Abstract
This paper presents the preparation of Z-cut near-stoichiometric lithium niobate (NSLN) wafers using a combined process of the lithium-rich Czochralski growth and diffusion methods. The fabricated Z-cut NSLN wafers exhibited outstanding comprehensive performance, including a high Curie temperature of up to 1200 °C, a refractive index gradient in the diameter direction below 1.5 × 10−4 cm−1, and a UV absorption edge shifted 14 nm toward the ultraviolet region compared to congruent lithium niobate crystals, with a coercive field of 1268 V/mm. Additionally, the wafers demonstrated excellent processing characteristics, with the bow of 4-inch wafers controlled within 55 μm, surpassing the machining standards of traditional lithium niobate wafers of the same size. These results indicated the highly uniform chemical stoichiometry and crystallization quality of the wafers. Leveraging the high uniformity and low coercive field of the wafers, periodic triangular domain structure arrays were successfully fabricated, laying the foundation for domain engineering design in electro-optic deflectors and switching devices. This study not only achieves the scalable preparation of NSLN wafers but also provides a reliable technical solution for their practical applications in high-performance electro-optic devices.
1. Introduction
Satellite laser communication has become a key technology for space communication by its high confidentiality and large-capacity transmission advantages. However, the limitation of the attitude adjustment of space platforms makes the dynamic networking of multiple optical signals a serious challenge [1]. Beam deflection technology, as a potential solution, is highly dependent on the properties of electro-optical materials [2]. Lithium niobate (LiNbO3, LN) crystals are ideal candidates for electro-optical deflectors due to their high response speed, large size manufacturability, high damage threshold, and mature processing [3,4,5,6]. In particular, its excellent electro-optical effect and flexible ferroelectric domain modulation capability allow it to achieve efficient beam deflection through a triangular domain structure [7]. Conventional same-component lithium niobate (CLN) crystals suffer from many intrinsic defects and a high coercive field (21.1 kV/mm), which severely limit the electro-optical performance and the preparation of triangular domain structures [8,9]. In contrast, near-stoichiometric lithium niobate (NSLN) significantly reduces defect concentration by optimizing the [Li]/[Nb] ratio to nearly 1:1 [10]. This optimization results in a reduced coercive field below 2 kV/mm [11,12] and a 22% enhancement in the electro-optic coefficient 33 (from 31.5 pm/V in CLN to 38.3 pm/V) [12]. These improvements enable low-voltage-driven domain inversion and provide an excellent solution for triangular-domain-based electro-optic deflectors.
For the growth of NSLN crystals, the traditional growth methods are the lithium-rich Czochralski method (LRCM), double crucible Czochralski (DCCZ) method, top-seeded solution growth (TSSG) method, and the Vapor Transport Equilibrium (VTE) method, also called the diffusion method [13,14,15,16,17,18]. The lithium-rich Czochralski growth method is simple, but the crystal composition deviation is large, the crystal quality is poor, and the crystals have no practical application value [19]. The double crucible Czochralski growth method is complex and costly, and no crystals of SLN grown over 4 inches have been reported [20,21]. The top-seeded crystal co-solvent solution growth method faces challenges such as many crystal defects and unstable components with slow growth rates, which makes it difficult to grow large-size crystals [22], while the diffusion method is a simple process but can only be applied to wafers [23] because the lattice constants shrink non-uniformly with the increase in the lithium content, which tends to lead to wafer bending. To address the critical scientific challenge of simultaneously achieving compositional uniformity and lattice integrity in conventional NSLN crystal growth, this study innovatively proposed a “lithium-rich Czochralski-diffusion synergistic growth method”, which enhances the compositional homogeneity compared to the pure lithium-rich Czochralski technique while improving surface flatness relative to conventional diffusion methods.
The domain polarization technique can selectively flip the spontaneous polarization to form patterned domain structures by applying an electric field with a super-corrective field [23,24]. Among them, the triangular domain structure has a unique advantage in beam deflection due to its symmetry. In this paper, the kinetic mechanism of domain inversion was revealed by modulating the crystal components and the polarization electric field, which provided theoretical and experimental support for the development of NSLN crystals in electro-optical devices.
