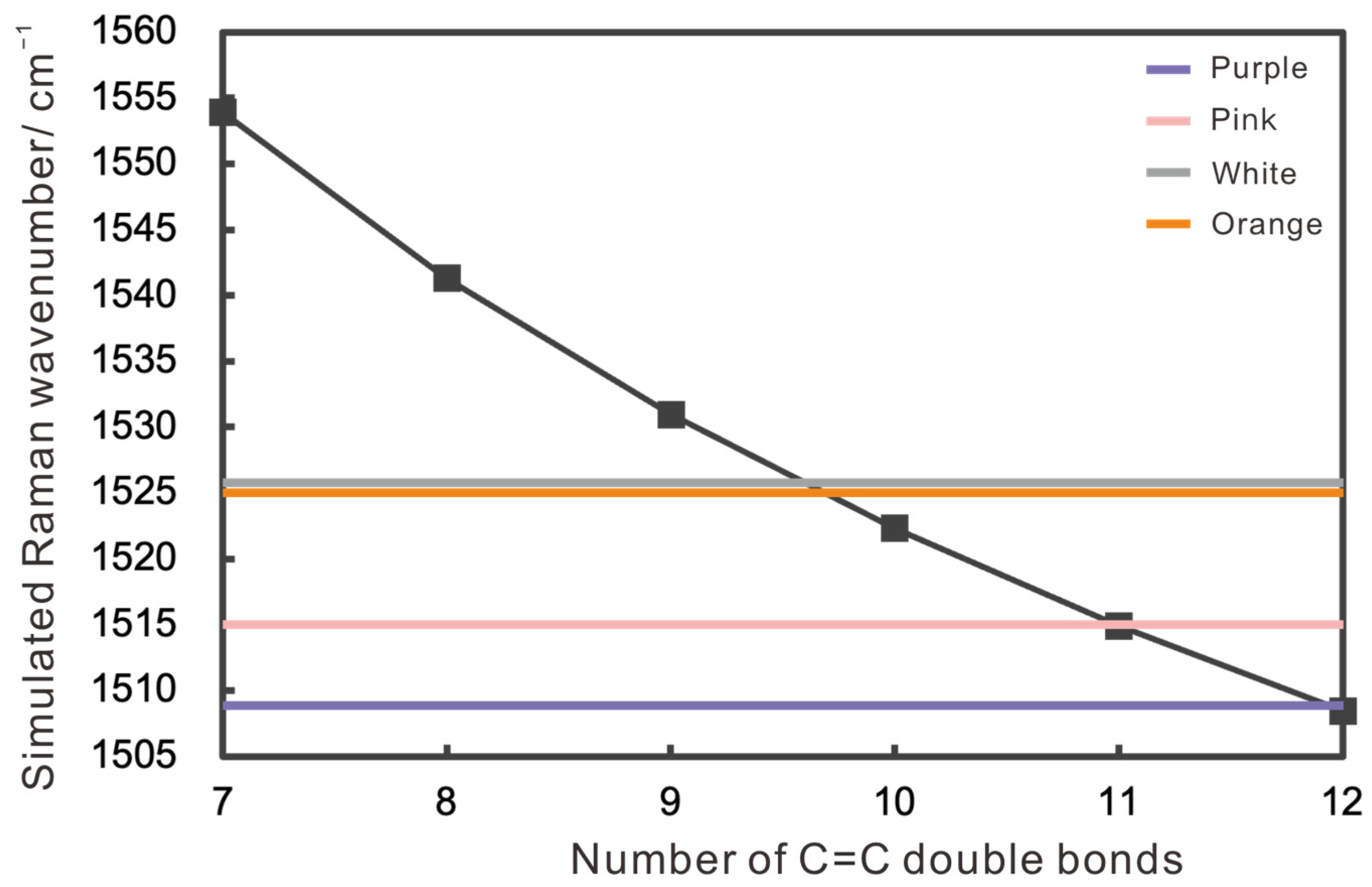
3.1.3. Raman Spectra
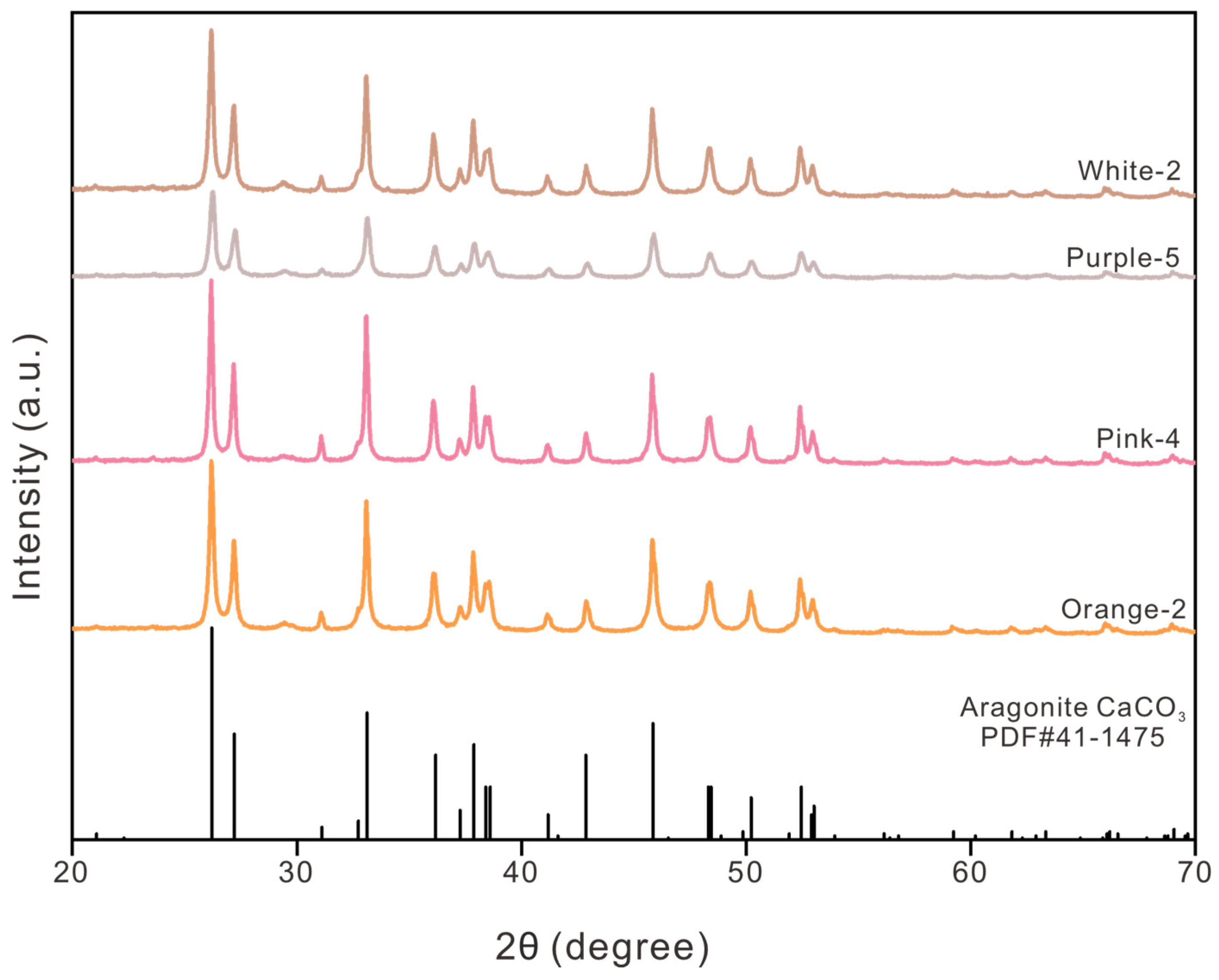
3.1.4. X-Ray Diffraction Analysis
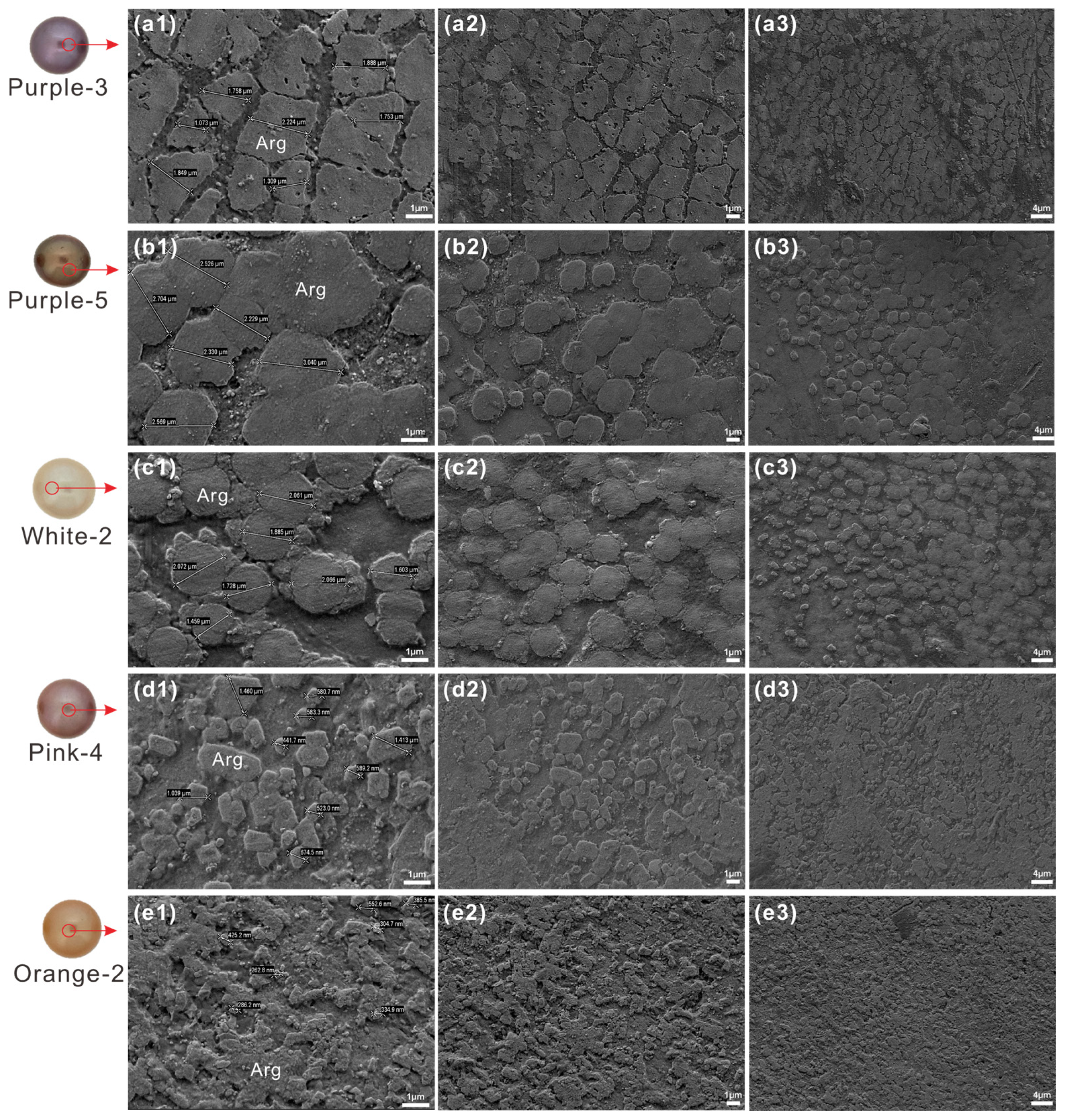
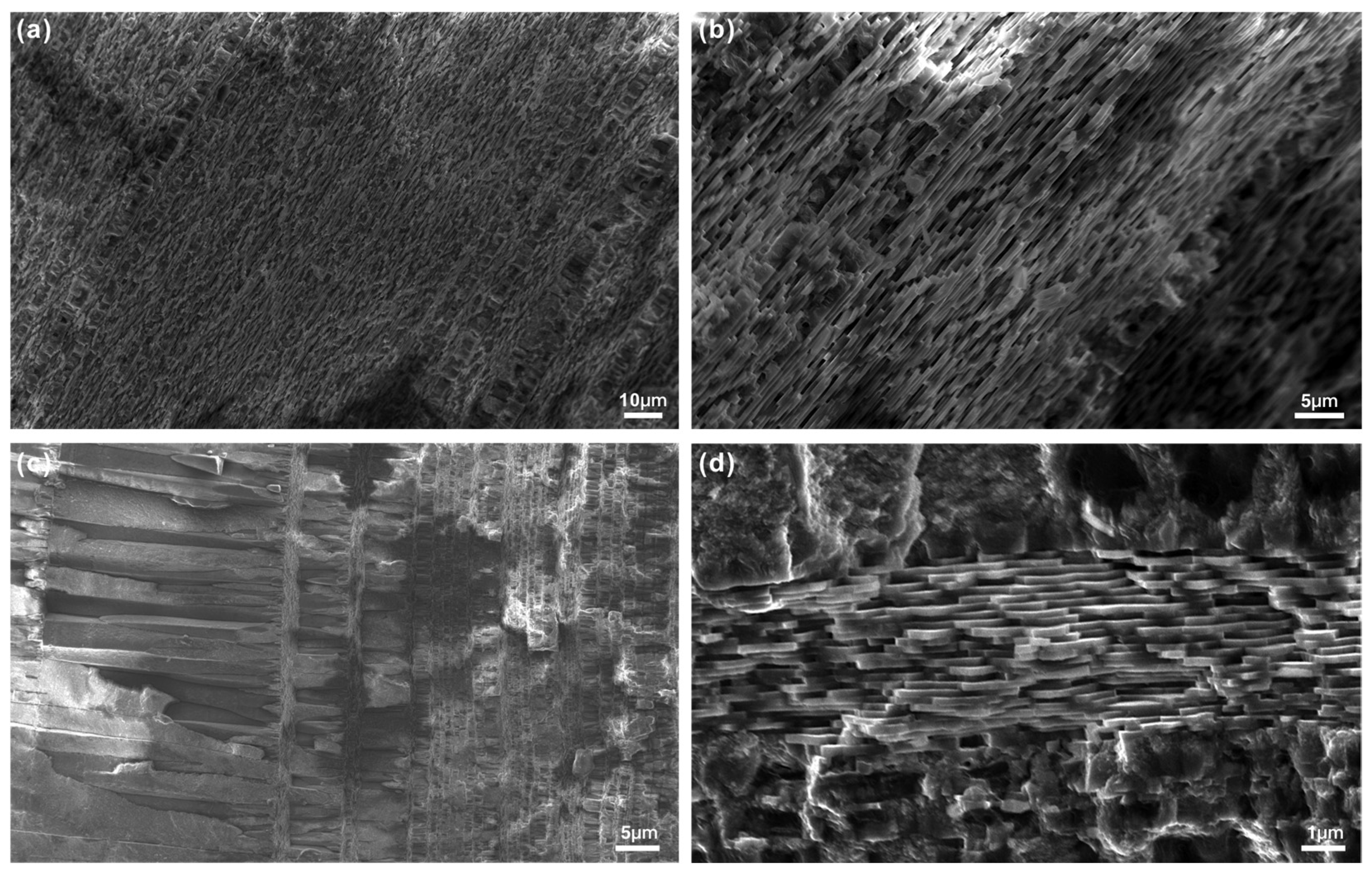
3.2. Scanning Electron Microscopy
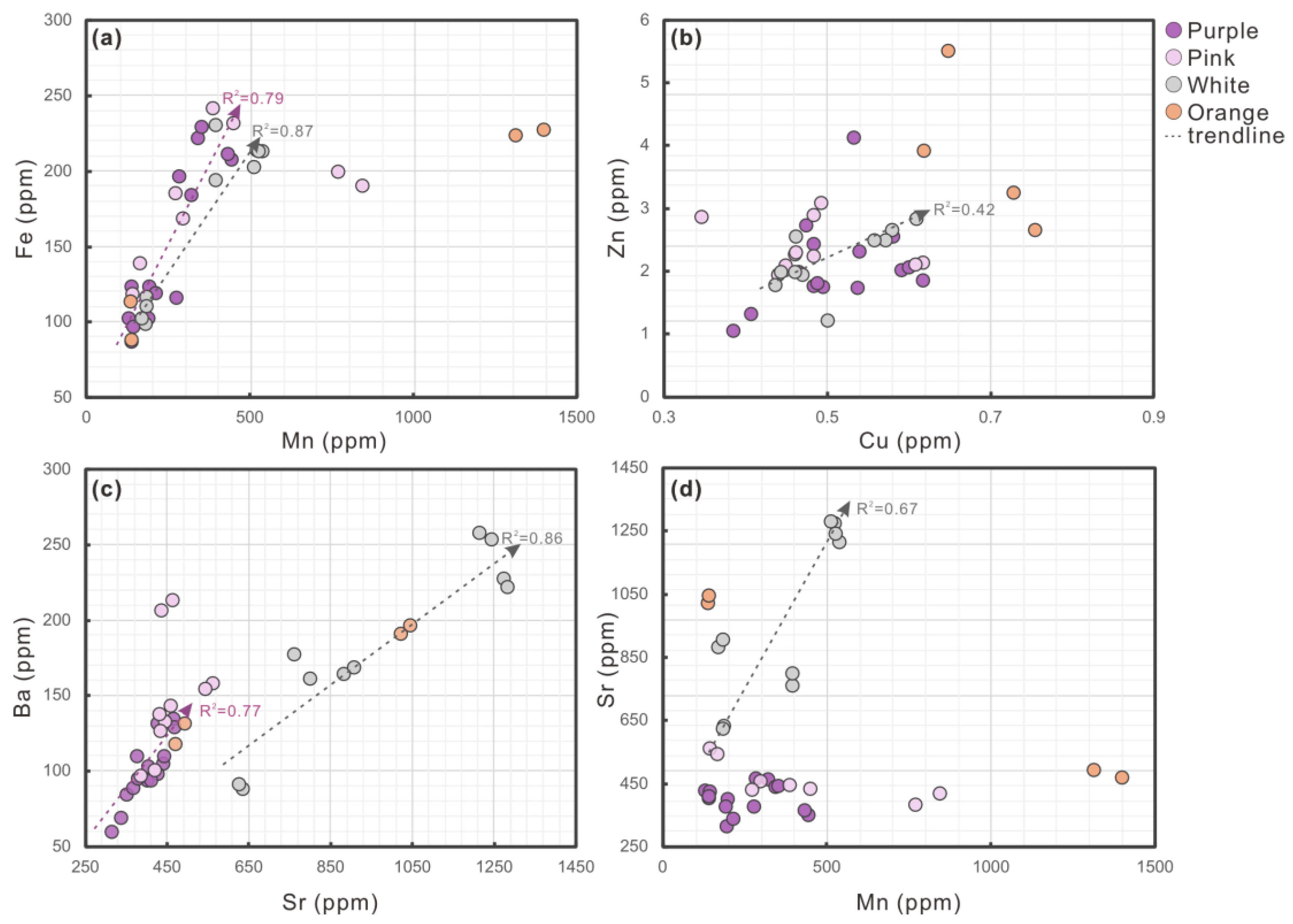
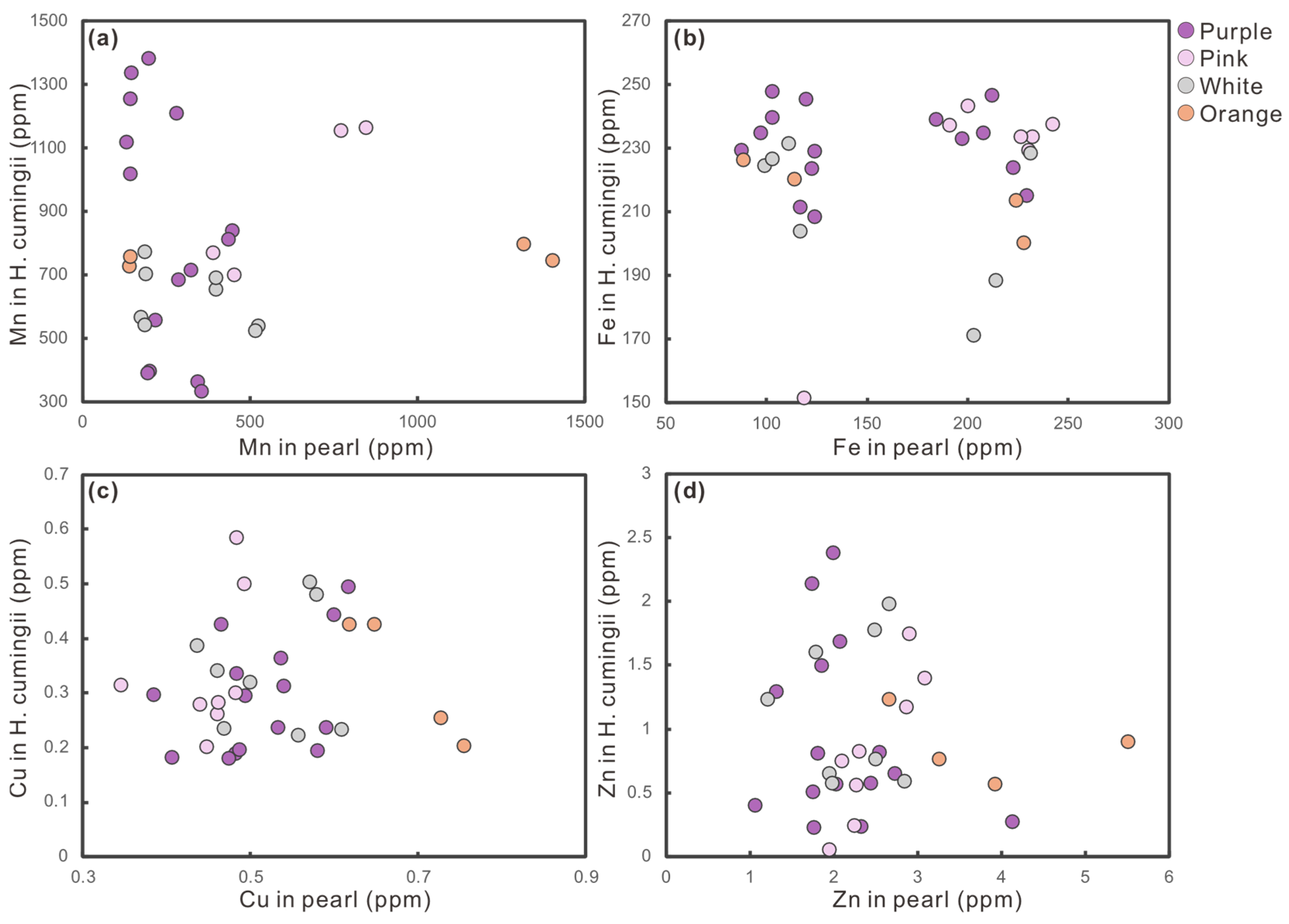
3.3. Trace Elements
4. Discussion
4.1. Biological Factors of Coloration in Freshwater Nucleated Cultured Pearl Mechanism
4.2. Factors Affecting Color Variation in Nucleated Cultured Pearls
4.2.1. Cooperative Color Regulation by Organic Matrix and Physical Structure
4.2.2. Metal Ions and Metalloporphyrin
4.3. Interaction Between Host Hyriopsis Cumingii and Pearl Coloration
5. Conclusions
- (1)
- Polyenic compounds are mainly primary chromophores, with C=C chain length dictating core hue. The purple pearls had 12 C=C bonds, pink pearls had 11 C=C bonds, and white and orange pearls about 10 C=C bonds. Peak intensities in Raman spectra (1125–1135 cm−1 and 1509–1525 cm−1) correlate positively with color saturation.
- (2)
- Nacre microstructure can modulate color expression. Compactly stacked lamellar tablets (1.07–1.88 μm) in purple pearls enhance light interference, while irregular polygonal tablets (0.26–1.46 μm) in pink and orange pearls promote scattering. Elevated protein content in dark pearls enhances pigment binding capacity, intensifying color saturation.
