Evaluation of Pre-Sterilization Cleaning Protocols on Endodontic Files Using SEM: Effects on Elemental Composition and Surface Roughness
Abstract
1. Introduction
2. Materials and Methods
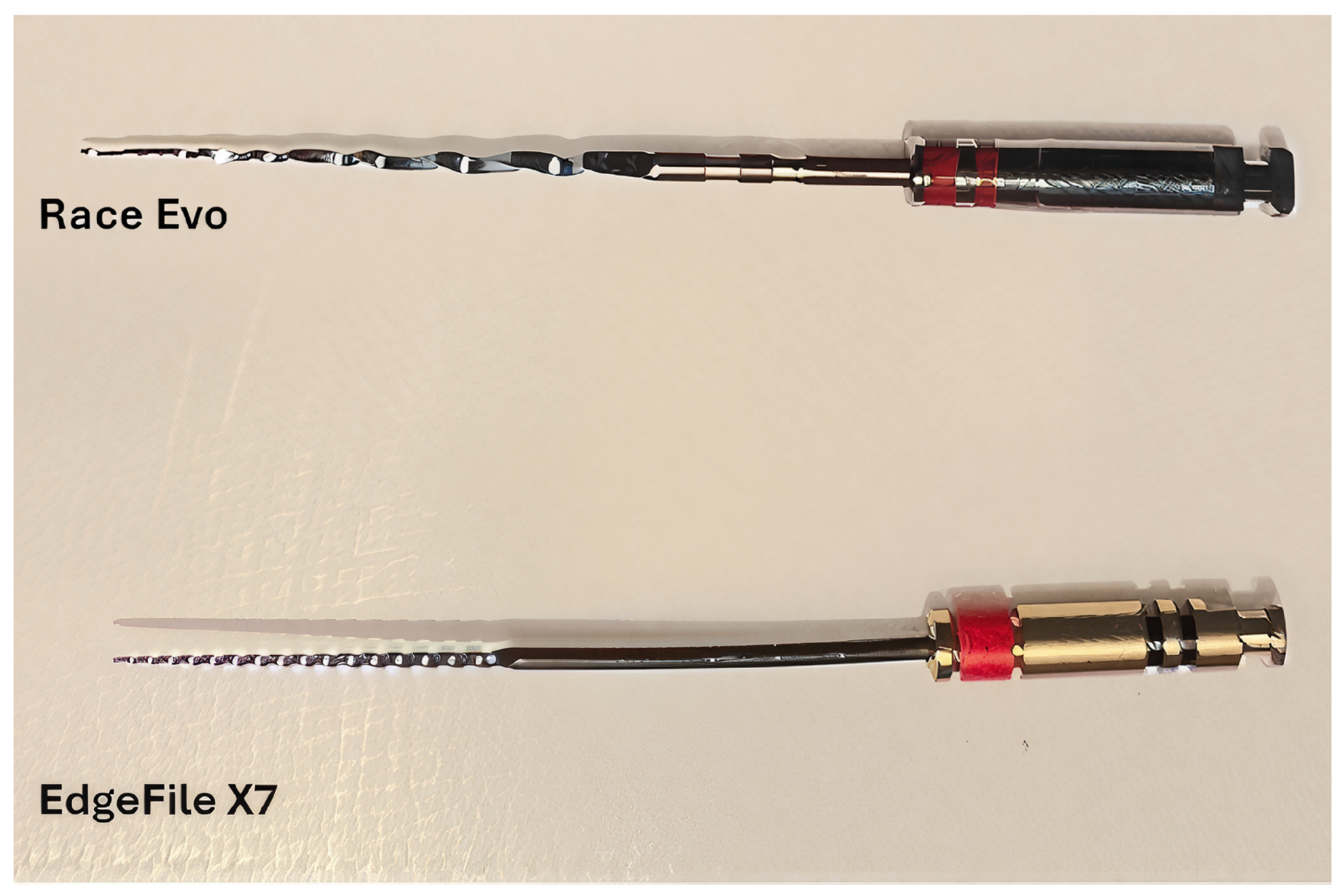
2.1. Sample Size and Preparation
- Group 1 (Negative Control): Sterilized, unused files.
