Inclusions, Chemical Composition, and Spectral Characteristics of Pinkish-Purple to Purple Spinels from Mogok, Myanmar
Abstract
1. Introduction
2. Regional Geological Setting
3. Materials and Methods
3.1. Materials
3.2. Methods
3.2.1. Conventional Gemological Features
3.2.2. Energy Dispersive X-Ray Fluorescence (EDXRF)
3.2.3. UV–Visible (UV–Vis) Spectrophotometry
3.2.4. Fourier Transform Infrared (FTIR) Spectroscopy
3.2.5. Raman Spectroscopy
3.2.6. Fluorescence Spectroscopy
4. Results and Discussion
4.1. Gemological Characteristics
4.2. Inclusions
4.3. Chemical Composition
4.4. Spectra Analysis
4.4.1. UV–Vis Spectra
4.4.2. Fluorescence Spectra
4.4.3. Raman Spectra
4.4.4. FTIR Spectra
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Malíčková, I.; Bačík, P.; Fridrichová, J.; Hanus, R.; Illášová, Ľ.; Štubňa, J.; Furka, D.; Furka, S.; Škoda, R. Optical and Luminescence Spectroscopy of Varicolored Gem Spinel from Mogok, Myanmar and Lục Yên, Vietnam. Minerals 2021, 11, 169. [Google Scholar] [CrossRef]
- Xu, Q.; Xu, B.; Gao, Y.; Li, S. Characterization of Red, Pink, Orange, and Purple Gem-Quality Spinel from Four Important Areas. Crystals 2024, 14, 50. [Google Scholar] [CrossRef]
- Zhang, L.; He, K.; Guo, Q.-F. Spectroscopic Characteristics and Coloring Mechanisms of Different Colored Spinels from Myanmar. Crystals 2023, 13, 575. [Google Scholar] [CrossRef]
- Wu, J.; Sun, X.; Ma, H.; Ning, P.; Tang, N.; Ding, T.; Li, H.; Zhang, T.; Ma, Y. Purple-Violet Gem Spinel from Tanzania and Myanmar: Inclusion, Spectroscopy, Chemistry, and Color. Minerals 2023, 13, 226. [Google Scholar] [CrossRef]
- Zhao, L.-P.; Li, G.; Weng, L.-Q. Gemological and Spectroscopic Characteristics of “Jedi” Spinel from Man Sin, Myanmar. Minerals 2022, 12, 1359. [Google Scholar] [CrossRef]
- Wang, M.-W.; Wang, M.-Y.; Qi, Y.-H.; Xue, Y.; Shi, G.-H. Chemical Composition and Spectral Characteristics of Different Colored Spinel Varieties from Myanmar. Minerals 2024, 14, 1124. [Google Scholar] [CrossRef]
