Lithosphere Modification Beneath the North China Craton: Geochemical Constraints of Water Contents from the Damaping Peridotite Xenoliths
Abstract
1. Introduction
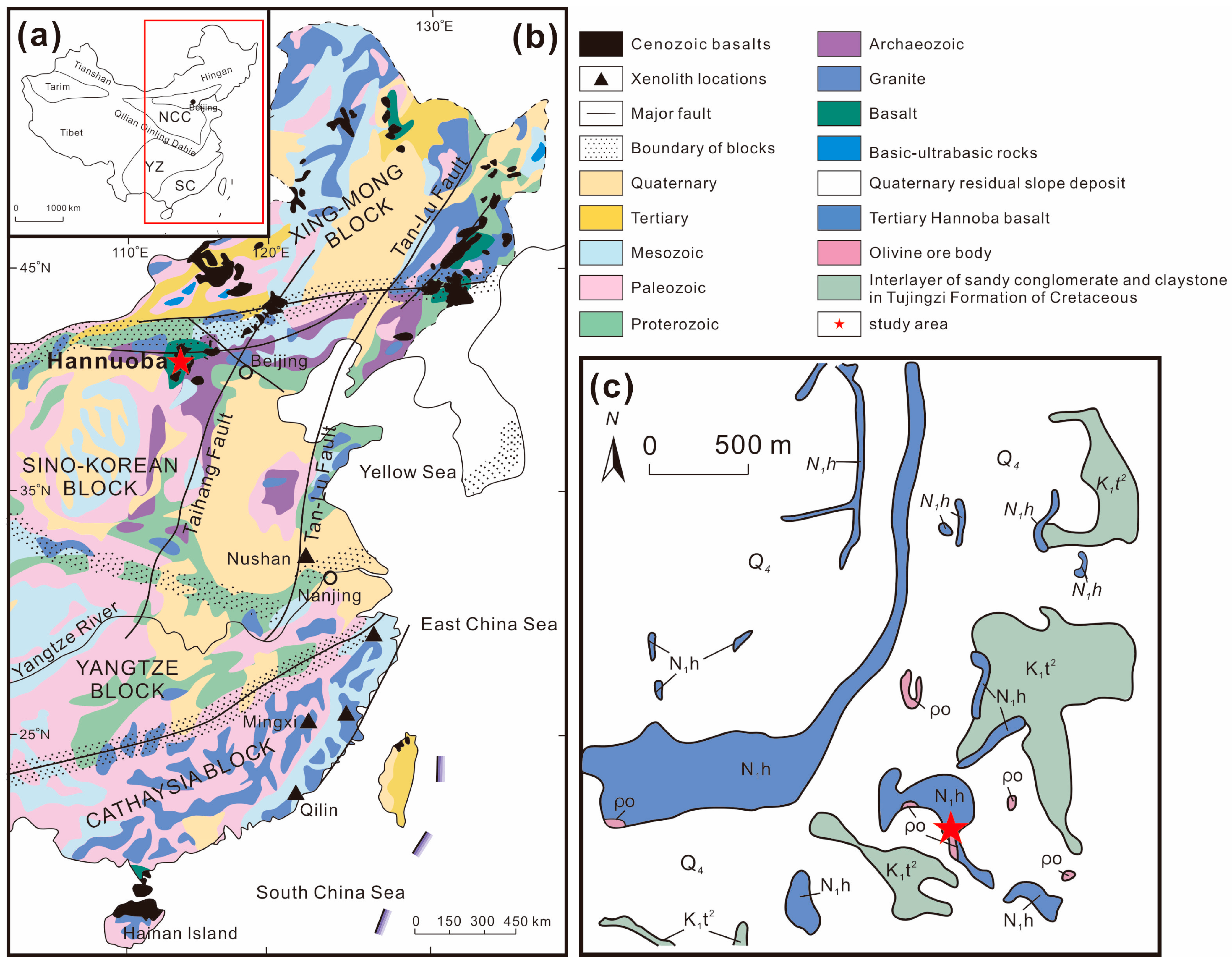
2. Geological Setting
3. Materials and Methods
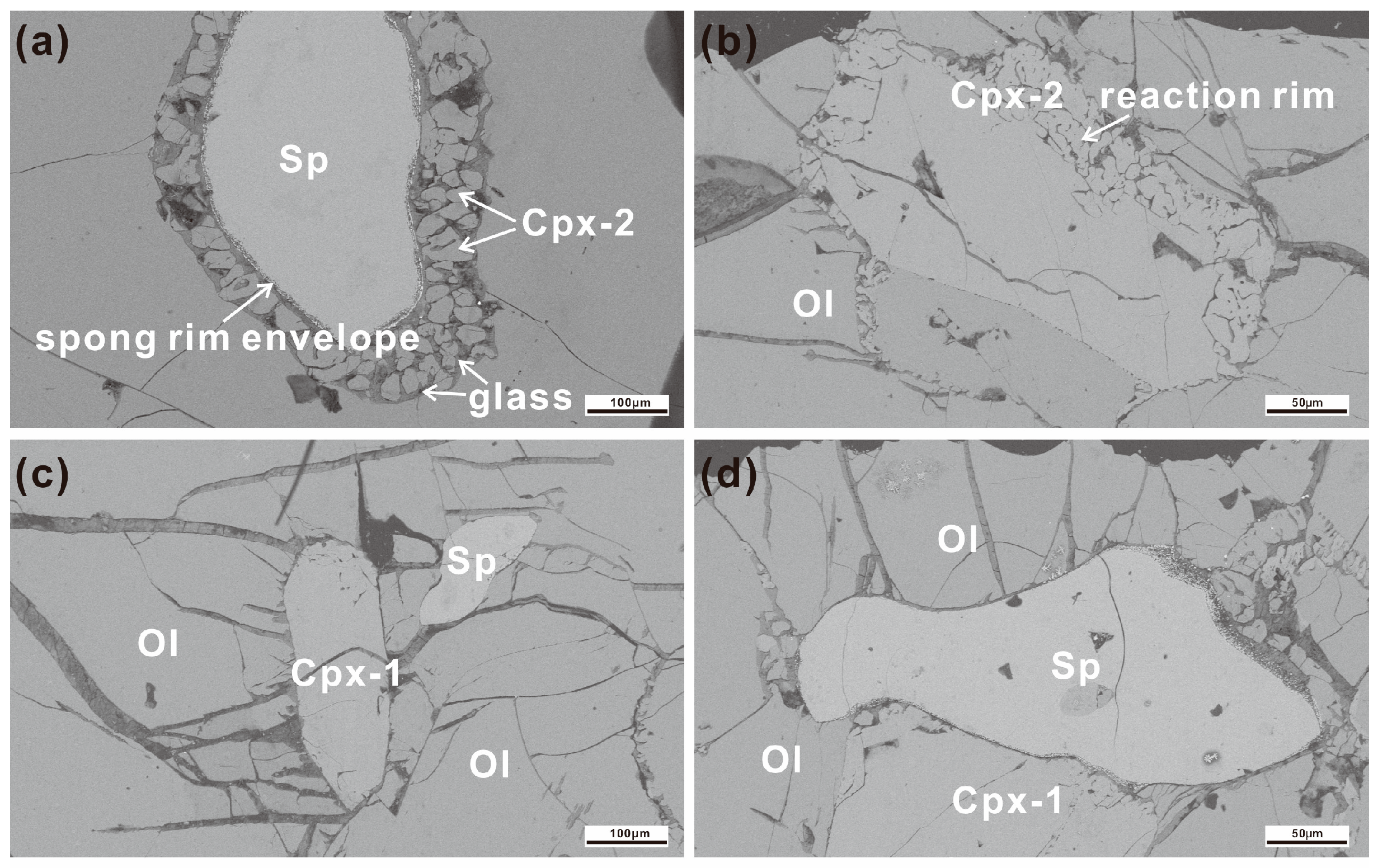