- Group 2 (Positive Control): Used files sterilized without pre-cleaning.
- Group 3: Files soaked in 30 mL of 5.25% sodium hypochlorite (NaOCl) for 5 min.
- Group 4: Files wiped with 100% ethanol without magnification. Using a small, soaked sponge, a single operator wiped the files using synchronized up-and-down movements coupled with screwing movements. If debris was still observed, the process was repeated.
- Group 5: Files wiped with 100% ethanol using 3.5× magnification dental loupes (JTL MEDIPLUS, Seoul, Republic of Korea). Using a small, soaked sponge, a single operator wiped the files using 5 up-and-down movements coupled with screwing-in movements. If debris was still observed, the process was repeated.
- Group 6: Files coated with enzymatic spray gel (Hu-Friedy, Chicago, IL, USA) for 5 min.
- Group 7: Files soaked in enzymatic instrument cleaner (Dürr Dental, Bietigheim-Bissingen, Germany) 2% solution. Files were placed in 30 mL of the solution for 5 min following manufacturer instructions [19].
2.2. SEM and EDX Analysis
2.3. Surface Roughness Analysis
- Rsk (Surface Skewness): This reflects the asymmetry of the surface profile and indicates whether the surface has more peaks or valleys [21].
2.4. Statistical Analysis
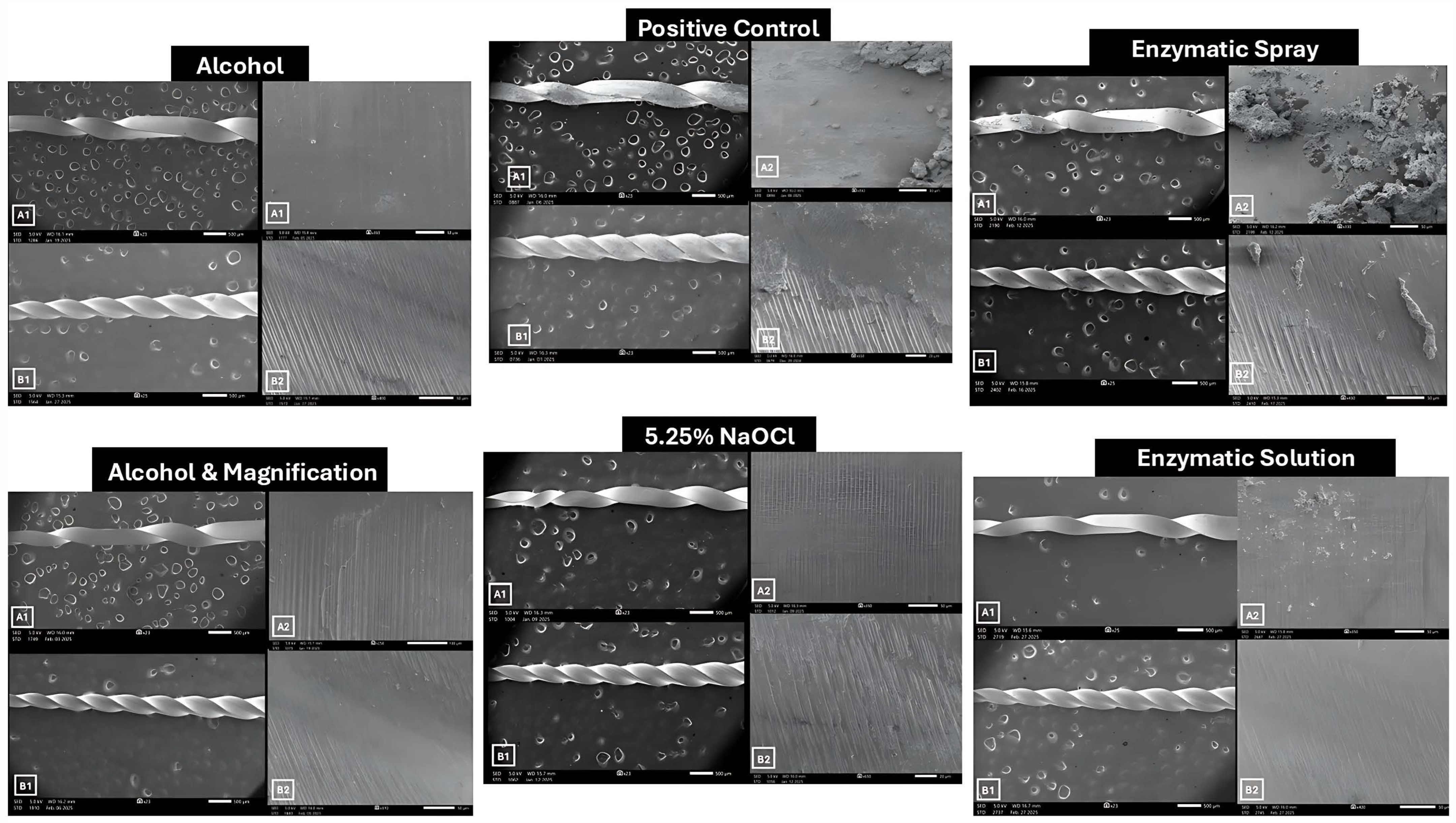
3. Results
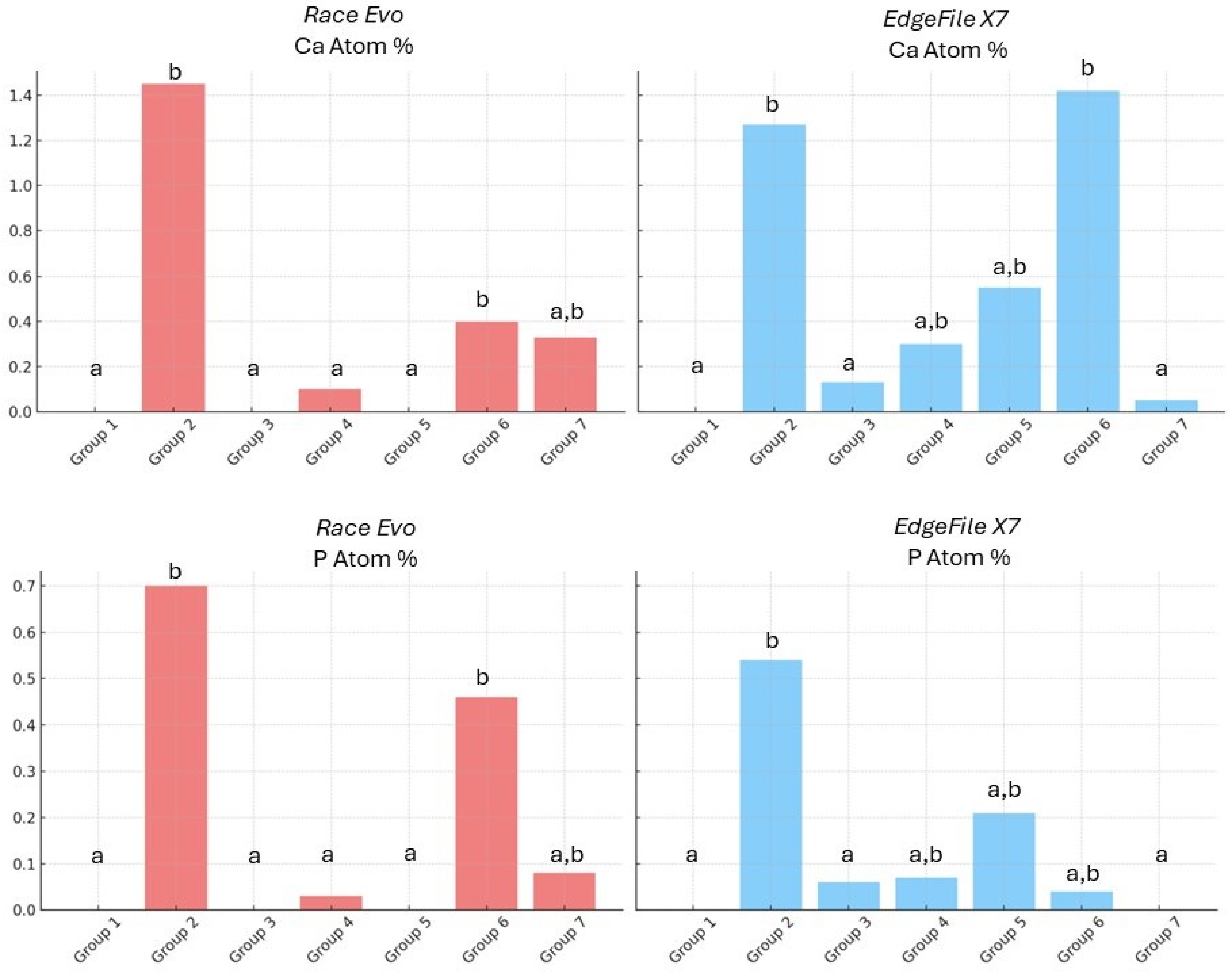
3.1. EDX Elemental Analysis
3.2. Surface Roughness
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Rutala, W.A.; Weber, D.J. Disinfection and Sterilization: An Overview. Am. J. Infect. Control 2013, 41, S2–S5. [Google Scholar] [CrossRef]