- Liu, Y.-C.; Qi, L.-J.; Schwarz, D.; Zhou, Z.-Y. Color Mechanism and Spectroscopic Thermal Variation of Pink Spinel Reportedly from Kuh-i-Lal, Tajikistan. Gems Gemol. 2022, 58, 338–352. [Google Scholar] [CrossRef]
- Belley, P.-M.; Palke, A.-C. Purple Gem Spinel from Vietnam and Afghanistan: Comparison of Trace Element Chemistry, Cause of Color, and Inclusions. Gems Gemol. 2021, 57, 228–237. [Google Scholar] [CrossRef]
- Du, Y.; Guo, Y. Study on the Chromogenic Mechanism of Violet-Red to Orange-Red Spinel. Crystals 2023, 13, 339. [Google Scholar] [CrossRef]
- Zhao, Y.; Xu, B.; Zhao, Z.-Y.; Xu, Q.; Li, Z.-Y. Gemological and Chemical Characterization of Varicolored Gem-Grade Spinel from Mogok, Myanmar. Crystals 2023, 13, 447. [Google Scholar] [CrossRef]
- Hänsel, S.; Lhuaamporn, T.; Suphan, C. Gem Notes: Update on Purple Spinel from Afghanistan. J. Gemmol. 2021, 37, 678–680. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhu, J.-R.; Yu, X.-Y. A Comparative Study of the Gemological Characteristics and Inclusions in Spinels from Myanmar and Tajikistan. Crystals 2022, 12, 617. [Google Scholar] [CrossRef]
- Renfro, N.; Laurs, B.M. Spinel from Bawma, Myanmar. Gems Gemol. 2010, 46, 154. [Google Scholar]
- Phyo, M.M.; Bieler, E.; Franz, L.; Balmer, W.; Krzemnicki, M.S. Spinel from Mogok, Myanmar—A Detailed Inclusion Study by Raman Microspectroscopy and Scanning Electron Microscopy. J. Gemmol. 2019, 36, 418–435. [Google Scholar] [CrossRef]
- Pardieu, V. Hunting for “Jedi” Spinels in Mogok. Gems Gemol. 2014, 50, 46–57. [Google Scholar] [CrossRef]
- Mitchell, A.; Htay, M.-T.; Htun, K.-M.; Win, M.N.; Oo, T.; Hlaing, T. Rock relationships in the Mogok metamorphic belt, Tatkon to Mandalay, central Myanmar. J. Asian Earth Sci. 2007, 29, 891–910. [Google Scholar] [CrossRef]