3.1. Sample Description
3.2. Methods
3.2.1. Microscopic Observation and Spectroscopy
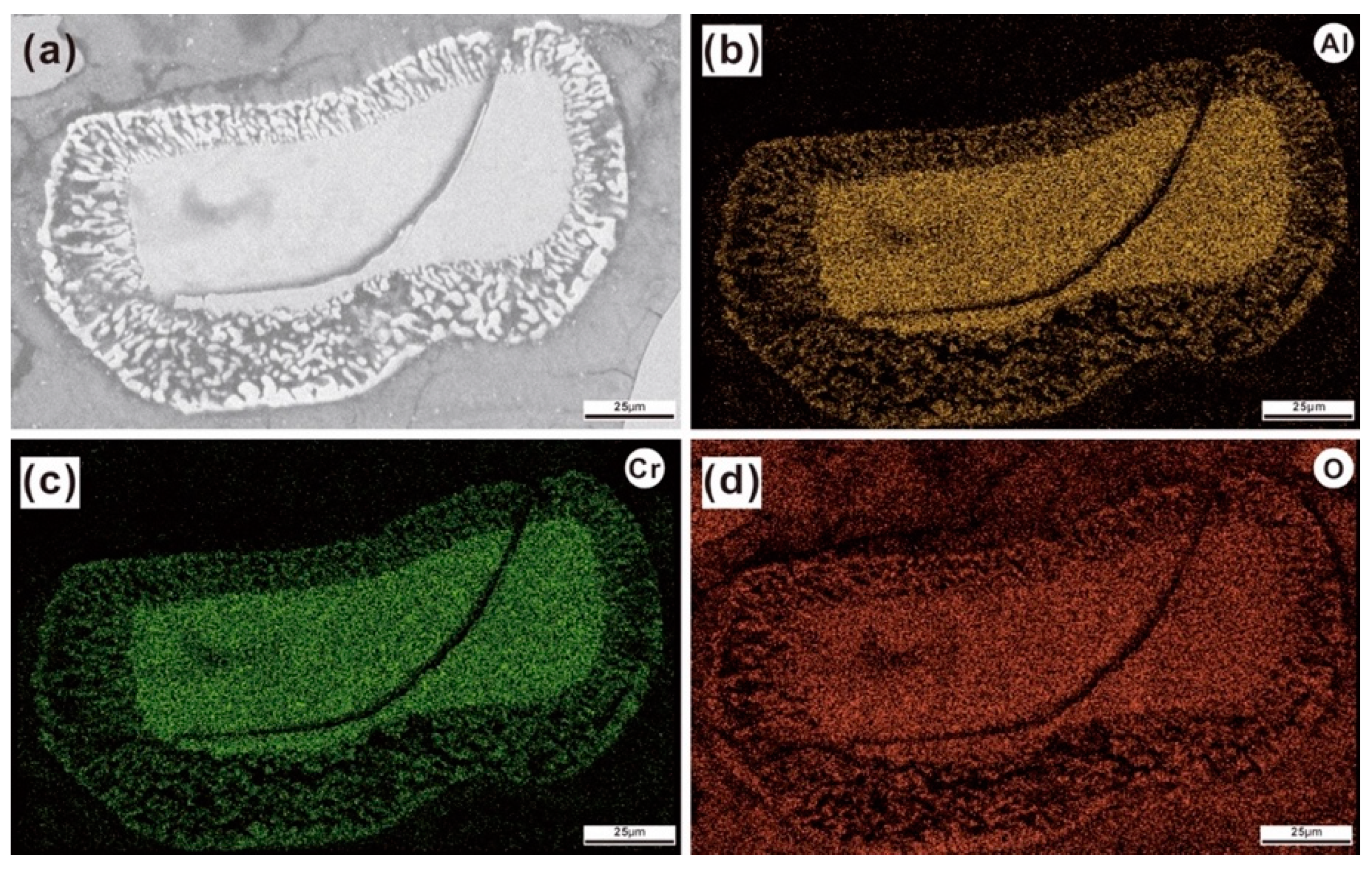
3.2.2. Scanning Electron Microscope
3.2.3. Chemical Analysis
3.2.4. Water Content Analysis
3.2.5. Isotope Analysis Methods
4. Results
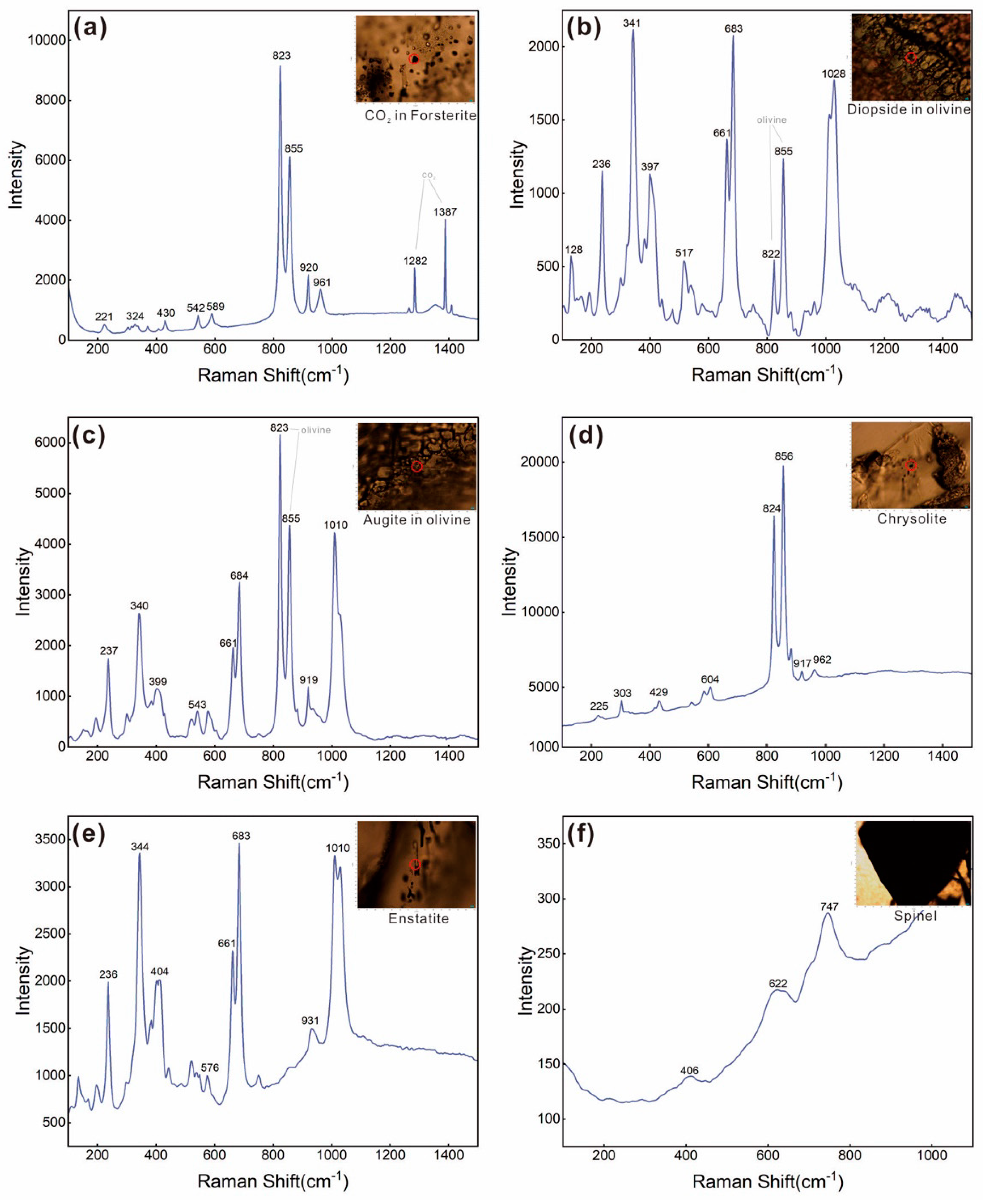