- Spaulding, E. Chemical Disinfection of Medical and Surgical Materials. In Disinfection, Sterilization, and Preservation; Lea & Febiger: Philadelphia, PA, USA, 1968; pp. 517–531. ISBN 0683307401. [Google Scholar]
- Dioguardi, M.; Laneve, E.; Di Cosola, M.; Cazzolla, A.P.; Sovereto, D.; Aiuto, R.; Laino, L.; Leanza, T.; Alovisi, M.; Troiano, G.; et al. The Effects of Sterilization Procedures on the Cutting Efficiency of Endodontic Instruments: A Systematic Review and Network Meta-Analysis. Materials 2021, 14, 1559. [Google Scholar] [CrossRef]
- Sureshchandra, B.; Malik, M.; Naik, R. Endodontic Files: Are Routine Sterilization Procedures Effective? Endodontology 2011, 23, 47. [Google Scholar] [CrossRef]
- Reams, G.J.; Baumgartner, J.C.; Kulid, J.C. Practical Application of Infection Control in Endodontics. J. Endod. 1995, 21, 281–284. [Google Scholar] [CrossRef]
- Jovanovic-Medojevic, M.; Opacic-Galic, V.; Geler, K.; Pavlica, D. Comparison of the Efficiency of Cleaning and Disinfection Protocols for Hand Endodontic Instruments. Vojnosanit. Pregl. 2023, 80, 596–603. [Google Scholar] [CrossRef]
- Popovic, J.; Gasic, J.; Zivkovic, S.; Petrovic, A.; Radicevic, G. Evaluation of Biological Debris on Endodontic Instruments after Cleaning and Sterilization Procedures. Int. Endod. J. 2010, 43, 336–341. [Google Scholar] [CrossRef]