- Searle, M.-P.; Noble, S.-R.; Cottle, J.-M.; Waters, D.J.; Mitchell, A.H.G.; Hlaing, T.; Horstwood, M.S.A. Tectonic evolution of the Mogok metamorphic belt, Burma (Myanmar) constrained by U-Th-Pb dating of metamorphic and magmatic rocks. Tectonics 2007, 26, 1–24. [Google Scholar] [CrossRef]
- Garnier, V.; Maluski, H.; Giuliani, G.; Ohnenstetter, D.; Schwarz, D. Ar–Ar and U–Pb ages of marble-hosted ruby deposits from central and southeast Asia. Can. J. Earth Sci. 2006, 43, 509–532. [Google Scholar] [CrossRef]
- Zaw, K.; Swe, W.; Barber, A.J.; Crow, M.J.; Nwe, Y.Y. Chapter 1: Introduction to the geology of Myanmar. Geol. Soc. Lond. Mem. 2017, 48, 1–17. [Google Scholar] [CrossRef]
- Zaw, K. Chapter 24: Overview of mineralization styles and tectonic–metallogenic setting in Myanmar. Geol. Soc. Lond. Mem. 2017, 48, 400–531. [Google Scholar] [CrossRef]
- Garnier, V.; Giuliani, G.; Ohnenstetter, D.; Fallick, A.E.; Dubessy, J.; Banks, D.; Vinh, H.Q.; Lhomme, T.; Maluski, H.; Pêcher, A.; et al. Marble-Hosted Ruby Deposits from Central and Southeast Asia: Towards a New Genetic Model. Ore Geol. Rev. 2008, 34, 169–191. [Google Scholar] [CrossRef]
- Gorghinian, A.; Mottana, A.; Rossi, A.; Oltean, F.M.; Esposito, A.; Marcelli, A. Investigating the color of Spinel: 1. Red Gem-Quality Spinels (“Balas”) from Ratnapura (Sri Lanka). Rend. Lincei 2013, 24, 127–140. [Google Scholar] [CrossRef]
- Malsy, A.; Klemm, L. Distinction of Gem Spinels from the Himalayan Mountain Belt. Mineralogy 2010, 64, 741–746. [Google Scholar] [CrossRef] [PubMed]
- Gubelin, E.J.; Koivula, J.I. Photoatlas of Inclusions in Gemstones; Gemological Inst of Amer: Carlsbad, CA, USA, 1986. [Google Scholar]
- Rankin, A.H.; Gubelin, E.J.; Koivula, J.I. Photoatlas of Inclusions in Gemstones. In Mineralogical Magazine; Opinio Publishers: Basel, Switzerland, 2005; Volume 2, p. 829. ISBN 3-03999029-2. [Google Scholar]
- Themelis, T. Gems and Mines of Mogok; A&T Publishers: Bangkok, Thailand, 2008. [Google Scholar]
