A Novel Zinc-Based MOF Featuring 2,4,6-Tris-(4-carboxyphenoxy)-1,3,5-triazine: Structure, Adsorption, and Photocatalytic Activity
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis
2.2.1. Preparation of Ligand
2.2.2. Preparation of MOF-S1 [Zn2(TCPT). × H2O]
2.3. Characterization Methods
2.3.1. Single-Crystal X-Ray Diffraction (SCXRD) Analysis
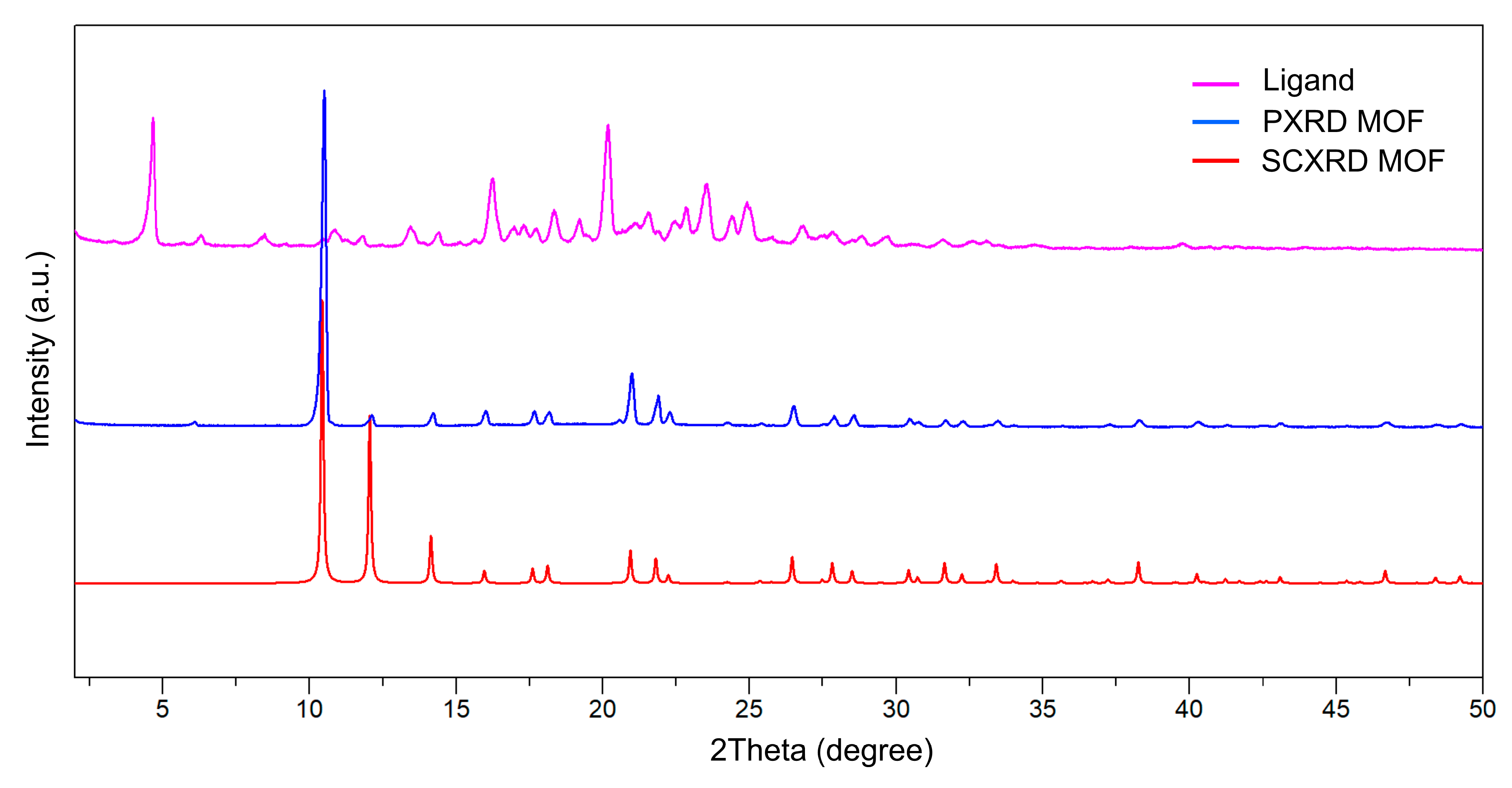
2.3.2. Powder X-Ray Diffraction (PXRD) Analysis
2.3.3. Nuclear Magnetic Resonance (NMR) Spectroscopy
2.3.4. Thermogravimetric Analysis
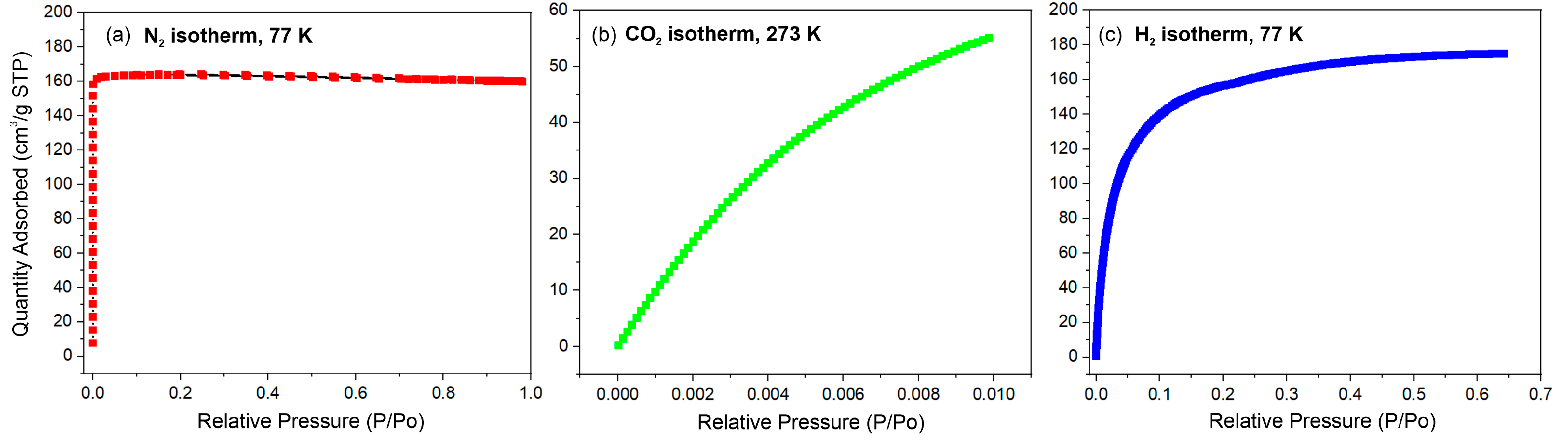
2.3.5. Specific Surface Area Analysis—N2, H2, and CO2 Physisorption
2.3.6. Adsorption of Dye Molecules on MOF-S1
2.3.7. Photodegradation Studies of Dye Molecules Using MOF-S1
3. Results and Discussion
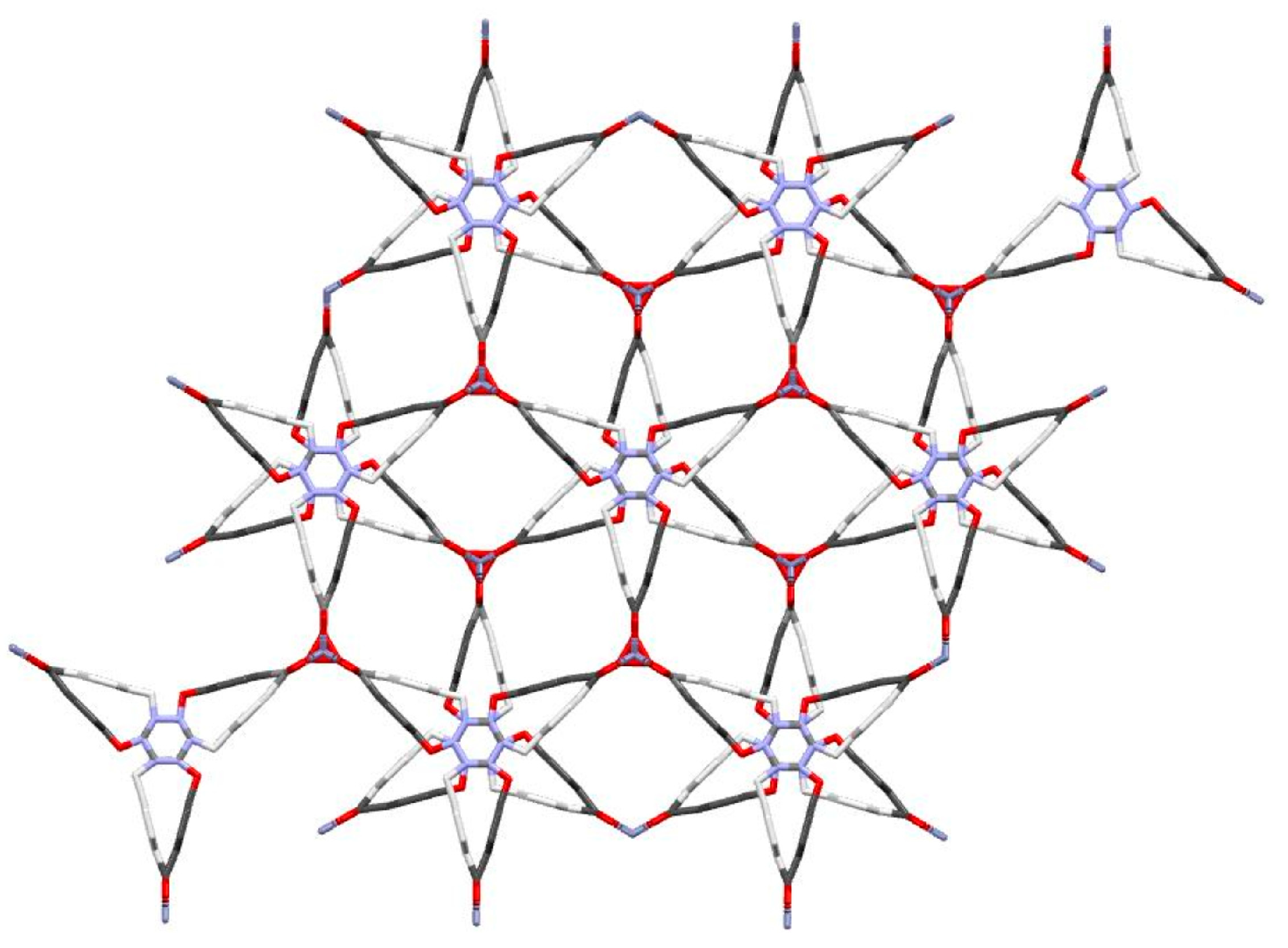
3.1. Physicochemical Characterization
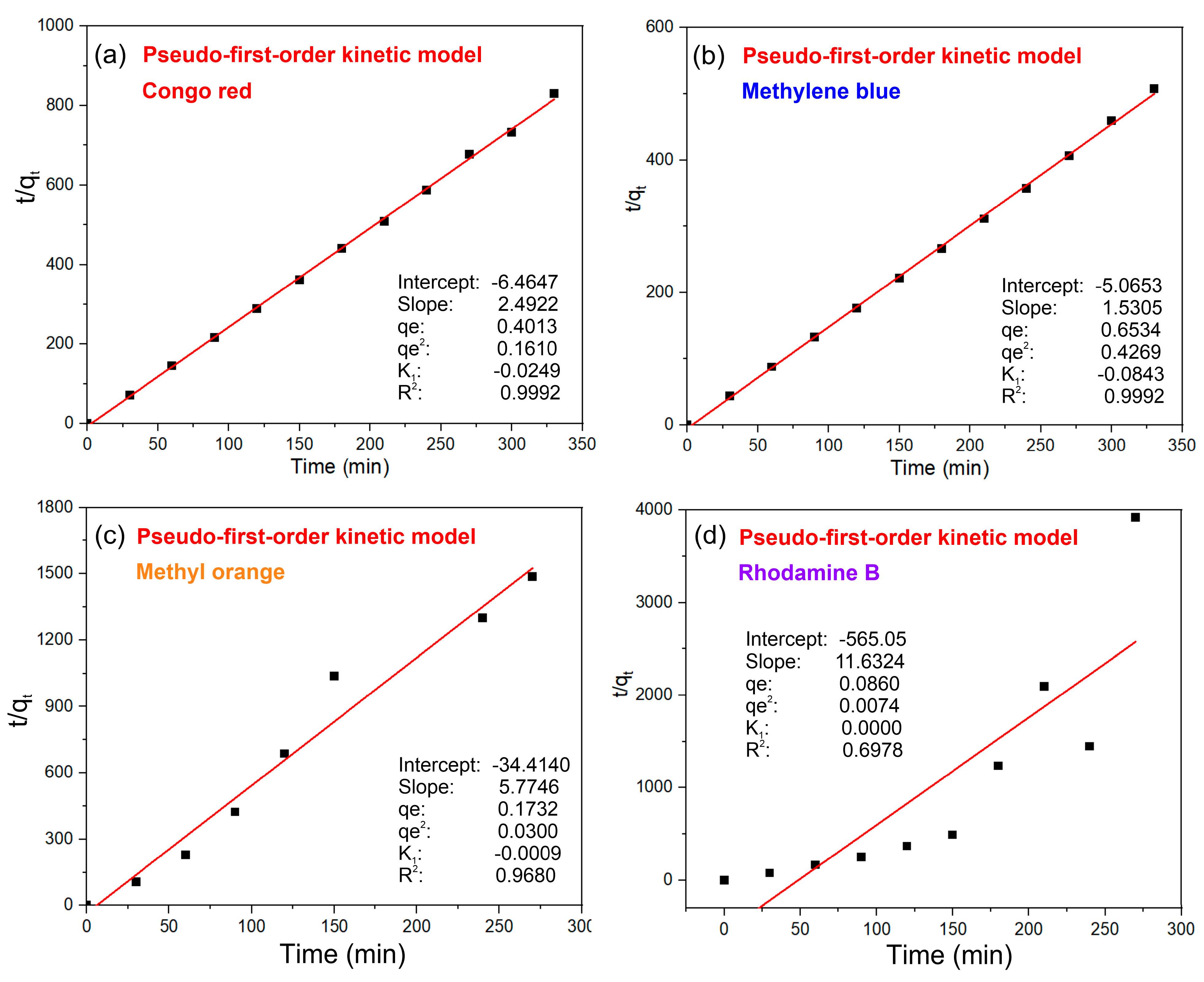
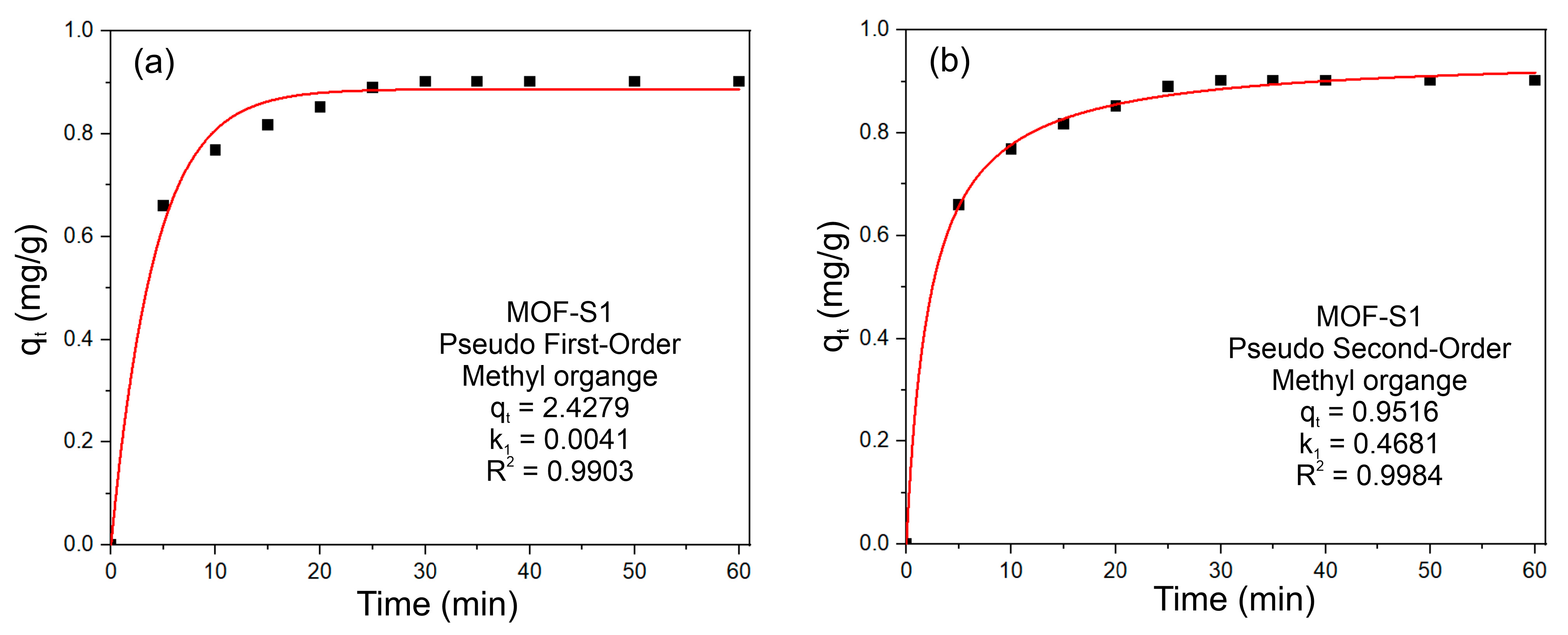
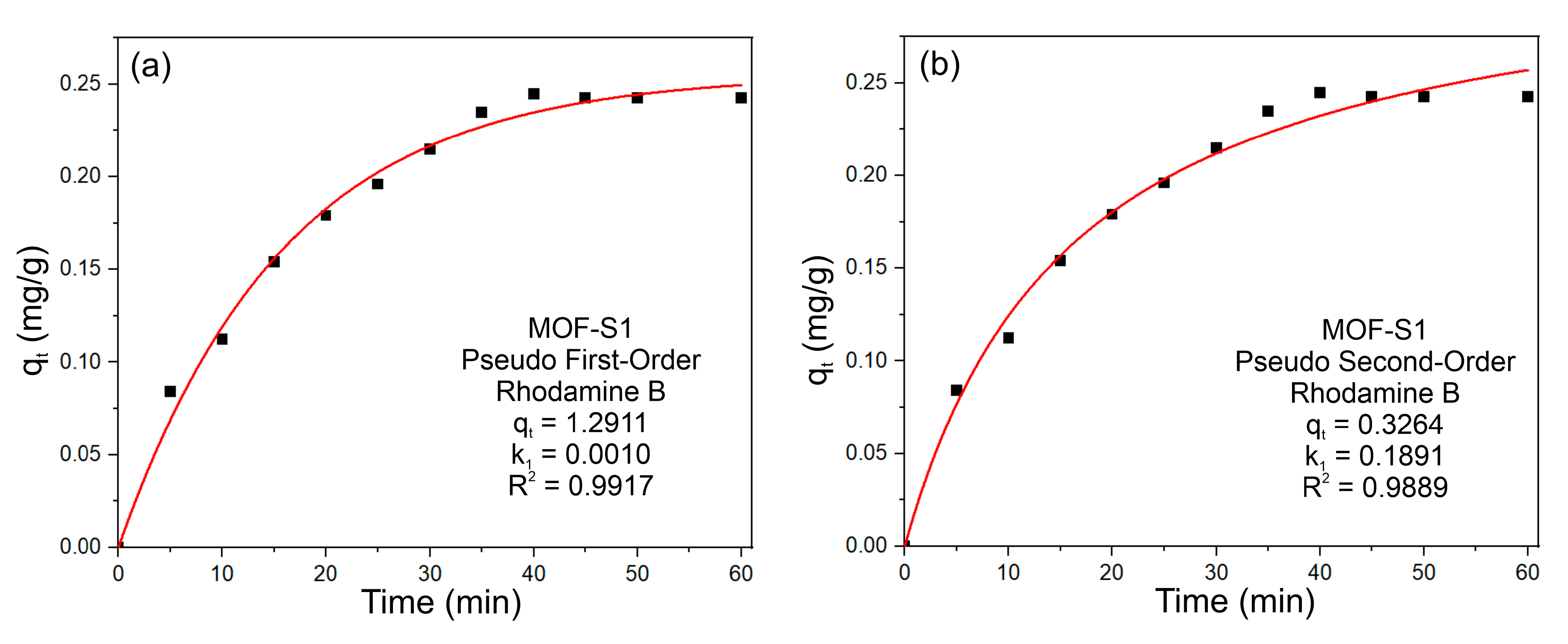
3.2. Adsorption of Dye Molecules on MOF-S1
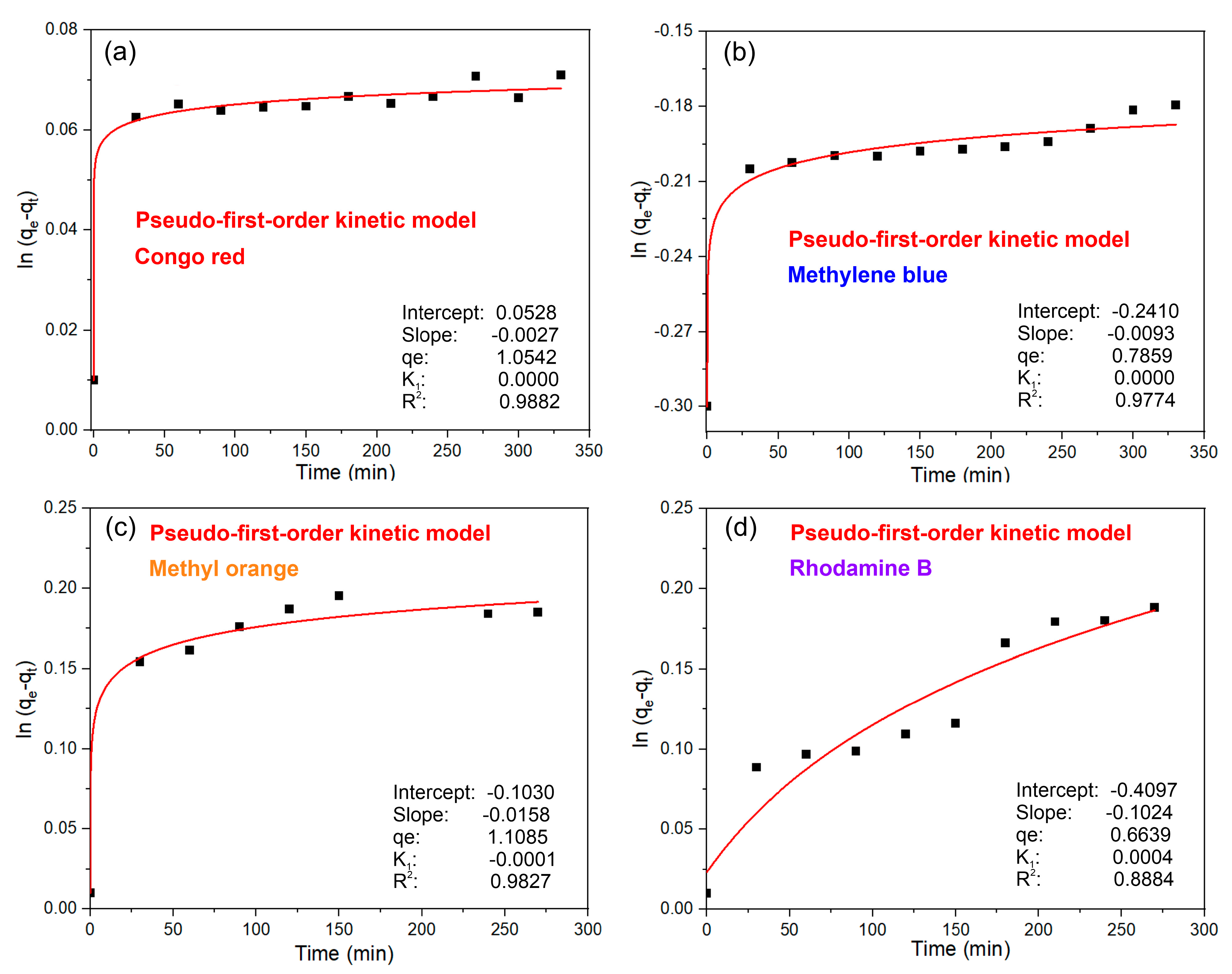
3.3. Adsorption Mechanism
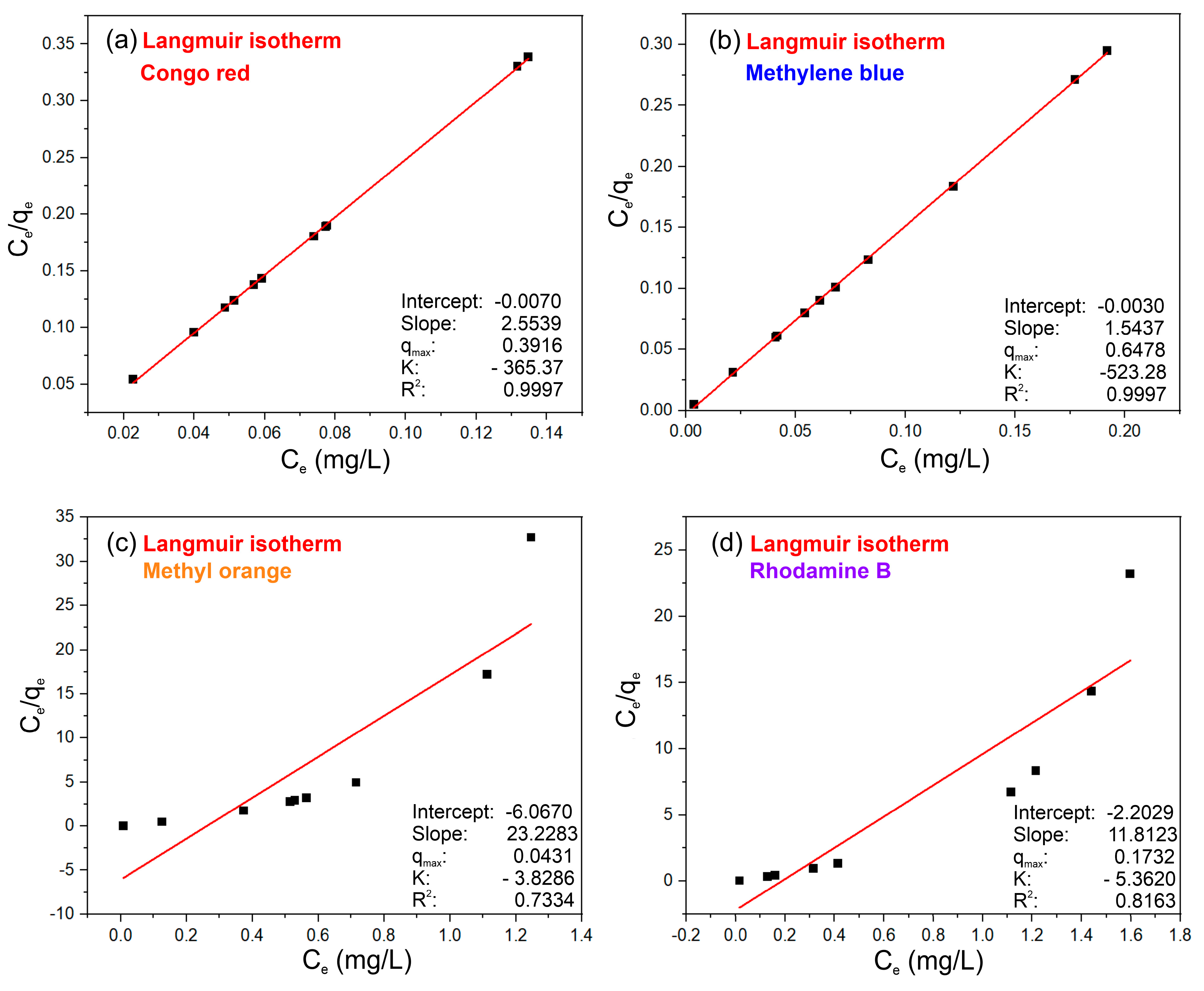
3.4. Adsorption Isotherms
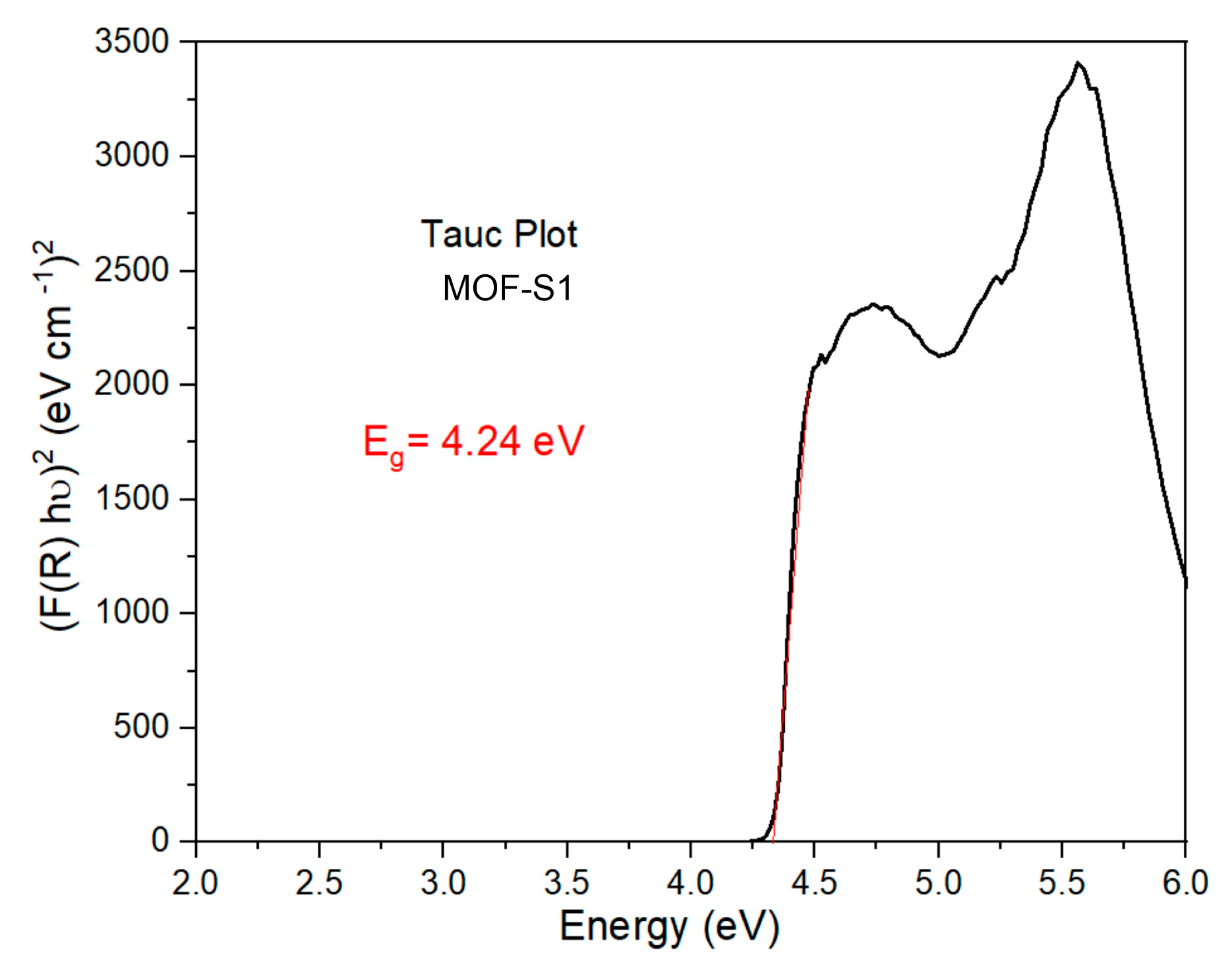
3.5. Determination of the Energy Band Gap
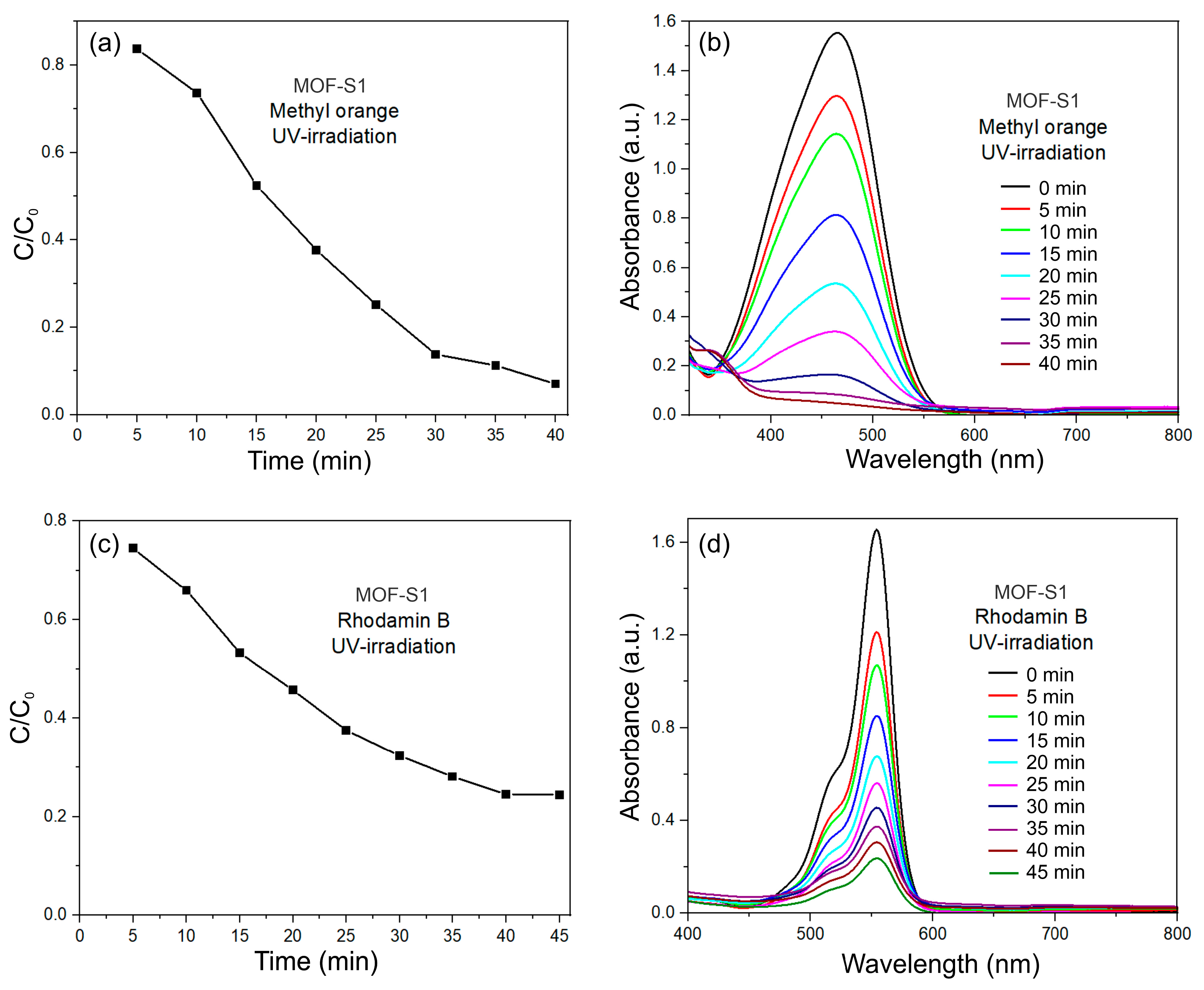
3.6. Photodegradation Studies of MO and RhB
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| BET | Brunauer–Emmett–Teller (method) |
| CR | Congo Red |
| DMF | Dimethylformamide |
| DR | Dubinin–Radushkevich (model) |
| DRS | Diffuse Reflectance Spectroscopy |
| DSC | Differential Scanning Calorimetry |
| HK | Horváth–Kawazoe (method) |
| HMBC | Heteronuclear Multiple Bond Correlation |
| HSQC | Heteronuclear Single Quantum Correlation |
| MB | Methylene Blue |
| MO | Methyl Orange |
| NMR | Nuclear Magnetic Resonance |
| PFO | Pseudo-first-order (model) |
| PSO | Pseudo-second-order (model) |
| PXRD | Powder X-ray Diffraction |
| RhB | Rhodamine B |
| SBUs | Secondary Building Units |
| SCXRD | Single-Crystal X-ray Diffraction |
| SSA | Specific Surface Area |
| TCPT | 2,4,6-Tris-(4-carboxyphenoxy)-1,3,5-triazine |
| TGA | Thermogravimetric Analysis |
| UV | Ultraviolet |
| Vis | visible |
References
- Ling, H.; Tian, B.; Hu, X.; Wang, W.; Zhang, J.; Liu, R.; Lu, Z.; Guo, Y.; Zhao, H. Applications of Metal–Organic Frameworks and Their Derivatives in Fuel Cells. Molecules 2025, 30, 981. [Google Scholar] [CrossRef] [PubMed]