- Khullar, P.; Raisingani, D.; Gupta, S.; Bishen, K.A. Evaluation of Biological Debris on Reusable Endodontic Instruments Subjected to Different Cleaning Methods Prior to Sterilization. Int. J. Infect. Control 2013, 9, 2. [Google Scholar] [CrossRef]
- Morrison, A.; Conrod, S. Dental Burs and Endodontic Files: Are Routine Sterilization Procedures Effective? Tex. Dent. J. 2010, 127, 295–300. [Google Scholar]
- Ziauddin, S.; Bhandary, S.; Pramod, J.; Srinivasan, R.; Mahesh, M. A Comparative Evaluation of the Effectiveness of Different Cleaning Protocols on Removal of Biological Debris on Endodontic Instruments—An in Vitro Study. Endodontology 2013, 25, 19. [Google Scholar] [CrossRef]
- Parashos, P.; Linsuwanont, P.; Messer, H. A Cleaning Protocol for Rotary Nickel-titanium Endodontic Instruments. Aust. Dent. J. 2004, 49, 20–27. [Google Scholar] [CrossRef]
- Generali, L.; Generali, P.; Bertani, P.; Cavani, F.; Checchi, V.; Filippini, T.; Veneri, F. Quantitative Evaluation of Debris Removal from NiTi Rotary Endodontic Instruments After Different Cleaning Procedures. Dent. J. 2025, 13, 49. [Google Scholar] [CrossRef]
- Linsuwanont, P.; Parashos, P.; Messer, H.H. Cleaning of Rotary Nickel–Titanium Endodontic Instruments. Int. Endod. J. 2004, 37, 19–28. [Google Scholar] [CrossRef]
- Arruda-Vasconcelos, R.; Chantre, L.G.F.; Lopes, R.S.C.; Lopes, C.C.; Barbosa-Ribeiro, M.; Gomes, B.P.F.D.A. Application of Forensic Luminol for Blood Detection in Endodontic Files. Rev. Odontol. UNESP 2017, 46, 227–231. [Google Scholar] [CrossRef]
