Abstract
Addressing global energy and environmental issues calls for the development of effective photocatalysts capable of enabling solar-driven water splitting, a key route toward sustainable hydrogen generation. In this work, we conducted a detailed density functional theory (DFT) study on three bilayer van der Waals (vdW) heterojunctions, Ga2SSe/GaP, Ga2SSe/PtSSe, and Ga2SSe/SnSSe, each explored in four distinct stacking configurations, with Ga2SSe serving as the base monolayer. We assessed their structural stability, electronic properties, and optical responses to determine their suitability for photocatalytic water splitting. The analysis showed that Ga2SSe/GaP and Ga2SSe/SnSSe exhibit type-II band alignment, while Ga2SSe/PtSSe displays a type-I alignment. Electrostatic potential profiles and Bader charge calculations identified SeGa2S/SSnSe and SeGa2S/SeSnS as direct Z-scheme systems, offering efficient charge carrier separation and robust redox potential. For effective water splitting, the band edges must straddle the water redox potentials. Our results indicate that configurations A and B in Ga2SSe/GaP, along with C and D in Ga2SSe/SnSSe, fulfill this requirement. These four configurations also exhibit strong absorption in both the visible and ultraviolet spectral ranges. Notably, configurations C and D of Ga2SSe/SnSSe achieve high solar-to-hydrogen (STH) efficiencies, reaching 38.44% and 21.75%, respectively. Overall, our findings suggest that these direct Z-scheme heterostructures are promising candidates for water splitting photocatalysis.
1. Introduction
The need for sustainable hydrogen production has become increasingly urgent due to rising concerns over environmental degradation and dwindling fossil fuel resources. Among the emerging strategies, photocatalytic water splitting using solar energy has proved particularly interesting for generating hydrogen in a clean, renewable way [1,2,3]. Hydrogen stands out as a desirable energy carrier because of its high energy content and carbon-free combustion. However, current industrial production methods, such as steam methane reforming, are still heavily reliant on fossil resources and generate large quantities of CO2 emissions [4,5]. As an alternative, solar-driven photocatalysis offers a low-emission, scalable route for yielding hydrogen by utilizing sunlight to decompose water molecules into hydrogen and oxygen [6,7].
Titanium dioxide (TiO2) [8] was among the first materials identified for photocatalytic applications and remains extensively studied. However, its wide bandgap (~3.2 eV) limits its absorption to a small portion of the solar spectrum, the ultraviolet, thus restricting its efficiency under solar illumination [9]. To address this limitation, transition metal dichalcogenides (TMDs) [10], particularly MoS2 [11] and WS2 [12], have been explored due to their favorable optical properties, layered structures, and adjustable electronic characteristics [13,14,15]. Despite these advantages, TMD monolayers often suffer from fast charge recombination, suboptimal charge mobility, and narrow absorption bandwidths, which undermine their overall performance in water-splitting reactions.
A promising strategy to overcome these issues is the design of semiconductor heterojunctions [16]. By engineering the interface between different materials, heterostructures can improve charge separation and broaden light absorption. Successful photocatalytic activity requires proper alignment of the band edges: the valence band maximum (VBM) must lie above the water oxidation potential [17,18], while the conduction band minimum (CBM) should lie below the reduction potential [19,20]. Hence, the relative positioning of energy bands critically influences the overall efficiency of the photocatalytic process [21,22].
Two heterojunction architectures, type-II [23] and direct Z-scheme [24], are particularly effective at enhancing charge separation by spatially separating photogenerated electrons and holes across different materials [25]. Z-scheme heterostructures are especially attractive, as they maintain strong redox potentials by localizing the oxidation and reduction reactions on distinct layers, thereby improving both charge separation and chemical reactivity [26]. Recent theoretical investigations have validated the advantages of such configurations. For example, Yang et al. [27] reported that Ga2SSe/Bi2O3 heterojunctions, featuring a Z-scheme-type mechanism for the transfer of charges, achieve efficient light harvesting and reduced charge recombination, resulting in solar-to-hydrogen (STH) conversion efficiencies surpassing 10%. Similarly, Kumar et al. [28] found that the C2N/WS2 heterojunction exhibits a favorable type-II alignment, with separated charge carriers enhancing photocatalytic performance under visible light. In contrast, type-I heterostructures are typically ineffective for complete water splitting because both electrons and holes tend to accumulate in a single material, hampering the redox reactions. For instance, Kim et al. [29] identified the limited activity of the BiVO4/Fe2O3 system as a result of its type-I band configuration, which obstructs hole transfer and lowers efficiency.
Building on this context, we selected GaP, PtSSe, and SnSSe as complementary materials to Ga2SSe, owing to their documented photocatalytic properties for visible-light-driven water splitting.
Sun et al. [30] confirmed sustained submicromolar hydrogen evolution from visible-light-irradiated suspensions of Pt-decorated GaP nanowires using methanol as a sacrificial electron donor. Meinhardová et al. [31] further fabricated GaP/TiO2 Z-scheme heterostructures via wet impregnation, leveraging methanol’s dual role as both sacrificial agent and current multiplier to enhance hydrogen production through photocatalytic methanol reforming.
PtSSe has also attracted attention for its excellent photocatalytic potential. Peng et al. [32], using first-principles calculations, demonstrated that monolayer PtSSe possesses excellent photocatalytic water-splitting potential, featuring high visible-light absorption, appropriate band edge positions, and efficient carrier separation and transport. Furthermore, Janus PtSSe-based van der Waals heterostructures have shown outstanding visible-light harvesting capabilities and high solar-to-hydrogen (STH) conversion efficiencies [33]. Similarly, Janus T-SnSSe monolayers exhibit robust photocatalytic activity for both the hydrogen evolution reaction (HER) and the oxygen evolution reaction (OER), with strong redox capabilities under illumination [34]. Moreover, ZrSSe/SnSSe heterostructures demonstrate significant water-splitting potential, featuring thermodynamically favorable OER activation in acidic conditions (pH = 0) and efficient visible-light absorption [35].
Ga2SSe itself has emerged as a highly promising photocatalyst. Li et al. [36] demonstrated that Janus Ga2SSe nanotubes exhibit exceptionally high hole mobility up to 2.89 × 104 cm2V−1s−1, significantly suppressed electron-hole recombination, exceptional oxidation capability, and strong visible-light absorption. These synergistic properties establish Ga2SSe as an outstanding photocatalyst candidate. Zhang et al. [37] established that Type II van der Waals GaSe/CN and Ga2SSe/CN heterostructures exhibit optimal band alignments, thereby satisfying the thermodynamic requirements for oxygen evolution reactions. Remarkably, these structures maintain thermodynamic feasibility across a broad pH range, while achieving remarkable solar-to-hydrogen conversion efficiencies of up to 15.11%. These properties establish their outstanding application prospects as photocatalysts for efficient water splitting. On the basis of the above studies, we employed density functional theory (DFT) calculations to investigate a series of van der Waals (vdW) heterojunctions [38] incorporating Ga2SSe as the base material. Specifically, we explored three combinations, namely Ga2SSe/GaP, Ga2SSe/PtSSe, and Ga2SSe/SnSSe, each modeled with four distinct stacking sequences. Our investigation encompassed structural stability, band structures, band alignment, interfacial charge redistribution, relative position of band edges with respect to redox potentials, optical absorption behavior, and solar-to-hydrogen (STH) efficiency [39]. The results highlight that stacking configurations C and D in the Ga2SSe/SnSSe system exhibit a direct Z-scheme alignment with superior STH performance, making them promising photocatalyst candidates for efficient solar water splitting.
