3.1. Laser Annealing Technology
Technological features make laser optical annealing an extremely valuable tool in the production of many electronic components, especially thin films for solar cells, transistors, and other semiconductor devices. In addition, UV-IR optical annealing heat treatment can significantly improve the stress relief and electrical conductivity of ZnO-based samples. A key benefit of these optical annealing processes is their shorter annealing times in comparison to other techniques. Specifically, the 10.6 μm laser annealing technique is noteworthy for its superior deep penetration depth and energy control capabilities. When films are deposited on substrates, there may be a difference in the lattice characteristics of the substrates and thin films. Lattice mismatch, the difference in the dimensions of the crystal structures of the substrate and the thin film, causes unwanted strain or deformation during the thin film fabrication process. The presence of stress or deformation in the thin film can degrade its optical and electrical conductivity properties. The annealing process allows for improved performance of thin films after deposition, effectively reducing the stress at the film–substrate interface.
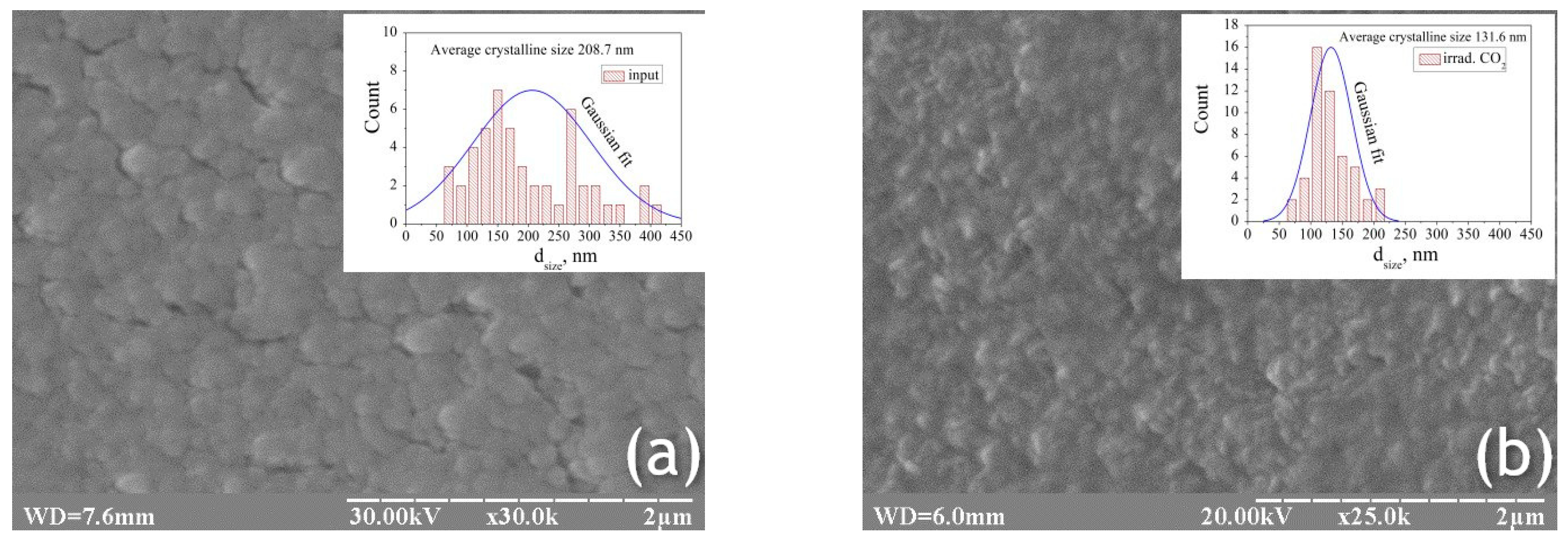
Figure 1a shows the results of microscopy of grown and laser-irradiated ZnNiO films on silicon substrate.