- Cayo-Rojas, C.F.; Brito-Ávila, E.; Aliaga-Mariñas, A.S.; Hernández-Caba, K.K.; Saenz-Cazorla, E.D.; Ladera-Castañeda, M.I.; Cervantes-Ganoza, L.A. Cleaning of Endodontic Files with and without Enzymatic Detergent by Means of the Manual Method versus the Ultrasonic Method. J. Int. Soc. Prev. Community Dent. 2021, 11, 307–315. [Google Scholar] [CrossRef]
- Zinelis, S.; Eliades, T.; Eliades, G. A Metallurgical Characterization of Ten Endodontic Ni-Ti Instruments: Assessing the Clinical Relevance of Shape Memory and Superelastic Properties of Ni-Ti Endodontic Instruments. Int. Endod. J. 2010, 43, 125–134. [Google Scholar] [CrossRef]
- RaceEvo|FKG Dentaire RaceEvo|FKG Dentaire. Available online: https://fkg.ch/download-center/ (accessed on 31 May 2025).
- Anonymous. Extremely Flexible Files. Br. Dent. J. 2021, 231, 311. [Google Scholar] [CrossRef]
- Dürr Dental. Available online: https://www.duerrdental.com/en/products/hygiene/hygiene-preparations/instruments/id-212-instrument-disinfection/ (accessed on 2 January 2025).
- Eldik, D.V.; Zilm, P.S.; Rogers, A.H.; Marin, P.D. A SEM Evaluation of Debris Removal from Endodontic Files after Cleaning and Steam Sterilization Procedures. Aust. Dent. J. 2004, 49, 128–135. [Google Scholar] [CrossRef]
- Fuss, F.K. The Effect of Surface Skewness on the Super/Postcritical Coefficient of Drag of Roughened Cylinders. Procedia Eng. 2011, 13, 284–289. [Google Scholar] [CrossRef]
- Finishing Surface. Comparing Surface Roughness Units and Standards. Available online: https://finishingsurface.com/comparing-surface-roughness-units-and-standards (accessed on 2 February 2025).