2. Materials and Methods
All first-principles calculations involved the Vienna Ab initio Simulation Package (VASP) [40,41]. Projector augmented-wave (PAW) pseudopotentials [42] were used to model ion–valence electron interactions. For the exchange-correlation functional, the Perdew–Burke–Ernzerhof (PBE) formulation within the generalized gradient approximation (GGA) framework was employed [43,44]. To improve the reliability of the predicted electronic structure (particularly the bandgap, band edge alignment, and optical behavior) the hybrid Heyd–Scuseria–Ernzerhof functional (HSE06) was used in subsequent calculations [45].
The plane-wave basis set was truncated at a kinetic energy cutoff of 450 eV. Brillouin zone integration was carried out with a 4 × 4 × 1 Monkhorst–Pack grid, which was applied consistently for structural relaxation and electronic property evaluations. We set convergence thresholds of 10−5 eV for the total energy and 0.05 eV/Å for the maximum force acting on atoms. To accurately capture the weak interlayer forces present in van der Waals (vdW) heterostructures, the DFT-D3 dispersion correction method was included [46]. To suppress artificial interactions between periodic images along the out-of-plane axis, a 20 Å vacuum spacing was applied along z. Furthermore, Bader charge analysis was used to quantify charge transfer across the heterostructure interface [47]. This method partitions the electron density in real space using zero-flux surfaces and is well-suited to plane-wave DFT calculations, as it requires only the total charge density on a fine grid. In contrast, Mulliken population analysis relies on a projection onto localized orbitals, which is not available in VASP. Moreover, Mulliken charges are known to be strongly basis-dependent and may overestimate charge transfer, making Bader analysis a more reliable choice in this context.
3. Results
3.1. Structural Configuration and Stability of the Heterostructures
The Janus Ga2SSe monolayer, which exhibits a hexagonal lattice structure, was selected as the foundational material for building bilayer heterostructures by pairing it with other Janus monolayers identified from the Materials Explorer database [48].
The relaxed lattice parameter of the Ga2SSe monolayer was determined to be 3.71 Å. To ensure structural compatibility and minimal strain at the interface, a lattice mismatch threshold of less than 0.5% was adopted. Applying this constraint, three monolayers, namely GaP, PtSSe, and SnSSe, were selected as appropriate counterparts for heterojunction construction. Their optimized lattice constants (3.89Å for GaP, 3.64 Å for PtSSe, and 3.76 Å for SnSSe), along with their respective indirect bandgaps (2.65 eV, 2.06 eV, and 1.50 eV), are detailed in Figure S1 and Table S1 [32,35,49,50].
Given the intrinsic asymmetry of Janus structures, each bilayer heterostructure can adopt four possible stacking arrangements (labeled A through D), distinguished by the specific atomic species positioned opposite each other across the van der Waals (vdW) interface. These geometrical configurations are illustrated in Figure 1.
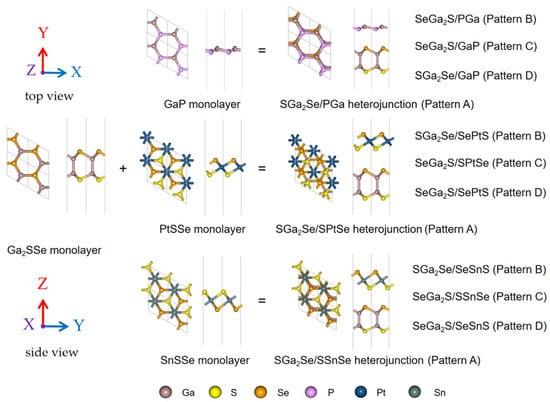
Figure 1.
Top and side views of the monolayers Ga2SSe, GaP, PtSSe, and SnSSe, along with the four possible stacking configurations (Patterns A–D) constructed for the Ga2SSe/GaP, Ga2SSe/PtSSe, and Ga2SSe/SnSSe heterostructures. The rhombus outlines indicate the unit cell of each structure.
To evaluate the energetic stability of these bilayer systems, the interfacial interaction energy (Eint) was computed for all stacking patterns, with values summarized in Table 1. The interaction energy is defined as follows [51]:
where EGa2SSe/monolayer, EGa2SSe, and Emonolayer represent the total energies of the heterojunction, the Ga2SSe monolayer, and the paired monolayer, respectively. In both Table 1 and the rest of the article, the nomenclature adopted to unambiguously name the different heterostructure patterns corresponds to the top-down atomic sequence. The negative Eint values across all configurations indicate that these heterostructures are energetically stable, primarily due to vdW interactions at the interface. This confirms their suitability for further electronic and optical property investigations.

Table 1.
Interfacial interaction energies (Eint) of the studied heterojunctions.
3.2. Electronic Properties
The projected band structures of the Ga2SSe/GaP, Ga2SSe/PtSSe, and Ga2SSe/SnSSe van der Waals heterostructures, computed using the HSE06 hybrid functional, are presented in Figure 2. Each heterostructure was analyzed in four distinct stacking arrangements. In the cases of Ga2SSe/GaP and Ga2SSe/SnSSe, the valence band maximum (VBM) and conduction band minimum (CBM) are located on different constituent layers, indicating a staggered (type-II) band alignment. This configuration facilitates spatial separation of photoexcited charge carriers, which is favorable for photocatalytic performance.
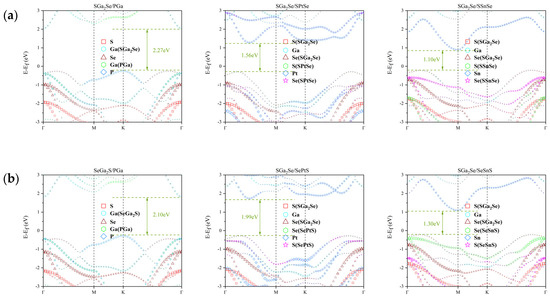
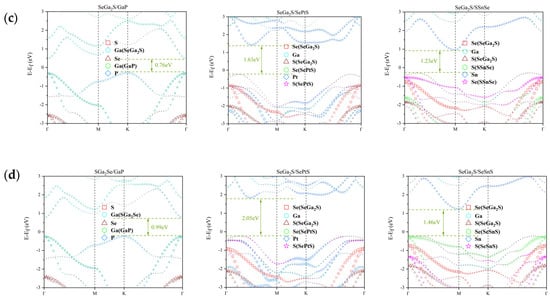
Figure 2.