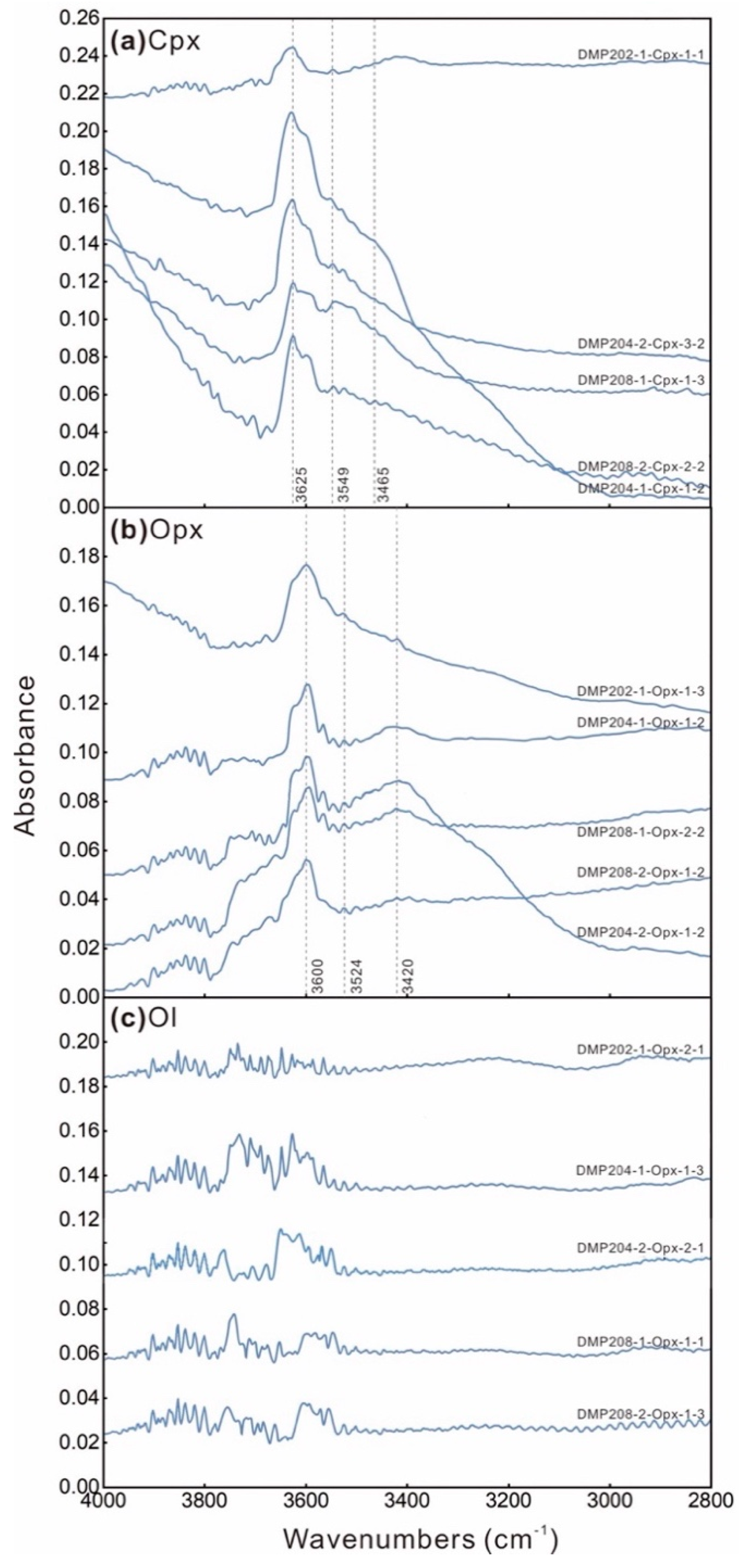
4.1. Raman Spectra
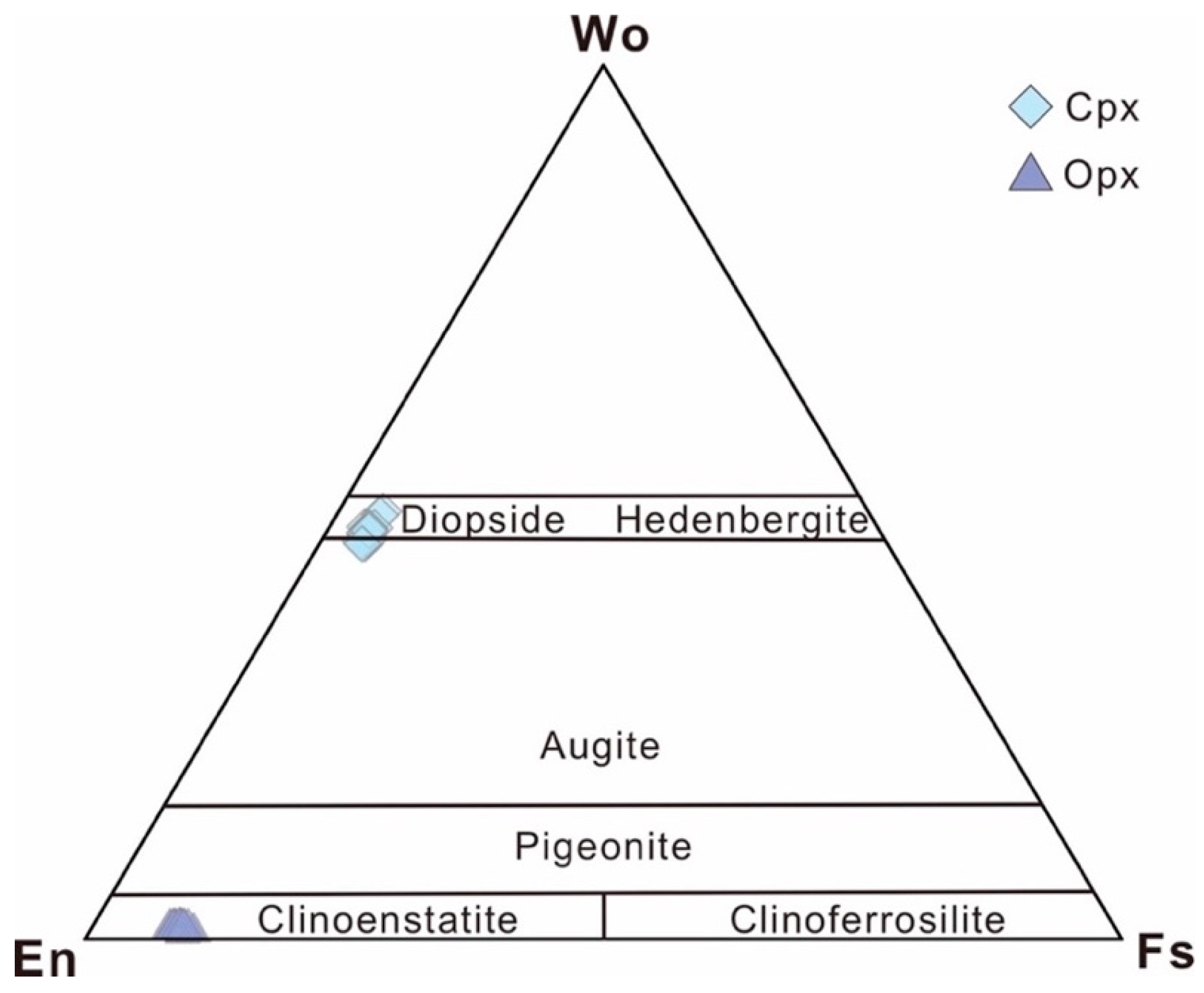
4.2. Major Elements
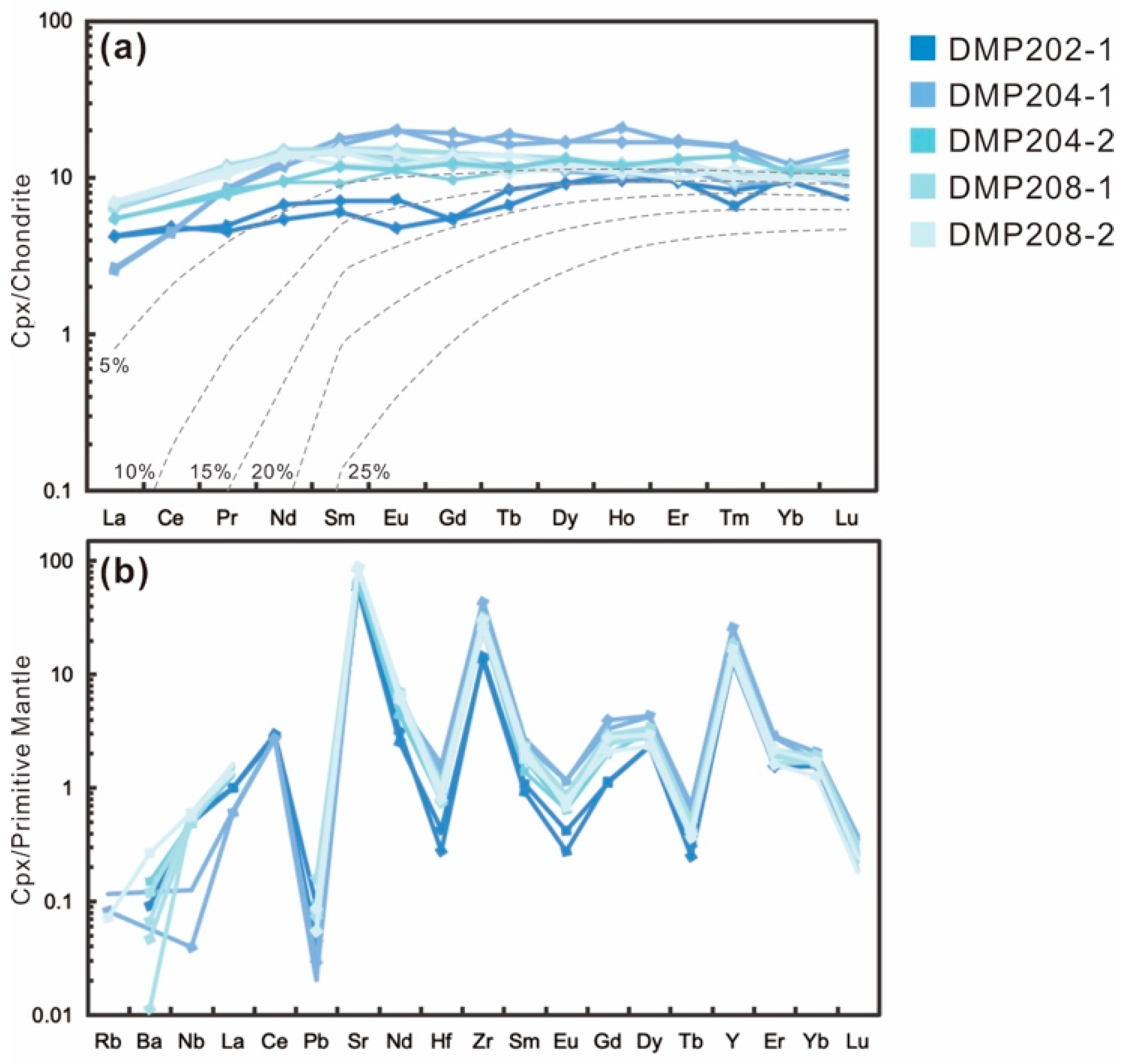
4.3. Trace Elements
4.4. Equilibrium Temperature of the Mantle Xenoliths
4.5. Water Contents
4.6. δ18O Values
5. Discussion
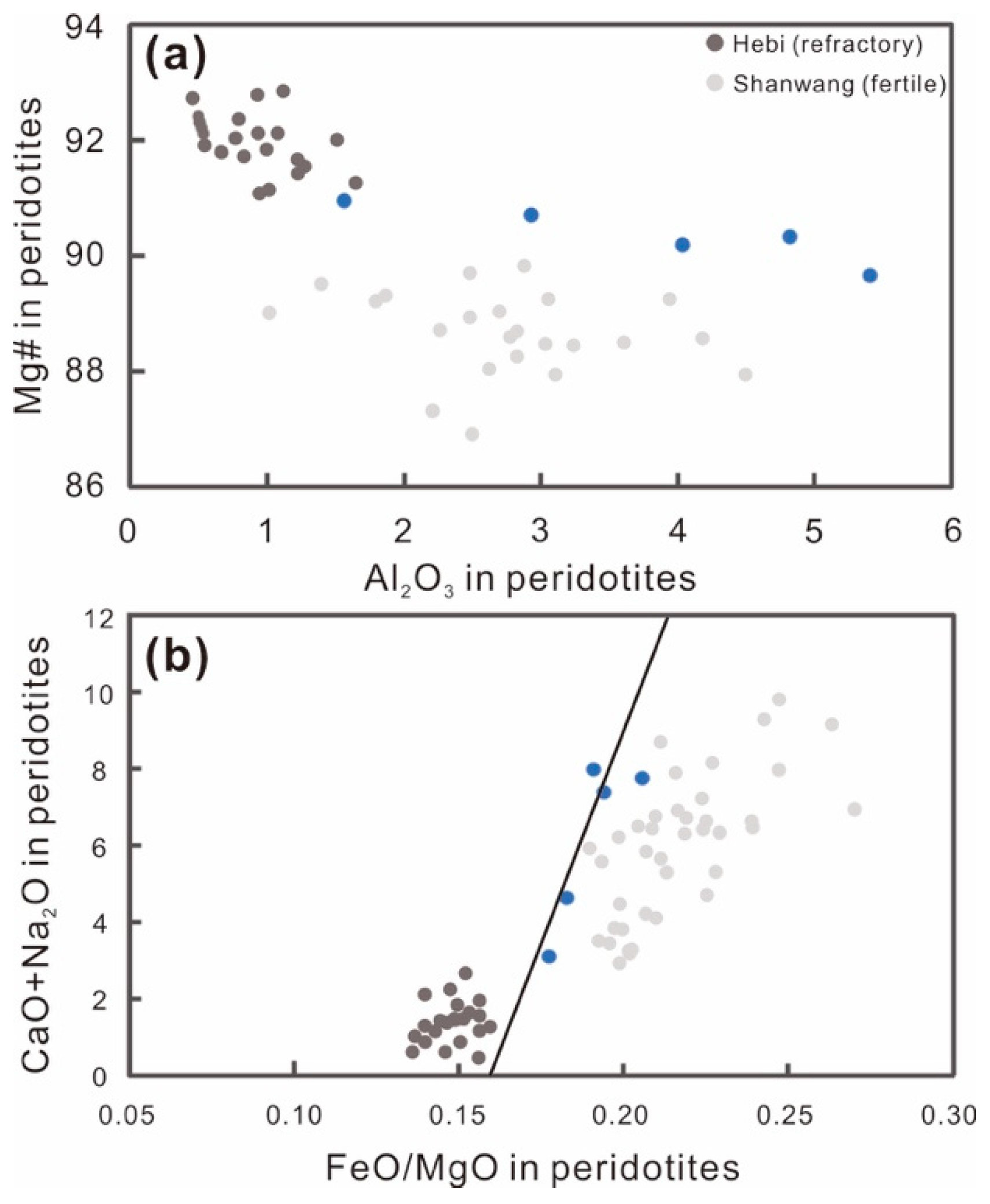