- Lopes, N.I.D.A.; Silva, L.Á.D.O.; Santos, L.D.A.; Buono, V.T.L. Surface Characterization of NiTi Superelastic and Shape Memory Alloys After Electrolytic Polishing. Mater. Res. 2017, 20, 572–579. [Google Scholar] [CrossRef]
- Plotino, G.; Costanzo, A.; Grande, N.M.; Petrovic, R.; Testarelli, L.; Gambarini, G. Experimental Evaluation on the Influence of Autoclave Sterilization on the Cyclic Fatigue of New Nickel-Titanium Rotary Instruments. J. Endod. 2012, 38, 222–225. [Google Scholar] [CrossRef]
- Koskinen, K.P.; Stenvall, H.; Uitto, V. Dissolution of Bovine Pulp Tissue by Endodontic Solutions. Eur. J. Oral Sci. 1980, 88, 406–411. [Google Scholar] [CrossRef]
- Hand, R.E.; Smith, M.L.; Harrison, J.W. Analysis of the Effect of Dilution on the Necrotic Tissue Dissolution Property of Sodium Hypochlorite. J. Endod. 1978, 4, 60–64. [Google Scholar] [CrossRef]
- Stokes, O.W.; Di Fiore, P.M.; Barss, J.T.; Koerber, A.; Gilbert, J.L.; Lautenschlager, E.P. Corrosion in Stainless-Steel and Nickel-Titanium Files. J. Endod. 1999, 25, 17–20. [Google Scholar] [CrossRef]
- Edie, J.W.; Andreasen, G.F.; Zaytoun, M.P. Surface Corrosion of Nitinol and Stainless Steel Under Clinical Conditions. Angle Orthod. 1981, 51, 319–324. [Google Scholar]
- Han-Hsing Lin, J.; Karabucak, B.; Lee, S.-M. Effect of Sodium Hypochlorite on Conventional and Heat-Treated Nickel-Titanium Endodontic Rotary Instruments—An in Vitro Study. J. Dent. Sci. 2021, 16, 738–743. [Google Scholar] [CrossRef]
- Uslu, G.; Özyürek, T.; Yılmaz, K. Effect of Sodium Hypochlorite and EDTA on Surface Roughness of HyFlex CM and HyFlex EDM Files. Microsc. Res. Tech. 2018, 81, 1406–1411. [Google Scholar] [CrossRef]
- Sood, K.; Mohan, B.; Lakshminarayanan, L. Effect of Cleaning and Sterilization Procedures on Niti Rotary Files—An Sem and Eds Study. Endodontology 2006, 18, 34. [Google Scholar] [CrossRef]
- EdgeEndo File X7. Available online: https://web.edgeendo.com/edgefile-x7/ (accessed on 6 January 2025).