- Pardieu, V.; Sangsawong, S.; Vertriest, W.; Raynaud, V. “Star of David” spinel twin crystal with multiphase inclusions from Mogok. Gems Gemmol. 2016, 52, 100–101. [Google Scholar]
- Peretti, A.; Mullis, J.; Franz, L.; de Capitani, C. Spinel formation by sulphur-rich saline brines from Mansin (Mogok area, Myanmar). In Proceedings of the 15th Swiss Geoscience Meeting, Davos, Switzerland, 17–18 November 2017. [Google Scholar]
- Zhu, J.; Yu, X. Inclusions of spinel from Burma. J. Gems Gemmol. 2018, 20, 18–23. [Google Scholar]
- Gunasekaran, S.; Anbalagan, G. Spectroscopic Characterization of Natural Calcite Minerals. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2007, 68, 656–664. [Google Scholar] [CrossRef] [PubMed]
- Giuliani, G.; Fallick, A.E.; Boyce, A.J.; Pardieu, V.; Pham, V.L. Pink and Red Spinels in Marble: Trace Elements, Oxygen Isotopes, and Sources. Can. Mineral. 2017, 55, 743–761. [Google Scholar] [CrossRef]
- Pardieu, V.; Hughes, R.W. Spinel: Resurrection of a Classic. InColor 2008, 2, 10–18. [Google Scholar]
- Sun, X.; Gao, Y.; Zhao, H. Interesting Inclusions in a Purple Spinel from Tanzania. J. Gemmol. 2022, 38, 315–317. [Google Scholar] [CrossRef]
- Fernando, G.W.A.R.; Pitawala, A.; Amaraweera, T.H.N.G. Emplacement and Evolution History of Pegmatites and Hydrothermal Deposits, Matale District, Sri Lanka. Int. J. Geosci. 2011, 02, 348–362. [Google Scholar] [CrossRef]
- Long, P.V.; Pardieu, V.; Giuliani, G. Update on Gemstone Mining in Luc Yen, Vietnam. Gems Gemmol. 2013, 49, 233–245. [Google Scholar] [CrossRef]
- Alonso-Perez, R.; Day, J.M.D.; Pearson, D.G.; Luo, Y.; Palacios, M.A.; Sudhakar, R.; Palke, A. Exploring Emerald Global Geochemical Provenance through Fingerprinting and Machine Learning Methods. Artif. Intell. Geosci. 2024, 5, 100085. [Google Scholar] [CrossRef]