Projected electronic band structures for the Janus Ga2SSe/GaP, Ga2SSe/PtSSe, and Ga2SSe/SnSSe vdW heterojunctions, obtained using the HSE06 functional. Results are shown for the four stacking configurations: (a) Pattern A, (b) Pattern B, (c) Pattern C, and (d) Pattern D.
Conversely, for Ga2SSe/PtSSe, both the VBM and CBM are confined to the same monolayer, signifying a straddling (type-I) band alignment. Such an arrangement tends to localize electrons and holes within the same layer, promoting recombination and reducing photocatalytic efficiency. Based on this analysis, only the heterostructures exhibiting type-II alignment were selected for further investigation.
To further explore the electronic behavior of the Ga2SSe/GaP and Ga2SSe/SnSSe van der Waals heterojunctions, we examined their electrostatic potential profiles along the out-of-plane (z) direction. As illustrated in Figure 3, each heterojunction exhibits an electrostatic potential difference (ΔΦ) across the interface. Based on the model introduced by Li et al. [52], the bandgap condition for polar materials can be modified as Eg > 1.23 − ΔΦ eV, where ΔΦ is the potential offset across the junction (with ΔΦ > 0).
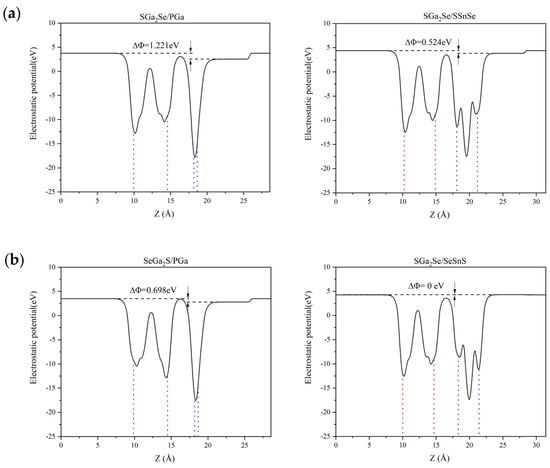
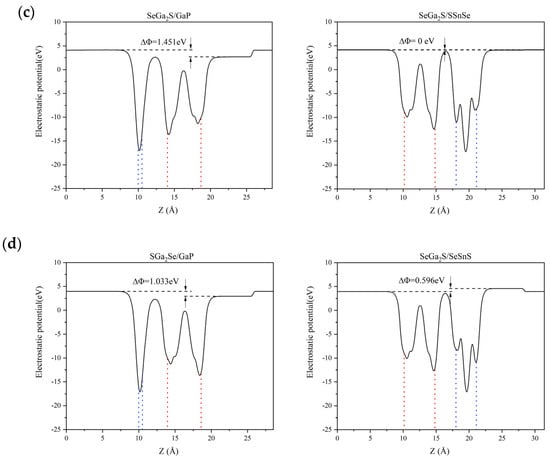
Figure 3.
Electrostatic potential profiles across the interfaces of Ga2SSe/GaP and Ga2SSe/SnSSe van der Waals heterojunctions, shown for the four stacking configurations: (a) Pattern A, (b) Pattern B, (c) Pattern C, and (d) Pattern D. The blue dotted lines delineate the GaP (left column) and SnSSe slabs (right column), and the red dotted lines delineate the Ga2SSe slab (left and right columns) in the heterostructure.
In addition, the standard redox potentials for the H+/H2 and O2/H2O half-reactions in aqueous solutions are given by the following expressions [53,54]:
For photocatalytic water splitting to occur efficiently, the positions of the conduction and valence band edges of the heterostructures must appropriately straddle these redox levels. At pH = 0, the reduction and oxidation potentials correspond to −5.67 eV and −4.44 eV, respectively. To evaluate the alignment of the heterojunction band edges with respect to these potentials, we used the following relations [55]:
Here, I is the ionization potential, A is the electron affinity, χ is the electronegativity, and Eg is the bandgap. These equations enable us to determine the conduction band minimum (CBM) and valence band maximum (VBM) positions of the heterostructures and assess whether the electronic structure of the materials is suitable for water-splitting reactions. The input quantities used for the band alignment were obtained from the fully relaxed heterostructure including van der Waals interactions.
The calculated VBM and CBM positions for the Ga2SSe/GaP and Ga2SSe/SnSSe heterojunctions are reported in Table S2. Bader charge analysis further reveals subtle but consistent interlayer charge transfers in the SeGa2S/SSnSe and SeGa2S/SeSnS systems, distinguishing them from conventional type-II alignments. Specifically, in SeGa2S/SSnSe, a net transfer of approximately 0.0004 |e| occurs from the SSnSe layer to the SeGa2S layer, while in SeGa2S/SeSnS, about 0.0038 |e| is transferred from SeSnS to SeGa2S. Although small in magnitude, these values are obtained from fully converged calculations using a dense k-point grid and high energy cutoffs, with total charge conservation accurate within 2 × 10−6|e|. These charge redistribution patterns, combined with the band alignment, support a direct Z-scheme mechanism in both heterostructures.
Unlike type-II heterojunctions, where photogenerated electrons and holes are separated between the layers, Z-scheme heterojunctions involve a unique interlayer recombination pathway, wherein electrons from one layer recombine with holes from the other in a Z-shaped transfer scheme. This approach preserves high-energy charge carriers, namely holes with strong oxidative power in one layer and electrons with strong reductive capability in the other, thus maintaining the overall redox potential and significantly boosting photocatalytic efficiency.
Depending on the heterojunction type, the band edge positions of both type-II and direct Z-scheme heterojunctions need to be adjusted using the ΔΦ value, as illustrated in Figure 4. Since the standard redox potentials of water are referenced to the highest vacuum energy level set as zero, and because the hydrogen evolution reaction (HER) and oxygen evolution reaction (OER) take place on different monolayers in type-II and direct Z-scheme heterojunctions, the standard redox potentials for the H+/H2 and O2/H2O redox couples must be corrected according to the following expressions:
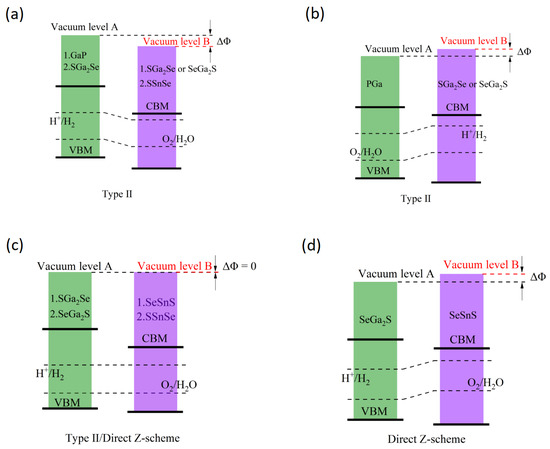
Figure 4.