5.1. Melt Depletion and Enrichment Processes
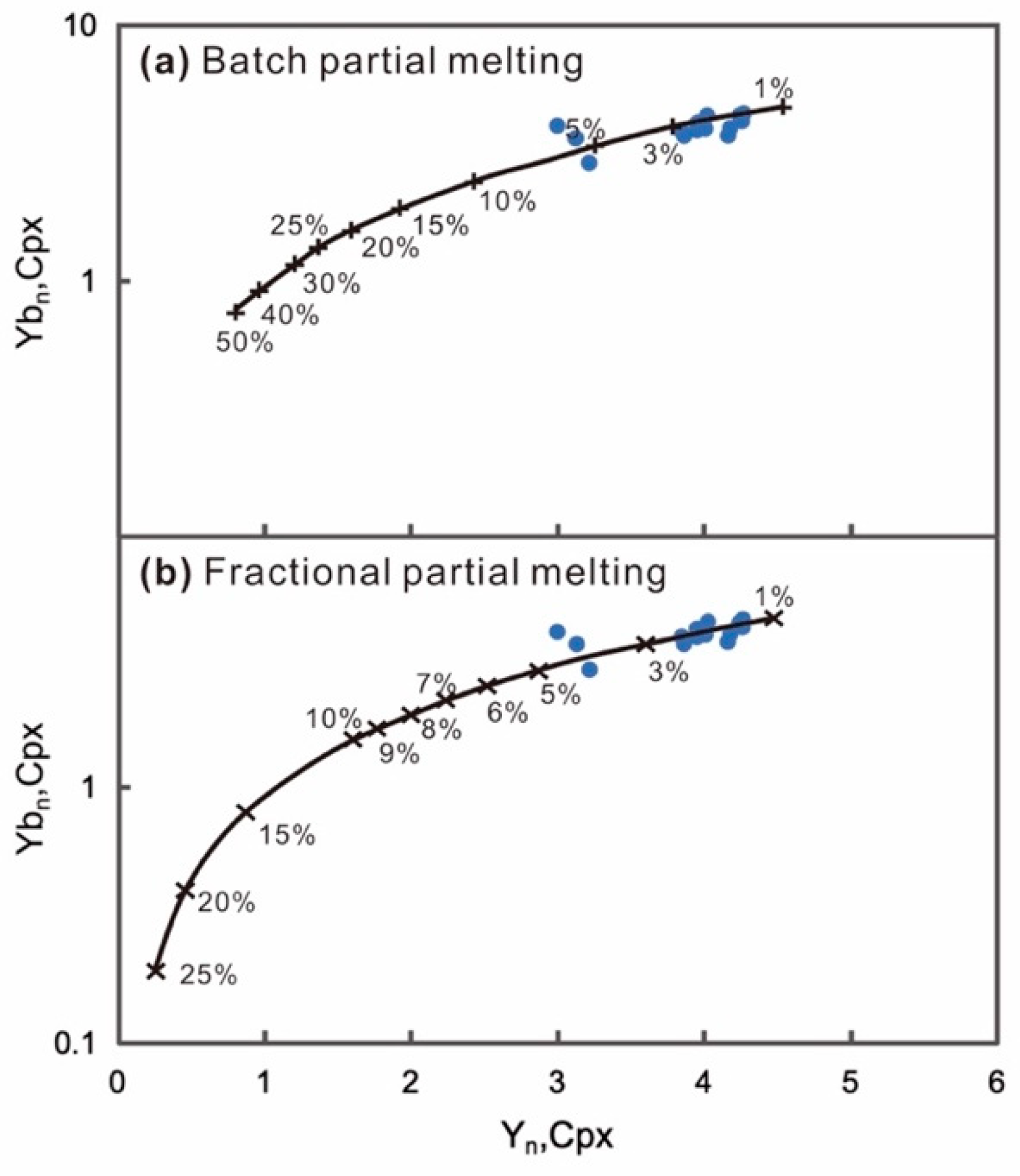
5.1.1. Partial Melting
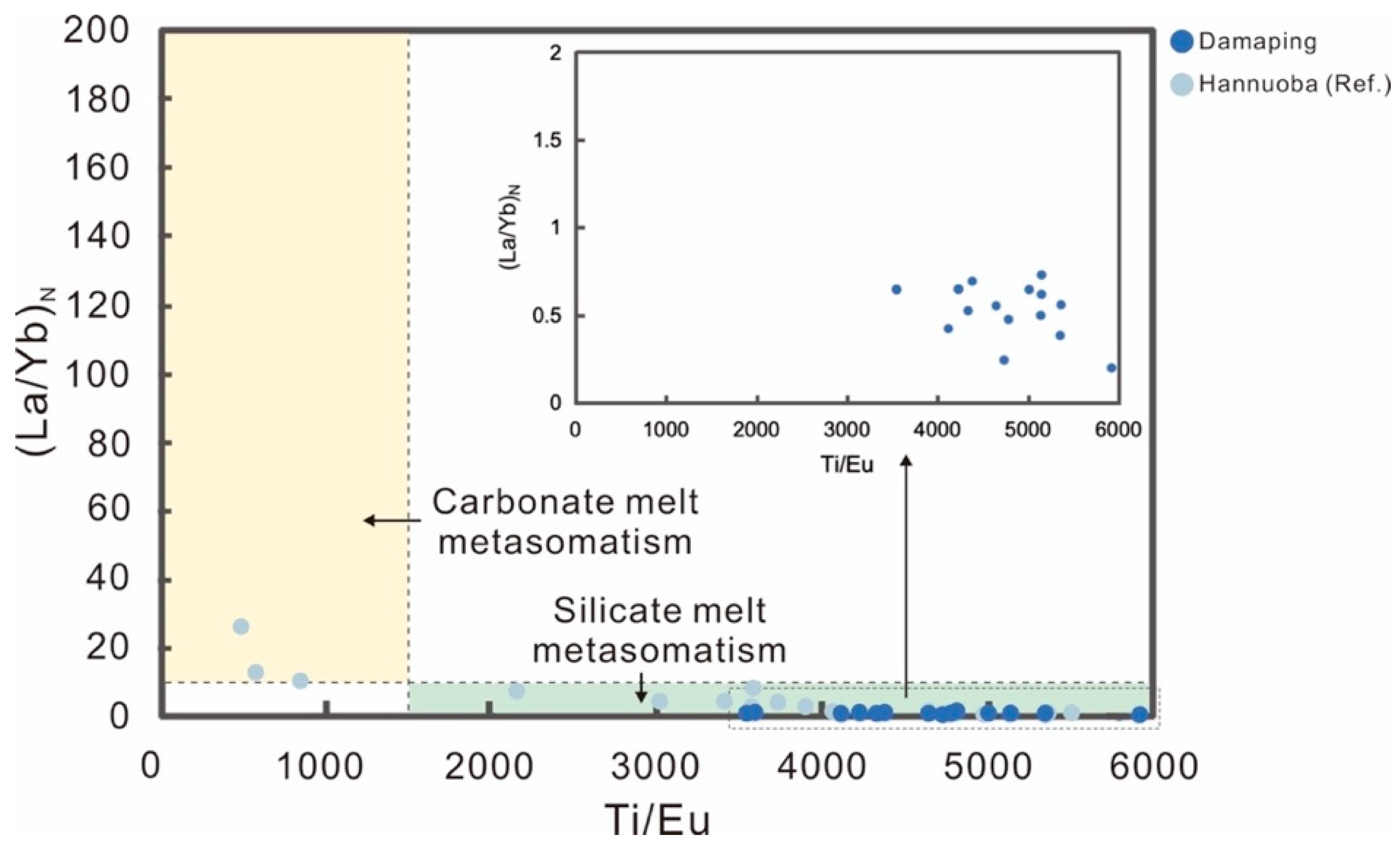
5.1.2. Silicate Melt Metasomatism
5.2. Preservation of the Initial Water Contents in the Mantle Source
5.3. Variations in H2O Contents Controlled by Partial Melting
5.4. Modification of the Lithospheric Mantle Beneath the Interior of the North China Craton
6. Conclusions
- (1)
- The Damaping spinel lherzolite shows moderately refractory characteristics (Mg# = 89.73–91.01). The lithospheric mantle has undergone 1–6% partial melting.