2. Experimental Process
This paper examined Z-cut NSLN wafers fabricated through three different approaches. The first method involved growing Z-cut NSLN crystals from lithium-rich polycrystalline feedstock (Li/Nb = 56/44) using the lithium-enriched Czochralski growth method and then processing them into Z-cut NSLN wafers. The second approach combined lithium-rich Czochralski growth with a diffusion process to produce Z-cut NSLN wafers. The third method utilized commercially available CLN wafers that were converted into Z-cut NSLN wafers through the same diffusion process employed in the second method. The specific experimental procedures are as follows:
2.1. Lithium-Rich Czochralski Growth Method
High-purity niobium oxide (Nb2O5, 99.99%) and lithium carbonate (Li2CO3, 99.99%) were used as the starting materials. The powders were placed in an oven and dried at 120 °C for 2 h to remove adsorbed moisture. Under the hot condition, the mixture was weighed accurately according to the molar ratio of Li/Nb = 56/44, and the raw materials were placed in a drum ball mill and ball milled for 12 h to ensure sufficient mixing; the mixed powder was loaded into a platinum crucible and sintered in a high-temperature furnace in phases: with the first phase, the temperature was raised to 800 °C at 50 °C/h and kept warm for 4 h to make the complete decomposition of Li2CO3; and, in the second phase, the temperature was raised to 1160 °C, holding the temperature for 4 h to synthesize the dense Li-rich polycrystalline material. In order to verify whether the lithium-rich polycrystalline material was formed correctly, the samples were subjected to an XRD test, and then compared with the standard PDF card. The XRD patterns of the Li-rich polycrystalline material, the LiNbO3 standard PDF card PDF#20-0631, and the Li3NbO4 standard PDF card PDF#16-0459 are shown in Figure 1a. From Figure 1a, it can be seen that the lithium-rich polycrystalline material had very obvious diffraction peaks, which indicated that the lithium-rich polycrystalline material had good crystallinity. In comparison with the standard PDF card, in addition to the standard LiNbO3, the position of the characteristic peaks were more consistent with the standard Li3NbO4 characteristic peaks in some locations, the two standard PDF cards contained all the lithium-rich polycrystalline material diffraction peaks, and there were no new diffraction peaks, so it was confirmed that this sample was indeed a lithium-rich polycrystalline material, including lithium-rich properties. The lithium-rich polycrystalline material was loaded into a platinum crucible with a diameter of 166 mm and a height of 80 mm, and an inductively heated direct-drawing single-crystal furnace was used, with the schematic diagram of the equipment is shown in Figure 1b. The lithium-rich polycrystalline material was melted in the platinum crucible, and since a too high rotational speed would lead to interfacial turbulence, a moderate rotational speed was chosen to keep it at 10–15 rpm, which could improve the melt convection and promote the homogeneity of the components. A slower lifting rate of 1.5 mm/h helped the orderly arrangement of lithium ions and reduced vacancy defects. After these parameters were set, the crystals were allowed to grow in the Z-axis direction. After the growth, the crystals were cooled to room temperature in the air at a rate of 50 °C/h. The crystal growth diagram is shown in Figure 1c. As can be seen from the figure, the length of the crystal was 130 mm and the diameter was 104 mm.
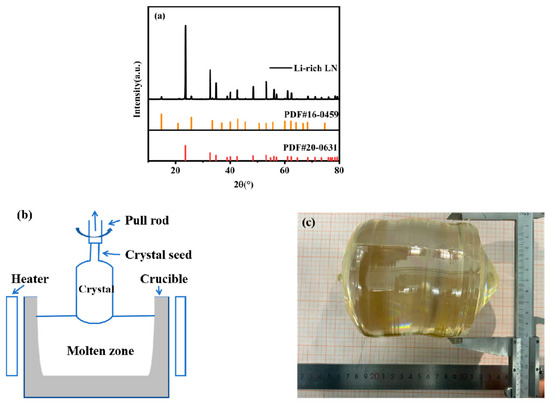
Figure 1.
(a) XRD of the Li-enriched polycrystalline material. (b) Schematic diagram of the Li-enriched Czochralski growth method device. (c) Crystal growth diagram.
2.2. Diffusion Method
2.2.1. Combination of Lithium-Rich Czochralski Growth and Diffusion Methods
The Z-cut NSLN crystal, grown via the lithium-rich Czochralski technique, was subjected to top removal, tail cutting, and precision slicing, and the Curie temperature was measured using a high-temperature dielectric spectrometer, Model DMS 1000. The crystal was also subjected to mechanical polishing to fabricate a standard Z-cut wafer with a diameter of 100.4 mm and a thickness of 1.16 mm. Firstly, the homogeneity of the crystals formed by the lithium-rich Czochralski growth method was tested. One wafer was taken from each of the head, middle, and tail parts and tested for its Curie temperature. Then, any piece of the Z-cut NSLN wafer grown and processed by the lithium-rich Czochralski growth method was used for the diffusion process, placed in a special ceramic crucible, using the same lithium-rich polycrystalline raw material employed during crystal growth as the diffusion source. The diffusion material was uniformly spread on the alumina ceramic cover with a thickness of about 5 mm, and assembled into a diffusion system using a sealed ceramic crucible, and the assembled crucible was placed into a programmed temperature-controlled muffle furnace; the temperature was raised to 1150 °C at a rate of 50 °C/h. Upon achieving the target temperature, the constant temperature was kept for 80 h (±0.5 °C temperature control accuracy) isothermal hold at 1150 °C. Following the isothermal treatment, the crucible was cooled down to room temperature at a rate of 50 °C/h. High-purity oxygen (99.999%) was introduced throughout the process, and the pressure in the furnace was maintained at 1.05 atmospheres. At the end of diffusion, the Z-cut NSLN wafers were tested for Curie temperature and bending.