Band alignment of the studied heterostructures relative to water redox potentials, corrected by vacuum level offset (ΔΦ): (a–c) Type-II heterojunctions—(a) “1” = GaP/SGa2Se, GaP/SeGa2S, “2” = SGa2Se/SSnSe; (b) PGa/SGa2Se, PGa/SeGa2S; (c) “1” = SGa2Se/SeSnS. (c,d) Direct Z-scheme heterostructures—(c) “2” = SeGa2S/SSnSe; (d) SeGa2S/SeSnS. Dashed lines show H+/H2 and O2/H2O redox levels. Red, dashed arrows indicate reaction sites and types.
Figure 5 presents the adjusted band edge positions for the Ga2SSe/GaP and Ga2SSe/SnSSe van der Waals heterojunctions. The arrows labeled hν indicate the absorption of photons leading to electron excitation from the valence to the conduction band. In type-II heterojunctions, photogenerated electrons in the donor layer (with higher CBM energy) transfer to the CB of the acceptor layer, while holes migrate in the opposite direction, enabling spatial separation of charge carriers and facilitating the HER and OER at different sites. In contrast, in direct Z-scheme heterostructures, electrons in the CB of the acceptor recombine with holes in the VB of the donor layer at the interface (shown with curved arrows in panels c and d). This recombination preserves the most reducing electrons and the most oxidizing holes in the respective layers, optimizing photocatalytic activity for water splitting. Therefore, the conduction and valence band edges of the SGa2Se/PGa, SeGa2S/PGa, SeGa2S/SSnSe, and SeGa2S/SeSnS heterojunctions straddle the redox potentials for both HER and OER, fulfilling the thermodynamic criteria required for water splitting within a range of acidic and alkaline environments. These four heterojunctions can serve as promising photocatalytic materials suitable for water splitting across a variety of pH conditions.
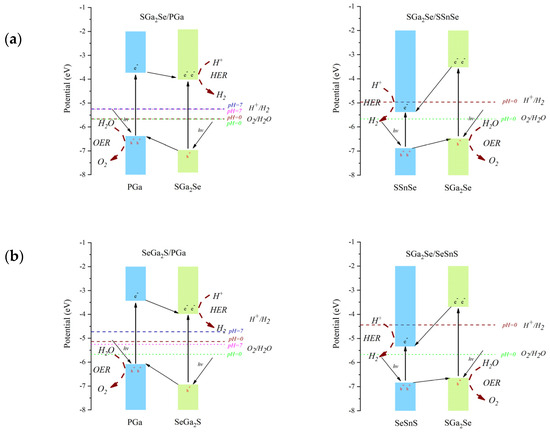
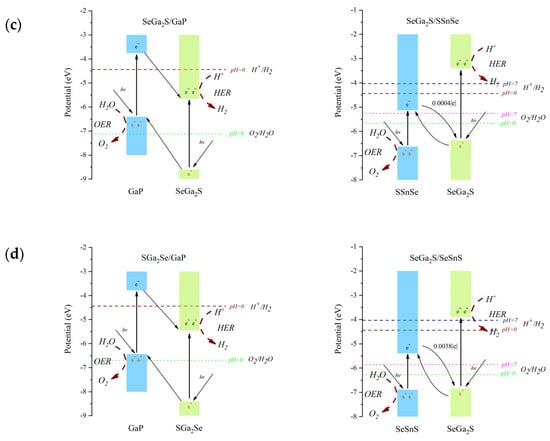
Figure 5.
Corrected band edge positions of the Ga2SSe/GaP and Ga2SSe/SnSSe vdW heterojunctions for (a) Pattern A, (b) Pattern B, (c) Pattern C, (d) Pattern D. The valence and conduction bands are represented by rectangles. Vertical arrows indicate electronic transitions from the valence band to the conduction band. Inclined arrows labeled hν represent photon absorption leading to electron excitation. Other inclined arrows indicate charge transfer between the heterostructure components. Red dashed arrows indicate reaction sites and types.
3.3. Optical and Photocatalytic Properties
Light absorption capability is widely recognized as a critical factor for efficient water-splitting photocatalysis. Figure 6a,b present the absorption coefficients of patterns A and B for the Ga2SSe/GaP vdW heterojunctions, as well as patterns C and D for the Ga2SSe/SnSSe vdW heterojunctions, alongside those of their respective monolayers. The absorption coefficient (α) was computed using the dielectric function formula [56]:
where the real (ε1) and imaginary (ε2) parts of the dielectric function are connected through the Kramers–Kronig relations, with ε2 derived from ε1. Compared to the isolated monolayers, particularly GaP and SnSSe, the heterostructures exhibit similar absorption profiles in the visible range, with a marked enhancement in the ultraviolet region. Despite the overall reduction in band gaps, a consistent red-shift of the absorption onset is not observed. This can be explained by analyzing the orbital contributions at the band edges. In the Ga2SSe/PGa heterostructure, the VBM is mainly composed of phosphorus orbitals, whereas the CBM does not involve p contributions. In contrast, in Ga2SSe alone, the VBM is dominated by sulfur and selenium orbitals, which are also absent in the CBM. This element-specific segregation of states across the band edges restricts efficient optical transitions near the gap and prevents a noticeable shift of the absorption edge. Moreover, the projected density of states (Figure 2) does not reveal the presence of mid-gap states or significant electronic reconstruction. In direct Z-scheme heterostructures, partial hybridization near the valence band indicates limited interlayer orbital mixing. These subtle interfacial effects impact the joint density of states and optical transition probabilities, particularly in the UV region, where enhanced absorption is observed. The changes in the absorption spectra are therefore governed not by the emergence of new states, but by the nature and spatial distribution of orbital contributions at the band edges, along with interface-driven modifications to the optical selection rules. This enhancement suggests that patterns A and B of Ga2SSe/GaP, along with patterns C and D of Ga2SSe/SnSSe, are strong candidates for photocatalytic water splitting applications.
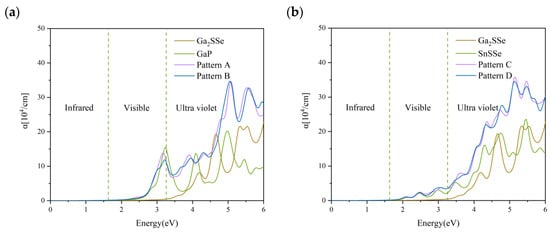
Figure 6.
Optical absorption spectra of the Ga2SSe/GaP vdW heterojunctions (a) and the Ga2SSe/SnSSe vdW heterojunctions (b), together with those of their corresponding monolayers, calculated using the HSE06 functional. The vertical green dotted lines delineate the boundaries of the visible wavelength region.
