Axinite, a Borosilicate with Extensive Fe-Mn Substitutions at the Scale of Monocrystal Revealed by Micro-XRF Imaging and In Situ Analysis: An Example from the Type Locality at Oisans (France)
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials: Brief Geological Context of Axinite Deposits in Oisans
2.2. Analytical Methods
3. Results
3.1. Petrography and Crystal Chemistry
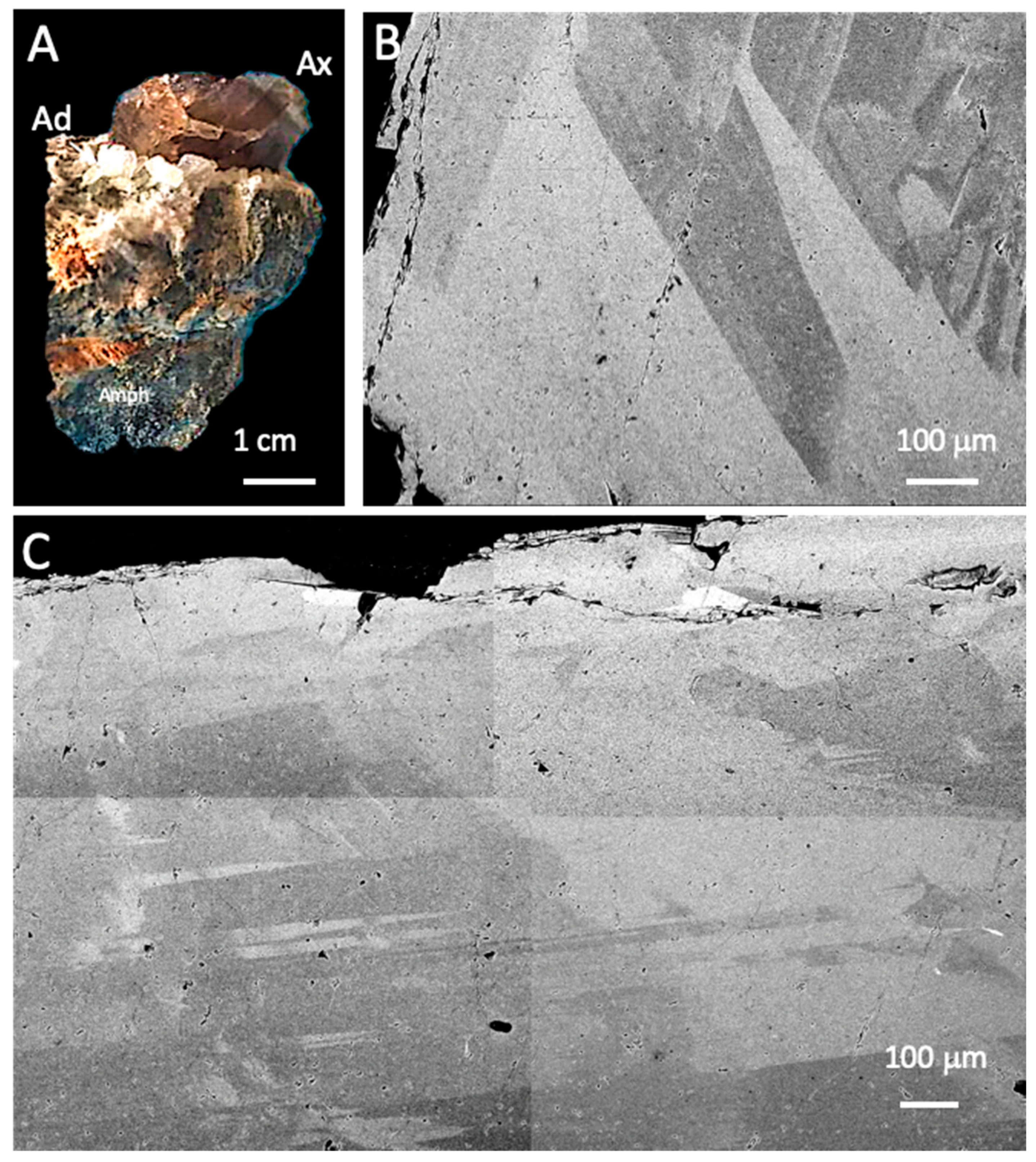
3.1.1. Petrographic Observations
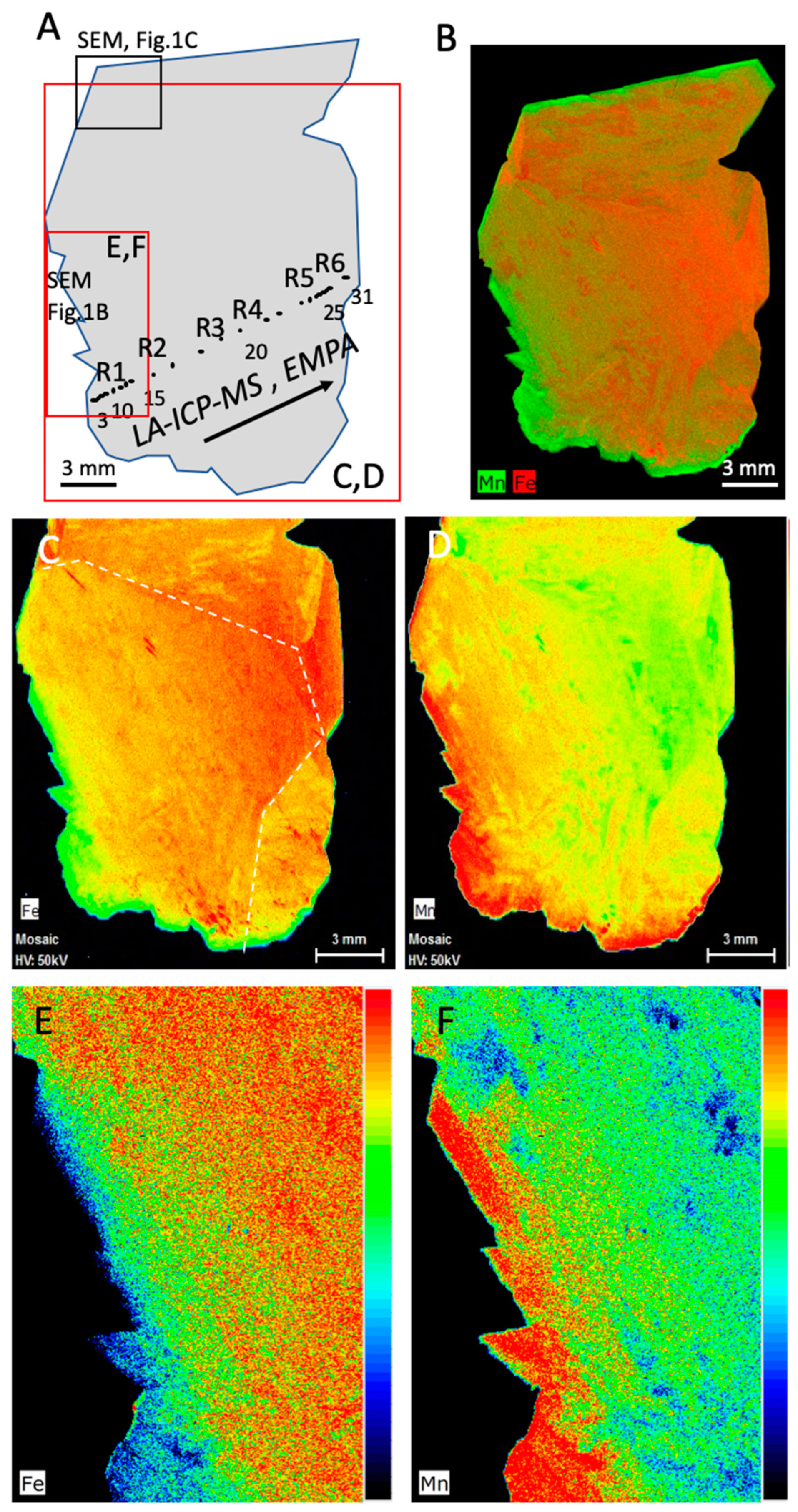
3.1.2. Micro-XRF Chemical Mapping
3.1.3. Crystal Chemistry—EMPA Analyses
3.2. XRD Patterns
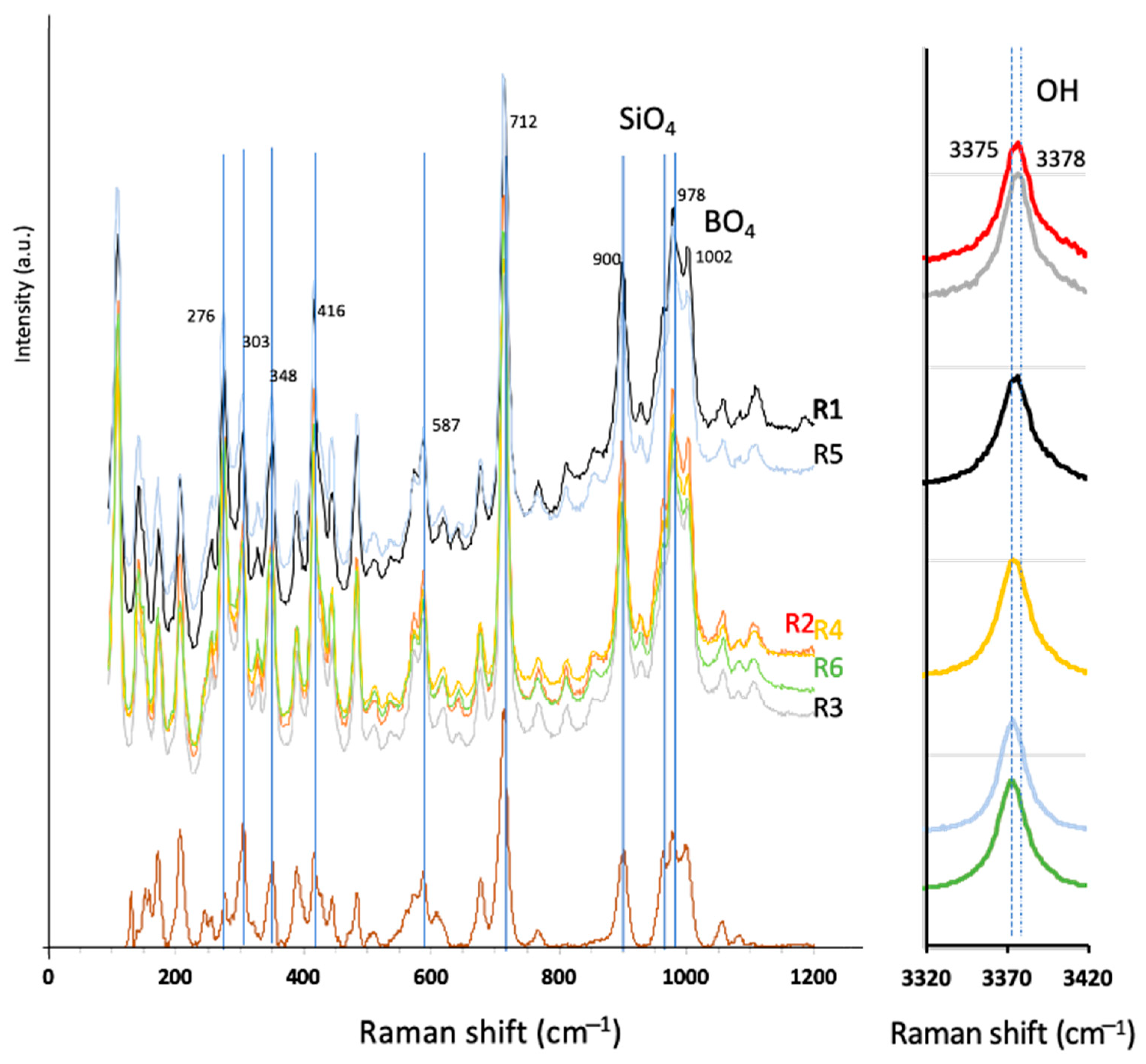
3.3. Raman Spectroscopy
3.4. LA-ICP-MS Data
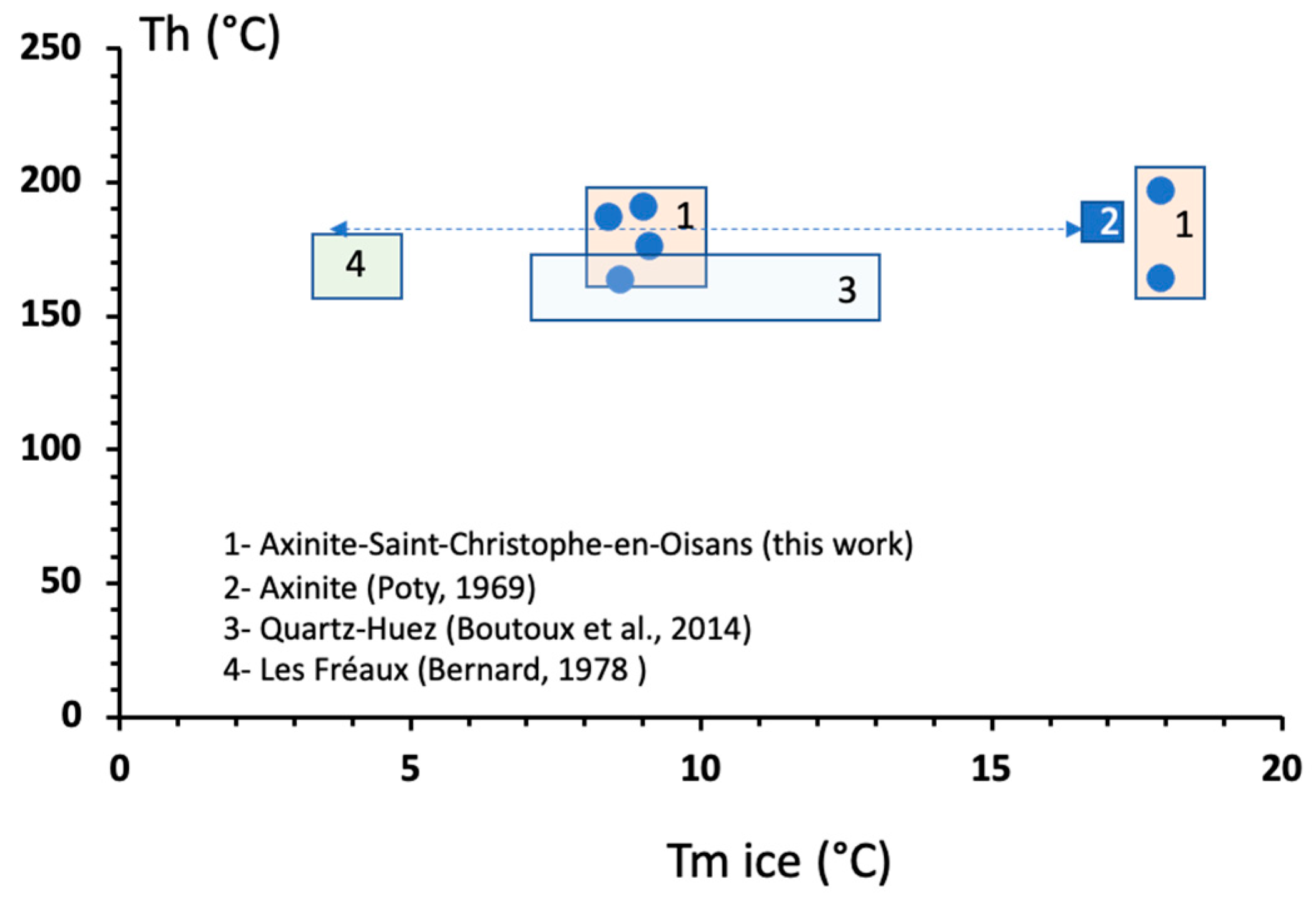
3.5. Fluid Inclusion Data
4. Discussion
4.1. Extensive Range of Composition at the Scale of Single Crystal
4.2. Insights of Crystallisation Conditions
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Andreozzi, G.B.; Lucchesi, S.; Graziani, G.; Russo, U. Site distribution of Fe2+ and Fe3+ in the axinite mineral group: New crystal-chemical formula. Am. Mineral. 2004, 89, 1763–1771. [Google Scholar] [CrossRef]
- Andreozzi, G.B.; Ottolini, L.; Lucchesi, S.; Graziani, G.; Russo, U. Crystal chemistry of the axinite-group minerals: A multi-analytical approach. Am. Mineral. 2000, 85, 698–706. [Google Scholar] [CrossRef]
- Salviulo, G.; Andreozzi, G.B.; Graziani, G. X-ray characterization of Mg, Fe, and Mn natural end-members of the axinite group. Powder Diffr. 2000, 15, 180–188. [Google Scholar] [CrossRef]
- Frost, R.; Bouzaid, J.; Martens, W.; Bayyareddy, J. Raman Spectroscopy of the borosilicate mineral Ferroaxinite. J. Raman Spectrosc. 2007, 38, 135–141. [Google Scholar] [CrossRef]