2.2.2. Conventional Diffusion Method
Under the same diffusion conditions described above, a commercial CLN wafer was used for diffusion experiments to form a Z-cut NSLN wafer; the thickness was measured using a thickness gauge, Model MINIAX DH-150, and its curvature was tested. A diagram of this diffusion setup is shown in Figure 2.
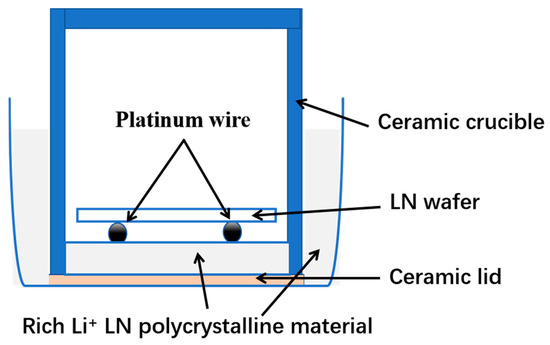
Figure 2.
Diagram of the diffusion device for the preparation of NSLN wafers.
2.3. Preparation of Periodic Domain Structures
The optical properties of the samples were characterized using a spectroscopic ellipsometer, Model ES01A. The Z-cut NSLN wafers with the best quality of the three wafers above were selected and subjected to a preliminary cleaning treatment to remove contaminants, impurities, and oxides from the surface. The wafer surface was ensured to be clean and flat. The photoresist (RDPPI-I/GOI) was uniformly coated on the front side of the Z-cut NSLN wafer. A certain amount of photoresist was first dropped at the center of the wafer, and then the wafer was rotated at 4000 rpm to spread the photoresist uniformly on the wafer surface by centrifugal force, forming a photoresist film with a thickness of 1.2 μm. Soft baking was performed after coating to remove the solvent in the photoresist and to improve the adhesion and stability of the photoresist. The coated wafer was placed on the photolithography table, aligned with the design triangle pattern of the mask plate, and exposed. Post-exposure baking was performed to cure the pattern, and then the developer removed the photoresist from the unexposed/exposed areas to form the target pattern. High temperature baking after development further enhanced adhesion of the adhesive film, and a flowchart of the insulating electrode preparation is shown in Figure 3a. Polarization of the wafer was performed after photolithography. A homemade plexiglass fixture containing saturated LiCl solution was used to connect a DC-regulated power supply to apply an electric field to the Z-cut NSLN wafer. When the strength of the external electric field exceeded the crystal coercive field, the critical voltage corresponding to the current surge was recorded, and the voltage was maintained until the current returned to zero, completing the domain inversion, and the polarization schematic is shown in Figure 3b. The completed domain inversion wafer was then placed in a mixed hydrofluoric acid–nitric acid solution (HF:HNO3 = 1:3) and corroded in a boiling water bath for 1 h, so that the inverted domain structure was visible.
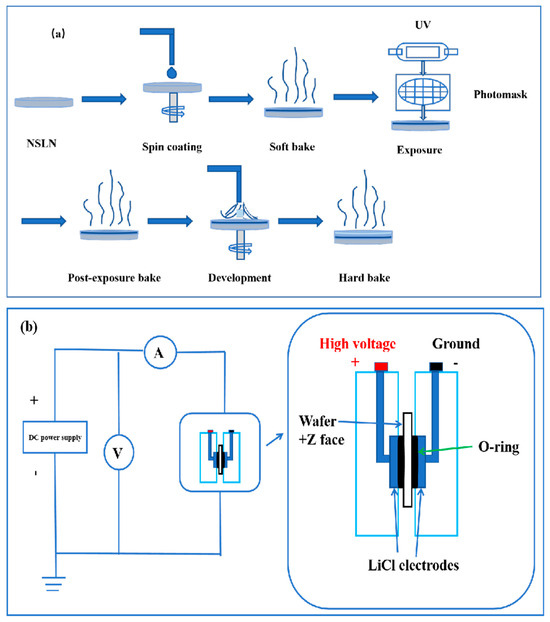
Figure 3.
(a) Flowchart for the preparation of insulating electrodes. (b) Schematic diagram of the external circuits and units used for electric field polarization.