To assess the effectiveness of the photocatalytic water-splitting process, the solar-to-hydrogen (STH) conversion efficiency is a key metric, measuring how well the photocatalyst converts sunlight into hydrogen production under zero applied bias. The STH efficiency (ηSTH) consists of two components: the light absorption efficiency (ηabs) and the carrier utilization efficiency (ηcu), which can be expressed as follows [57,58]:
where P(ℏω) represents the AM1.5G solar irradiance at photon energy ℏω, and Eg denotes the bandgap energy. ΔG corresponds to the minimum potential difference needed for water splitting (1.23 eV), while E stands for the photon energy involved in the photocatalytic process, defined as follows [57,58]:
where χ(H2) and χ(O2) represent the overpotentials for the hydrogen evolution reaction (HER) and oxygen evolution reaction (OER), respectively. The solar-to-hydrogen efficiency (ηSTH) is then calculated using the following formula [57,58]:
Moreover, since the intrinsic electric field significantly influences the electron–hole separation, the solar-to-hydrogen efficiency (ηSTH) should be corrected accordingly, as follows [57,58]:
Here, ΔΦ represents the electrostatic potential difference. As reported in Table 2, the corrected solar-to-hydrogen efficiencies (η’STH) for patterns A and B in the Ga2SSe/GaP heterojunctions are 5.99% and 8.48%, respectively. By contrast, patterns C and D in the Ga2SSe/SnSSe heterostructures shows significantly higher values of 38.44% and 21.75%, respectively.

Table 2.
Overpotentials (in eV) for the hydrogen evolution reaction (χ(H2)) and oxygen evolution reaction (χ(O2)) at pH = 7, along with the efficiencies for light absorption (ηabs), carrier utilization (ηcu), solar-to-hydrogen conversion (ηSTH), and the corrected solar-to-hydrogen efficiency (η’STH).
These enhanced efficiencies in the Z-scheme arrangements not only outperform the type-II counterparts but also surpass the conventional 10% benchmark commonly cited for practical solar-to-hydrogen applications. For comparison, widely studied reference materials typically report significantly lower STH efficiencies. TiO2 (anatase), for instance, generally achieves only around 0.1 to 1.0% [59], mainly due to its wide band gap and UV-limited absorption. MoS2 in monolayer or few-layer form performs slightly better, with reported values in the range of approximately 1.0 to 2.0%, particularly when combined with heterostructures or co-catalysts [60]. Perovskites-based photocatalysts such as MoS2-NiFe-MAPbBr3 may reach efficiencies between 10 and 15% [61], though their operational instability under realistic conditions remains a critical limitation. These values are based on representative experimental and theoretical studies available in the literature, although reported STH values often vary depending on specific experimental setups or device architectures.
In terms of overpotentials, values for traditional photocatalysts are not always consistently reported, but it is generally acknowledged that TiO2 and MoS2 systems often require relatively high HER overpotentials, above 0.2–0.5 V [62,63,64], particularly in the absence of co-catalysts. The Z-scheme configurations examined in this study exhibit HER overpotentials of the same order of magnitude, in the range of 0.2–1.4 V, indicating similar energy efficiency and charge separation. For OER, similar overpotentials of about 0.2 V have been reported for optimized MoS2/Ni3S2 heterostructures [63].
In this context, our predicted efficiencies, especially for the Ga2SSe/SnSSe Z-scheme, are not only competitive with but also significantly higher than those of most conventional photocatalysts, clearly demonstrating the promise of such 2D heterostructures for next-generation solar hydrogen production. Furthermore, other theoretically investigated 2D heterojunction systems have also shown notable STH values, such as CdS/SPtSe with 37.50% [65], SiSe/SnSSe with 14.61% [66], ZrSSe/Ga2SSe with 17.64% [67], and GaN/InS with 26.33% [68]. These comparative results further support the relevance and strong potential of the systems explored in our study.
4. Conclusions
This work underscores the promising capabilities of Ga2SSe-based van der Waals heterojunctions for efficient solar-driven photocatalytic water splitting. Using comprehensive DFT simulations, we show that the Ga2SSe/SnSSe (patterns C and D) and Ga2SSe/GaP (patterns A and B) heterostructures feature ideal type-II band alignments and strong redox properties, meeting the essential criteria for water splitting. Importantly, patterns C and D exhibit a direct Z-scheme charge transfer mechanism, facilitating effective separation of photogenerated electron–hole pairs while maintaining powerful reduction and oxidation potentials. Each of these four heterojunctions satisfies the water-splitting conditions and demonstrates strong light absorption in both the visible and ultraviolet spectra. Particularly, the Ga2SSe/SnSSe heterostructures in patterns C and D achieve exceptional solar-to-hydrogen conversion efficiencies, surpassing the commercial benchmark of 10% with values of 38.44% and 21.75%, respectively, outperforming many previously reported photocatalysts. These findings establish these Z-scheme heterojunctions as highly promising candidates for future photocatalytic water splitting applications.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/cryst15080728/s1, Figure S1: Band structures of the Ga2SSe monolayer (a), GaP monolayer (b), PtSSe monolayer (c), and SnSSe monolayer (d) calculated using the HSE06 functional. The Fermi energy is set to 0 eV; Table S1: Lattice parameter (a in Å) and band gap (Eg in eV) of the four monolayers, calculated using the HSE06 functional, along with a comparison with available literature data; Table S2: Electrostatic potential difference (ΔΦ), valence band maximum (VBM), and conduction band minimum (CBM) of the Ga2SSe/GaP and Ga2SSe/SnSSe van der Waals heterojunctions.
Author Contributions
Conceptualization, P.B. and M.-C.R.; methodology, F.Y., P.B. and M.-C.R.; software, F.Y.; validation, F.Y., P.B. and M.-C.R.; formal analysis, F.Y.; investigation, F.Y.; resources, P.B. and M.-C.R.; data curation, F.Y.; writing—original draft preparation, F.Y.; writing—review and editing, P.B. and M.-C.R.; visualization, F.Y.; supervision, P.B. and M.-C.R.; project administration, P.B. and M.-C.R.; funding acquisition, P.B. and M.-C.R. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
Data will be made available through Zenodo depository upon request to the corresponding author.