- Zagorsky, V.Y.; Peretyazhko, I.S.; Dmitrieva, A.S. Axinite-(Mn) from miarolitic granitic pegmatites of the Malkhan gem-tourmaline deposit (Transbaikalia, Russia): Composition, paragenesis and conditions of formation. Eur. J. Min. 2016, 28, 811–824. [Google Scholar] [CrossRef]
- Hauy, R.J. Extrait du Traité de Minéralogie que le Cen Haüy s’occupe de rédiger. J. Des Mines 1797, 5, 239–334. [Google Scholar]
- Basso, R.; Della Giusta, A.; Vlaic, G. La structura della tinzenite. Per. Mineral. 1973, 42, 369–379. [Google Scholar]
- Takeuchi, Y.; Ozawa, T.; Ito, T.; Araki, T.; Zoltai, T.; Finney, J.J. The B2Si8O30 groups of tetrahedra in axinite and comments on the deformation of Si tetrahedra in silicates. Z. Kristallogr. 1974, 140, 289–312. [Google Scholar] [CrossRef]
- Gatta, G.D.; Redhammer, G.J.; Guastoni, A.; Guastella, G.; Meven, M.; Pavese, A. H-bonding scheme and cation partitioning in axinite: A single-crystal neutron diffraction and Mössbauer spectroscopic study. Phys. Chem. Miner. 2016, 43, 341–352. [Google Scholar] [CrossRef]
- Sanero, E.; Gottardi, G. Nomenclature and crystal-chemistry of axinite. Am. Mineral. 1968, 53, 1407–1411. [Google Scholar]
- Filip, J.; Kolitsch, U.; Novák, M.; Schneeweiss, O. The crystal structure of near-end-member ferroaxinite from an iron-contaminated pegmatite at Malesov, Czech Republic. Can. Min. 2006, 44, 1159–1170. [Google Scholar] [CrossRef]
- Lumpkin, G.R.; Ribbe, P.H. Chemistry and physical properties of axinites. Am. Mineral. 1979, 64, 635–645. [Google Scholar]
- Pieczka, A.; Kraczka, J. Crystal chemistry of Fe2+—Axinite from Strzegom. Mineral. Polonica 1994, 25, 43–49. [Google Scholar]
- Bellahsen, N.; Jolivet, L.; Lacombe, O.; Bellanger, M.; Boutoux, A.; Garcia, S.; Mouthereau, F.; Le Pourhiet, L.; Gumiaux, C. Mechanisms of margin inversion in the external Western Alps: Implications for crustal rheology. Tectonophysics 2012, 560–561, 62–83. [Google Scholar] [CrossRef]