3. Results and Discussion
3.1. Curie Temperatures of the Li-Rich Czochralski Growth Method
A high-temperature dielectric temperature spectrometer was used to determine the Curie temperatures of the head, middle, and tail of the Li-rich Czochralski growth method-grown and processed Z-cut NSLN wafers and the control CLN wafers, by detecting the sudden change characteristics of capacitance constants in the process of ferroelectric–parabolic phase transition, and the results are shown in Figure 4. The “ferroelectric–paraelectric” phase transition in ferroelectric materials specifically refers to the critical process where the material transforms from the ferroelectric phase (with spontaneous polarization) to the paraelectric phase (without spontaneous polarization) near the Curie temperature. This phase transition fundamentally represents a physical phenomenon involving structural symmetry change and abrupt property variation at specific temperatures. As can be seen from the figure, the Curie temperatures were 1168 °C, 1180 °C, 1195 °C, and 1140 °C, which correspond to (a), (b), (c) and (d) in Figure 4, respectively. Due to lithium being a light element, directly measuring its concentration often yields imprecise results. Researchers have therefore developed indirect characterization methods, such as the Curie temperature measurement [25]. According to the theoretical equation (1) for Curie temperature versus lithium content presented in reference [26], where Tc and C are the Curie temperature and Li2O concentration (mole%), respectively. The lithium content was calculated as 49.1%, 49.5%, and 49.9% for the head, middle, and tail, and 48.5% for the CLN control, respectively. It could be concluded that there was a significant compositional inhomogeneity (>0.4% difference in head/middle/tail lithium content) in the crystals grown by the lithium-rich Czochralski growth method. This inhomogeneity may affect the performance of the crystals and therefore further studies are needed to optimize the growth process to reduce this compositional difference.
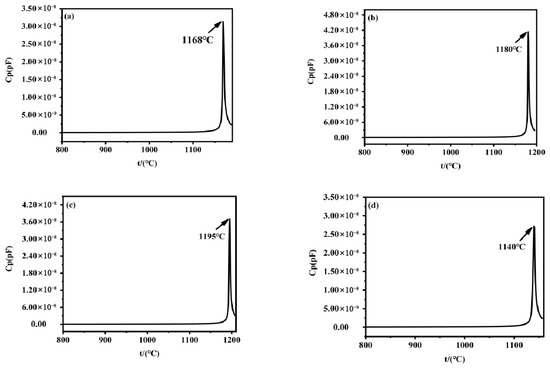
Figure 4.
Curie temperature of the different parts of Z-cut NSLN wafers: (a) head; (b) middle; (c) tail. (d) Curie temperature of the CLN wafers.
3.2. Bow of Crystals After Diffusion
A thickness measuring instrument was used to measure the bow of the following samples: Z-cut NSLN wafers prepared by the direct diffusion of CLN wafers and Z-cut NSLN wafers prepared by combining the lithium-rich Czochralski growth and diffusion methods, utilizing the center-point thickness measurement method. The wafer warpage was caused by the anisotropic changes in lattice constants (a and c) induced by the diffusion process. The measurement results are shown in Figure 5. From the results, the wafers with CLN direct diffusion had a significantly large bow (>1 mm), which could not meet the requirements of practical applications because the deformation was too large and difficult to be repaired by conventional grinding/polishing processes, where the industrial processing standards usually require bows < 100 μm. For the wafers prepared by the combination of the lithium-rich Czochralski growth and diffusion methods, the average bow was about 55 μm, meeting industrial processing standards and making them directly suitable for subsequent device fabrication. This result showed that the combination of the lithium-rich Czochralski growth and diffusion processes can effectively control the deformation of wafers and has significant advantages in industrial applications.

Figure 5.
(a) Bow of the CLN wafers by diffusion. (b) Bow of the NSLN wafers prepared by the combination of the lithium-rich Czochralski growth and diffusion methods.
To further evaluate the component homogeneity of the Z-cut NSLN wafers prepared by the combination of the lithium-rich Czochralski growth and diffusion methods, we also tested the Curie temperature of the wafers. The samples at the center and both sides of the wafer were selected for measurement, and the results are shown in Figure 6. From the Figure 6, it can be seen that the Curie temperatures of the three sites were 1200 °C, 1198 °C, and 1199 °C, which correspond to (a), (b) and (c) in Figure 6, respectively. Keeping a high degree of consistency, this indicated that the distribution of lithium elements in the crystal had excellent homogeneity without obvious component segregation. This result fully verified the stability of product from the combined lithium-rich Czochralski growth and diffusion processes, indicating that the optimized growth method can effectively suppress lithium volatilization and ensure the component uniformity of the wafers. This excellent flatness and component uniformity provides an important guarantee for subsequent device preparation, such as for an electro-optical deflector, ensuring the reliability and consistency of the device performance, and also shows the good application prospects of this process in the field of electro-optical functional material preparation.