- Guillong, M.; Günther, D. Quasi ‘non-destructive’ laser ablation-inductively coupled plasma-mass spectrometry fingerprinting of sapphires. Spectrochim. Acta Part B At. Spectrosc. 2001, 56, 1219–1231. [Google Scholar] [CrossRef]
- Dutta, R.; Mandal, N. Effects of Fe2+ and Zn2+ substitution on the structure and high-pressure stability of MgAl2O4 spinel from DFT calculations. J. Phys. Chem. Solids 2012, 73, 1099–1105. [Google Scholar] [CrossRef]
- Carta, D.; Marras, C.; Loche, D.; Mountjoy, G.; Ahmed, S.I.; Corrias, A. An X-ray absorption spectroscopy study of the inversion degree in zinc ferrite nanocrystals dispersed on a highly porous silica aerogel matrix. J. Chem. Phys. 2013, 138, 054702. [Google Scholar] [CrossRef] [PubMed]
- Andreozzi, G.B.; D’Ippolito, V.; Skogby, H.; Hålenius, U.; Bosi, F. Color mechanisms in spinel: A multi-analytical investigation of natural crystals with a wide range of coloration. Phys. Chem. Min. 2019, 46, 343–360. [Google Scholar] [CrossRef]
- Kleišmantas, A.; Daukšytė, A. The Influence of Vietnam and Sri Lanka Spinel Mineral Chemical Elements on color. Chemija 2016, 27, 45–51. [Google Scholar] [CrossRef]
- Chankhantha, C.; Amphon, R.; Rehman, H.U.; Shen, A.H. Characterisation of Pink-to-Red Spinel from Four Important Localities. J. Gemmol. 2020, 37, 393–403. [Google Scholar] [CrossRef]
- D’Ippolito, V.; Andreozzi, G.B.; Hålenius, U.; Skogby, H.; Hametner, K.; Günther, D. Color mechanisms in spinel: Cobalt and iron interplay for the blue color. Phys. Chem. Min. 2015, 42, 431–439. [Google Scholar] [CrossRef]
- Tang, J.; Guo, Y.; Zhang, J. Colorimetry characteristics and color contribution of fluorescence in natural Cr-containing spinel. Sci. Rep. 2023, 13, 2426. [Google Scholar] [CrossRef] [PubMed]
- Pluthametwisute, T.; Wanthanachaisaeng, B.; Saiyasombat, C.; Sutthirat, C. Minor Elements and Color Causing Role in Spinel: Multi-Analytical Approaches. Minerals 2022, 12, 928. [Google Scholar] [CrossRef]