- Bellanger, M.; Augier, R.; Bellahsen, N.; Jolivet, L.; Moniè, P.; Baudin, T.; Beyssac, O. Shortening of the European Dauphinois margin (Oisans Massif, Western Alps): New insights from RSCM maximum temperature estimates and 40Ar/39Ar in situ dating. J. Geodyn. 2015, 83, 37–64. [Google Scholar] [CrossRef]
- Gasquet, D.; Bertrand, J.-M.; Paquette, J.-L.; Lehmann, J.; Ratzov, G.; Guedes, R.D.A.; Tiepolo, M.; Boullier, A.-M.; Scaillet, S.; Nomade, S. Miocene to Messinian deformation and hydrothermal activity in a pre-Alpine basement massif of the French western Alps: New U-Th-Pb and argon ages from the Lauzière massif. Bull. Soc. Geol. Fr. 2010, 181, 227–241. [Google Scholar] [CrossRef]
- Cathelineau, M.; Boiron, M.-C.; Lecomte, A.; Martins, O.; Dias da Silva, I. Mateus A. Lithium, Phosphorus, Fluorine-Rich Intrusions and the Phosphate Sequence at Segura (Portugal): A Comparison with other Hyper-Differentiated Magmas. Minerals 2024, 14, 287. [Google Scholar] [CrossRef]
- Patton, C.; Hellstrom, J.; Paul, B.; Woodhead, J.; Hergt, J. Iolite: Freeware for the visualisation and processing of mass spectrometric data. J. Anal. At. Spectrom. 2011, 26, 2508–2518. [Google Scholar] [CrossRef]
- Longerich, H.P.; Jackson, S.E.; Günther, D. Inter-laboratory note. Laser ablation inductively coupled plasma mass spectrometric transient signal data acquisition and analyte concentration calculation. J. Anal. At. Spectrom. 1996, 11, 899–904. [Google Scholar] [CrossRef]
- Cathelineau, M.; Boiron, M.-C.; Marignac, C.; Dour, M.; Dejean, M.; Carocci, E.; Truche, L.; Pinto, F. High pressure and temperatures during the early stages of tungsten deposition at Panasqueira revealed by fluid inclusions in topaz. Ore Geol. Rev. 2020, 126, 103741. [Google Scholar] [CrossRef]
- Ruff. Fe and Mn. Available online: https://rruff.info/Axinite (accessed on 15 February 2025).