- (2)
- Water contents of clinopyroxene and orthopyroxene in the xenoliths are 13 to 19 ppm and 5 to 8 ppm. The calculated whole-rock water content ranges from 3 to 5.44 ppm. The heterogeneity in the water content of the lithospheric mantle is attributed to the effects of partial melting.
- (3)
- The MORB-like δ¹⁸O values (5.27–5.59‰) of Damaping spinel lherzolites reflect a mantle source similar to mid-ocean ridge basalts. Low water contents may result from Late Mesozoic–Early Cenozoic lithospheric reheating, which drove hydrogen diffusion from minerals like olivine.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Sample | SiO2 | Na2O | Cr2O3 | TiO2 | MgO | FeO | K2O | MnO | Al2O3 | NiO | CaO | Total | Mg# |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| DMP202-1-OL-1 | 41.76 | 0.02 | 0.07 | 0.00 | 49.21 | 8.91 | 0.01 | 0.10 | 0.04 | 0.29 | 0.07 | 100.47 | 90.78 |
| DMP202-1-OL-2 | 40.98 | 0.02 | 0.00 | 0.00 | 49.02 | 9.38 | 0.00 | 0.03 | 0.00 | 0.35 | 0.01 | 99.78 | 90.31 |
| DMP202-1-OL-3 | 41.04 | 0.03 | 0.00 | 0.06 | 48.61 | 9.02 | 0.02 | 0.03 | 0.05 | 0.26 | 0.04 | 99.14 | 90.58 |
| DMP202-1-OL-4 | 40.79 | 0.05 | 0.02 | 0.00 | 48.57 | 9.05 | 0.00 | 0.01 | 0.04 | 0.29 | 0.06 | 98.87 | 90.54 |
| DMP202-1-OL-5 | 41.54 | 0.02 | 0.02 | 0.01 | 48.50 | 9.28 | 0.03 | 0.12 | 0.00 | 0.22 | 0.07 | 99.82 | 90.31 |
| DMP204-1-OL-1 | 40.99 | 0.04 | 0.01 | 0.00 | 47.88 | 9.78 | 0.00 | 0.08 | 0.00 | 0.30 | 0.00 | 99.07 | 89.73 |
| DMP204-1-OL-2 | 40.37 | 0.03 | 0.00 | 0.00 | 48.64 | 9.89 | 0.00 | 0.14 | 0.00 | 0.24 | 0.00 | 99.32 | 89.76 |
| DMP204-1-OL-3 | 40.24 | 0.00 | 0.02 | 0.05 | 48.39 | 9.88 | 0.01 | 0.10 | 0.02 | 0.27 | 0.02 | 98.98 | 89.73 |
| DMP204-1-OL-4 | 40.47 | 0.03 | 0.00 | 0.00 | 48.15 | 9.46 | 0.00 | 0.14 | 0.00 | 0.33 | 0.04 | 98.61 | 90.08 |
| DMP204-1-OL-5 | 40.43 | 0.02 | 0.02 | 0.00 | 47.93 | 9.59 | 0.01 | 0.08 | 0.00 | 0.27 | 0.00 | 98.35 | 89.91 |
| DMP204-2-OL-1 | 40.58 | 0.02 | 0.03 | 0.05 | 48.49 | 8.86 | 0.01 | 0.21 | 0.01 | 0.25 | 0.01 | 98.52 | 90.71 |
| DMP204-2-OL-2 | 41.62 | 0.01 | 0.02 | 0.04 | 48.91 | 9.03 | 0.00 | 0.13 | 0.00 | 0.46 | 0.07 | 100.28 | 90.62 |
| DMP204-2-OL-3 | 41.41 | 0.02 | 0.02 | 0.01 | 48.48 | 8.54 | 0.00 | 0.16 | 0.00 | 0.34 | 0.00 | 98.98 | 91.01 |
| DMP204-2-OL-4 | 40.41 | 0.05 | 0.00 | 0.01 | 48.91 | 9.20 | 0.01 | 0.20 | 0.05 | 0.32 | 0.05 | 99.21 | 90.46 |
| DMP204-2-OL-5 | 40.77 | 0.01 | 0.05 | 0.06 | 49.24 | 9.24 | 0.01 | 0.16 | 0.00 | 0.24 | 0.05 | 99.81 | 90.48 |
| DMP208-1-OL-1 | 39.62 | 0.02 | 0.01 | 0.00 | 47.99 | 9.30 | 0.00 | 0.03 | 0.03 | 0.32 | 0.08 | 97.41 | 90.20 |
| DMP208-1-OL-2 | 40.99 | 0.02 | 0.04 | 0.00 | 48.12 | 9.34 | 0.01 | 0.11 | 0.00 | 0.25 | 0.03 | 98.90 | 90.19 |
| DMP208-1-OL-3 | 41.36 | 0.03 | 0.02 | 0.01 | 49.36 | 9.95 | 0.00 | 0.18 | 0.01 | 0.24 | 0.05 | 101.21 | 89.84 |
| DMP208-1-OL-4 | 41.17 | 0.00 | 0.04 | 0.02 | 48.44 | 9.31 | 0.03 | 0.01 | 0.03 | 0.26 | 0.08 | 99.38 | 90.27 |
| DMP208-1-OL-5 | 41.19 | 0.03 | 0.00 | 0.00 | 48.56 | 9.52 | 0.01 | 0.13 | 0.03 | 0.26 | 0.04 | 99.76 | 90.10 |
| DMP208-2-OL-1 | 40.29 | 0.02 | 0.00 | 0.04 | 48.08 | 9.17 | 0.00 | 0.21 | 0.03 | 0.28 | 0.11 | 98.22 | 90.34 |
| DMP208-2-OL-2 | 40.16 | 0.03 | 0.00 | 0.00 | 48.61 | 9.08 | 0.00 | 0.13 | 0.00 | 0.35 | 0.01 | 98.37 | 90.51 |
| DMP208-2-OL-3 | 40.79 | 0.02 | 0.00 | 0.01 | 48.61 | 9.13 | 0.01 | 0.18 | 0.00 | 0.41 | 0.07 | 99.23 | 90.47 |
| DMP208-2-OL-4 | 41.06 | 0.03 | 0.01 | 0.00 | 48.17 | 9.45 | 0.04 | 0.10 | 0.02 | 0.23 | 0.07 | 99.18 | 90.09 |
| DMP208-2-OL-5 | 41.07 | 0.03 | 0.03 | 0.03 | 47.95 | 9.38 | 0.00 | 0.17 | 0.00 | 0.21 | 0.03 | 98.90 | 90.11 |
| Sample | SiO2 | Na2O | Cr2O3 | TiO2 | MgO | FeO | K2O | MnO | Al2O3 | CaO | Total | Mg# | Cr# | En | Fs | Wo |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| DMP202-1-OPX-1 | 54.74 | 0.09 | 0.55 | 0.06 | 33.10 | 4.99 | 0.02 | 0.03 | 3.78 | 0.60 | 97.96 | 92.20 | 8.81 | 0.91 | 0.08 | 0.01 |
| DMP202-1-OPX-2 | 55.65 | 0.08 | 0.47 | 0.03 | 32.52 | 5.49 | 0.01 | 0.16 | 3.77 | 0.61 | 98.79 | 91.35 | 7.73 | 0.90 | 0.09 | 0.01 |