- Kapoor, R.T.; Mohd, R. Chapter 20—Recent Advances in Application of Metal Organic Frameworks for Adsorption of Dye from Wastewater. In Engineered Biocomposites for Dye Adsorption; Jagaba, A.H., Kutty, S.R.M., Isa, M.H., Birniwa, A.H., Eds.; Elsevier: Amsterdam, The Netherlands, 2025. [Google Scholar] [CrossRef]
- Laeim, H.; Molahalli, V.; Prajongthat, P.; Pattanaporkratana, A.; Pathak, G.; Phettong, B.; Hongkarnjanakul, N.; Chattham, N. Porosity Tunable Metal-Organic Framework (Mof)-Based Composites for Energy Storage Applications: Recent Progress. Polymers 2025, 17, 130. [Google Scholar] [CrossRef]
- Zhu, Q.; Sun, W.; Zhou, H.; Mao, D. A Review of Lithium–Sulfur Batteries Based on Metal–Organic Frameworks: Progress and Prospects. Batteries 2025, 11, 89. [Google Scholar] [CrossRef]
- Liang, Y.; Zhu, J.-Z.; Jin, S.-Y.; Meng, Y.-R.; Li, S.-F.; Zuo, J.-L.; Zhang, G.; Su, J. X-Ray Luminescent Metal-Organic Frameworks: Design Strategies and Functional Applications. J. Mater. Chem. C 2025, 13, 4836–4860. [Google Scholar]
- Han, Z.; Yang, Y.; Rushlow, J.; Huo, J.; Liu, Z.; Hsu, Y.-C.; Yin, R.; Wang, M.; Liang, R.; Wang, K.-Y. Development of the Design and Synthesis of Metal–Organic Frameworks (Mofs)–from Large Scale Attempts, Functional Oriented Modifications, to Artificial Intelligence (Ai) Predictions. Chem. Soc. Rev. 2025, 54, 367–395. [Google Scholar]
- Rapeyko, A.; i Xamena, F.X.L. Zirconium-Containing Metal–Organic Frameworks (Mofs) as Catalysts for Biomass Conversion. Appl. Sci. 2025, 15, 2609. [Google Scholar] [CrossRef]
- Liu, M.; Wang, Y.F.; Xu, F.; Zhang, N.; Hou, C.Y.; Sun, L.X.; Xing, Y.H.; Bai, F.Y. High-Symmetry Co/Ni Triazine Polycarboxylate Diverse Frameworks Constructed by M X (Coo) Y Building Blocks: Characterization and Catalytic Performance Evaluation of P-Nitrophenol. Inorg. Chem. 2022, 61, 19951–19960. [Google Scholar]
- Zhao, J.; Zhang, Y.; Luo, Y.; Zheng, W.; Xu, X.; Luo, F. Amorphous Metal–Organic Frameworks: Pioneering Frontiers in Cancer Diagnostics and Therapeutics. Chem. Eng. J. 2024, 492, 152295. [Google Scholar]
- Yu, L.; Ullah, S.; Wang, H.; Xia, Q.; Thonhauser, T.; Li, J. High-Capacity Splitting of Mono-and Dibranched Hexane Isomers by a Robust Zinc-Based Metal–Organic Framework. Angew. Chem. Int. Ed. 2022, 61, e202211359. [Google Scholar]