- Kiryakov, A.N.; Zatsepin, A.F.; Osipov, V.V. Optical properties of polyvalent iron ions and anti-site defects in transparent MgAl2O4 ceramics. J. Lumin. 2021, 239, 118390. [Google Scholar] [CrossRef]
- Saeseaw, S.; Wang, W.; Scarratt, K.; Emmett, J.; Douthit, T. Distinguishing Heated Spinels from Unheated Natural Spinels and from Synthetic Spinels—A short review of on-going research. GIA Res. News 2009, 13. Available online: https://www.gia.edu/doc/Heated-spinel-Identification-at-April-02-2009.pdf (accessed on 10 July 2025).
- Wang, C.; Shen, A.H.; Liu, Y. Characterization of order-disorder transition in MgAl2O4: Cr3+ spinel using photoluminescence. J. Lumin. 2020, 227, 117552. [Google Scholar] [CrossRef]
- Hainschwang, T.; Karampelas, S.; Fritsch, E.; Notari, F. Luminescence spectroscopy and microscopy applied to study gem materials: A case study of C centre containing diamonds. Mineral. Petrol. 2013, 107, 393–413. [Google Scholar] [CrossRef]
- Preudhomme, J.; Tarte, P. Infrared studies of spinels—III: The normal II–III spinels. Spectrochim. Acta Part A Mol. Spectrosc. 1971, 27, 1817–1835. [Google Scholar] [CrossRef]
- Boguslavska, N.N.; Venger, E.F.; Vernidub, N.M.; Pasechnik, Y.A.; Shportko, K.V. Reststrahlen Spectroscopy of MgAl2O4 Spinel. Semicond. Phys. Quantum Electron. Optoelectron. 2002, 5, 95–100. [Google Scholar] [CrossRef]
- White, W.B.; DeAngelis, B.A. Interpretation of the Vibrational Spectra of Spinels. Spectrochim. Acta Part Mol. Spectrosc. 1967, 23, 985–995. [Google Scholar] [CrossRef]


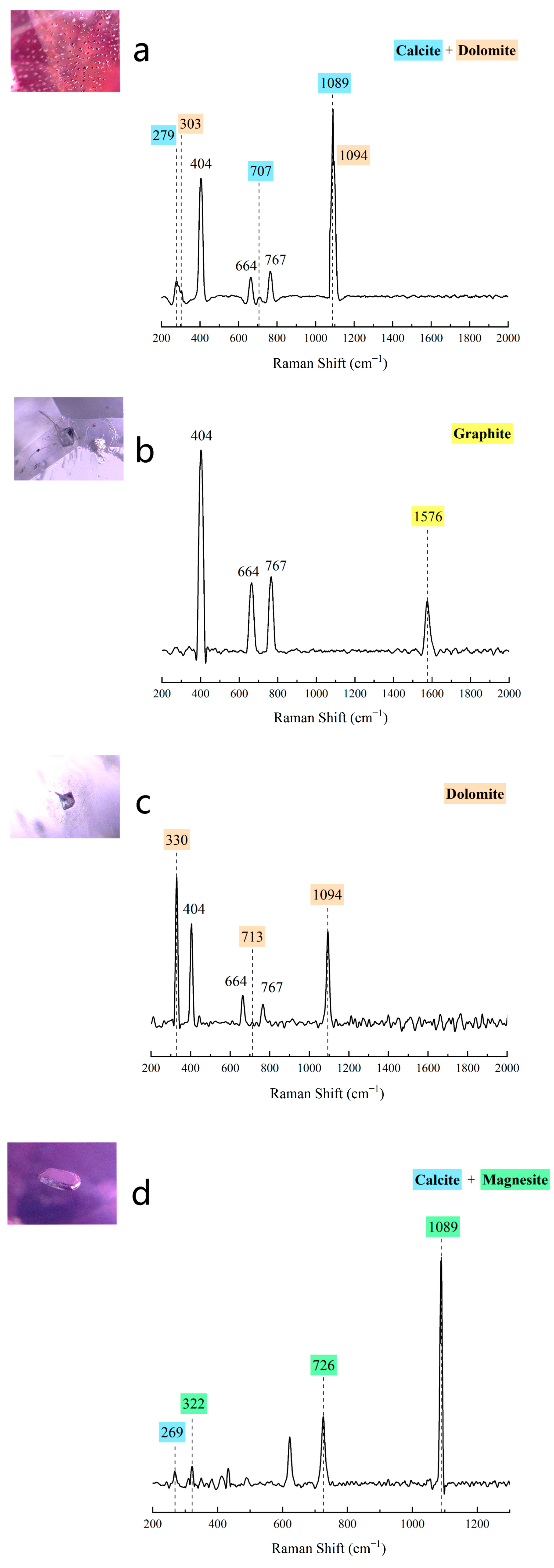




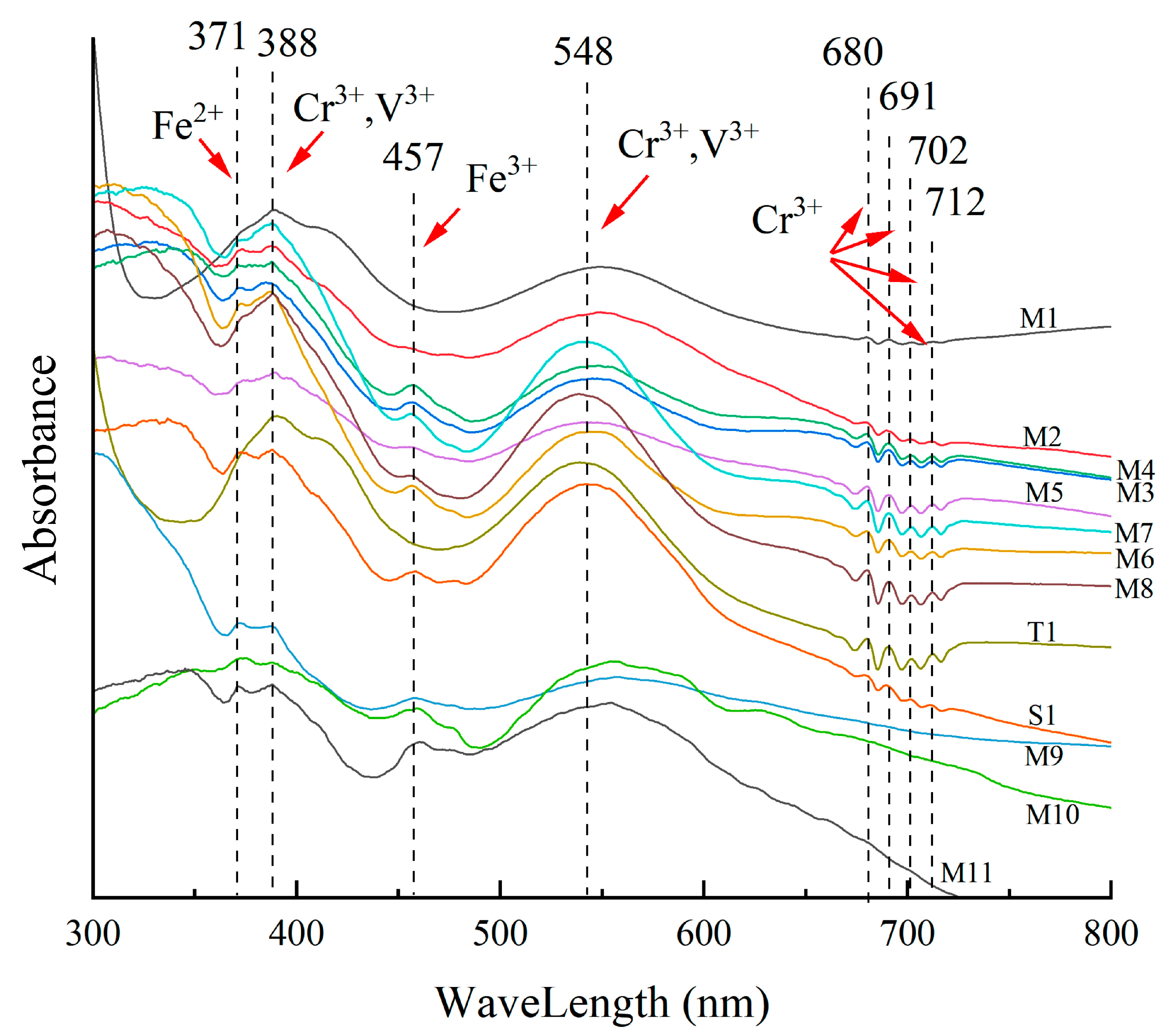



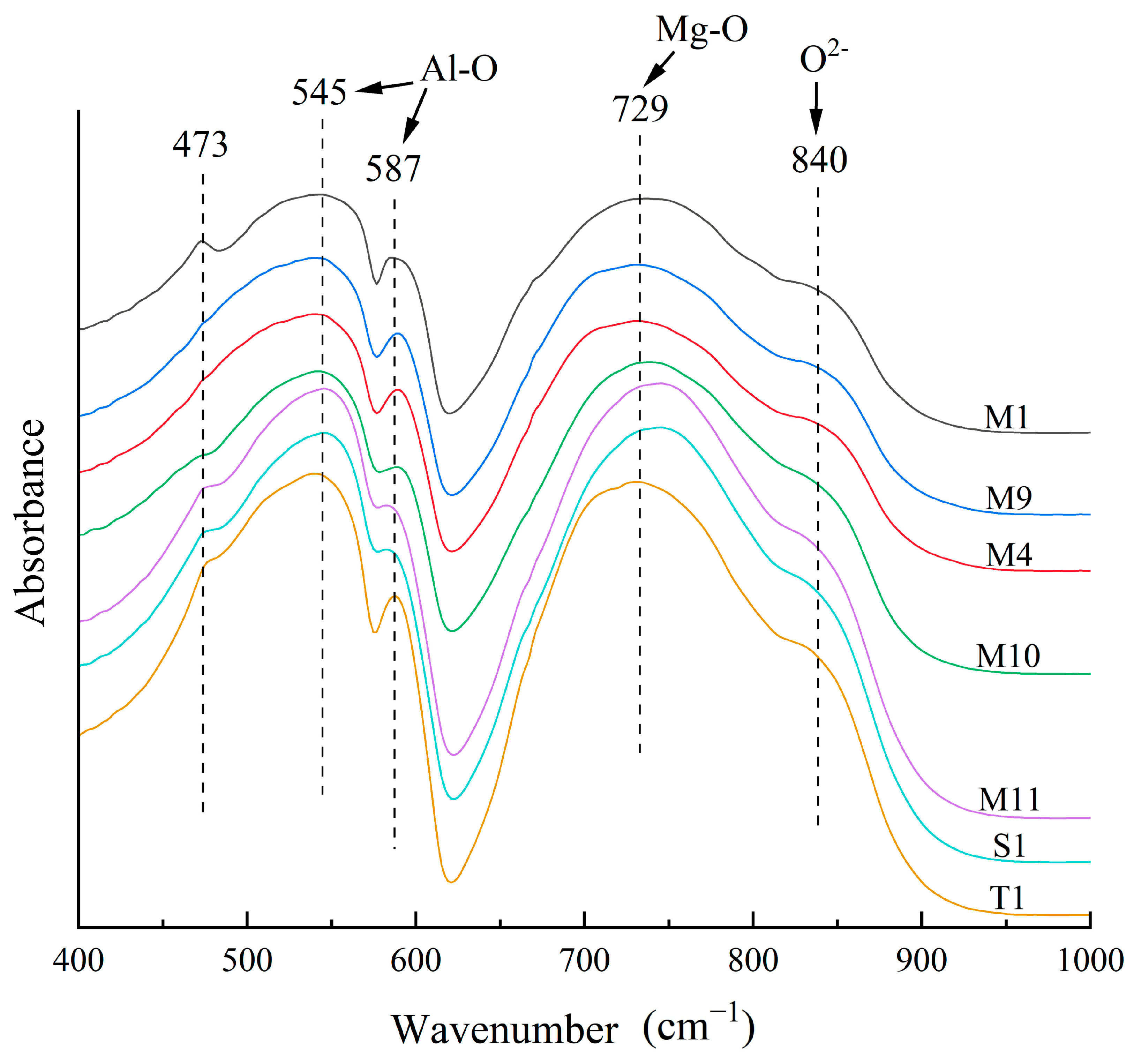
| Sample | Color | SG | RI | LWUV | SWUV |
|---|---|---|---|---|---|
| Pinkish-purple spinels | |||||
| M1 | very light pinkish-purple | 3.549 | 1.711 | red, medium | red, weak |
| M5 | light pinkish-purple | 3.527 | 1.718 | red, medium | inert |
| M6 | light pinkish-purple | 3.592 | 1.716 | red, weak | inert |