| DMP202-1-OPX-3 | 54.64 | 0.10 | 0.51 | 0.06 | 33.10 | 5.02 | 0.00 | 0.18 | 3.63 | 0.61 | 97.84 | 92.17 | 8.61 | 0.91 | 0.08 | 0.01 |
| DMP202-1-OPX-4 | 55.89 | 0.06 | 0.54 | 0.07 | 33.12 | 4.88 | 0.04 | 0.01 | 3.82 | 0.68 | 99.12 | 92.37 | 8.67 | 0.91 | 0.08 | 0.01 |
| DMP202-1-OPX-5 | 55.09 | 0.09 | 0.43 | 0.04 | 33.18 | 5.02 | 0.00 | 0.13 | 3.68 | 0.71 | 98.37 | 92.18 | 7.22 | 0.91 | 0.08 | 0.01 |
| DMP204-1-OPX-1 | 56.00 | 0.12 | 0.37 | 0.12 | 33.27 | 5.54 | 0.00 | 0.09 | 3.91 | 0.73 | 100.15 | 91.46 | 5.98 | 0.90 | 0.09 | 0.01 |
| DMP204-1-OPX-2 | 55.63 | 0.12 | 0.43 | 0.09 | 33.37 | 5.79 | 0.00 | 0.12 | 3.83 | 0.68 | 100.06 | 91.13 | 7.03 | 0.90 | 0.09 | 0.01 |
| DMP204-1-OPX-3 | 56.41 | 0.10 | 0.51 | 0.16 | 33.94 | 5.84 | 0.00 | 0.13 | 3.90 | 0.64 | 101.64 | 91.20 | 8.06 | 0.90 | 0.09 | 0.01 |
| DMP204-1-OPX-4 | 55.95 | 0.07 | 0.36 | 0.06 | 33.71 | 6.07 | 0.00 | 0.05 | 3.84 | 0.58 | 100.68 | 90.82 | 5.88 | 0.90 | 0.09 | 0.01 |
| DMP204-2-OPX-1 | 56.00 | 0.12 | 0.37 | 0.12 | 33.27 | 5.54 | 0.00 | 0.09 | 3.91 | 0.73 | 100.15 | 91.46 | 5.98 | 0.90 | 0.09 | 0.01 |
| DMP204-2-OPX-2 | 55.63 | 0.12 | 0.43 | 0.09 | 33.37 | 5.79 | 0.00 | 0.12 | 3.83 | 0.68 | 100.06 | 91.13 | 7.03 | 0.90 | 0.09 | 0.01 |
| DMP204-2-OPX-3 | 56.41 | 0.10 | 0.51 | 0.16 | 33.94 | 5.84 | 0.00 | 0.13 | 3.90 | 0.64 | 101.64 | 91.20 | 8.06 | 0.90 | 0.09 | 0.01 |
| DMP204-2-OPX-4 | 55.95 | 0.07 | 0.36 | 0.06 | 33.71 | 6.07 | 0.00 | 0.05 | 3.84 | 0.58 | 100.68 | 90.82 | 5.88 | 0.90 | 0.09 | 0.01 |
| DMP208-1-OPX-1 | 55.27 | 0.08 | 0.44 | 0.15 | 33.08 | 5.02 | 0.01 | 0.26 | 3.81 | 0.68 | 98.79 | 92.16 | 7.18 | 0.91 | 0.08 | 0.01 |
| DMP208-1-OPX-2 | 55.85 | 0.07 | 0.44 | 0.17 | 33.02 | 5.13 | 0.00 | 0.20 | 3.84 | 0.63 | 99.36 | 91.99 | 7.19 | 0.91 | 0.08 | 0.01 |
| DMP208-1-OPX-3 | 55.23 | 0.05 | 0.39 | 0.15 | 33.36 | 5.22 | 0.01 | 0.10 | 3.90 | 0.60 | 99.00 | 91.94 | 6.27 | 0.91 | 0.08 | 0.01 |
| DMP208-1-OPX-4 | 56.39 | 0.05 | 0.43 | 0.11 | 33.61 | 5.16 | 0.00 | 0.13 | 3.88 | 0.58 | 100.34 | 92.07 | 6.85 | 0.91 | 0.08 | 0.01 |
| DMP208-1-OPX-5 | 55.78 | 0.07 | 0.43 | 0.12 | 33.27 | 4.98 | 0.02 | 0.17 | 3.84 | 0.66 | 99.34 | 92.25 | 6.95 | 0.91 | 0.08 | 0.01 |
| DMP208-2-OPX-2 | 54.49 | 0.09 | 0.43 | 0.02 | 33.01 | 6.03 | 0.00 | 0.10 | 3.64 | 0.67 | 98.46 | 90.71 | 7.33 | 0.89 | 0.09 | 0.01 |
| DMP208-2-OPX-3 | 54.72 | 0.08 | 0.45 | 0.04 | 33.09 | 5.40 | 0.00 | 0.08 | 3.73 | 0.75 | 98.33 | 91.62 | 7.50 | 0.90 | 0.08 | 0.01 |
| DMP208-2-OPX-4 | 55.33 | 0.10 | 0.41 | 0.16 | 33.36 | 5.76 | 0.00 | 0.12 | 3.85 | 0.69 | 99.77 | 91.18 | 6.72 | 0.90 | 0.09 | 0.01 |
| Sample | SiO2 | Na2O | Cr2O3 | TiO2 | MgO | FeO | K2O | MnO | Al2O3 | CaO | Total | Mg# | Cr# | En | Fs | Wo |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| DMP202-1-CPX-1 | 52.96 | 1.10 | 1.14 | 0.15 | 16.22 | 2.38 | 0.00 | 0.09 | 4.60 | 20.61 | 99.25 | 92.39 | 6.55 | 0.50 | 0.04 | 0.46 |
| DMP202-1-CPX-2 | 52.20 | 1.03 | 1.20 | 0.17 | 15.87 | 1.96 | 0.03 | 0.01 | 4.70 | 21.11 | 98.27 | 93.51 | 14.64 | 0.49 | 0.03 | 0.47 |
| DMP202-1-CPX-3 | 52.68 | 1.13 | 1.11 | 0.19 | 16.34 | 2.22 | 0.00 | 0.01 | 4.92 | 21.21 | 99.81 | 92.92 | 13.10 | 0.50 | 0.04 | 0.46 |
| DMP204-1-CPX-1 | 52.22 | 1.56 | 0.95 | 0.63 | 15.50 | 2.35 | 0.00 | 0.05 | 6.20 | 19.39 | 98.85 | 92.15 | 9.36 | 0.50 | 0.04 | 0.45 |
| DMP204-1-CPX-2 | 51.70 | 1.57 | 0.84 | 0.68 | 14.59 | 2.28 | 0.00 | 0.10 | 5.95 | 21.27 | 98.97 | 91.94 | 8.62 | 0.47 | 0.04 | 0.49 |
| DMP204-1-CPX-3 | 52.35 | 1.84 | 0.97 | 0.55 | 14.58 | 2.18 | 0.00 | 0.14 | 6.72 | 20.81 | 100.13 | 92.28 | 8.85 | 0.47 | 0.04 | 0.49 |
| DMP204-2-CPX-1 | 52.62 | 1.32 | 1.02 | 0.26 | 16.23 | 2.17 | 0.01 | 0.08 | 5.36 | 20.59 | 99.66 | 93.03 | 11.29 | 0.50 | 0.04 | 0.46 |
| DMP204-2-CPX-2 | 52.53 | 1.27 | 0.94 | 0.33 | 15.76 | 2.23 | 0.00 | 0.13 | 5.28 | 20.90 | 99.37 | 92.66 | 10.70 | 0.49 | 0.04 | 0.47 |
| DMP204-2-CPX-3 | 52.75 | 1.35 | 1.23 | 0.31 | 15.88 | 2.22 | 0.00 | 0.00 | 5.51 | 20.58 | 99.83 | 92.72 | 13.04 | 0.50 | 0.04 | 0.46 |
| DMP208-1-CPX-1 | 53.30 | 1.34 | 1.11 | 0.38 | 15.82 | 2.34 | 0.02 | 0.00 | 5.43 | 20.51 | 100.25 | 92.33 | 12.07 | 0.50 | 0.04 | 0.46 |