- Poty, B. La Croissance des Cristaux de Quartz sur L’exemple du Filon de La Gardette (Bourg d’Oisans) et des Filons du Massif du Mont-Blanc. Ph.D. Thesis, Nancy-Université, Nancy, France, 1967. NNT: 1967NAFS0052. [Google Scholar]
- Poty, B.P.; Stalder, H.-A.; Weisbrod, A.M. Fluid inclusion studies in quartz from fissures of western and central Alps. Schweiz. Mineral. Petrogr. Mitt. 1974, 54, 717–752. [Google Scholar]
- Boutoux, A.; Verlaguet, A.; Bellahsen, N.; Lacombe, O.; Villemant, B.; Caron, B.; Martin, E.; Assayag, N.; Cartigny, P. Fluid systems above basement shear zones during inversion of pre-orogenic sedimentary basins (External Crystalline Massifs, Western Alps). Lithos 2014, 206–207, 435–453. [Google Scholar] [CrossRef]
- Bernard, D. Microthermométrie des inclusions fluides de cristaux syncinématiques. Application à la couverture sédimentaire du Nord Pelvoux. Ph.D. Thesis, Grenoble University, Grenoble, France, 1978. [Google Scholar]
- Grew, E.S. Borosilicates (exclusive of tourmaline) and boron in rock-forming minerals in metamorphic environments. In Mineralogical Society of America. Rev. Mineral. 1996, 33, 387–502. [Google Scholar]
- Pringle, I.J.; Kawachi, Y. Axinite mineral group in low-grade regionally metamorphosed rocks in southern New Zealand. Am. Mineral. 1980, 65, 1119–1129. [Google Scholar]
- Lacombe, J.-C. Etude pétrographique de la partie orientale du massif des Ecrins-Pelvoux- Le complexe volcano-sédimentaire—Alpes Françaises. Ph.D. Thesis, University Grenoble, Grenoble, France, 1970; 123p. [Google Scholar]
- Dubinina, E.O.; Baskina, V.A.; Avdeenko, A.S. Nature of ore-forming fluids of the Dal’negorsk deposit: Isotopic and geochemical parameters of the altered host rocks. Geol. Ore Depos. 2011, 53, 58–73. [Google Scholar] [CrossRef]