- Almohareb, R.A.; Barakat, R.; Albakri, A.; Altamimi, M. Effect of Autoclaving Cycles on the Cyclic Fatigue Resistance of Race and Race Evo Nickel-Titanium Endodontic Rotary Files: An In Vitro Study. Metals 2021, 11, 1947. [Google Scholar] [CrossRef]
- Liang, Y.; Yue, L. Evolution and Development: Engine-Driven Endodontic Rotary Nickel-Titanium Instruments. Int. J. Oral Sci. 2022, 14, 12. [Google Scholar] [CrossRef]
- Faus-Llácer, V.; Hamoud-Kharrat, N.; Marhuenda Ramos, M.T.; Faus-Matoses, I.; Zubizarreta-Macho, Á.; Ruiz Sánchez, C.; Faus-Matoses, V. Influence of the Geometrical Cross-Section Design on the Dynamic Cyclic Fatigue Resistance of NiTi Endodontic Rotary Files—An In Vitro Study. J. Clin. Med. 2021, 10, 4713. [Google Scholar] [CrossRef]
- Ruiz-Sánchez, C.; Faus-Llácer, V.; Faus-Matoses, I.; Zubizarreta-Macho, Á.; Sauro, S.; Faus-Matoses, V. The Influence of NiTi Alloy on the Cyclic Fatigue Resistance of Endodontic Files. J. Clin. Med. 2020, 9, 3755. [Google Scholar] [CrossRef]
- Barakat, R.M.; Almohareb, R.A.; Algahtani, F.N.; Altamimi, A.A.; Alfuraih, J.I.; Bahlol, L.S.; Jamleh, A. Comprehensive Characterization of Blue Wire NiTi File Failure: A Comparative Analysis of Cyclic Fatigue and Torsional Resistance Properties. Coatings 2024, 14, 361. [Google Scholar] [CrossRef]
- Guandalini, B.; Vendramini, I.; Leonardi, D.P.; Tomazinho, F.S.F.; Tomazinho, P.H. Comparative Analysis of Four Cleaning Methods of Endodontic Files. RSBO 2015, 11, 154–158. [Google Scholar] [CrossRef]
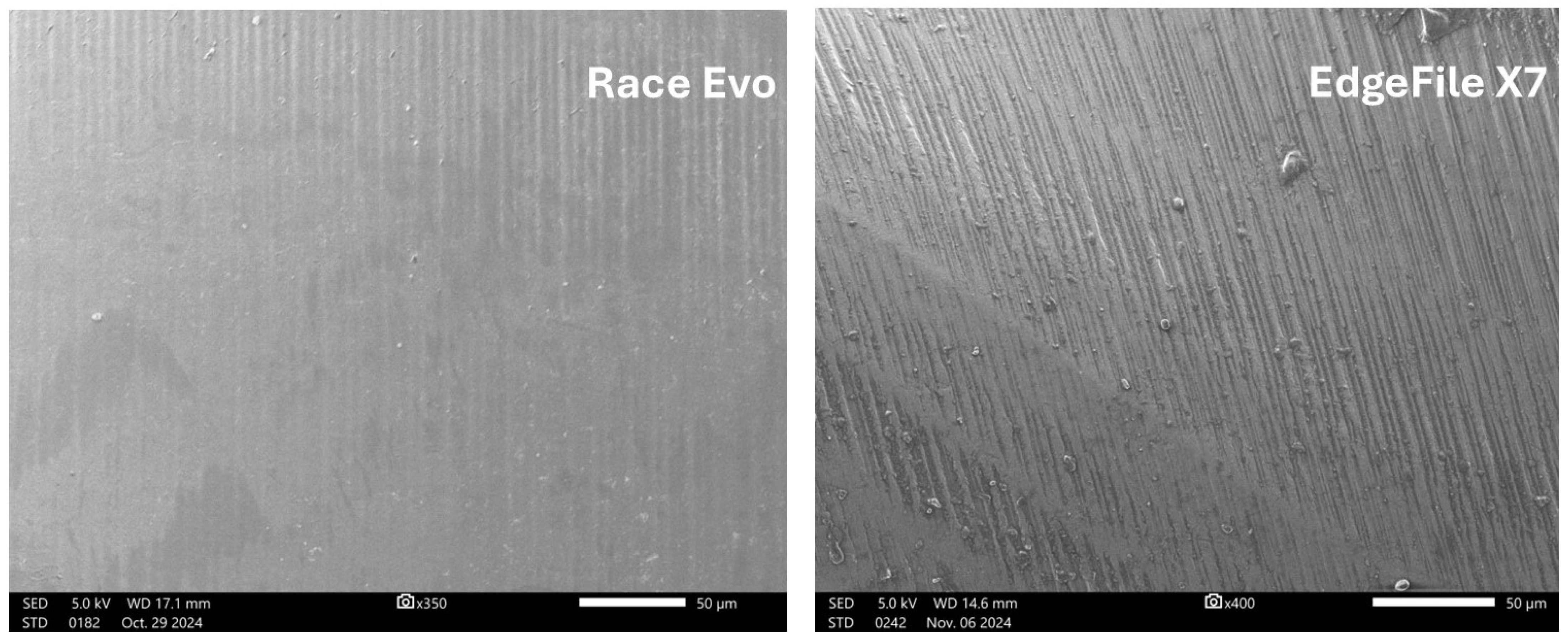
| File System | Group | Rq | Rsk | ||
|---|---|---|---|---|---|
| Mean ± SD | Median | Mean ± SD | Median | ||
| Race Evo | 1 | 22.69 ± 5.79 | 22.41 a | 0.41 ± 0.22 | 0.40 a |
| 2 | 30.51 ± 5.76 | 29.16 b | 0.58 ± 0.19 | 0.53 b | |
| 3 | 27.74 ± 6.77 | 28.52 a,b | 0.49 ± 0.13 | 0.44 a,b | |
| 4 | 29.26 ± 0.22 | 29.53 b | 0.45 ± 0.18 | 0.44 a,b | |