Figure 6.
(a) Curie temperatures of the different parts of the Z-cut NSLN wafers: (a) center; (b) left side; (c) right side.
3.3. Dispersion Curve Measurements
A spectroscopic ellipsometer was used to characterize the optical properties of the samples, with the test conditions using wavelengths of 245–1700 nm and an incidence angle of 70 °C. The measurement position is shown in Figure 7a, where it can be seen that the distance between the adjacent test points was 10 mm [27]. The following results were obtained by ellipsometric angle () measurements and Gaussian simulation fitting, and the numerical results of the refractive index (n) and extinction coefficient (k) are shown in Table 1. It is evident from Table 1 that the Z-cut NSLN wafers had small transverse and longitudinal refractive index gradients, indicating excellent optical homogeneity. This property provides significant application benefits for the fabrication of electro-optical devices. The curves of n and k of the wafers are shown in Figure 7b. It can be seen in the figure that n decreased with increasing wavelength, indicating that the crystal had no anomalous optical jumps in the tested wavelength bands. The overall low value of k indicated that the crystal had good transparency and low light scattering/absorption loss, which makes it suitable for optical device applications. Both UV and visible light induce electronic transitions by promoting valence electrons to higher energy states through photon absorption. Therefore, the UV–visible spectrum mainly reflects changes in the energy level of electrons within a molecule. The composition of a crystal can be determined by analyzing its UV absorption spectrum [28,29], and the UV absorption edge is shown in Figure 7c. It can be seen in the figure that the UV absorption edge of the Z-cut NSLN wafers was shifted towards the UV region by about 14 nm compared to the CLN wafers. It was confirmed that the lithium-rich process reduces the defects, and the crystal structure was ordered, which is in line with the characteristics of high-quality NSLN wafers.

Figure 7.
(a) Positions of wafers for testing optical properties. (b) n and k curves of wafers. (c) UV absorption edge of wafers.

Table 1.
Refractive index (n) and extinction coefficient (k) values for the NSLN wafers.
3.4. Ferroelectric Hysteresis Loop
The ferroelectric hysteresis loop characterizes the polarization electric field dependence in ferroelectric materials. In the ferroelectric phase of lithium niobate crystals, there exist two low-energy potential wells near the oxygen planes where Li ions can reside in either stable state [30]. Since the spontaneous polarization direction is determined by the Li ion positions, two polarization states (“up-polarization” and “down-polarization”) exist. Polarization switching occurs when external energy enables Li ions to overcome the potential barrier across the oxygen plane and transition to the opposite stable site, achieving “polarization reversal.” The domain structure state was verified by testing the hysteresis return line of the Z-cut NSLN wafer at a test frequency of 5 Hz, and the test results are shown in Figure 8. It can be seen in the figure that the electric ferroelectric hysteresis loop of the Z-cut NSLN wafer showed obvious asymmetry, and the coercive field in the positive and negative directions were significantly different, which indicated that there was an internal field in the crystal. The results showed that the measured coercive field was 1268 V/mm, which was about 94% lower than that of the CLN wafers (typical value ~21 kV/mm). The CLN wafers contained numerous intrinsic defects that generated localized built-in electric fields, which hindered domain switching and consequently increased the coercive field (Ec). In contrast, the NSLN crystals exhibited significantly reduced defect concentrations, leading to weakened built-in fields and correspondingly lower Ec values required for domain switching. Therefore, the reduction in Ec primarily originated from the decreased intrinsic defect concentration, while the homogenization of nanodomain structures resulting from near-stoichiometric optimization also played a synergistic role [31]. This result provides an ideal substrate for the subsequent preparation of regular triangular domain structures.
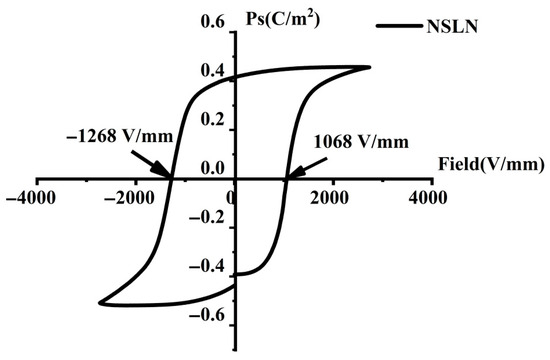
Figure 8.
Z-cut NSLN wafers with a ferroelectric hysteresis loop.