- Weibel, M.; Graeser, S.; Oberholzer, W.F.; Stalder, H.-A.; Gabriel, W. Die Mineralien der Schweiz, 5th ed.; Birkhäuser: Basel, Switzerland, 1990. [Google Scholar] [CrossRef]
- Meisser, N.; Brugger, J.; May, R.; Cheseaux, D. Der Axinit vom Catogne (VS). Schweiz. Strahler 1996, 10, 325–337, (In German and French). [Google Scholar]




| Rim | Core | |||||
|---|---|---|---|---|---|---|
| 3 | 10 | 15 | 20 | 25 | 31 | |
| SiO2 | 40.7 | 41.76 | 42.06 | 42.24 | 42.15 | 42 |
| TiO2 | 0.04 | 0.04 | 0 | 0.04 | 0 | 0.02 |
| Al2O3 | 17.83 | 17.86 | 18.06 | 17.91 | 17.86 | 17.7 |
| FeO | 3.74 | 4.45 | 4.54 | 4.83 | 4.81 | 4.95 |
| MnO | 8.37 | 6.25 | 6.2 | 5.89 | 5.69 | 5.48 |
| MgO | 0.51 | 0.95 | 1.13 | 1.15 | 1.16 | 1.22 |
| CaO | 19.6 | 19.37 | 19.56 | 19.41 | 19.7 | 19.3 |
| Na2O | 0.02 | 0 | 0.01 | 0 | 0.03 | 0.02 |
| K2O | 0.04 | 0.01 | 0.01 | 0.04 | 0.01 | 0.01 |
| BaO | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| B2O3 * | 6.00 | 6.06 | 6.11 | 6.11 | 6.11 | 6.07 |
| H2O * | 1.55 | 1.57 | 1.58 | 1.58 | 1.58 | 1.57 |
| Total | 98.40 | 98.31 | 99.27 | 99.21 | 99.10 | 98.34 |
| O=F. Cl * | 1.60 | 1.69 | 0.73 | 0.79 | 0.90 | 1.66 |
| B * | 2.00 | 2.00 | 2.00 | 2.00 | 2.00 | 2.00 |
| Si | 7.86 | 7.99 | 7.97 | 8.00 | 8.00 | 8.02 |
| Al total | 4.06 | 4.03 | 4.03 | 4.00 | 3.99 | 3.98 |
| Ti | 0.01 | 0.01 | 0.00 | 0.01 | 0.00 | 0.00 |
| Fe | 0.60 | 0.71 | 0.72 | 0.77 | 0.76 | 0.79 |
| Mn | 1.37 | 1.01 | 1.00 | 0.95 | 0.91 | 0.89 |
| Mg | 0.15 | 0.27 | 0.32 | 0.32 | 0.33 | 0.35 |
| Ca | 4.06 | 3.97 | 3.97 | 3.94 | 4.00 | 3.95 |
| Na | 0.01 | 0.00 | 0.00 | 0.00 | 0.01 | 0.01 |
| K | 0.01 | 0.00 | 0.00 | 0.01 | 0.00 | 0.00 |
| Ba | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| (OH. F. Cl) * | 2.00 | 2.00 | 2.00 | 2.00 | 2.00 | 2.00 |
| Total | 22.12 | 21.99 | 22.02 | 22.00 | 22.01 | 21.99 |