It is evident that each of the ZnNiO films consists of a single ZnO phase, exhibiting a high degree of orientation with the c-axis perpendicular to the substrate surface. ZnO crystallizes in the space group C6v4 (wurtzite) and has two atoms in the unit cell. The lowest surface energy density in the ZnO crystal has the (002) orientation. The tendency for crystal grains with low surface energy to increase in size drives the development of a growth orientation along the crystallographic direction with the lowest surface energy. The observation indicates that the textured film is established under conditions of effective thermodynamic equilibrium. Sufficient surface mobility for the contacting atoms is provided specifically in the (002) direction. Therefore, the film growth process occurs under certain deposition conditions. The images for laser annealing with a wavelength of λ = 10.64 μm in the intensity range I = 10–50 W/cm
2 were analyzed. There is a limit on the intensity of Zn nanocrystal formation (I
b = 50 W/cm
2) and destruction of the ZnO
x film (I > 100 W/cm
2). Our findings indicate that laser annealing in the studied range modifies the surface topology. Specifically, as illustrated in
Figure 1b, an intensity of 50 W/cm
2 during laser annealing results in the formation of nanoislands with a height of around 50 nm and maintains a consistent surface density distribution. The height of the nanoislands was estimated using the standard method of changing sharpness at points along the image depth. However, as a rule, a higher irradiation intensity forms higher nanoislands with lower density.
The preceding information suggests a direct interdependence among mismatch strain, lattice mismatch, and stress. Therefore, the stress was reduced through laser annealing, which decreased the lattice mismatch. Comparing images of different annealing processes, it follows that the crystallites slightly decreased in size after laser annealing. This was attributable to the effects induced by the annealing of the thin film using low-energy laser radiation. Multiple precipitation crystallite deposition of ZnNiO thin films absorbs laser radiation energy, and the crystallite size may also become smaller [
25]. As shown in
Figure 1b, CO
2 laser irradiation results in the features depicted in the diagram, indicating grain growth and a shift in the (002) peak. This peak shift is a consequence of the relaxation of internal stress within the irradiated films [
26]. All of the initial films grown in our experiment show some divergence. This demonstrates a correlation between the input energy and both the film deformation on the substrate and the grain size. These changes are also observed in annealed samples [
27]. With decreasing irradiation time, both grain sizes and absolute peak intensities increase, and film stresses decrease. Surprisingly, annealing ZnNiO thin films in air can lead to a reduction, instead of an elevation, of the oxygen concentration near the surface. This implies that variations in oxygen content could contribute to the stress reduction seen during annealing. Significant residual stresses are commonly present in deposited thin films. The main source of these stresses is internal stresses, which is determined by the density and structure of the film. It is likely that the high compressive stress inherent in these grown films may be the driving force for grain growth during the laser irradiation process. The compressive stress of the film arises from the high atomic packing density. It can also be a consequence of frozen crystallographic defects in the grains. Given that intragrain defects and grain boundaries have a greater free energy compared to the crystalline structure, grain growth occurs to minimize the system’s free energy.
Laser exposure supplies the thermal energy necessary to reduce the energy in thin layers. During irradiation, the following reactions are possible in them ZnO films: ZnO→ZnZn + VO + 1/2O2, ZnZn→Zni + VZn. Therefore, in irradiated ZnO films, we observed a decrease in stoichiometry, where oxygen vacancies acted as the main donors (VO) and zinc interstitials (Zni). Moreover, the application of sufficient thermal energy induced recrystallization in the ZnO thin films, resulting in enhanced quality. The electrical conductivity properties were also used as an indirect measure of the film’s stoichiometry. Laser irradiation affects the electrical properties of ZnNiO films, demonstrating a reduction in the band gap width. The band gap energy shift can result from the relaxation of mechanical stresses or an increase in defects during laser irradiation, specifically oxygen vacancies or interstitial Zn atoms. It is known that at temperatures close to room temperature, electrical conductivity is mainly the cause of the presence of defects associated with deep-level participation, such as interstitial Zn and oxygen vacancies. The conduction activation energy of ZnO material changes due to crystal defects (dislocations, grain boundaries, point defects). The chemical composition of ZnNiO films obtained by laser sputtering is non-stoichiometric and characterized by an excess of zinc. As a result, these films contain a significant number of lattice defects and surface defects. After irradiation with a CO2 laser, these non-radiative defects can be restructured. Such restructuring makes the crystal structure more perfect and leads to a decrease in the contribution of the conduction mechanism due to defects. Due to laser irradiation, the density of traps in the film is reduced and its crystalline quality is improved. In the case of laser irradiation, a deviation from ideal stoichiometry is observed.