- (3)
- Trace metal ions contribute via metalloporphyrin formation and lattice substitution. Identical culturing conditions and elemental profiles in pearls and host mussels indicate negligible nacre influence, confirming that color is endogenously regulated.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Samples | Na | Mg | Al | K | Ca | Ti | V | Cr | |
| SRM 610 | 104,108.09 | 484.79 | 11,260.00 | 505.76 | 85,713.25 | 453.93 | 457.71 | 427.34 | |
| SRM 610 | 104,650.02 | 487.73 | 11,318.66 | 510.70 | 86,184.35 | 453.75 | 458.27 | 424.00 | |
| BCR-2G | 24,587.51 | 23,579.47 | 78,104.57 | 16,230.88 | 51,914.93 | 13,171.81 | 437.01 | 17.13 | |
| BHVO-2G | 17,471.64 | 46,062.77 | 77,597.77 | 4759.73 | 81,916.99 | 15,883.83 | 331.79 | 289.41 | |
| Purple-1—1 | 2393.99 | 11.04 | 1.06 | 63.00 | 392,252.45 | 0.00 | 0.05 | 2.52 | |
| Purple-1—2 | 2505.98 | 7.78 | 0.00 | 71.52 | 390,714.18 | 0.42 | 0.00 | 2.37 | |
| Purple-2—1 | 2829.37 | 26.92 | 0.00 | 146.20 | 390,009.76 | 0.00 | 0.04 | 2.74 | |
| Purple-2—2 | 2668.12 | 27.73 | 0.00 | 136.22 | 391,570.44 | 0.00 | 0.00 | 2.57 | |
| Purple-3—1 | 2530.84 | 13.47 | 0.00 | 80.73 | 390,795.96 | 0.00 | 0.00 | 2.29 | |
| Purple-3—2 | 2489.74 | 10.50 | 0.00 | 71.50 | 390,739.34 | 0.00 | 0.00 | 1.90 | |
| Purple-4—1 | 2477.86 | 9.05 | 0.00 | 84.32 | 392,137.22 | 0.00 | 0.00 | 2.10 | |
| Purple-4—2 | 2521.44 | 10.54 | 0.02 | 114.98 | 392,056.00 | 0.00 | 0.00 | 2.38 | |
| Purple-5—1 | 2467.05 | 39.36 | 0.00 | 65.05 | 391,006.99 | 0.00 | 0.00 | 2.60 | |
| Purple-5—2 | 2741.24 | 36.61 | 0.00 | 64.93 | 392,160.73 | 0.00 | 0.07 | 3.09 | |
| Purple-6—1 | 2869.07 | 10.55 | 0.00 | 95.72 | 391,850.05 | 0.00 | 0.00 | 1.42 | |
| Purple-6—2 | 2862.04 | 7.12 | 0.31 | 104.58 | 392,102.80 | 0.00 | 0.08 | 2.01 | |
| Purple-7—1 | 3025.20 | 13.12 | 0.97 | 19.37 | 390,637.16 | 0.36 | 0.05 | 3.52 | |
| Purple-8—1 | 2620.58 | 0.60 | 0.00 | 94.90 | 392,702.85 | 0.00 | 0.00 | 3.03 | |
| Purple-8—2 | 2688.84 | 2.86 | 0.00 | 94.59 | 391,884.66 | 0.00 | 0.00 | 2.62 | |
| Pink-1—1 | 2417.91 | 11.30 | 0.07 | 89.99 | 391,483.75 | 0.00 | 0.00 | 3.75 | |
| Pink-1—2 | 2459.45 | 13.73 | 0.31 | 86.21 | 392,249.98 | 0.00 | 0.06 | 3.62 | |
| Pink-2—1 | 1777.37 | 38.91 | 0.00 | 43.63 | 391,785.21 | 0.00 | 0.00 | 3.39 | |
| Pink-2—2 | 1870.65 | 37.60 | 0.14 | 32.94 | 392,389.28 | 0.25 | 0.00 | 1.29 | |
| Pink-3—1 | 2551.77 | 29.37 | 0.00 | 51.45 | 392,773.28 | 0.19 | 0.00 | 2.96 | |
| Pink-3—2 | 2581.64 | 32.86 | 0.00 | 58.52 | 392,882.63 | 0.00 | 0.00 | 3.65 | |
| Pink-4—1 | 2143.45 | 32.33 | 0.02 | 97.32 | 392,855.92 | 0.00 | 0.00 | 2.11 | |
| Pink-4—2 | 2144.68 | 37.91 | 0.00 | 80.90 | 392,670.15 | 0.00 | 0.00 | 2.99 | |
| Pink-5—1 | 2777.71 | 28.58 | 0.00 | 101.60 | 392,366.04 | 0.00 | 0.00 | 2.87 | |
| Pink-5—2 | 2573.91 | 27.01 | 0.00 | 90.02 | 393,047.38 | 0.00 | 0.04 | 2.55 | |
| White-1—1 | 2743.72 | 17.41 | 0.00 | 231.53 | 391,299.90 | 0.00 | 0.00 | 3.13 | |
| White-1—2 | 2767.43 | 16.68 | 0.00 | 230.42 | 391,384.17 | 0.00 | 0.00 | 3.01 | |
| White-2—1 | 2907.06 | 17.65 | 0.00 | 153.01 | 392,088.44 | 0.00 | 0.07 | 3.01 | |
| White-2—2 | 2950.30 | 18.16 | 0.20 | 149.76 | 392,585.69 | 0.00 | 0.00 | 3.93 | |
| White-3—1 | 2868.58 | 7.56 | 0.52 | 84.54 | 393,301.93 | 0.00 | 0.00 | 3.55 | |
| White-3—2 | 3026.37 | 8.84 | 0.07 | 92.92 | 392,761.96 | 0.00 | 0.00 | 2.34 | |
| White-4—1 | 2587.88 | 4.31 | 0.36 | 141.11 | 393,446.81 | 0.00 | 0.00 | 2.12 | |
| White-4—2 | 2588.96 | 1.58 | 0.47 | 142.33 | 392,119.94 | 0.00 | 0.05 | 2.97 | |
| White-5—1 | 2383.75 | 23.69 | 0.00 | 127.30 | 392,171.24 | 0.00 | 0.02 | 1.15 | |
| White-5—2 | 2253.94 | 27.09 | 0.00 | 116.11 | 391,807.09 | 0.00 | 0.00 | 3.02 | |
| Orange-1—1 | 2563.35 | 30.35 | 0.00 | 354.20 | 390,848.32 | 0.00 | 0.01 | 3.09 | |
| Orange-1—2 | 2653.02 | 33.68 | 0.00 | 313.24 | 390,219.51 | 0.34 | 0.00 | 3.85 | |
| Orange-2—1 | 2673.63 | 13.81 | 0.00 | 110.46 | 393,672.54 | 0.00 | 0.00 | 1.82 | |
| Orange-2—2 | 2659.22 | 10.61 | 0.00 | 113.14 | 391,491.59 | 0.00 | 0.02 | 2.91 | |
| Samples | Mn | Fe | Ni | Cu | Zn | Sr | Mo | Cs | Ba |
| SRM 610 | 508.45 | 467.11 | 477.05 | 452.44 | 450.93 | 550.17 | 425.68 | 383.11 | 476.02 |
| SRM 610 | 505.90 | 491.03 | 477.19 | 445.97 | 453.03 | 547.04 | 422.28 | 372.05 | 470.52 |
| BCR-2G | 1616.22 | 58,382.05 | 11.31 | 19.04 | 175.67 | 343.35 | 257.99 | 1.13 | 679.77 |
| BHVO-2G | 1382.69 | 50,988.08 | 127.71 | 128.52 | 132.31 | 391.05 | 4.39 | 0.10 | 128.51 |
| Purple-1—1 | 197.46 | 122.23 | 0.00 | 0.54 | 1.73 | 401.79 | 0.17 | 0.01 | 93.70 |
| Purple-1—2 | 191.42 | 102.89 | 0.00 | 0.46 | 1.99 | 377.18 | 0.13 | 0.00 | 110.09 |
| Purple-2—1 | 130.32 | 102.67 | 0.00 | 0.59 | 2.02 | 427.28 | 0.22 | 0.00 | 131.61 |
| Purple-2—2 | 139.73 | 123.69 | 0.00 | 0.54 | 2.32 | 404.10 | 0.19 | 0.00 | 103.43 |
| Purple-3—1 | 322.09 | 184.38 | 0.00 | 0.49 | 1.75 | 465.22 | 0.06 | 0.00 | 134.76 |
| Purple-3—2 | 283.89 | 197.02 | 0.00 | 0.48 | 1.76 | 467.96 | 0.40 | 0.00 | 129.28 |
| Purple-4—1 | 444.38 | 207.90 | 0.00 | 0.48 | 2.44 | 350.80 | 0.07 | 0.00 | 84.60 |
| Purple-4—2 | 433.79 | 211.80 | 0.22 | 0.53 | 4.12 | 366.39 | 0.12 | 0.01 | 89.10 |
| Purple-5—1 | 342.97 | 222.34 | 0.00 | 0.60 | 2.07 | 440.16 | 0.44 | 0.00 | 105.30 |
| Purple-5—2 | 353.91 | 229.38 | 0.75 | 0.62 | 1.85 | 443.37 | 0.05 | 0.00 | 109.93 |