Acknowledgments
The authors are thankful to the China Scholarship Council for financing the PhD thesis of F. Yang. This work was granted access to the HPC resources A0150806881 made by the “Grand Equipement National de Calcul Intensif (GENCI)”. The “Centre de Calcul Intensif d’Aix-Marseille” is acknowledged for granting access to its high-performance computing resources.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Hoang, A.T.; Pandey, A.; Chen, W.-H.; Ahmed, S.F.; Nižetić, S.; Ng, K.H.; Said, Z.; Duong, X.Q.; Ağbulut, Ü.; Hadiyanto, H.; et al. Hydrogen Production by Water Splitting with Support of Metal and Carbon-Based Photocatalysts. ACS Sustain. Chem. Eng. 2023, 11, 1221–1252. [Google Scholar] [CrossRef]
- Ng, K.H.; Lai, S.Y.; Cheng, C.K.; Cheng, Y.W.; Chong, C.C. Photocatalytic Water Splitting for Solving Energy Crisis: Myth, Fact or Busted? Chem. Eng. J. 2021, 417, 128847. [Google Scholar] [CrossRef]
- Acar, C.; Dincer, I.; Naterer, G.F. Review of Photocatalytic Water-Splitting Methods for Sustainable Hydrogen Production: Review: Photocatalysis for Sustainable Hydrogen. Int. J. Energy Res. 2016, 40, 1449–1473. [Google Scholar] [CrossRef]
- Ghorbani, B.; Zendehboudi, S.; Afrouzi, Z.A. Multi-Objective Optimization of an Innovative Integrated System for Production and Storage of Hydrogen with Net-Zero Carbon Emissions. Energy Convers. Manag. 2023, 276, 116506. [Google Scholar] [CrossRef]
- Oni, A.O.; Anaya, K.; Giwa, T.; Di Lullo, G.; Kumar, A. Comparative Assessment of Blue Hydrogen from Steam Methane Reforming, Autothermal Reforming, and Natural Gas Decomposition Technologies for Natural Gas-Producing Regions. Energy Convers. Manag. 2022, 254, 115245. [Google Scholar] [CrossRef]
- Wang, Y.; Suzuki, H.; Xie, J.; Tomita, O.; Martin, D.J.; Higashi, M.; Kong, D.; Abe, R.; Tang, J. Mimicking Natural Photosynthesis: Solar to Renewable H2 Fuel Synthesis by Z-Scheme Water Splitting Systems. Chem. Rev. 2018, 118, 5201–5241. [Google Scholar] [CrossRef]
- Jafari, T.; Moharreri, E.; Amin, A.; Miao, R.; Song, W.; Suib, S. Photocatalytic Water Splitting—The Untamed Dream: A Review of Recent Advances. Molecules 2016, 21, 900. [Google Scholar] [CrossRef]
- Dal Santo, V.; Naldoni, A. Titanium Dioxide Photocatalysis. Catalysts 2018, 8, 591. [Google Scholar] [CrossRef]
- Irfan, F.; Tanveer, M.U.; Moiz, M.A.; Husain, S.W.; Ramzan, M. TiO2 as an Effective Photocatalyst Mechanisms, Applications, and Dopants: A Review. Eur. Phys. J. B 2022, 95, 184. [Google Scholar] [CrossRef]
- Manzeli, S.; Ovchinnikov, D.; Pasquier, D.; Yazyev, O.V.; Kis, A. 2D Transition Metal Dichalcogenides. Nat. Rev. Mater. 2017, 2, 17033. [Google Scholar] [CrossRef]
- Wu, C.; Zhang, J.; Tong, X.; Yu, P.; Xu, J.; Wu, J.; Wang, Z.M.; Lou, J.; Chueh, Y. A Critical Review on Enhancement of Photocatalytic Hydrogen Production by Molybdenum Disulfide: From Growth to Interfacial Activities. Small 2019, 15, 1900578. [Google Scholar] [CrossRef]
- Sridharan, M.; Maiyalagan, T. Recent Progress in Tungsten Disulphide Based Photocatalyst for Hydrogen Production and Environmental Remediation. Chem. Eng. J. 2021, 424, 130393. [Google Scholar] [CrossRef]
- Yang, R.; Fan, Y.; Zhang, Y.; Mei, L.; Zhu, R.; Qin, J.; Hu, J.; Chen, Z.; Hau Ng, Y.; Voiry, D.; et al. 2D Transition Metal Dichalcogenides for Photocatalysis. Angew. Chem. 2023, 62, e202218016. [Google Scholar] [CrossRef]
- Rosman, N.N.; Mohamad Yunus, R.; Jeffery Minggu, L.; Arifin, K.; Salehmin, M.N.I.; Mohamed, M.A.; Kassim, M.B. Photocatalytic Properties of Two-Dimensional Graphene and Layered Transition-Metal Dichalcogenides Based Photocatalyst for Photoelectrochemical Hydrogen Generation: An Overview. Int. J. Hydrogen Energy 2018, 43, 18925–18945. [Google Scholar] [CrossRef]
- Zheng, W.; Jiang, Y.; Hu, X.; Li, H.; Zeng, Z.; Wang, X.; Pan, A. Light Emission Properties of 2D Transition Metal Dichalcogenides: Fundamentals and Applications. Adv. Opt. Mater. 2018, 6, 1800420. [Google Scholar] [CrossRef]
- Li, H.; Zhou, Y.; Tu, W.; Ye, J.; Zou, Z. State-of-the-Art Progress in Diverse Heterostructured Photocatalysts toward Promoting Photocatalytic Performance. Adv. Funct. Mater. 2015, 25, 998–1013. [Google Scholar] [CrossRef]
- Zhang, G.; Wang, X. Oxysulfide Semiconductors for Photocatalytic Overall Water Splitting with Visible Light. Angew. Chem. Int. Ed. 2019, 58, 15580–15582. [Google Scholar] [CrossRef]
- Wang, X.; Zhong, H.; Xi, S.; Lee, W.S.V.; Xue, J. Understanding of Oxygen Redox in the Oxygen Evolution Reaction. Adv. Mater. 2022, 34, 2107956. [Google Scholar] [CrossRef]
- Li, J.; Slassi, A.; Han, X.; Cornil, D.; Ha-Thi, M.; Pino, T.; Debecker, D.P.; Colbeau-Justin, C.; Arbiol, J.; Cornil, J.; et al. Tuning the Electronic Bandgap of Graphdiyne by H-Substitution to Promote Interfacial Charge Carrier Separation for Enhanced Photocatalytic Hydrogen Production. Adv. Funct. Mater. 2021, 31, 2100994. [Google Scholar] [CrossRef]
- Lasia, A. Mechanism and Kinetics of the Hydrogen Evolution Reaction. Int. J. Hydrogen Energy 2019, 44, 19484–19518. [Google Scholar] [CrossRef]
- Hu, W.; Lin, L.; Zhang, R.; Yang, C.; Yang, J. Highly Efficient Photocatalytic Water Splitting over Edge-Modified Phosphorene Nanoribbons. J. Am. Chem. Soc. 2017, 139, 15429–15436. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.; Tongay, S.; Zhou, J.; Li, J.; Wu, J. Band Offsets and Heterostructures of Two-Dimensional Semiconductors. Appl. Phys. Lett. 2013, 102, 012111. [Google Scholar] [CrossRef]