3.2. Electrical Conductivity Properties of Nanostructured ZnNiO Thin Film
To determine the properties of thin films, we measured how their electrical characteristics change with temperature. The four-point probe method was employed to measure the electrical parameters and characteristics. We calculated the electrical conductivity (σ) of the samples from the measured electrical resistance (R).
Figure 2 illustrates the variation of ln(σ) with respect to the inverse temperature (1000/T). For comparison, the Arrhenius plot of a laser-irradiated ZnNiO thin film is shown alongside that of an unirradiated film in this figure. As follows from this, the semiconducting nature of all tested films is confirmed by the decrease in their resistance with increasing temperature, in the temperature range of 20–200 °C. The error in measuring electrical resistance was 1%. However, the temperature measurement error, which was 3% in this range, has a greater contribution to the non-monotonicity of the curves.
The room temperature electrical conductivity was measured to be a relatively high 6 × 10−2 S/cm. This research film is classified as an n-type semiconductor material. This n-type conductivity arises from the presence of inherent defects, specifically interstitial zinc atoms and oxygen vacancies. The increased conductivity in the investigated ZnNiO thin film can be understood by considering that the conductivity of ZnO films originates from electron capture at defects located along the grain boundaries. The observation that the estimated grain size (208 nm) of the synthesized nanostructured ZnNiO is greater than the Debye length (approximately 30 nm for nano ZnO) is indicative of electron accumulation at the grain boundaries. The observed enhancement in electrical conductivity can be attributed to the phenomenon of charge accumulation occurring at the grain boundaries.
The electrical conductivity of the ZnNiO film can be roughly analyzed using the following well-known relationship [
28]:
where σ—conductance, σ
0—pre-exponential factor, ΔE—activation energy governing electrical conductivity, T—temperature, and k—Boltzmann constant, respectively.
The graphs illustrate a two-stage temperature dependence of the electrical conductivity. To better describe the conductivity mechanism in zinc oxide, the conductivity curve is divided into three different ranges (I, II, and III). Linear region I (low-temperature region) refers to the linear conductivity mechanism, region II is the transition region, and linear region III represents the activation region involved in electrical conductivity. To analyze the electrical conductivity in these areas, the Arrhenius equation can be used, which describes the dependence of electrical conductivity on temperature. The values of E were calculated for regions I and III, respectively, from the linear slopes of the Arrhenius dependence in
Figure 2a. The two different conduction mechanisms correspond to two different donor levels at different bandgap positions, which likely explains the observation of the two slopes. Regions I and III correspond to different conduction mechanisms, and in these regions the electrical conductivity increases with increasing temperature. In turn, more and more charge carriers overcome the energy barrier and can be estimated using the Arrhenius equation, which relates electrical conductivity and temperature; this can be represented by the following expression:
where σ
0L and σ
0H—pre-exponential factors and ΔE
L and ΔE
H—low-temperature and high-temperature activation energies (for low-temperature and high-temperature stages of conductivity, respectively). The activation energies were calculated using the Arrhenius plots, and these values are presented in
Table 1 for region I (the low-temperature range of 300–400 K) and region III (the high-temperature range of 400–500 K). The obtained values of ΔE
L and ΔE
H were 71 meV and 354 meV, respectively. We estimated the activation energy ΔE
H to be 354 meV for conduction. This value is much smaller than the optical band gap E
g, which is 3.30 eV. The activation energy values in different temperature regions (e.g., I and III) indicate the presence of two energy levels of donor nature. The values of 71 meV and 354 meV are associated with the energy levels of two shallow donors, respectively, in the band gap of the ZnO semiconductor. Thus, it is estimated that in region III, the electrical conductivity of the ZnNiO thin film is thermally activated by the excitation of electrons from the deeper donor level to the conduction band minimum. The transition region, region II, is characterized by ionization of additional charge carriers from the shallow donor level and ends with their depletion at the transition temperature (T
i). As a result, the electrical conductivity decreases with increasing temperature (
Figure 2b), until the activation of charge carriers from the relatively deeper donor level begins [
29]. The participation of the jump mechanism through energy barriers created by inhomogeneities (e.g., grain boundaries) at temperatures above room temperature exists, but its contribution is quite small.