| Purple-6—1 | 143.42 | 97.16 | 0.09 | 0.49 | 1.81 | 426.53 | 0.04 | 0.00 | 98.23 |
| Purple-6—2 | 139.93 | 87.36 | 0.06 | 0.58 | 2.55 | 411.12 | 0.00 | 0.00 | 93.91 |
| Purple-7—1 | 277.50 | 116.58 | 1.20 | 0.47 | 2.73 | 379.11 | 0.04 | 0.00 | 95.11 |
| Purple-8—1 | 194.08 | 123.67 | 0.00 | 0.41 | 1.31 | 314.79 | 0.04 | 0.01 | 59.84 |
| Purple-8—2 | 214.19 | 119.46 | 0.46 | 0.38 | 1.06 | 338.05 | 0.07 | 0.00 | 69.37 |
| Pink-1—1 | 386.43 | 241.96 | 0.00 | 0.45 | 2.10 | 444.76 | 0.12 | 0.02 | 133.03 |
| Pink-1—2 | 450.85 | 232.14 | 0.87 | 0.46 | 2.27 | 433.19 | 0.02 | 0.02 | 126.80 |
| Pink-2—1 | 2130.58 | 226.31 | 0.00 | 0.35 | 2.86 | 434.95 | 0.27 | 0.00 | 206.35 |
| Pink-2—2 | 2245.31 | 230.42 | 0.67 | 0.46 | 2.30 | 463.37 | 0.45 | 0.00 | 213.11 |
| Pink-3—1 | 143.13 | 118.68 | 0.13 | 0.49 | 3.08 | 562.07 | 0.03 | 0.00 | 158.28 |
| Pink-3—2 | 165.42 | 139.42 | 0.00 | 0.48 | 2.89 | 544.26 | 0.03 | 0.00 | 154.46 |
| Pink-4—1 | 845.24 | 190.91 | 0.26 | 0.48 | 2.25 | 419.38 | 0.07 | 0.00 | 100.63 |
| Pink-4—2 | 771.04 | 200.20 | 0.18 | 0.44 | 1.94 | 384.67 | 0.14 | 0.01 | 97.18 |
| Pink-5—1 | 297.47 | 169.19 | 0.75 | 0.62 | 2.13 | 458.54 | 0.02 | 0.00 | 143.38 |
| Pink-5—2 | 273.17 | 185.54 | 0.39 | 0.61 | 2.10 | 431.37 | 0.07 | 0.00 | 137.92 |
| White-1—1 | 523.58 | 213.92 | 0.54 | 0.58 | 2.66 | 1273.61 | 0.16 | 0.00 | 227.87 |
| White-1—2 | 513.06 | 202.69 | 0.27 | 0.57 | 2.49 | 1282.02 | 0.06 | 0.00 | 222.09 |
| White-2—1 | 395.10 | 194.41 | 0.24 | 0.56 | 2.50 | 760.13 | 0.23 | 0.00 | 177.58 |
| White-2—2 | 395.66 | 230.98 | 0.20 | 0.61 | 2.84 | 799.35 | 0.26 | 0.00 | 161.43 |
| White-3—1 | 186.01 | 116.72 | 0.52 | 0.47 | 1.94 | 634.10 | 0.38 | 0.01 | 88.17 |
| White-3—2 | 183.07 | 99.17 | 0.05 | 0.46 | 1.98 | 625.11 | 0.06 | 0.00 | 91.43 |
| White-4—1 | 170.88 | 102.92 | 0.35 | 0.50 | 1.21 | 882.10 | 0.00 | 0.01 | 164.49 |
| White-4—2 | 184.08 | 111.10 | 0.77 | 0.44 | 1.78 | 907.66 | 0.21 | 0.00 | 168.67 |
| White-5—1 | 539.10 | 213.27 | 0.35 | 0.44 | 1.98 | 1215.09 | 0.28 | 0.01 | 257.73 |
| White-5—2 | 528.40 | 213.52 | 0.00 | 0.46 | 2.54 | 1243.40 | 0.00 | 0.00 | 253.59 |
| Orange-1—1 | 1314.64 | 224.05 | 0.00 | 0.62 | 3.92 | 494.17 | 0.10 | 0.01 | 131.53 |
| Orange-1—2 | 1400.90 | 228.02 | 0.13 | 0.65 | 5.51 | 471.03 | 0.14 | 0.00 | 118.02 |
| Orange-2—1 | 137.16 | 113.96 | 0.00 | 0.75 | 2.65 | 1021.79 | 0.09 | 0.00 | 190.90 |
| Orange-2—2 | 140.32 | 88.32 | 0.32 | 0.73 | 3.25 | 1045.82 | 0.09 | 0.01 | 196.40 |
| Samples | Na | Mg | K | Ca | Ti | V | Cr | ||
| purple-b1—1 | 2782.09 | 1.06 | 9.42 | 386,135.45 | 0.00 | 0.00 | 1.91 | ||
| purple-b1—2 | 2807.73 | 0.12 | 13.34 | 385,828.10 | 0.00 | 0.00 | 2.05 | ||
| purple-b2—1 | 2117.81 | 18.07 | 11.21 | 388,335.95 | 0.00 | 0.08 | 2.26 | ||
| purple-b2—2 | 2144.64 | 15.93 | 11.81 | 389,402.20 | 0.00 | 0.00 | 1.15 | ||
| purple-b3—1 | 2466.27 | 2.74 | 7.57 | 386,567.84 | 0.00 | 0.02 | 2.41 | ||
| purple-b3—2 | 2444.53 | 2.04 | 8.26 | 386,004.60 | 0.00 | 0.00 | 2.43 | ||
| purple-b4—1 | 2466.31 | 3.26 | 8.16 | 383,117.80 | 0.22 | 0.01 | 1.98 | ||
| purple-b4—2 | 2480.24 | 0.72 | 6.76 | 386,841.01 | 0.00 | 0.00 | 2.15 | ||
| gary-b1—1 | 2612.05 | 16.51 | 31.20 | 389,928.33 | 0.00 | 0.08 | 2.72 | ||
| gary-b1—2 | 2441.14 | 16.21 | 27.20 | 386,538.04 | 0.00 | 0.00 | 1.73 | ||
| gary-b2—1 | 1788.39 | 23.54 | 10.34 | 385,854.93 | 0.00 | 0.00 | 2.24 | ||
| gary-b2—2 | 1726.86 | 22.09 | 11.14 | 383,039.90 | 0.00 | 0.00 | 2.23 | ||
| gary-b3—1 | 1776.89 | 25.66 | 25.33 | 384,840.78 | 0.22 | 0.00 | 1.28 | ||
| gary-b3—2 | 1872.35 | 28.96 | 25.59 | 384,277.14 | 0.29 | 0.00 | 2.07 | ||
| gary-b4—1 | 2408.39 | 8.81 | 4.49 | 382,570.68 | 0.00 | 0.05 | 2.63 | ||
| gary-b4—2 | 2421.84 | 5.15 | 4.80 | 384,593.28 | 0.04 | 0.00 | 2.74 | ||
| pink-b4—1 | 2446.22 | 1.52 | 19.88 | 386,620.11 | 0.01 | 0.06 | 2.78 | ||
| pink-b4—2 | 2484.90 | 3.03 | 32.51 | 383,433.15 | 0.00 | 0.00 | 1.38 | ||
| PINKb-3—1 | 2403.87 | 0.08 | 20.91 | 389,764.58 | 0.00 | 0.00 | 3.62 | ||
| PINKb-3—2 | 2368.95 | 0.00 | 13.12 | 388,687.73 | 0.00 | 0.00 | 1.73 | ||
| PINKb-1—1 | 2614.90 | 10.72 | 15.04 | 392,060.16 | 0.04 | 0.00 | 3.05 | ||
| PINKb-1—2 | 2701.41 | 12.81 | 14.19 | 393,621.93 | 0.00 | 0.01 | 2.94 | ||
| PINKb-2—1 | 1951.08 | 19.44 | 9.04 | 392,408.43 | 0.00 | 0.03 | 3.58 | ||
| PINKb-2—2 | 2021.78 | 20.02 | 5.19 | 393,945.55 | 0.00 | 0.02 | 2.97 | ||
| orange-b1—1 | 1994.52 | 28.98 | 7.41 | 385,531.25 | 0.00 | 0.00 | 1.36 | ||
| orange-b1—2 | 1953.38 | 22.71 | 5.33 | 386,445.21 | 0.00 | 0.00 | 1.61 | ||
| orange-b2—1 | 1735.82 | 16.35 | 9.20 | 385,508.34 | 0.00 | 0.04 | 1.65 | ||
| orange-b2—2 | 1796.36 | 18.10 | 8.87 | 384,758.48 | 0.00 | 0.06 | 1.19 | ||
| orange-b3—1 | 2372.39 | 0.00 | 8.24 | 383,737.73 | 0.00 | 0.00 | 2.84 | ||
| orange-b3—2 | 2307.00 | 0.00 | 8.65 | 383,969.10 | 0.00 | 0.05 | 1.38 | ||
| orange-b4—1 | 2495.96 | 0.62 | 7.39 | 384,190.83 | 0.50 | 0.02 | 1.81 | ||
| orange-b4—2 | 2538.99 | 2.10 | 8.50 | 382,632.62 | 0.00 | 0.08 | 2.76 | ||
| White-b1—1 | 2277.08 | 0.00 | 4.94 | 393,630.00 | 0.00 | 0.00 | 2.13 | ||
| White-b1—2 | 2291.63 | 0.00 | 10.18 | 392,185.07 | 0.00 | 0.00 | 2.18 | ||
| White-b2—1 | 2321.91 | 0.00 | 5.15 | 390,402.59 | 0.00 | 0.07 | 2.91 | ||
| White-b2—2 | 2245.95 | 0.00 | 5.57 | 389,099.51 | 0.05 | 0.00 | 2.69 | ||
| White-b3—1 | 2147.26 | 0.00 | 5.08 | 389,814.59 | 0.00 | 0.01 | 2.31 | ||