- Sheng, J.-G.; Tang, M.; Qin, Q.; Li, D.-Q.; Chen, W.-W. Two Co(II) Coordination Polymers: Treatment Activity on Non-Small Cell Lung Cancer by Inducing the Cancer Cell Apoptosis through High-Intensity Focused Ultrasound. J. Iran. Chem. Soc. 2021, 18, 2323–2330. [Google Scholar]
- Xiao, Y.; You, Z.X.; Xing, Y.H.; Bai, F.Y.; Shi, Z. Three-Pole Wheel Paddle Luminescent Metal Organic Frameworks (Lmofs) Based on the Oxygen Substituted Triazine Tricarboxylic Acid Ligand: Recognition and Detection of Small Drug Molecules and Aromatic Amine Molecules. Dalton Trans. 2022, 51, 9336–9347. [Google Scholar] [PubMed]
- Zhang, X.; Zhang, Y.-Z.; Zhang, D.-S.; Zhu, B.; Li, J.-R. A Hydrothermally Stable Zn(II)-Based Metal–Organic Framework: Structural Modulation and Gas Adsorption. Dalton Trans. 2015, 44, 15697–15702. [Google Scholar] [CrossRef]
- Park, H.J.; Suh, M.P. Enhanced Isosteric Heat, Selectivity, and Uptake Capacity of CO2 Adsorption in a Metal-Organic Framework by Impregnated Metal Ions. Chem. Sci. 2013, 4, 685–690. [Google Scholar] [CrossRef]
- Silva, A.R.; Alexandre, J.Y.; Souza, J.E.; Neto JG, L.; de Sousa Júnior, P.G.; Rocha, M.V.; Dos Santos, J.C. The Chemistry and Applications of Metal–Organic Frameworks (Mofs) as Industrial Enzyme Immobilization Systems. Molecules 2022, 27, 4529. [Google Scholar] [CrossRef] [PubMed]
- Yuan, S.; Feng, L.; Wang, K.; Pang, J.; Bosch, M.; Lollar, C.; Sun, Y.; Qin, J.; Yang, X.; Zhang, P. Stable Metal–Organic Frameworks: Design, Synthesis, and Applications. Adv. Mater. 2018, 30, 1704303. [Google Scholar]
- Tang, C.; Li, X.; Hu, Y.; Du, X.; Wang, S.; Chen, B.; Wang, S. Porphyrin-Based Metal-Organic Framework Materials: Design, Construction, and Application in the Field of Photocatalysis. Molecules 2024, 29, 467. [Google Scholar] [CrossRef]
- Gandara-Loe, J.; Pastor-Perez, L.; Bobadilla, L.F.; Odriozola, J.A.; Reina, T.R. Understanding the Opportunities of Metal–Organic Frameworks (Mofs) for CO2 Capture and Gas-Phase CO2 Conversion Processes: A Comprehensive Overview. React. Chem. Eng. 2021, 6, 787–814. [Google Scholar] [CrossRef]
- Freund, R.; Zaremba, O.; Arnauts, G.; Ameloot, R.; Skorupskii, G.; Dincă, M.; Bavykina, A.; Gascon, J.; Ejsmont, A.; Goscianska, J.; et al. The Current Status of Mof and Cof Applications. Angew. Chem. Int. Ed. 2021, 60, 23975–24001. [Google Scholar] [CrossRef]
- Mahmoud, E. Evolution of the Design of Ch4 Adsorbents. Surfaces 2020, 3, 433–466. [Google Scholar] [CrossRef]