- Ng, B.; Putri, L.K.; Kong, X.Y.; Teh, Y.W.; Pasbakhsh, P.; Chai, S. Z-Scheme Photocatalytic Systems for Solar Water Splitting. Adv. Sci. 2020, 7, 1903171. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, Q.; Zhan, X.; Wang, F.; Safdar, M.; He, J. Visible Light Driven Type II Heterostructures and Their Enhanced Photocatalysis Properties: A Review. Nanoscale 2013, 5, 8326. [Google Scholar] [CrossRef]
- Fu, C.; Wu, X.; Yang, J. Material Design for Photocatalytic Water Splitting from a Theoretical Perspective. Adv. Mater. 2018, 30, 1802106. [Google Scholar] [CrossRef]
- Su, T.; Shao, Q.; Qin, Z.; Guo, Z.; Wu, Z. Role of Interfaces in Two-Dimensional Photocatalyst for Water Splitting. ACS Catal. 2018, 8, 2253–2276. [Google Scholar] [CrossRef]
- Yang, F.; Boulet, P.; Record, M.-C. DFT Investigation of a Direct Z-Scheme Photocatalyst for Overall Water Splitting: Janus Ga2SSe/Bi2O3 Van Der Waals Heterojunction. Materials 2025, 18, 1648. [Google Scholar] [CrossRef]
- Kumar, R.; Das, D.; Singh, A.K. C2N/WS2 van Der Waals Type-II Heterostructure as a Promising Water Splitting Photocatalyst. J. Catal. 2018, 359, 143–150. [Google Scholar] [CrossRef]
- Kim, J.H.; Jang, J.-W.; Jo, Y.H.; Abdi, F.F.; Lee, Y.H.; Van De Krol, R.; Lee, J.S. Hetero-Type Dual Photoanodes for Unbiased Solar Water Splitting with Extended Light Harvesting. Nat. Commun. 2016, 7, 13380. [Google Scholar] [CrossRef]
- Sun, J.; Liu, C.; Yang, P. Surfactant-Free, Large-Scale, Solution Liquid Solid Growth of Gallium Phosphide Nanowires and Their Use for Visible-Light-Driven Hydrogen Production from Water Reduction. J. Am. Chem. Soc. 2011, 133, 19306–19309. [Google Scholar] [CrossRef]
- Meinhardová, V.; Dubnová, L.; Kobielusz, M.; Kouba, D.; Slang, S.; Huo, P.; Matvieiev, O.; Macyk, W.; Kočí, K.; Čapek, L. Electron Migration Pathways in S-Scheme GaP-TiO2 Photocatalysts and Their Implications for Photocatalytic Hydrogen Production. Acta Mater. 2025, 296, 121274. [Google Scholar] [CrossRef]
- Peng, R.; Ma, Y.; Huang, B.; Dai, Y. Two-Dimensional Janus PtSSe for Photocatalytic Water Splitting under the Visible or Infrared Light. J. Mater. Chem. A 2019, 7, 603–610. [Google Scholar] [CrossRef]
- Jamdagni, P.; Kumar, A.; Srivastava, S.; Pandey, R.; Tankeshwar, K. Janus PtSSe-Based van Der Waals Heterostructures for Direct Z-Scheme Photocatalytic Water Splitting. Int. J. Hydrogen Energy 2024, 66, 268–277. [Google Scholar] [CrossRef]
- Xie, M.; Shang, Y.; Li, X.; Da, Y.; Liu, X. An Ab Initio Study of Two Dimensional SnX2 and Janus SnXY (X = S, Se) Nanosheets as Potential Photocatalysts for Water Splitting. ACS Appl. Nano Mater. 2023, 6, 10569–10580. [Google Scholar] [CrossRef]
- Anjum, N.; Kashif, M.; Shahzad, A.; Rasheed, A.; Ren, G. 2D Janus ZrSSe/SnSSe Heterostructure: A Promising Candidate for Photocatalytic Water Splitting. ACS Omega 2024, 9, 19848–19858. [Google Scholar] [CrossRef]
- Li, L.; Zhao, C.; Zhang, Y.; Zhang, L.; Zhu, Y. Janus Ga2SSe Nanotubes as Efficient Photocatalyst for Overall Water Splitting. Nanotechnology 2022, 33, 465703. [Google Scholar] [CrossRef]
- Zhang, W.X.; Yin, Y.; He, C. Spontaneous Enhanced Visible-Light-Driven Photocatalytic Water Splitting on Novel Type-II GaSe/CN and Ga2SSe/CN vdW Heterostructures. J. Phys. Chem. Lett. 2021, 12, 5064–5075. [Google Scholar] [CrossRef]
- Geim, A.K.; Grigorieva, I.V. Van Der Waals Heterostructures. Nature 2013, 499, 419–425. [Google Scholar] [CrossRef]
- Chen, J.; Yang, D.; Song, D.; Jiang, J.; Ma, A.; Hu, M.Z.; Ni, C. Recent Progress in Enhancing Solar-to-Hydrogen Efficiency. J. Power Sources 2015, 280, 649–666. [Google Scholar] [CrossRef]
- Orio, M.; Pantazis, D.A.; Neese, F. Density Functional Theory. Photosynth. Res. 2009, 102, 443–453. [Google Scholar] [CrossRef]
- Hafner, J. Ab-Initio Simulations of Materials Using VASP: Density-functional Theory and Beyond. J. Comput. Chem. 2008, 29, 2044–2078. [Google Scholar] [CrossRef] [PubMed]
- Kresse, G.; Joubert, D. From Ultrasoft Pseudopotentials to the Projector Augmented-Wave Method. Phys. Rev. B 1999, 59, 1758–1775. [Google Scholar] [CrossRef]
- Cooper, V.R. Van Der Waals Density Functional: An Appropriate Exchange Functional. Phys. Rev. B 2010, 81, 161104. [Google Scholar] [CrossRef]
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized Gradient Approximation Made Simple. Phys. Rev. Lett. 1996, 77, 3865–3868. [Google Scholar] [CrossRef]
- Heyd, J.; Scuseria, G.E.; Ernzerhof, M. Hybrid Functionals Based on a Screened Coulomb Potential. J. Chem. Phys. 2003, 118, 8207–8215. [Google Scholar] [CrossRef]
- Moellmann, J.; Grimme, S. DFT-D3 Study of Some Molecular Crystals. J. Phys. Chem. C 2014, 118, 7615–7621. [Google Scholar] [CrossRef]
- Tang, W.; Sanville, E.; Henkelman, G. A Grid-Based Bader Analysis Algorithm without Lattice Bias. J. Phys. Condens. Matter 2009, 21, 084204. [Google Scholar] [CrossRef]
- Jain, A.; Ong, S.P.; Hautier, G.; Chen, W.; Richards, W.D.; Dacek, S.; Cholia, S.; Gunter, D.; Skinner, D.; Ceder, G.; et al. Commentary: The Materials Project: A Materials Genome Approach to Accelerating Materials Innovation. APL Mater. 2013, 1, 011002. [Google Scholar] [CrossRef]