| White-b3—2 | 2363.85 | 0.00 | 7.40 | 389,678.97 | 0.42 | 0.00 | 2.67 | ||
| White-b4—1 | 2536.40 | 0.42 | 3.32 | 388,803.49 | 0.00 | 0.00 | 2.58 | ||
| White-b4—2 | 2527.99 | 0.14 | 5.92 | 387,538.42 | 0.52 | 0.00 | 1.71 | ||
| Samples | Mn | Fe | Ni | Cu | Zn | Sr | Mo | Cs | Ba |
| purple-b1—1 | 395.51 | 223.64 | 0.78 | 0.36 | 2.14 | 179.89 | 0.09 | 0.00 | 55.09 |
| purple-b1—2 | 389.82 | 247.76 | 0.44 | 0.43 | 2.38 | 188.40 | 0.08 | 0.00 | 63.41 |
| purple-b2—1 | 1118.31 | 239.62 | 0.38 | 0.24 | 0.57 | 615.33 | 0.16 | 0.00 | 181.59 |
| purple-b2—2 | 1016.54 | 208.31 | 0.00 | 0.31 | 0.23 | 537.14 | 0.13 | 0.00 | 166.90 |
| purple-b3—1 | 713.33 | 238.98 | 0.21 | 0.29 | 0.50 | 278.32 | 0.12 | 0.01 | 82.90 |
| purple-b3—2 | 683.98 | 232.92 | 0.28 | 0.34 | 0.23 | 271.57 | 0.16 | 0.00 | 80.23 |
| purple-b4—1 | 838.46 | 234.86 | 0.20 | 0.19 | 0.57 | 358.03 | 0.14 | 0.00 | 115.68 |
| purple-b4—2 | 812.36 | 246.74 | 0.36 | 0.24 | 0.27 | 351.10 | 0.19 | 0.01 | 114.05 |
| gary-b1—1 | 362.56 | 223.82 | 0.17 | 0.44 | 1.69 | 312.14 | 0.08 | 0.00 | 73.90 |
| gary-b1—2 | 332.91 | 215.12 | 0.02 | 0.50 | 1.49 | 308.14 | 0.04 | 0.00 | 62.84 |
| gary-b2—1 | 1333.95 | 234.73 | 0.39 | 0.20 | 0.81 | 863.44 | 0.07 | 0.00 | 251.25 |
| gary-b2—2 | 1254.22 | 229.46 | 0.46 | 0.19 | 0.81 | 838.03 | 0.12 | 0.00 | 266.04 |
| gary-b3—1 | 1208.89 | 211.61 | 0.00 | 0.18 | 0.65 | 618.11 | 0.20 | 0.00 | 164.26 |
| gary-b3—2 | 1381.39 | 229.07 | 0.57 | 0.18 | 1.29 | 694.67 | 0.25 | 0.00 | 182.72 |
| gary-b4—1 | 557.63 | 245.31 | 0.11 | 0.30 | 0.40 | 304.80 | 0.14 | 0.00 | 75.48 |
| gary-b4—2 | 568.32 | 255.93 | 0.44 | 0.25 | 0.53 | 306.53 | 0.17 | 0.01 | 77.68 |
| pink-b4—1 | 768.59 | 237.45 | 0.12 | 0.20 | 0.75 | 363.21 | 0.11 | 0.00 | 105.05 |
| pink-b4—2 | 699.98 | 233.69 | 0.03 | 0.26 | 0.56 | 361.06 | 0.08 | 0.02 | 94.07 |
| PINKb-3—1 | 587.61 | 233.57 | 1.18 | 0.31 | 1.17 | 239.45 | 0.11 | 0.00 | 58.79 |
| PINKb-3—2 | 560.20 | 229.23 | 0.74 | 0.28 | 0.82 | 237.48 | 0.13 | 0.00 | 57.69 |
| PINKb-1—1 | 227.28 | 151.51 | 0.86 | 0.50 | 1.40 | 259.37 | 0.04 | 0.01 | 61.40 |
| PINKb-1—2 | 224.17 | 122.47 | 0.17 | 0.58 | 1.74 | 293.24 | 0.00 | 0.01 | 75.05 |
| PINKb-2—1 | 1161.84 | 237.27 | 0.32 | 0.30 | 0.24 | 571.78 | 0.56 | 0.00 | 136.98 |
| PINKb-2—2 | 1152.91 | 243.27 | 0.38 | 0.28 | 0.05 | 588.96 | 0.32 | 0.00 | 148.21 |
| orange-b1—1 | 796.86 | 213.74 | 0.23 | 0.43 | 0.56 | 1187.09 | 0.20 | 0.00 | 218.46 |
| orange-b1—2 | 745.64 | 200.16 | 0.00 | 0.43 | 0.89 | 1082.86 | 0.18 | 0.01 | 183.27 |
| orange-b2—1 | 725.13 | 220.15 | 0.38 | 0.20 | 1.23 | 1828.47 | 0.19 | 0.01 | 411.49 |
| orange-b2—2 | 757.14 | 226.27 | 0.23 | 0.25 | 0.76 | 1939.84 | 0.22 | 0.00 | 454.13 |
| orange-b3—1 | 617.54 | 248.12 | 0.00 | 0.35 | 0.62 | 871.66 | 0.00 | 0.00 | 135.53 |
| orange-b3—2 | 602.11 | 243.61 | 0.40 | 0.33 | 1.26 | 892.08 | 0.41 | 0.00 | 133.98 |
| orange-b4—1 | 853.89 | 265.41 | 0.83 | 0.33 | 0.57 | 1031.78 | 0.07 | 0.00 | 158.40 |
| orange-b4—2 | 886.57 | 262.07 | 0.57 | 0.27 | 0.55 | 1099.48 | 0.09 | 0.00 | 169.52 |
| White-b1—1 | 538.20 | 188.61 | 0.40 | 0.48 | 1.98 | 735.29 | 0.24 | 0.00 | 148.50 |
| White-b1—2 | 521.70 | 171.35 | 0.00 | 0.50 | 1.77 | 722.61 | 0.34 | 0.00 | 143.03 |
| White-b2—1 | 651.96 | 0.00 | 0.00 | 0.22 | 0.76 | 715.23 | 0.27 | 0.00 | 146.50 |
| White-b2—2 | 689.29 | 228.30 | 0.00 | 0.23 | 0.59 | 722.30 | 0.36 | 0.02 | 148.69 |
| White-b3—1 | 700.95 | 204.06 | 0.00 | 0.23 | 0.65 | 805.23 | 0.52 | 0.00 | 163.44 |
| White-b3—2 | 772.33 | 224.65 | 0.00 | 0.34 | 0.58 | 856.75 | 0.30 | 0.01 | 172.47 |
| White-b4—1 | 563.99 | 226.65 | 0.84 | 0.32 | 1.23 | 635.89 | 0.20 | 0.01 | 120.78 |
| White-b4—2 | 540.10 | 231.38 | 0.06 | 0.39 | 1.60 | 604.65 | 0.29 | 0.00 | 109.88 |
References
- Karampelas, S.; Fritsch, E.; Mevellec, J.-Y.; Sklavounos, S.; Soldatos, T. Role of polyenes in the coloration of cultured freshwater pearls. Eur. J. Mineral. 2009, 21, 85–97. [Google Scholar] [CrossRef]
- Sato, A.; Cartier, L.E. The Value of Pearls. Gemguide 2022, 1–8. [Google Scholar]
- Wang, Z.; Adzigbli, L.; Zheng, Z.; Yang, C.; Deng, Y. How cultured pearls acquire their colour. Aquac. Res. 2020, 51, 3925–3934. [Google Scholar] [CrossRef]
- Muhammad, G.; Fujimura, T.; Komaru, A. The effects of nacre microstructure on green and pink interference colors in Pinctada fucata martensii pearls. Aquaculture 2021, 535, 736424. [Google Scholar] [CrossRef]
- Li, L.P.; Yan, W.X. The relationship between the structure and luster of cultured pearls. China Gems 1998, 4, 68–70. [Google Scholar]
- Karampelas, S.; Fritsch, E.; Mevellec, J.Y.; Gauthier, J.P.; Sklavounos, S.; Soldatos, T. Determination by Raman scattering of the nature of pigments in cultured freshwater pearls from the mollusk Hyriopsis cumingi. J. Raman Spectrosc. 2007, 38, 217–230. [Google Scholar] [CrossRef]
- Yan, G.A.O.; Beili, Z. Research on Relationship between Colour and Raman Spectrum of Freshwater Cultured Pearl. J. Gems Gemmol. 2001, 3, 17–20. [Google Scholar]
- Zwaan, J.H.; Karampelas, S. An unusual black natural pearl from Mytilidae. J. Gemmol. 2021, 37, 461–463. [Google Scholar] [CrossRef]
- Shi, L.; Liu, X.; Mao, J.; Han, X. Study of coloration mechanism of cultured freshwater pearls from mollusk Hyriopsis cumingii. J. Appl. Spectrosc. 2014, 81, 97–101. [Google Scholar] [CrossRef]