- Gorbounov, M.; Halloran, P.; Soltani, S.M. Hydrophobic and Hydrophilic Functional Groups and Their Impact on Physical Adsorption of CO2 in Presence of H2O: A Critical Review. J. CO2 Util. 2024, 86, 102908. [Google Scholar] [CrossRef]
- Kalhor, S.; Sepehrmansourie, H.; Zarei, M.; Zolfigol, M.A.; Shi, H. Application of Functionalized Zn-Based Metal–Organic Frameworks (Zn-Mofs) with Cuo in Heterocycle Synthesis Via Azide–Alkyne Cycloaddition. Inorg. Chem. 2024, 63, 4898–4914. [Google Scholar] [CrossRef] [PubMed]
- Lin, Z.-J.; Lü, J.; Hong, M.; Cao, R. Metal–Organic Frameworks Based on Flexible Ligands (Fl-Mofs): Structures and Applications. Chem. Soc. Rev. 2014, 43, 5867–5895. [Google Scholar]
- ZareKarizi, F.; Joharian, M.; Morsali, A. Pillar-Layered Mofs: Functionality, Interpenetration, Flexibility and Applications. J. Mater. Chem. A 2018, 6, 19288–19329. [Google Scholar]
- Sheldrick, G.M. A Short History of Shelx. Found. Crystallogr. 2008, 64, 112–122. [Google Scholar] [CrossRef]
- Sheldrick, G.M. Crystal Structure Refinement with Shelxl. Cryst. Struct. Commun. 2015, 71, 3–8. [Google Scholar]
- De Lange, M.F.; Lin, L.C.; Gascon, J.; Vlugt, T.J.; Kapteijn, F. Assessing the Surface Area of Porous Solids: Limitations, Probe Molecules, and Methods. Langmuir 2016, 32, 12664–12675. [Google Scholar]
- Dombrowski, R.J.; Lastoskie, C.M.; Hyduke, D.R. The Horvath–Kawazoe Method Revisited. Colloids Surf. A Physicochem. Eng. Asp. 2001, 187, 23–39. [Google Scholar]
- Nguyen, C.; Do, D. The Dubinin–Radushkevich Equation and the Underlying Microscopic Adsorption Description. Carbon 2001, 39, 1327–1336. [Google Scholar]
- Zhao, H.; Ni, J.; Zhang, J.-J.; Liu, S.-Q.; Sun, Y.-J.; Zhou, H.; Li, Y.-Q.; Duan, C.-Y. A Trichromatic Mof Composite for Multidimensional Ratiometric Luminescent Sensing. Chem. Sci. 2018, 9, 2918–2926. [Google Scholar]
- Walton, K.S.; Snurr, R.Q. Applicability of the Bet Method for Determining Surface Areas of Microporous Metal− Organic Frameworks. J. Am. Chem. Soc. 2007, 129, 8552–8556. [Google Scholar]
- Boer, J.H. Studies on Pore Systems in Catalysts: V. The T Method. J. Catal. 1964, 4, 319–323. [Google Scholar]
- Rouquerol, J.; Llewellyn, P.; Rouquerol, F.J.S.C. Is the Bet Equation Applicable to Microporous Adsorbents? In Studies in Surface Science and Catalysis; Elsevier: Amsterdam, The Netherlands, 2007; pp. 49–56. [Google Scholar]