| ppm | Li | Be | Sc | Ti | V | Cr | Co | Zn | Ga | Ge | Sr | In | Sn | Tb | Dy | Ho | Er | Tm | Yb | Lu | Y |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2.1 | 1002.0 | 75.9 | 51.2 | 100.7 | 111.6 | 1.2 | 72.2 | 13.3 | 5.6 | 11.6 | 1.3 | 0.6 | 13.6 | 8.9 | 61.5 | 13.4 | 119.7 | 18.9 | 315.8 | |
| 2 | 3.4 | 1021.4 | 13.5 | 65.9 | 37.5 | 17.1 | 1.2 | 94.7 | 9.0 | 6.1 | 12.9 | 1.1 | 0.3 | 8.5 | 5.5 | 34.0 | 6.4 | 46.2 | 6.0 | 210.9 | |
| 3 | 1073.7 | 3.5 | 124.4 | 23.3 | 3.2 | 1.3 | 101.8 | 10.3 | 13.7 | 11.7 | 1.0 | 0.4 | 8.8 | 5.3 | 33.2 | 6.7 | 46.9 | 5.9 | 222.2 | ||
| 4 | 5.2 | 928.7 | 4.4 | 73.7 | 23.9 | 5.3 | 1.3 | 100.0 | 7.9 | 4.8 | 13.9 | 0.6 | 0.0 | 3.9 | 2.8 | 18.9 | 4.0 | 28.5 | 3.7 | 114.0 | |
| 5 | 7.0 | 612.3 | 8.6 | 67.2 | 66.2 | 8.5 | 1.6 | 114.0 | 10.7 | 4.9 | 10.8 | 0.6 | 0.1 | 3.6 | 2.1 | 11.7 | 1.9 | 11.2 | 1.4 | 77.7 | |
| 6 | 6.4 | 742.3 | 8.5 | 80.4 | 67.5 | 5.8 | 1.5 | 111.9 | 9.0 | 5.4 | 12.5 | 0.7 | 0.0 | 3.7 | 2.1 | 11.5 | 1.9 | 12.0 | 1.5 | 82.0 | |
| 7 | 8.7 | 683.8 | 5.5 | 60.0 | 65.5 | 4.2 | 1.6 | 118.4 | 7.1 | 14.7 | 0.6 | 0.0 | 2.2 | 1.3 | 7.2 | 1.3 | 8.2 | 0.9 | 48.6 | ||
| 8 | 3.8 | 732.9 | 0.9 | 533.8 | 64.6 | 2.7 | 145.8 | 13.7 | 3.5 | 14.1 | 0.0 | 0.0 | 0.0 | 0.2 | 0.9 | 0.2 | 0.0 | 0.0 | 7.6 | ||
| 9 | 9.1 | 704.2 | 2.4 | 67.1 | 83.5 | 1.5 | 133.2 | 6.9 | 3.0 | 13.7 | 0.6 | 0.0 | 2.2 | 1.1 | 6.3 | 1.1 | 7.2 | 0.8 | 45.1 | ||
| 10 | 4.3 | 359.0 | 1.6 | 101.4 | 99.7 | 1.9 | 139.5 | 10.1 | 5.0 | 10.0 | 0.3 | 0.0 | 2.2 | 1.0 | 5.8 | 0.9 | 5.1 | 0.6 | 43.1 | ||
| 11 | 3.7 | 289.6 | 2.5 | 79.8 | 65.9 | 1.9 | 130.4 | 4.3 | 23.8 | 0.5 | 0.0 | 0.0 | 0.0 | 1.9 | 0.4 | 3.3 | 0.5 | 10.0 | |||
| 12 | 4.2 | 482.3 | 2.3 | 99.8 | 111.4 | 2.0 | 147.2 | 7.9 | 4.7 | 11.7 | 0.5 | 2.3 | 0.0 | 1.0 | 0.7 | 4.2 | 0.8 | 5.8 | 0.7 | 28.3 | |
| 13 | 2.4 | 449.2 | 1.4 | 272.6 | 88.5 | 3.2 | 2.1 | 121.7 | 8.5 | 6.8 | 8.6 | 0.0 | 13.2 | 0.0 | 0.0 | 0.0 | 0.0 | 1.3 | |||