In an irradiated ZnNiO thin film, the activation energy (ΔEL and ΔEH) decreases due to changes in electron density from donors. At higher temperatures, electrons gain enough thermal energy to move from the donor levels into the conduction band minimum. Clearly, the thermal activation observed in region I is attributed to deep donor ions. The displacement of O2− ions from their regular lattice sites is expected to result in the creation of oxygen vacancies or zinc interstitials. As donors, these vacancies can also cause lattice deformation to maintain charge neutrality. The measured activation energies for the two regions indicate different mechanisms of trapped or scattered ions. With increasing donor concentration, the activation energy will decrease, which explains the increase in atomic electroactive defects at crystal grain boundaries. Therefore, laser annealing causes a decrease in both the potential barrier height and the activation energy. The reduction in the barrier height at the grain boundaries facilitates charge transfer. However, in the transition region, electrons lack the energy to move from the donor levels to the conduction band. Therefore, conduction occurs due to the movement of electrons in the impurity band, which reduces the contribution of free electrons to the conductivity.
The low-temperature activation energy of ZnNiO samples is likely related to one of the following two donor ionization processes: oxygen vacancy, (V
O) V
O → V
+O + e
−; or zinc interstitial ionization, Zn + i → Zn
+ + i + e
−. The desorption of O
2 particles, which can be described by the reaction O
2− → O
2 + e
−, can be a source of activation energy at high temperature. From DC conductivity studies, it is believed that the potential barrier, donor concentration, point defects, and oxygen adsorption–desorption control the conductivity of the ZnO system [
30].
3.3. Analysis of Laser Radiation Effects on Crystal Structure, Electrical Properties, and Photoelectric Properties
Laser irradiation had an impact on the electrical properties of ZnNiO films, resulting in increased conductivity. The electrical conductivity of thin films after laser irradiation increases significantly.
Table 1 shows the values of the specific electrical conductivity and activation energy for ZnNiO films.
The obtained values are consistent with the ΔE values found in the literature for ZnO [
31,
32,
33]. Laser irradiation causes a decrease in activation energy. The observed decrease in activation energy in the irradiated film can be explained by the increase in the concentration of vacancy-type defects. The increase in the concentration of cations leads to stronger localization of electrons, which directly reduces the activation energy due to the increase in the number of donor centers. In addition, the improved crystallinity of the films may also be a factor in this decrease [
34].
The absorption of laser energy occurs mainly due to local accumulations of the metal-containing phase (zinc). The absorbed energy induces defects down to the depth to which the photons reach [
35]. The estimation of the radiation absorption I
abs in the grain at parameters α = 120 cm
−1 [
24] I
abs = I
0ꞏ[1 − exp(−αd)] according to Beer’s law gives I
abs = 80 mV/cm
2, which is an unacceptably small value compared to the absorption at the grain boundaries. On the other hand, under given irradiation regimes, the diffusion outflow of atoms from the grain boundaries can be estimated by the expression of the diffusion length L
diff = (D
Znꞏt
imp)
1/2, which is a few nanometers for zinc (D
Zn = 1.07ꞏ10
−14 cm
2/s—diffusion coefficient for zinc below melting point [
36], t
imp = 1 s—irradiation pulse duration). That is, it can be stated that all processes occur at existing or potential (defect-enriched) grain boundaries. As a result of local heating, the grain boundaries become more pronounced. Since the heating–cooling cycle of the film is quite short, diffusion of atomic particles, or defects, is possible only on a nanometer scale [
37]. The changes caused by the laser are stable when measured again after a few weeks.
The findings from these measurements indicate that the properties of ZnO thin films are contingent upon several parameters, including their stoichiometry and crystallinity. A positive correlation was observed between the intensity of laser irradiation and the electrical conductivity. The trends evident in the experimental conductivity curves can be elucidated by variations in the concentration of electron traps within the film. The energy level and spatial distribution of these traps within the band gap also constitute contributing factors. Specifically, an increase in laser radiation intensity was associated with a decrease in the trap density.