- Jura, G.; Harkins, W.D. Surfaces of Solids. Xi. Determination of the Decrease (Π) of Free Surface Energy of a Solid by an Adsorbed Film. J. Am. Chem. Soc. 1944, 66, 1356–1362. [Google Scholar]
- Revellame, E.D.; Fortela, D.L.; Sharp, W.; Hernandez, R.; Zappi, M.E. Adsorption Kinetic Modeling Using Pseudo-First Order and Pseudo-Second Order Rate Laws: A Review. Clean. Eng. Technol. 2020, 1, 100032. [Google Scholar] [CrossRef]
- Tan, K.L.; Hameed, B.H. Insight into the Adsorption Kinetics Models for the Removal of Contaminants from Aqueous Solutions. J. Taiwan Inst. Chem. Eng. 2017, 74, 25–48. [Google Scholar]
- Murphy, O.P.; Vashishtha, M.; Palanisamy, P.; Kumar, K.V. A Review on the Adsorption Isotherms and Design Calculations for the Optimization of Adsorbent Mass and Contact Time. ACS Omega 2023, 8, 17407–17430. [Google Scholar] [CrossRef]
- Myrick, M.L.; Simcock, M.N.; Baranowski, M.; Brooke, H.; Morgan, S.L.; McCutcheon, J.N. The Kubelka-Munk Diffuse Reflectance Formula Revisited. Appl. Spectrosc. Rev. 2011, 46, 140–165. [Google Scholar] [CrossRef]
- Wang, C.C.; Zhang, Y.Q.; Zhu, T.; Wang, P.; Gao, S.J. Photocatalytic Degradation of Methylene Blue and Methyl Orange in a Zn(II)-Based Metal–Organic Framework. Desalination Water Treat. 2016, 57, 17844–17851. [Google Scholar]
- Thi, Q.V.; Tamboli, M.S.; Ta, Q.T.H.; Kolekar, G.B.; Sohn, D. A Nanostructured Mof/Reduced Graphene Oxide Hybrid for Enhanced Photocatalytic Efficiency under Solar Light. Mater. Sci. Eng. B 2020, 261, 114678. [Google Scholar]
- Liu, N.; Jing, C.; Li, Z.; Huang, W.; Gao, B.; You, F.; Zhang, X. Effect of Synthesis Conditions on the Photocatalytic Degradation of Rhodamine B of Mil-53 (Fe). Mater. Lett. 2019, 237, 92–95. [Google Scholar]
- Pu, S.; Xu, L.; Sun, L.; Du, H. Tuning the Optical Properties of the Zirconium–Uio-66 Metal–Organic Framework for Photocatalytic Degradation of Methyl Orange. Inorg. Chem. Commun. 2015, 52, 50–52. [Google Scholar]
- Qian, L.-L.; Wang, Z.-X.; Tian, H.-X.; Li, M.; Li, B.-L.; Li, H.-Y. Synthesis, Structure and Photocatalytic Degradation of Organic Dyes of a Copper (II) Metal–Organic Framework (Cu–Mof) with a 4-Coordinated Three-Dimensional Cdso4 Topology. Cryst. Struct. Commun. 2019, 75, 1053–1059. [Google Scholar]