| 14 | 3.2 | 512.6 | 1.0 | 205.0 | 91.8 | 3.5 | 2.3 | 133.3 | 8.7 | 4.9 | 9.2 | 0.0 | 6.2 | 0.0 | 0.0 | 0.0 | 0.0 | 1.0 | |||
| 15 | 2.5 | 1501.7 | 1.1 | 296.8 | 97.5 | 2.5 | 151.4 | 9.0 | 11.8 | 0.0 | 3.0 | 0.0 | 0.0 | 1.0 | 0.0 | 2.7 | |||||
| 16 | 4.0 | 509.6 | 1.1 | 378.5 | 123.6 | 2.0 | 161.3 | 11.8 | 4.7 | 11.5 | 0.3 | 4.2 | 1.6 | 0.0 | 2.6 | 0.0 | 9.5 | ||||
| 17 | 687.5 | 1.3 | 53.8 | 61.7 | 1.7 | 111.6 | 10.0 | 3.3 | 12.3 | 1.5 | 0.4 | 3.0 | 0.4 | 9.0 | |||||||
| 18 | 2.3 | 1629.2 | 2.7 | 161.7 | 111.0 | 2.3 | 133.6 | 7.1 | 12.7 | 0.0 | 5.1 | 1.2 | 0.4 | 3.6 | 0.5 | 6.6 | |||||
| 19 | 1.8 | 1433.0 | 3.4 | 138.4 | 108.7 | 2.2 | 133.9 | 6.5 | 12.8 | 0.5 | 5.3 | 1.8 | 0.5 | 4.9 | 0.7 | 8.7 | |||||
| 20 | 1397.4 | 3.3 | 136.4 | 101.0 | 2.2 | 134.4 | 6.1 | 13.5 | 0.6 | 5.1 | 1.7 | 0.5 | 5.1 | 0.8 | 7.8 | ||||||
| 21 | 3.4 | 1572.9 | 0.7 | 284.9 | 81.3 | 2.6 | 157.7 | 8.4 | 12.4 | 0.0 | 2.3 | 0.0 | 0.0 | 0.0 | 0.0 | 1.4 | |||||
| 22 | 1.8 | 830.4 | 1.8 | 221.2 | 111.8 | 2.0 | 118.2 | 8.8 | 5.2 | 10.8 | 0.5 | 10.3 | 2.0 | 0.5 | 4.1 | 0.6 | 11.8 | ||||
| 23 | 3.0 | 1474.0 | 1.2 | 182.6 | 80.2 | 2.6 | 142.7 | 6.6 | 12.5 | 3.0 | 0.0 | 0.0 | 1.2 | 0.0 | 2.3 | ||||||
| 24 | 2.2 | 1575.1 | 2.6 | 165.5 | 99.0 | 2.4 | 137.9 | 6.7 | 2.0 | 11.7 | 0.4 | 5.3 | 1.1 | 0.3 | 3.4 | 0.5 | 6.3 | ||||
| 25 | 3.1 | 568.9 | 1.0 | 116.1 | 63.5 | 2.3 | 158.8 | 5.3 | 17.2 | 0.1 | 0.0 | 0.0 | 0.0 | 0.0 | 2.5 | ||||||
| 26 | 1.7 | 415.8 | 0.7 | 144.7 | 62.6 | 2.4 | 177.4 | 6.2 | 14.0 | 0.0 | 1.7 | 0.0 | 0.0 | 0.0 | 0.0 | 0.9 | |||||
| 27 | 1.7 | 162.0 | 1.2 | 85.4 | 43.4 | 2.5 | 185.6 | 3.2 | 19.3 | 0.1 | 0.0 | 0.0 | 0.0 | 0.0 | 1.2 | ||||||
| 28 | 3.5 | 1454.5 | 1.2 | 265.0 | 57.5 | 1.4 | 2.8 | 181.9 | 6.0 | 11.9 | 0.0 | 2.1 | 0.0 | 0.0 | 0.0 | 0.0 | 0.6 | ||||
| 29 | 3.5 | 1773.2 | 0.5 | 487.8 | 63.2 | 3.0 | 206.1 | 5.7 | 12.1 | 0.0 | 1.7 | 0.0 | 0.0 | 0.0 | 0.0 | 0.4 | |||||