3.5. Triangular Domain Structures
An array of equilateral triangular domain structures was prepared on the surface of a Z-cut NSLN wafer using photolithography, as shown in Figure 9a. The pattern orientation was strictly aligned along the X-axis direction of the wafer to maintain precise parallelism with the intrinsic hexagonal symmetry of the crystal, and the edge roughness of the etching mask was controlled within ±20 nm to provide a precise template for subsequent polarization. The domains were inverted by applying a 1268 V/mm electric field at room temperature, and the optimized HF:HNO3 = 1:3 etching solution was used. Macroscopic observation showed that the wafer surface exhibited a highly ordered array of triangular domains, as shown in Figure 9b, and the angle between the boundaries of the triangular domains and the substrate crystallographic direction was precisely 60°. Optical microscope observation showed that the boundaries of the triangular domains were perfectly aligned with the domain walls of the lattice, as shown in Figure 9c, where the application of an electric field perpendicular to the paper plane resulted in sharply defined domain walls with distinct positive and negative domains, while no microstructural defects such as dislocations or cracks were observed.

Figure 9.
(a) Equilateral triangular domain structure pattern. (b) Corroded equilateral triangular domain structure pattern. (c) Boundary of triangular domains with lattice domain walls.
4. Summary
This paper focuses on the preparation method, performance characterization, and domain structure modulation of the Z-cut NSLN crystals. Through the optimized combination of the lithium-rich Czochralski growth and diffusion method processes, key issues such as the component uniformity and mechanical deformation of the wafers were successfully solved. In terms of the crystal properties, firstly, the Curie temperature test results showed that the deviation of the measured values in different parts of the wafer was less than 1 °C, confirming the highly uniform distribution of lithium elements in the crystal. Second, compared with the direct diffusion method for CLN wafers, the NSLN wafers prepared by the combination of the lithium-rich Czochralski growth and diffusion methods showed significantly improved mechanical properties, with a bow of only 55 μm, which fully meets the stringent requirements for wafer flatness in industrial processing. Optical and electrical performance tests further verified the material’s superiority. Ellipsometry measurements showed that the wafers had excellent optical uniformity, with a refractive index gradient of less than 1.5 × 10−4 cm−1; hysteresis loop tests showed that the coercive field was reduced to 1268 V/mm, which demonstrated excellent ferroelectric properties. In terms of domain structure modulation, equilateral triangular domain arrays strictly aligned with the lattice were successfully prepared on the surface of NSLN wafers by photolithography, which realized the precise control of the domain structure at the submicron level. This breakthrough lays an important foundation for the development of high-performance ferroelectric devices. The research results not only provide a reliable material solution for the application of NSLN wafers in high-speed electro-optical modulators and electro-optical deflectors, but also establish a systematic process method for the subsequent development of more complex domain structure devices. Future research can further explore the potential of this material system for applications in integrated optics and quantum optics devices.
Author Contributions
Conceptualization, X.X., Y.Z., and X.Z.; Data curation, Y.Z. and H.Z. (Han Zhang); Formal analysis, Y.Z.; Funding acquisition, X.X., H.Z. (Huan Zhang), and X.Z.; Investigation, X.X., J.C., Y.H., J.S., S.L., Q.X., H.Z. (Huan Zhang), L.M., C.Y., and X.Z.; Supervision, X.X.; Validation, X.X.; Writing—original draft preparation, Y.Z.; Writing—review and editing, Y.Z. and X.X. All authors have read and agreed to the published version of the manuscript.
Funding
This work is supported by the Ningxia Key Natural Science Foundation Project (2023AAC02045); the Scientific Research Project of the Ningxia Education Department (NYG2024067); the National Natural Science Foundation of China (61965001, 11864001, and 61461001); the Fundamental Research Funds for the Central Universities, North Minzu University (2021KJCX07); the Ningxia Province Key Research and Development Program (2018BEE03015, 2021BEE03005, and 2022BFE02009); the Natural Science Foundation of Ningxia (2019AAC03103, 2020AAC03239, and 2023AAC03304); and the Ningxia First-class Discipline and Scientific Research projects (Electronic Science and Technology, No. NXYLXK2017A07—DKPD2023C10 and DKPD2023D01).
Data Availability Statement
The original contributions presented in this study are included in the article. Further inquiries can be directed to the corresponding author(s).
Acknowledgments
The authors thank the Key Laboratory of North Minzu University (Physics and Photoelectric Information Functional Materials Sciences and Technology), the Ningxia Advanced Intelligent Perception Control Innovation Team, the Ningxia Acousto-optic Crystals Industrialization Innovation Team, and the Ningxia New Solid Electronic Materials and Devices Research and Development Innovation Team (2020CXTDLX12).
Conflicts of Interest
The author Xuefeng Zhang was employed by the company Ningxia Ju Jing Yuan Crystal Technology, Co., Ltd. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as potential conflicts of interest.