- Wei, F.; Chen, D.; Liang, Z.; Zhao, S. Comparison Study on the Adsorption Capacity of Rhodamine B, Congo Red, and Orange II on Fe-Mofs. Nanomaterials 2018, 8, 248. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.-Y.; Zhang, M.-L.; Ren, Y.-X.; Wang, J.-J.; Yang, X.-G. Design of Co-Mof Nanosheets for Efficient Adsorption and Photocatalytic Degradation of Organic Dyes. J. Mol. Struct. 2023, 1288, 135796. [Google Scholar]
- Li, Y.; Gao, C.; Jiao, J.; Cui, J.; Li, Z.; Song, Q. Selective Adsorption of Metal–Organic Framework toward Methylene Blue: Behavior and Mechanism. ACS Omega 2021, 6, 33961–33968. [Google Scholar] [PubMed]
- Zhou, E.-H.; Li, B.-H.; Chen, W.-X.; Luo, Z.; Liu, J.; Singh, A.; Kumar, A.; Jin, J.-C. Photocatalytic Degradation of Organic Dyes by a Stable and Biocompatible Zn(II) Mof Having Ferulic Acid: Experimental Findings and Theoretical Correlation. J. Mol. Struct. 2017, 1149, 352–356. [Google Scholar]
- Kitchamsetti, N.; Chakra, C.S.; De Barros, A.L.; Kim, D. Development of Mof Based Recyclable Photocatalyst for the Removal of Different Organic Dye Pollutants. Nanomaterials 2023, 13, 336. [Google Scholar] [CrossRef]
- Abdollahi, B.; Farshnama, S.; Asl, E.A.; Najafidoust, A.; Sarani, M. Cu (Bdc) Metal–Organic Framework (Mof)-Based Ag2cro4 Heterostructure with Enhanced Solar-Light Degradation of Organic Dyes. Inorg. Chem. Commun. 2022, 138, 109236. [Google Scholar]
- Zhang, X.; Wang, L.-J.; Han, Z.; Meng, X.; Wang, H.-N.; Zhou, Z.-Y.; Su, Z.-M. Degradation of Azo Dyes under Visible Light with Stable Mof Based on Tetrastyrene Imidazole Ligand. Dalton Trans. 2020, 49, 4352–4357. [Google Scholar]
- Zhang, C.; Ma, D.; Zhang, X.; Ma, J.; Liu, L.; Xu, X. Preparation, Structure and Photocatalysis of Metal-Organic Frameworks Derived from Aromatic Carboxylate and Imidazole-Based Ligands. J. Coord. Chem. 2016, 69, 985–995. [Google Scholar]
- Nguyen, V.H.; Pham, A.L.H.; Lee, T.; Nguyen, T.D. Facile Synthesis of Bismuth (III) Based Metal-Organic Framework with Difference Ligands Using Microwave Irradiation Method. Chem. Eng. Res. Des. 2022, 177, 321–330. [Google Scholar]
- Guesh, K.; Caiuby, C.A.D.; Mayoral, Á.; Díaz-García, M.; Díaz, I.; Sanchez-Sanchez, M. Sustainable Preparation of Mil-100 (Fe) and Its Photocatalytic Behavior in the Degradation of Methyl Orange in Water. Cryst. Growth Des. 2017, 17, 1806–1813. [Google Scholar]
- Ao, D.; Zhang, J.; Liu, H. Visible-Light-Driven Photocatalytic Degradation of Pollutants over Cu-Doped Nh2-Mil-125 (Ti). J. Photochem. Photobiol. A Chem. 2018, 364, 524–533. [Google Scholar]