- Ul Haq, B.; AlFaify, S.; Ahmed, R.; Khan, M.H.; Alsardia, M.M.; Khadka, S.-H. First-Principles Study of the Physical Properties of Novel Polytypes of Gallium Phosphide. Cryst. Growth Des. 2021, 21, 6417–6424. [Google Scholar] [CrossRef]
- Da Silva, R.; Barbosa, R.; Mançano, R.R.; Durães, N.; Pontes, R.B.; Miwa, R.H.; Fazzio, A.; Padilha, J.E. Metal Chalcogenides Janus Monolayers for Efficient Hydrogen Generation by Photocatalytic Water Splitting. ACS Appl. Nano Mater. 2019, 2, 890–897. [Google Scholar] [CrossRef]
- Yang, F.; Boulet, P.; Record, M.-C. Electronic Structure and Photocatalytic Performance of Janus MoSSe/Ga2SSe van Der Waals Heterostructures. Int. J. Hydrogen Energy 2024, 73, 536–546. [Google Scholar] [CrossRef]
- Li, X.; Li, Z.; Yang, J. Proposed Photosynthesis Method for Producing Hydrogen from Dissociated Water Molecules Using Incident Near-Infrared Light. Phys. Rev. Lett. 2014, 112, 018301. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.X.; Hou, J.T.; Bai, M.; He, C.; Wen, J.R. Construction of Novel ZnO/Ga2SSe (GaSe) vdW Heterostructures as Efficient Catalysts for Water Splitting. Appl. Surf. Sci. 2023, 634, 157648. [Google Scholar] [CrossRef]
- Ahmad, I.; Shahid, I.; Ali, A.; Ruan, Z.; Yan, C.; Ali, J.; Gao, L.; Cai, J. Two Dimensional Janus SGaInSe(SeGaInS)/PtSe2 van Der Waals Heterostructures for Optoelectronic and Photocatalytic Water Splitting Applications. Int. J. Hydrogen Energy 2022, 47, 28833–28844. [Google Scholar] [CrossRef]
- Yang, F.; Record, M.-C.; Boulet, P. Self-Defined Monolayers, Bilayers and Trilayers of Two-Dimensional Janus MoSSe and Ga2SSe van Der Waals Homojunctions as Potential Photocatalysts for Overall Water Splitting. Renew. Energy 2025, 245, 122829. [Google Scholar] [CrossRef]
- Lin, H.-F.; Liu, H.-Y.; Wang, M.; Wang, S.-S.; Hou, T.-P.; Wu, K.-M. Janus Ga2SeTe/In2SSe Heterostructures: Tunable Electronic, Optical, and Photocatalytic Properties. Phys. Chem. Chem. Phys. 2022, 24, 4425–4436. [Google Scholar] [CrossRef]
- Fu, C.-F.; Sun, J.; Luo, Q.; Li, X.; Hu, W.; Yang, J. Intrinsic Electric Fields in Two-Dimensional Materials Boost the Solar-to-Hydrogen Efficiency for Photocatalytic Water Splitting. Nano Lett. 2018, 18, 6312–6317. [Google Scholar] [CrossRef]
- Liu, L.-L.; Li, D.-F.; Tang, R.-F.; Tang, M.-X.; Zhang, X.-Y.; Liu, M.-L.; Hu, L.; Wang, S.-F.; Wu, X.-Z. Derivative Ga2S3 Monolayers as Water-Splitting Photocatalysts: Enhanced Solar to Hydrogen Conversion for Reduced Dipole. Results Phys. 2023, 52, 106831. [Google Scholar] [CrossRef]
- Gunawan, D.; Zhang, J.; Li, Q.; Cui, Y.T.; Scott, J.; Antonietti, M.; Guo, J.; Amal, R. Materials Advances in Photocatalytic Solar Hydrogen Production: Integrating Systms and Economics for a Sustainable Future. Adv. Mater. 2024, 36, 2404618. [Google Scholar] [CrossRef]
- Zhao, X.; Chen, S.; Yin, H.; Jiang, S.; Zhao, K.; Kang, J.; Liu, P.F.; Jiang, L.; Zhu, Z.; Cui, D.; et al. Perovskite Microcrystals with Intercalated Monolayer MoS2 Nanosheets as Advanced Photocatalyst for Solar-Powered Hydrogen Generation. Matter 2025, 3, 935–949. [Google Scholar] [CrossRef]
- Asiri, A.M.; Ren, D.; Zhang, H.; Khan, S.B.; Alamry, K.A.; Marwani, H.M.; Khan, M.S.J.; Adeosun, W.A.; Zakeeruddin, S.M.; Grätzel, M. Solar Water Splitting Using Earth-Abundant Electrocatalysts Driven by High-Efficiency Perovskite Solar Cells. ChemSusChem 2022, 15, e202102471. [Google Scholar] [CrossRef]
- Liu, B.; Chen, H.M.; Liu, C.; Andrews, S.C.; Hahn, C.; Yang, P. Large-Scale Synthesis of Transition-Metal-Doped TiO2 nanowires with Controllable Overpotential. J. Am. Chem. Soc. 2013, 135, 9995–9998. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, T.; Pohl, D.; Rellinghaus, B.; Dong, R.; Liu, S.; Zhuang, X.; Deng, X. Interface Engineering of MoS2/Ni3S2 Heterostructures for Highly Enhanced Electrochemical Overall-Water-Splitting Activity. Angew. Chem. 2016, 128, 6814–6819. [Google Scholar] [CrossRef]
- Nayana, K.; Sunitha, A.P. MoS2-x/GCD-MoS2-x nanostructures for tuning the overpotential of Volmer-Heyrovsky reaction of electrocatalytic hydrogen evolution. Int. J. Hydrogen Energy 2024, 55, 422–431. [Google Scholar] [CrossRef]
- Wang, G.; Tang, W.; Xie, W.; Tang, Q.; Wang, Y.; Guo, H.; Gao, P.; Dang, S.; Chang, J. Type-II CdS/PtSSe Heterostructures Used as Highly Efficient Water-Splitting Photocatalysts. Appl. Surf. Sci. 2022, 589, 152931. [Google Scholar] [CrossRef]
- Wang, F.; Yang, C.-L.; Li, X.-H.; Liu, Y.-L.; Zhao, W.-K. Hydrogen Generation from Direct Z-Scheme for Photocatalytic Overall Water Splitting with the SiSe/SnSe2 and SiSe/SnSSe Heterostructures. J. Catal. 2024, 432, 115429. [Google Scholar] [CrossRef]
- Allaoui, I.; El Kenz, A.; Benyoussef, A.; Khuili, M.; Fazouan, N. Strain-Engineered ZrSSe/Ga2SSe vdW Heterostructure with Enhanced Visible Light Harvesting and High Solar-to-Hydrogen Efficiency. Micro Nanostruct. 2025, 205, 208174. [Google Scholar] [CrossRef]
- Qiao, H.; Zhang, Y.; Yan, Z.-H.; Duan, L.; Ni, L.; Fan, J.-B. A Type-II GaN/InS van Der Waals Heterostructure with High Solar-to-Hydrogen Efficiency of Photocatalyst for Water Splitting. Appl. Surf. Sci. 2022, 604, 154602. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).