| T1 | light pinkish-purple | 3.586 | 1.713 | red, strong | inert |
| M7 | deep pinkish-purple | 3.579 | 1.714 | red, medium | inert |
| M8 | deep pinkish-purple | 3.572 | 1.713 | red, strong | inert |
| Purple spinels | |||||
| M9 | very light purple | 3.522 | 1.714 | inert | inert |
| M2 | light grayish-purple | 3.542 | 1.713 | red, weak | inert |
| M3 | light grayish-purple | 3.583 | 1.715 | red, weak | inert |
| M4 | light grayish-purple | 3.560 | 1.717 | red, weak | inert |
| S1 | purple | 3.579 | 1.714 | inert | inert |
| M10 | deep purple | 3.619 | 1.721 | inert | inert |
| M11 | deep purple | 3.590 | 1.715 | inert | inert |
| Locality | Distinctive Inclusions |
|---|---|
| Mogok, Myanmar | Amphibole, anatase, anhydrite, apatite, baddeleyite, boehmite, brucite, calcite, chlorite, chondrodite, clinopyroxene, dolomite, geikielite, graphite, ilmenite, magnesite, phlogopite, rutile, sulfur, zircon |
| Tajikistan | Apatite, rutile, talc, zircon |
| Sri Lanka | Apatite, boehmite, calcite, dolomite, graphite, zircon |
| Vietnam | Apatite, calcite, dolomite, graphite, magnesite, talc |
| Tanzania | Apatite, dolomite, ilmenite, magnesite, phlogopite, rutile |
| Madagascar | Apatite, clinopyroxene, rutile |
| Sample | Al2O3 | MgO | SiO2 | Cr2O3 | V2O5 | Fe2O3 | ZnO | TiO2 | MnO | Ga2O3 | K2O | CaO | NiO |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| M1 | 68.537 | 28.170 | 1.765 | 0.078 | 0.288 | 0.276 | 0.543 | 0.007 | - | 0.070 | 0.005 | 0.025 | 0.003 |
| M2 | 67.194 | 28.402 | 1.830 | 0.139 | 0.422 | 1.903 | 0.085 | 0.007 | 0.001 | 0.036 | 0.012 | 0.046 | 0.006 |
| M3 | 68.049 | 25.883 | 1.936 | 0.175 | 0.006 | 1.691 | 2.133 | - | 0.008 | 0.094 | - | 0.015 | - |
| M4 | 66.656 | 27.533 | 1.743 | 0.215 | 0.056 | 1.946 | 1.518 | 0.026 | 0.054 | 0.178 | 0.007 | 0.014 | - |
| M5 | 66.578 | 25.155 | 1.640 | 0.191 | 0.127 | 1.332 | 5.205 | 0.005 | 0.019 | 0.208 | 0.010 | 0.017 | - |