- Abdi, J.; Banisharif, F.; Khataee, A. Amine-Functionalized Zr-Mof/Cnts Nanocomposite as an Efficient and Reusable Photocatalyst for Removing Organic Contaminants. J. Mol. Liq. 2021, 334, 116129. [Google Scholar]
- Roy, S.; Darabdhara, J.; Ahmaruzzaman, M. MoS2 Nanosheets@ Metal Organic Framework Nanocomposite for Enhanced Visible Light Degradation and Reduction of Hazardous Organic Contaminants. J. Clean. Prod. 2023, 430, 139517. [Google Scholar]
- Liu, J.; Zhan, H.; Wang, P.; Chen, M.; Zhu, X.; Han, J.; Fu, B. Assembling Biobr Nanoplates on Mil-125 (Ti)–Nh2 Via Group Linkage Towards Effective Dye-Contaminated Water Purification. J. Solid State Chem. 2024, 329, 124408. [Google Scholar]



| Identification Code | MOF-S1 |
|---|---|
| Empirical formula | C24H14N3O10.5Zn2 |
| Formula weight | 643.12 |
| Temperature/K | 273.15 |
| Crystal system | trigonal |
| Space group | P-31c |
| a/Å | 16.9557 |
| b/Å | 16.9557 |
| c/Å | 6.9191 |
| α/° | 90 |
| β/° | 90 |
| γ/° | 120 |
| Volume/Å3 | 1722.71 |
| Z | 2 |
| ρcalc g/cm3 | 1.240 |
| μ/mm−1 | 1.440 |
| F(000) | 646.0 |
| Crystal size/mm3 | 0.3 × 0.2 × 0.1 |
| Radiation | MoKα (λ = 0.71073) |
| 2Θ range for data collection/° | 6.51 to 50.896 |
| Index ranges | −20 ≤ h ≤ 20, −20 ≤ k ≤ 20, −8 ≤ l ≤ 8 |
| Reflections collected/independent | 33,897/1074 |
| Symmetry consistency and data precision indices | Rint = 0.0577, Rsigma = 0.0141 |
| Data/restraints/parameters | 1074/8/93 |
| Goodness-of-fit on F2 | 1.103 |
| Final R indices [I ≥ 2σ (I)] | R1 = 0.0635, wR2 = 0.1867 |
| Final R indices [all data] | R1 = 0.0713, wR2 = 0.1945 |
| Largest diff. peak/hole/e Å−3 | 0.79/−1.58 |
| CCDC number | 2,431,147 |
| MOF | Solvent | Addition | Temp | Duration | Yield | Reference |
|---|---|---|---|---|---|---|
| (°C) | (h) | % | ||||
| S1 | H2O/DMF | - | 105 | 14 | 78 | this work |
| 0.6/10 v/v | ||||||
| SNU-100’ | DMF | - | 90 | 24 | NA | [14] |
| DMA/ACN/H2O | HBF4 | 90 | 48 | 43.5 | [13] | |
| 2/2/2 v/v/v | ||||||
| Zn-TCPT | DMA/ACN/H2O | HBF4 | 90 | 48 | 44 | [10] |
| LMOF | DMF/EtOH/H2O | - | 100 | 3 | 74 | [12] |
| 2.5/2.5/1 v/v/v |
| SSA, m2/g, Langmuir | Vmicro, cm3/g | Vtotal, cm3/g (P/P0 = 0.96) | Smicro, m2/g, t-Plot | Pore Width Å, HK | Ads. E kJ/mol, DR | CO2 Uptake at 273 K, mmol/g | H2 Uptake at 77 K, mmol/g | |
|---|---|---|---|---|---|---|---|---|
| t-Plot | HK | |||||||
| 711 | 0.25 | 0.25 | 0.25 | 706 | 6.5 | 58 | 2.4 | 7.6 |