3.4. Photocurrent
Figure 3a,b show the photoelectric current signal waveforms for a thin-film photodetector based on ZnNiO/Al
2O
3. The bias voltage is 10 V under ultraviolet illumination with a wavelength of 280 nm.
The dark currents are about 2.5 and 4.1 μA for the original and laser-annealed films, and the photocurrents are about 6.5 and 6.7 μA, respectively. The photocurrent increases rapidly and then gradually saturates (
Figure 4a). The decay of the photodetector’s photosignal under a 5 V bias is a rather lengthy process and is influenced by the structural quality of the film. The time-dependent behavior of the photocurrent for the identical thin-film structure is illustrated in
Figure 4b using a semi-logarithmic representation. The photocurrent characteristics look asymmetrical. Light exposure causes a gradual loss of oxygen from the ZnO surface, resulting in the development of a surface region characterized by an increasing density of oxygen vacancies (donors). Turning on the light causes the oxygen vacancies to donate electrons. By employing surface doping, electron concentration is accumulated in the surface region. In the absence of light, atmospheric oxygen reacts with the surface, causing the zinc-rich surface to gradually oxidize over time. When ZnO films are irradiated with ultraviolet light, free carriers (electron–hole pairs) are formed. The application of an external electric field causes the movement of these charges towards the electrodes. The polycrystalline structure of the ZnO film promotes the formation of capture centers. They can capture carriers before they are scattered and collected by the electrodes. Initially, there are a large number of capture centers that are slowly filled with carriers generated by ultraviolet light. Consequently, the photocurrent exhibits an increase with longer irradiation times, a phenomenon of carrier capture also referred to as the polarization effect.
The photocurrent exhibits an almost linear increase with increasing applied voltage, since the number of collected charge carriers is linearly proportional to the magnitude of the applied electric field. This observation aligns with a fundamental model explaining the photogeneration and collection of charge carriers [
38], and the photocurrent can be mathematically represented as
where q—electron charge, N
ph—number of incident photons per unit time, η
abs—optical absorption efficiency, μ·τ—product of the mobility of photogenerated charge carriers by the lifetime, d—interelectrode distance, and E—electric field applied to the thin film. The photoconductivity decay analysis indicates that the time constant for the photocurrent to fall to 20% of its maximum is approximately 39 min for the pristine film and extends to around 133 min for the CO
2-laser-treated film. Changes in the density of capture centers, which immobilize photogenerated carriers, explain this process. With a lower density of these centers, the probability of carrier recombination decreases, resulting in a prolonged photocurrent decay time. To determine the steady-state sensitivity of the film as a photoresistor, a charge carrier mobility of 80 cm
2/V·s was used in the calculation [
39]. These calculated values, as well as the estimated lifetime of the charge carriers generated within the film, are provided in
Table 2. The approximate value of the μ·τ parameter for the films was calculated to be (1.6 ÷ 1.0)·10
−7 cm
2/V using Equation (3). ZnNiO films’ photoconductivity parameters are also listed in
Table 2.
Therefore, a difference exists in the photocurrent decay behavior between the original and laser-annealed films. This difference can be explained by differences in their structural perfection. Since the grain boundaries are full of traps (defects or impurities) that trap free carriers, the irradiated film has a smaller grain size. After irradiation, the grain boundaries in the original become much larger. Correspondingly, the dark current increases with grain size, while the photocurrent changes only slightly.
Analyzing the temporal kinetics of the photocurrent provides a deeper understanding of the photoprocess. The rise and fall of the photocurrent for excitation with a 4.4 eV (280 nm) LED extends beyond the absorption band gap. At room temperatures, time-differentiated photocurrent dependences dI(t)/dt were plotted for a series of 20 s pulses of excitation of the LEDs. The photocurrent rise is initially relatively fast, passes through a maximum, and then, over a very long time, exhibits a slight decrease. The differentiated temporal kinetics (rise and decay) of the photocurrent under LED excitation are illustrated in
Figure 5a. For the decay of the collective of generated charge carriers, which follows exponential dependences of the form I = I
0ꞏexp(–t/τ), this representation creates peaks at t = τ. The number of peaks in these graphs corresponds to different relaxation times. By analyzing these curves, it is possible to determine the number of characteristic processes and their corresponding values of τ (time constants).