References
- Grein, M.E.; Kerman, A.J.; Dauler, E.A.; Shatrovoy, O.; Molnar, R.J.; Rosenberg, D.; Boroson, D.M. Design of a ground-based optical receiver for the lunar laser communications demonstration. In Proceedings of the 2011 International Conference on Space Optical Systems and Applications (ICSOS) IEEE, Santa Monica, CA, USA, 11–13 May 2011; pp. 78–82. [Google Scholar]
- Chen, P.; Wang, X.; Liu, B.; Yan, L.; Du, X.; Zhang, J.; Zhao, J. Cu-doped KTN crystal with controllable, reversible, and fast photochromic properties: A superior electro-optical material for improving beam deflection performance. Ceram. Int. 2024, 50, 32645–32654. [Google Scholar] [CrossRef]
- Yan, A.M.; Zhi, Y.N.; Sun, J.F.; Liu, L.R. Design and experiment of a large aperture digital beam deflector based on electro-optic crystal switch array. Appl. Phys. B 2012, 107, 421–427. [Google Scholar] [CrossRef]
- Ren, H.; Liu, L.; Song, Z.; Zhang, J.; Liu, D.A. Single-LiNbO3-slab-integrated 1xN electro-optic switch. In Photonic Devices and Algorithms for Computing V; SPIE: Bellingham, DC, USA, 2003; Volume 5201, pp. 180–189. [Google Scholar]
- Djukic, D.; Roth, R.; Yardley, J.T.; Osgood, R.M., Jr.; Bakhru, S.; Bakhru, H. Low-voltage planar-waveguide electrooptic prism scanner in Crystal-Ion-Sliced thin-film LiNbO3. Opt. Express 2004, 12, 6159–6164. [Google Scholar] [CrossRef] [PubMed]
- Bosco, A.; Boogert, S.T.; Boorman, G.E.; Blair, G.A. A large aperture electro-optic deflector. Appl. Phys. Lett. 2009, 94, 211104. [Google Scholar] [CrossRef]
- Yang, F.; Wu, Y.; Cai, C.; Fang, H. Designing an Electro-Optical Tunable Racetrack Microring Resonator on a Diamond–Lithium Niobate Thin-Film Hybrid Platform. Electronics 2003, 12, 4616. [Google Scholar] [CrossRef]
- Xiong, X.; Cao, Q.-T.; Xiao, Y.-F. Thin-film lithium niobate photonic integrated devices: Advances and oppotunities. Acta Phys. Sin. 2023, 72. [Google Scholar] [CrossRef]
- Ganesamoorthy, S.; Nakamura, M.; Takekawa, S.; Kumaragurubaran, S.; Terabe, K.; Kitamura, K. A comparative study on the domain switching characteristics of near stoichiometric lithium niobate and lithium tantalate single crystals. Mater. Sci. Eng. B 2005, 120, 125–129. [Google Scholar] [CrossRef]
- Xiao, X.; Si, J.; Liang, S.; Xu, Q.; Zhang, H.; Ma, L.; Zhang, X. Preparation, Properties, and Applications of Near Stoichiometric Lithium Tantalate Crystals. Crystals 2023, 13, 1031. [Google Scholar] [CrossRef]
- Tan, S.F.; Li, J.T.; Zhang, L. Progress in the preparation of near stoichiometric ratio lithium niobate crystals. Sci. Technol. Inf. 2009, 11, 6. [Google Scholar]
- Furukawa, Y. In Proceedings of the 1st NIMS International Conference on Material Solutions for Photonics Program. Tsukuba, Japan, 14–16 October 2003; pp. 83–84. [Google Scholar]
- Furukawa, Y.; Sato, M.; Kitamura, K.; Nitanda, F. Growth and characterization of off-congruent LiNbO3 single crystals grown by the double crucible method. J. Cryst. Growth 1993, 128, 909–914. [Google Scholar] [CrossRef]
- Yatsenko, A.; Yevdokimov, S.; Palatnikov, M.; Sidorov, N. NMR Spectra Particularities in LiNbO3 Crystals with a Near-Stoichiometric Composition. Ceramics 2023, 6, 432–446. [Google Scholar] [CrossRef]
- Palatnikov, M.; Sidorov, N.; Kadetova, A.; Titov, R.; Biryukova, I.; Makarova, O.; Efremov, I. Growing, structure and optical properties of LiNbO3: B crystals, a material for laser radiation transformation. Materials 2023, 16, 732. [Google Scholar] [CrossRef]
- Greshnyakov, E.D.; Lisjikh, B.I.; Akhmatkhanov, A.R.; Shur, V.Y. Charged domain walls in lithium niobate and lithium tantalate crystals with composition gradients. Ferroelectrics 2023, 604, 32–39. [Google Scholar] [CrossRef]