- Karampelas, S.; Fritsch, E.; Gauthier, J.-P.; Hainschwang, T. UV-Vis-NIR reflectance spectroscopy of natural-color saltwater cultured pearls from Pinctada margaritifera. Gems Gemol. 2011, 47, 31–35. [Google Scholar] [CrossRef]
- Karampelas, S. Spectral characteristics of natural-color saltwater cultured pearls from Pinctada maxima. Gems Gemol. 2012, 48, 193–197. [Google Scholar] [CrossRef]
- Yan, X.; Jiang, Y.; Jin, H.; Chen, T.; Zhou, Y.; Liu, J.; Yan, J. Unique spectral characteristics of natural-color Edison pearls cultured in Hyriopsis cumingii, and its formation mechanisms. Micron 2022, 160, 103324. [Google Scholar] [CrossRef] [PubMed]
- Parker, A.R. The diversity and implications of animal structural colours. J. Exp. Biol. 1998, 201, 2343–2347. [Google Scholar] [CrossRef]
- Zhang, W.; Zhang, G. Dynamic structural color in the nacre of Hyriopsis Cumingii and its cause. Optik 2017, 135, 252–255. [Google Scholar] [CrossRef]
- McDougall, C.; Moase, P.; Degnan, B.M. Host and donor influence on pearls produced by the silver-lip pearl oyster, Pinctada maxima. Aquaculture 2016, 450, 313–320. [Google Scholar] [CrossRef]
- Shinohara, M.; Kinoshita, S.; Tang, E.; Funabara, D.; Kakinuma, M.; Maeyama, K.; Nagai, K.; Awaji, M.; Watabe, S.; Asakawa, S. Comparison of two pearl sacs formed in the same recipient oyster with different genetic background involved in yellow pigmentation in Pinctada fucata. Mar. Biotechnol. 2018, 20, 594–602. [Google Scholar] [CrossRef] [PubMed]
- Deng, Y.H.; Yuan, K.R. The factors for restricting quality of Chinese pearl. J. Guilin Univ. Technol. 2001, 21, 6–12. [Google Scholar]
- Chen, L.; Liu, Y.; Hu, Z.; Gao, S.; Zong, K.; Chen, H. Accurate determinations of fifty-four major and trace elements in carbonate by LA–ICP-MS using normalization strategy of bulk components as 100%. Chem. Geol. 2011, 284, 283–295. [Google Scholar] [CrossRef]
- Li, B.; Shen, A.H. Effect of Fe2O3@ SiO2 Core–Shell Nanoparticle Doping Ratio on Color Appearance of Synthetic Opal Films Inspired by Natural Fire Opal. Coatings 2025, 15, 646. [Google Scholar] [CrossRef]
- Xu, Z.; Li, R.; Guo, Q.; Cao, S.W. Discussion about the Test of Aragonite Crystal Orientation by IR Spectral Reflection. Infrared Technol. 2015, 37, 171–175. [Google Scholar]
- Urmos, J.; Sharma, S.; Mackenzie, F. Characterization of some biogenic carbonates with Raman spectroscopy. Am. Mineral. 1991, 76, 641–646. [Google Scholar]
- Zhang, G.; Xie, X.; Wang, Y. Raman spectra of nacre from shells of main pearl-culturing mollusk in China. Guang Pu Xue Yu Guang Pu Fen Xi Guang Pu 2001, 21, 193–196. [Google Scholar]
- Chen, C.; Yu, J.; Zhang, C.; Ye, X.; Shen, A.H. Nature of Pigments in Orange and Purple Coloured Chinese Freshwater Cultured Pearls: Insights from Experimental Raman Spectroscopy and DFT Calculations. Minerals 2023, 13, 959. [Google Scholar] [CrossRef]
- Du, X.; Fan, G.; Jiao, Y.; Zhang, H.; Guo, X.; Huang, R.; Zheng, Z.; Bian, C.; Deng, Y.; Wang, Q. The pearl oyster Pinctada fucata martensii genome and multi-omic analyses provide insights into biomineralization. Gigascience 2017, 6, gix059. [Google Scholar] [CrossRef] [PubMed]
- Qin, Z.; Ma, H.; Mu, S.; Tong, Z. Research on relationship between color and Raman Spectrum of freshwater cultured pearl of good quality. Acta Mineral. Sin. 2007, 27, 73–76. [Google Scholar]
- Hu, Y.; Fan, L.W.; Huang, Y.L. Research on Raman Spectra of Organic Ingredients on Colored Pearls. Spectrosc. Spectr. Anal. 2014, 34, 98–102. [Google Scholar]
- Schügerl, F.; Kuzmany, H. Optical modes of trans-polyacetylene. J. Chem. Phys. 1981, 74, 953–958. [Google Scholar] [CrossRef]
- Schaffer, H.; Chance, R.; Silbey, R.; Knoll, K.; Schrock, R. Conjugation length dependence of Raman scattering in a series of linear polyenes: Implications for polyacetylene. J. Chem. Phys. 1991, 94, 4161–4170. [Google Scholar] [CrossRef]
- Barnard, W.; De Waal, D. Raman investigation of pigmentary molecules in the molluscan biogenic matrix. J. Raman Spectrosc. 2006, 37, 342–352. [Google Scholar] [CrossRef]
- Jin, S.; Renfro, N.D.; Palke, A.C.; Ardon, T.; Homkrajae, A. Application of UV-VIS-NIR Spectroscopy to Gemology. Gems Gemol. 2024, 60, 456–473. [Google Scholar] [CrossRef]
- Jun, Y.; Shi, B.F.; Xue, J.Y.; Jia, W.S.; Jiang, X.; Chong, X.; Jian, Z. Study on the common effect of heat treatment, dyeing or irradiation treatment on UV-Vis diffuse reflectance spectra of pearls. Spectrosc. Spectr. Anal. 2022, 42, 3697–3702. [Google Scholar]
- Jachowicz, J.; McMullen, R.L. Tryptophan fluorescence in hair—Examination of contributing factors. J. Cosmet. Sci. 2011, 62, 291. [Google Scholar]
- Falk, H.; Mayr, E.; Richter, A.E. Simple diffuse reflectance UV-Vis spectroscopic determination of organic pigments (fringelites) in fossils. Microchim. Acta 1994, 117, 1–5. [Google Scholar] [CrossRef]
- Snow, M.R.; Pring, A.; Self, P.; Losic, D. The origin of the color of pearls in iridescence from nano-composite structures of the nacre. Am. Mineral. 2004, 89, 1353–1358. [Google Scholar] [CrossRef]
- Zhao, J.M.; Mei, K.S.; Zhang, W.B.; Xu, W.; Tan, B.P.; Ma, H.M.; Liufu, Z.G.; Ai, Q.H. A Review on Characteristic of Nacre and Its Potential for Biomimetic Applications. Chin. High Technol. Lett. 2003, 13, 94–98. [Google Scholar]