| 5 | 29.11 ± 6.62 | 29.11 a,b | 0.52 ± 0.15 | 0.52 a,b | |
| 6 | 35.28 ± 11.73 | 30.86 b | 0.57 ± 0.21 | 0.57 b | |
| 7 | 28.23 ± 5.76 | 29.06 a,b | 0.51 ± 0.17 | 0.53 a,b | |
| EdgeFile X7 | 1 | 31.98 ± 8.81 | 32.53 a | 0.44 ± 0.17 | 0.46 a |
| 2 | 31.87 ± 5.60 | 32.54 a | 0.70 ± 0.21 | 0.72 b | |
| 3 | 28.61 ± 7.67 | 28.67 a | 0.86 ± 0.15 | 0.88 b | |
| 4 | 28.98 ± 6.31 | 28.30 a | 0.76 ± 0.18 | 0.80 b | |
| 5 | 28.28 ± 5.99 | 26.78 a | 0.68 ± 0.18 | 0.72 b | |
| 6 | 30.28 ± 3.28 | 29.98 a | 0.75 ± 0.17 | 0.76 b | |
| 7 | 29.73 ± 5.13 | 30.34 a | 0.68 ± 0.16 | 0.67 b | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Almohareb, R.A.; Barakat, R.M.; Alzahrani, H.; Alkhattabi, R.; Alsaeed, R.; Faludah, S.; Alsaqat, R. Evaluation of Pre-Sterilization Cleaning Protocols on Endodontic Files Using SEM: Effects on Elemental Composition and Surface Roughness. Crystals 2025, 15, 684. https://doi.org/10.3390/cryst15080684
Almohareb RA, Barakat RM, Alzahrani H, Alkhattabi R, Alsaeed R, Faludah S, Alsaqat R. Evaluation of Pre-Sterilization Cleaning Protocols on Endodontic Files Using SEM: Effects on Elemental Composition and Surface Roughness. Crystals. 2025; 15(8):684. https://doi.org/10.3390/cryst15080684
Chicago/Turabian StyleAlmohareb, Rahaf A., Reem M. Barakat, Hadeel Alzahrani, Raghad Alkhattabi, Renad Alsaeed, Sarah Faludah, and Reem Alsaqat. 2025. "Evaluation of Pre-Sterilization Cleaning Protocols on Endodontic Files Using SEM: Effects on Elemental Composition and Surface Roughness" Crystals 15, no. 8: 684. https://doi.org/10.3390/cryst15080684
APA StyleAlmohareb, R. A., Barakat, R. M., Alzahrani, H., Alkhattabi, R., Alsaeed, R., Faludah, S., & Alsaqat, R. (2025). Evaluation of Pre-Sterilization Cleaning Protocols on Endodontic Files Using SEM: Effects on Elemental Composition and Surface Roughness. Crystals, 15(8), 684. https://doi.org/10.3390/cryst15080684