A positive average dI/dt value upon the onset of illumination confirms the photoconductive behavior of the heterostructure, signifying that the photocurrent increases with time when exposed to UV light. Conversely, the negative average dI/dt value assumes a decrease in photocurrent when the light source is turned off. This observation implies incomplete recombination of charge carriers, which subsequently contributes to the level of dark current associated with the photoconductive phenomenon. The observed differences in dI/dt values between the ON and OFF states reveal the photocurrent response’s sensitivity to light switching. Conversely, the low average dI/dt values for the ZnO structures indicate a slower photocurrent response and less efficient charge carrier movement. These results emphasize the significance of dI/dt behavior in understanding the dynamic photovoltaic characteristics and photoresponse of thin film structures.
The photoconductivity relaxation curves exhibit a stretched exponential behavior, indicating fluctuations in the potential barrier due to carrier trapping:
In Equation (4), I
0—value of saturation current, the value does not change over time; the elongation coefficient γ is within 0 < γ ≤ 1. Equation (4) describes how the relaxation time τ changes as the decay progresses over time t. The time dependence of the life expectancy parameter can be expressed by the following equation:
(τ
0-constant). Specifically, this parameter reflects the broadening of the curve, and its value is governed by the magnitude of the potential barrier fluctuations involved in carrier capture [
40]. In this particular case, we plotted the derivative of the photocurrent with respect to the logarithm of time: dI(t)/dln(t). Differentiation with respect to the parameter ln(t) yields peaks at t = τ (
Figure 5b), but these peaks exhibit asymmetry and some broadening. This deviates from the case of ideal exponential decay. The extension parameter γ can be quantitatively estimated by fitting the first derivative to the experimentally observed peak profile [
41,
42].
The relaxation process’s defining features are presented in
Figure 5a,b. When the photocurrent increases under 280 nm illumination, the excitation consists of three main processes: a rapid initial increase with a characteristic relaxation time of 0.13 s; a slower increase preceded by the passage of the photocurrent through a maximum value (relaxation time: 13 s); and, with further illumination, a prolonged decrease in the photocurrent (relaxation time: 207 s). The peaks are broad, broader than expected for simple exponential growth processes. The photocurrent initially decayed rapidly, exhibiting a characteristic decay time of 19 s, which is similar to its rapid rise time. Slow peaks can be approximately represented by stretched exponentials rather than simple exponentials, and the expansion factor was a = 0.5. Subsequent to the rapid initial decay, the photocurrent displays a prolonged tail, which is characterized by a time constant of roughly 128 s and is well-described by a stretched exponential function.
The detector structure exhibits a relatively high external quantum efficiency (EQE >> 1). This phenomenon is explained by the strong capture of one type of carrier. Such carriers are identified as holes in our system. This process increases the lifetime of the other type of charge carrier (electrons). These electrons circulate in the circuit and cause an increase in photocurrent. This represents a process that releases trapped charge carriers, making them available for recombination events. Under these conditions, the photocurrent is enhanced. Therefore, the experimental relaxation curves are stretched exponentials, not simple exponentials. This transport mechanism involves the operation of centers with a barrier to electron capture and/or a substantial potential for their fluctuations. Building a detailed model of recombination in semi-insulating photoconductive detectors requires additional research. The remarkably high photosensitivity and EQE exhibited by such photodetectors are attributable to the contribution of trapped photogenerated charge carriers, a phenomenon that inherently results in prolonged relaxation. Growth under Zn-enriched conditions suppresses the formation of VZn. A potential outcome of this is a decrease in trap concentration, which would subsequently diminish the hole trapping effect. However, this can in turn make it challenging to achieve high resistivity and low leakage current due to the increased contribution of zinc interstitials acting as donors.
The use of polycrystalline ZnO films with a high concentration of defects, particularly grain boundary traps, leads to a transient UV response in the photoconductor, characterized by long rise and fall times of the photocurrent. The increase in the fraction of the photocurrent due to the non-radiative decay mechanism can serve as additional direct evidence of this. In dark conditions, atmospheric O2 can adsorb on the ZnOx surface and extract free electrons with the formation of O2. To increase the photoresponse rate, a high-quality ZnO film with a large grain size should be used.