- Li, G.; Lin, L.; Sha, N.L.; Zhao, H.Y. A preliminary study on the relationship between luster, color and organic matter of freshwater cultured pearls. J. Guilin Univ. Technol. 2007, 27, 569–571. [Google Scholar]
- Leung, H.; Sinha, S.K. Scratch and indentation tests on seashells. Tribol. Int. 2009, 42, 40–49. [Google Scholar] [CrossRef]
- Li, X. Nanoscale structural and mechanical characterization of natural nanocomposites: Seashells. JOM 2007, 59, 71–74. [Google Scholar] [CrossRef]
- Ma, H.; Wei, Q.; Lu, S.; Mu, D.; Wu, M. The ultrastructure characterization of high-quality freshwater cultured pearls modified by color using physical method. Acta Mineral. Sin. 2012, 32, 139–145. [Google Scholar]
- Liu, Y.; Hurwit, K.N.; Tian, L. Relationship between the groove density of the grating structure and the strength of iridescence in mollusc shells. J. Aust. Gemmol. 2003, 21, 405–407. [Google Scholar]
- Yan, X.; Liu, X.; Zhou, W.; Zhou, D.; Liu, P.; Chen, T.; Yan, J. The gemological characteristics of yellow seawater bead-cultured pearl farming in Beihai City, southern China. Micron 2024, 176, 103558. [Google Scholar] [CrossRef] [PubMed]
- Satitkune, S.; Monarumit, N.; Boonmee, C.; Phlayrahan, A.; Promdee, K.; Won-In, K. Combination of FTIR and SEM for identifying freshwater-cultured pearls from different quality. Opt. Spectrosc. 2016, 120, 500–504. [Google Scholar] [CrossRef]
- Comfort, A. Acid-soluble pigments of molluscan shells. 5. Identity of some subsidiary fractions derived from Pinctada vulgaris. Biochem. J. Appl. Spectrosc. 1950, 47, 254–255. [Google Scholar] [CrossRef]
- Jackson, D.J.; McDougall, C.; Green, K.; Simpson, F.; Wörheide, G.; Degnan, B.M. A rapidly evolving secretome builds and patterns a sea shell. BMC Biol. 2006, 4, 40. [Google Scholar] [CrossRef]
- Joubert, C.; Linard, C.; Le Moullac, G.; Soyez, C.; Saulnier, D.; Teaniniuraitemoana, V.; Ky, C.L.; Gueguen, Y. Temperature and food influence shell growth and mantle gene expression of shell matrix proteins in the pearl oyster Pinctada margaritifera. PLoS ONE 2014, 9, e103944. [Google Scholar] [CrossRef]
- Dorant, Y.; Quillien, V.; Le Luyer, J.; Ky, C.-L. Comparative transcriptomics identifies genes underlying growth performance of the Pacific black-lipped pearl oyster Pinctada margaritifera. BMC Genom. 2024, 25, 717. [Google Scholar] [CrossRef]
- Dauphin, Y.; Cuif, J.-P. Trichromatic characterization of the “black pearls” from aquaculture centers of French Polynesia. Aquaculture 1995, 133, 113–121. [Google Scholar] [CrossRef]
- Dauphin, Y.; Cuif, J.P.; Cotte, M.; Salomé, M. Structure and composition of the boundary zone between aragonitic crossed lamellar and calcitic prism layers in the shell of Concholepas concholepas (Mollusca, Gastropoda). Invertebr. Biol. 2012, 131, 165–176. [Google Scholar] [CrossRef]
- Zhang, G.; Xu, S.; Fang, A. Graft tissue on the genesis of color of denuclearize pearl in mantle of Hyriopsis cumingii. Shuisheng Shengwu Xuebao 2013, 37, 581–587. [Google Scholar]

| Number of C = C Double Bonds (N) | Raman Shift ν1 (According to Schügerl et al., 1981) [27] | Raman Shift ν1 (According to Schafer et al., 1991) [28] | Raman Shift ν1 (According to Barnard et al., 2006) [29] |
|---|---|---|---|
| ν1 = 1459 + 720/(N + 1) | ν1 = 1438 + 830/N (7 ≦ N ≦ 12) | ν1 = 97.07 × ln(1/N) + 1745 (3 ≦ N ≦ 12) | |
| 4 | 1603 | / | 1610 |
| 5 | 1579 | / | 1589 |
| 6 | 1562 | / | 1571 |
| 7 | 1549 | 1557 | 1556 |
| 8 | 1539 | 1542 | 1543 |
| 9 | 1531 | 1530 | 1532 |
| 10 | 1524 | 1521 | 1521 |
| 11 | 1519 | 1513 | 1512 |
| 12 | 1514 | 1507 | 1504 |
| 13 | 1510 | / | / |
| 14 | 1507 | / | / |
| Samples | 1125 cm−1–1135 cm−1 | 1509 cm−1–1525 cm−1 |
|---|---|---|
| H | H | |
| Purple-1 | 5624.76 | 7091.08 |
| Purple-2 | 12,092.56 | 16,575.38 |
| Purple-3 | 11,210.73 | 14,973.70 |
| Purple-4 | 8495.13 | 10,648.60 |
| Purple-5 | 12,572.45 | 18,670.11 |
| Purple-6 | 12,090.53 | 12,965.54 |
| Purple-7 | 12,875.10 | 20,404.74 |
| Purple-8 | 16,369.74 | 16,480.06 |
| Pink-1 | 1570.05 | 1390.24 |
| Pink-2 | 2524.35 | 2535.04 |
| Pink-3 | 3881.85 | 4967.69 |
| Pink-4 | 4205.80 | 4952.88 |
| Pink-5 | 9958.14 | 15,164.71 |
| White-1 | 1215.03 | 215.64 |
| White-2 | 426.27 | 560.86 |
| White-3 | 1622.24 | 1295.58 |
| White-4 | 1438.42 | 1458.21 |
| White-5 | 1485.76 | 1493.50 |
| Orange-1 | 2975.89 | 4450.27 |
| Orange-2 | 3367.52 | 4182.97 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, B.; Xu, B.; Zhao, Y.; Zhang, C.; Zhao, S.; Zhao, Z. Integrated Chromogenic Analysis of Freshwater Pearls: Revealing the Internal Factors Driving Color Variation. Crystals 2025, 15, 797. https://doi.org/10.3390/cryst15090797
Yang B, Xu B, Zhao Y, Zhang C, Zhao S, Zhao Z. Integrated Chromogenic Analysis of Freshwater Pearls: Revealing the Internal Factors Driving Color Variation. Crystals. 2025; 15(9):797. https://doi.org/10.3390/cryst15090797
Chicago/Turabian StyleYang, Baoyi, Bo Xu, Yi Zhao, Chenxi Zhang, Siyi Zhao, and Zheyi Zhao. 2025. "Integrated Chromogenic Analysis of Freshwater Pearls: Revealing the Internal Factors Driving Color Variation" Crystals 15, no. 9: 797. https://doi.org/10.3390/cryst15090797
APA StyleYang, B., Xu, B., Zhao, Y., Zhang, C., Zhao, S., & Zhao, Z. (2025). Integrated Chromogenic Analysis of Freshwater Pearls: Revealing the Internal Factors Driving Color Variation. Crystals, 15(9), 797. https://doi.org/10.3390/cryst15090797