| 30 | 4.9 | 1002.0 | 2.0 | 305.5 | 54.7 | 3.6 | 3.3 | 196.7 | 4.6 | 12.9 | 0.0 | 2.1 | 0.0 | 0.0 | 0.0 | 0.0 | 0.6 | ||||
| 31 | 1.5 | 1577.1 | 2.8 | 235.8 | 82.2 | 2.4 | 186.1 | 6.0 | 12.2 | 0.0 | 6.2 | 0.0 | 0.0 | 1.6 | 0.0 | 1.5 | |||||
| 32 | 3.0 | 595.9 | 0.8 | 250.6 | 75.4 | 2.5 | 169.5 | 7.2 | 2.8 | 9.5 | 0.0 | 4.8 | 0.0 | 0.0 | 0.0 | 0.0 | 0.3 | ||||
| 33 | 2.5 | 773.5 | 1.0 | 229.9 | 82.0 | 2.3 | 2.5 | 164.2 | 7.2 | 2.5 | 10.0 | 5.2 | 0.0 | 0.0 | 0.0 | 0.0 | 1.1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cathelineau, M.; Gerbeaud, O.; Peiffert, C. Axinite, a Borosilicate with Extensive Fe-Mn Substitutions at the Scale of Monocrystal Revealed by Micro-XRF Imaging and In Situ Analysis: An Example from the Type Locality at Oisans (France). Crystals 2025, 15, 402. https://doi.org/10.3390/cryst15050402
Cathelineau M, Gerbeaud O, Peiffert C. Axinite, a Borosilicate with Extensive Fe-Mn Substitutions at the Scale of Monocrystal Revealed by Micro-XRF Imaging and In Situ Analysis: An Example from the Type Locality at Oisans (France). Crystals. 2025; 15(5):402. https://doi.org/10.3390/cryst15050402
Chicago/Turabian StyleCathelineau, Michel, Olivier Gerbeaud, and Chantal Peiffert. 2025. "Axinite, a Borosilicate with Extensive Fe-Mn Substitutions at the Scale of Monocrystal Revealed by Micro-XRF Imaging and In Situ Analysis: An Example from the Type Locality at Oisans (France)" Crystals 15, no. 5: 402. https://doi.org/10.3390/cryst15050402
APA StyleCathelineau, M., Gerbeaud, O., & Peiffert, C. (2025). Axinite, a Borosilicate with Extensive Fe-Mn Substitutions at the Scale of Monocrystal Revealed by Micro-XRF Imaging and In Situ Analysis: An Example from the Type Locality at Oisans (France). Crystals, 15(5), 402. https://doi.org/10.3390/cryst15050402