| C% | Light | Eg | t | C% | Light | Eg | t | Ref. | |
|---|---|---|---|---|---|---|---|---|---|
| Zn-MOF | 91.7 | UV | 3.6 | 40 | [39] | ||||
| MOF-5/rGO | 93 | vis | 3.4 | 20 | 97 | vis | 3.4 | 20 | [40] |
| MIL-53(Fe) with H2O2 | 98 | vis | 2.98 | 50 | [41] | ||||
| UiO-66(AN) | 65 | vis | 2.47 | 90 | [42] | ||||
| Cu–MOF | 90.3 | vis | 2.30 | 120 | [43] | ||||
| Fe-MOFs | 99.38 | vis | 300 | [44] | |||||
| Co-MOF | 23.4 | vis | 1.86 | 180 | 8.7 | vis | 1.86 | 180 | [45] |
| Cu-BTC | 47 | 13 | - | [46] | |||||
| {[Zn2(fer)2]∙0.5H2O}n | 88 | UV | 100 | [47] | |||||
| MOFs (Zn, Co, Ni, Fe, and Ag) | 74.5 | vis | 3.0 | 120 | [48] | ||||
| Ag2CrO4/Cu(BDC)(50) | 50 | vis | 2.92 | 40 | [49] | ||||
| Co-MOF | 95 | vis | 1.9 | 200 | [50] | ||||
| Zn(II)-imidazole MOF | 82 | UV | 4.97 | 180 | [51] | ||||
| Bi-MOF | 99.1 | vis | 180 | [52] | |||||
| MIL-100(Fe) | 64 | UV | 420 | [53] | |||||
| NH2-MIL-125(Ti) | 37 | vis | 90 | [54] | |||||
| UiO-66 | 42 | vis | 30 | [55] | |||||
| UiO-66-NH2 | 60 | vis | 30 | [55] | |||||
| MoS2-HKUST-1 | 96.4 | vis | 30 | [56] | |||||
| BiOBr/MIL-125-(NH2) | 91 | vis | 180 | [57] | |||||
| MOF-S1 | 93 | UV | 4.24 | 74 | UV | 4.24 | This work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Angelova, M.; Lazarova, H.; Kurteva, V.; Nikolova, R.; Rusew, R.; Shivachev, B. A Novel Zinc-Based MOF Featuring 2,4,6-Tris-(4-carboxyphenoxy)-1,3,5-triazine: Structure, Adsorption, and Photocatalytic Activity. Crystals 2025, 15, 348. https://doi.org/10.3390/cryst15040348
Angelova M, Lazarova H, Kurteva V, Nikolova R, Rusew R, Shivachev B. A Novel Zinc-Based MOF Featuring 2,4,6-Tris-(4-carboxyphenoxy)-1,3,5-triazine: Structure, Adsorption, and Photocatalytic Activity. Crystals. 2025; 15(4):348. https://doi.org/10.3390/cryst15040348
Chicago/Turabian StyleAngelova, Magdalena, Hristina Lazarova, Vanya Kurteva, Rositsa Nikolova, Rusi Rusew, and Boris Shivachev. 2025. "A Novel Zinc-Based MOF Featuring 2,4,6-Tris-(4-carboxyphenoxy)-1,3,5-triazine: Structure, Adsorption, and Photocatalytic Activity" Crystals 15, no. 4: 348. https://doi.org/10.3390/cryst15040348
APA StyleAngelova, M., Lazarova, H., Kurteva, V., Nikolova, R., Rusew, R., & Shivachev, B. (2025). A Novel Zinc-Based MOF Featuring 2,4,6-Tris-(4-carboxyphenoxy)-1,3,5-triazine: Structure, Adsorption, and Photocatalytic Activity. Crystals, 15(4), 348. https://doi.org/10.3390/cryst15040348