- Lengyel, K.; Péter, Á.; Kovács, L.; Corradi, G.; Pálfalvi, L.; Hebling, J.; Polgar, K. Growth, defect structure, and THz application of stoichiometric lithium niobate. Appl. Phys. Rev. 2015, 2, 40601. [Google Scholar] [CrossRef]
- Bhatt, R.; Bhaumik, I.; Ganesamoorthy, S.; Karnal, A.K.; Gupta, P.K.; Swami, M.K.; Upadhyay, A. Study of structural defects and crystalline perfection of near stoichiometric LiNbO3 crystals grown from flux and prepared by VTE technique. J. Mol. Struct. 2014, 1075, 377–383. [Google Scholar] [CrossRef]
- Elkus, B.S.; Abdelsalam, K.; Rao, A.; Velev, V.; Fathpour, S.; Kumar, P.; Kanter, G.S. Generation of broadband correlated photon-pairs in short thin-film lithium-niobate waveguides. Opt. Express 2019, 27, 38521–38531. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Sun, D.; Liu, Q.; Song, Y.; Zhang, F.; Zhou, W.; Liu, H. Growth of large size near-stoichiometric lithium niobate single crystals with low coercive field for manufacturing high quality periodically poled lithium niobate. Opt. Mater. 2022, 125, 112058. [Google Scholar] [CrossRef]
- Sun, J.; Kong, Y.; Zhang, L.; Yan, W.; Wang, X.; Xu, J.; Zhang, G. Growth of large-diameter nearly stoichiometric lithium niobate crystals by continuous melt supplying system. J. Cryst. Growth 2006, 292, 351–354. [Google Scholar] [CrossRef]
- Furukawa, Y.; Kitamura, K.; Ji, Y.; Montemezzani, G.; Zgonik, M.; Medrano, C.; Günter, P. Photorefractive properties of iron-doped stoichiometric lithium niobate. Opt. Lett. 1997, 22, 501–503. [Google Scholar] [CrossRef]
- Baron, C.; Cheng, H.; Gupta, M.C. Domain inversion in LiTaO3 and LiNbO3 by electric field application on chemically patterned crystals. Appl. Phys. Lett. 1996, 68, 481–483. [Google Scholar] [CrossRef]
- Gahagan, K.T.; Scrymgeour, D.A.; Casson, J.L.; Gopalan, V.; Robinson, J.M. Integrated high-power electro-optic lens and large-angle deflector. Appl. Opt. 2001, 40, 5638–5642. [Google Scholar] [CrossRef]
- Gonzalez, M.; Margueron, S.; Murauskas, T.; Boulet, P.; Gauthier-Manuel, L.; Dulmet, B.; Bartasyte, A. Influence of parameters in vapor transport equilibration treatment on composition and homogeneity of LiTaO3 single crystals. Phys. Status Solidi A 2025, 222, 2400129. [Google Scholar] [CrossRef]
- Carruthers, J.R.; Peterson, G.E.; Grasso, M.; Bridenbaugh, P.M. Nonstoichiometry and crystal growth of lithium niobate. J. Appl. Phys. 1971, 42, 1846–1851. [Google Scholar] [CrossRef]
- Dong, Z.; Xu, Q.; Liang, S.; Si, J.; Wang, M.; Zhang, X.; He, J. Research on the Fabrication of X-Cut Near Stoichiometric Lithium Niobate Wafers. Crystals 2025, 15, 282. [Google Scholar] [CrossRef]
- Nakamura, M.; Takekawa, S.; Kumaragurubaran, S.; Kitamura, K. Curie temperature and [Li]/([Li]+[Nb]) ratio of near-stoichiometric LiNbO3 crystal grown from different Li-rich solutions. Jpn. J. Appl. Phys. 2008, 47, 3476. [Google Scholar] [CrossRef]
- Kovács, L.; Ruschhaupt, G.; Polgár, K.; Corradi, G.; Wöhlecke, M. Composition dependence of the ultraviolet absorption edge in lithium niobate. Appl. Phys. Lett. 1997, 70, 2801–2803. [Google Scholar] [CrossRef]
- Liang, L. Research of Up-Conversion Single-Photon Detectors Based on Periodically Poled Lithium Niobate Waveguides. Ph.D. Thesis, Shandong University, Jinan, China, 2019. [Google Scholar]
- Gopalan, V.; Mitchell, T.E.; Furukawa, Y.; Kitamura, K. The role of nonstoichiometry in 180° domain switching of LiNbO3 crystals. Appl. Phys. Lett. 1998, 72, 1981–1983. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).