| M6 | 68.628 | 21.984 | - | 0.066 | 0.009 | 1.351 | 7.564 | - | 0.003 | 0.226 | 0.011 | 0.034 | - |
| M7 | 68.466 | 27.189 | 1.161 | 0.383 | 0.002 | 1.995 | 1.089 | 0.008 | 0.086 | 0.109 | - | 0.017 | - |
| M8 | 68.449 | 27.017 | 2.029 | 0.435 | 0.056 | 1.434 | 0.650 | 0.015 | 0.056 | 0.079 | 0.022 | - | - |
| M9 | 68.535 | 27.566 | 1.633 | 0.041 | 0.084 | 1.370 | 1.069 | 0.094 | 0.004 | 0.060 | - | 0.014 | 0.002 |
| M10 | 80.046 | 0.535 | 0.998 | 0.050 | 1.725 | 14.723 | 1.765 | - | 0.126 | 0.084 | - | 0.025 | - |
| M11 | 73.813 | 17.157 | 4.096 | 0.045 | 0.138 | 4.056 | 0.317 | 0.118 | 0.069 | 0.147 | - | - | - |
| S1 | 69.465 | 24.694 | 1.785 | 0.367 | 0.048 | 3.011 | 0.393 | 0.100 | 0.088 | 0.118 | 0.774 | 0.014 | - |
| T1 | 71.117 | 24.741 | 1.221 | 0.289 | 0.618 | 1.194 | 0.469 | 0.085 | 0.215 | 0.152 | 0.159 | 0.039 | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guo, D.; Li, G.; Weng, L.; Zhang, M.; Schmitz, F.D. Inclusions, Chemical Composition, and Spectral Characteristics of Pinkish-Purple to Purple Spinels from Mogok, Myanmar. Crystals 2025, 15, 659. https://doi.org/10.3390/cryst15070659
Guo D, Li G, Weng L, Zhang M, Schmitz FD. Inclusions, Chemical Composition, and Spectral Characteristics of Pinkish-Purple to Purple Spinels from Mogok, Myanmar. Crystals. 2025; 15(7):659. https://doi.org/10.3390/cryst15070659
Chicago/Turabian StyleGuo, Danyu, Geng Li, Liqun Weng, Meilun Zhang, and Fabian Dietmar Schmitz. 2025. "Inclusions, Chemical Composition, and Spectral Characteristics of Pinkish-Purple to Purple Spinels from Mogok, Myanmar" Crystals 15, no. 7: 659. https://doi.org/10.3390/cryst15070659
APA StyleGuo, D., Li, G., Weng, L., Zhang, M., & Schmitz, F. D. (2025). Inclusions, Chemical Composition, and Spectral Characteristics of Pinkish-Purple to Purple Spinels from Mogok, Myanmar. Crystals, 15(7), 659. https://doi.org/10.3390/cryst15070659