| DMP208-1-CPX-2 | 51.72 | 1.33 | 1.09 | 0.35 | 15.91 | 2.37 | 0.04 | 0.03 | 5.47 | 20.58 | 98.88 | 92.28 | 11.81 | 0.50 | 0.04 | 0.46 |
| DMP208-1-CPX-3 | 52.41 | 1.18 | 1.29 | 0.43 | 15.68 | 2.39 | 0.00 | 0.14 | 5.95 | 21.15 | 100.63 | 92.14 | 12.74 | 0.49 | 0.04 | 0.47 |
| DMP208-1-CPX-4 | 52.95 | 1.28 | 1.04 | 0.40 | 15.79 | 2.09 | 0.04 | 0.10 | 5.38 | 21.09 | 100.16 | 93.08 | 11.52 | 0.49 | 0.04 | 0.47 |
| DMP208-1-CPX-5 | 52.07 | 1.13 | 1.04 | 0.38 | 15.87 | 2.08 | 0.00 | 0.07 | 5.26 | 20.63 | 98.53 | 93.16 | 11.68 | 0.50 | 0.04 | 0.46 |
| DMP208-1-CPX-6 | 52.44 | 1.31 | 0.88 | 0.29 | 16.01 | 2.49 | 0.00 | 0.06 | 5.18 | 20.45 | 99.10 | 91.98 | 10.25 | 0.50 | 0.04 | 0.46 |
| DMP208-2-CPX-1 | 52.27 | 1.23 | 1.17 | 0.33 | 15.79 | 2.22 | 0.00 | 0.07 | 5.07 | 21.04 | 99.18 | 92.70 | 13.41 | 0.49 | 0.04 | 0.47 |
| DMP208-2-CPX-2 | 53.54 | 1.15 | 1.24 | 0.41 | 16.09 | 2.14 | 0.00 | 0.05 | 5.09 | 20.12 | 99.83 | 93.06 | 14.06 | 0.51 | 0.04 | 0.46 |
| DMP208-2-CPX-3 | 51.97 | 1.20 | 0.82 | 0.29 | 15.86 | 2.28 | 0.01 | 0.03 | 5.13 | 20.95 | 98.54 | 92.55 | 9.69 | 0.49 | 0.04 | 0.47 |
| DMP208-2-CPX-4 | 53.63 | 1.24 | 0.91 | 0.30 | 16.17 | 2.28 | 0.01 | 0.06 | 4.98 | 20.10 | 99.67 | 92.66 | 10.89 | 0.51 | 0.04 | 0.45 |
| Sample | SiO2 | Na2O | Cr2O3 | TiO2 | MgO | FeO | K2O | MnO | Al2O3 | CaO | Total | Mg# | Cr# |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| DMP202-1-SP-1 | 0.12 | 0.02 | 19.19 | 0.15 | 17.43 | 10.70 | 0.02 | 0.12 | 45.65 | 0.01 | 93.41 | 74.38 | 22.00 |
| DMP202-1-SP-3 | 0.10 | 0.00 | 20.95 | 0.07 | 18.72 | 11.12 | 0.01 | 0.11 | 48.43 | 0.00 | 99.50 | 75.01 | 22.50 |
| DMP202-1-SP-5 | 0.12 | 0.02 | 21.12 | 0.08 | 18.98 | 10.71 | 0.00 | 0.13 | 49.67 | 0.00 | 100.84 | 75.96 | 22.20 |
| DMP204-1-SP-1 | 0.08 | 0.07 | 12.19 | 0.15 | 19.52 | 10.77 | 0.03 | 0.18 | 57.42 | 0.02 | 100.41 | 76.36 | 12.47 |
| DMP204-1-SP-2 | 0.05 | 0.04 | 12.59 | 0.19 | 19.49 | 11.47 | 0.02 | 0.07 | 56.85 | 0.00 | 100.77 | 75.18 | 12.94 |
| DMP204-2-SP-1 | 0.07 | 0.02 | 18.07 | 0.08 | 18.98 | 11.10 | 0.00 | 0.10 | 50.44 | 0.02 | 98.87 | 75.30 | 19.38 |
| DMP204-2-SP-2 | 0.11 | 0.02 | 17.76 | 0.20 | 19.29 | 10.89 | 0.00 | 0.08 | 51.16 | 0.00 | 99.50 | 75.94 | 18.90 |
| DMP208-1-SP-1 | 0.10 | 0.06 | 17.56 | 0.22 | 19.16 | 11.31 | 0.01 | 0.07 | 51.46 | 0.01 | 99.96 | 75.13 | 18.63 |
| DMP208-1-SP-2 | 0.08 | 0.03 | 19.88 | 0.19 | 18.92 | 11.51 | 0.00 | 0.07 | 49.89 | 0.00 | 100.57 | 74.56 | 21.10 |
| DMP208-1-SP-3 | 0.06 | 0.00 | 19.38 | 0.23 | 19.09 | 11.89 | 0.00 | 0.13 | 49.66 | 0.00 | 100.43 | 74.11 | 20.75 |
| DMP208-2-SP-1 | 0.05 | 0.03 | 20.41 | 0.18 | 18.44 | 10.98 | 0.01 | 0.10 | 48.04 | 0.00 | 98.24 | 74.97 | 22.18 |
| DMP208-2-SP-2 | 0.07 | 0.01 | 20.70 | 0.21 | 18.66 | 12.24 | 0.00 | 0.03 | 48.52 | 0.02 | 100.46 | 73.11 | 22.26 |
| DMP208-2-SP-3 | 0.07 | 0.01 | 18.82 | 0.17 | 19.28 | 11.85 | 0.00 | 0.03 | 49.36 | 0.00 | 99.59 | 74.37 | 20.37 |
| Sample | SiO2 | Na2O | Cr2O3 | TiO2 | MgO | FeO | K2O | MnO | Al2O3 | CaO | Total | Mg# |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| DMP202-1 | 46.40 | 0.11 | 0.20 | 0.04 | 41.64 | 7.39 | 0.01 | 0.07 | 1.57 | 1.52 | 99.13 | 90.94 |
| DMP204-1 | 43.71 | 0.21 | 1.00 | 0.12 | 38.48 | 7.92 | 0.00 | 0.11 | 5.41 | 2.33 | 99.45 | 89.65 |
| DMP204-2 | 46.80 | 0.14 | 0.62 | 0.08 | 40.00 | 7.32 | 0.00 | 0.13 | 2.93 | 1.68 | 99.87 | 90.70 |
| DMP208-1 | 45.66 | 0.21 | 1.34 | 0.11 | 33.82 | 6.46 | 0.01 | 0.10 | 4.82 | 3.14 | 95.78 | 90.32 |
| DMP208-2 | 43.45 | 0.22 | 1.23 | 0.09 | 37.79 | 7.34 | 0.01 | 0.12 | 4.04 | 3.33 | 97.77 | 90.18 |
| Sample | DMP202-1-1 | DMP202-1-2 | DMP204-1-2 | DMP204-1-3 | DMP204-2-2 | DMP204-2-3 | DMP208-1-1 | DMP208-1-2 | ||||||
| Sc | 100.64 | 111.24 | 99.75 | 95.61 | 91.75 | 96.59 | 93.73 | 95.25 | ||||||
| Ti | 1470 | 1720 | 6780 | 5500 | 3270 | 3090 | 3790 | 3470 | ||||||
| V | 332.04 | 333.83 | 323.19 | 317.02 | 326.51 | 323.44 | 322.03 | 319.57 | ||||||
| Cr | 10,464.22 | 9400.62 | 8128.57 | 8317.80 | 10,346.61 | 10,108.11 | 8964.65 | 9521.37 | ||||||
| Co | 27.62 | 28.87 | 21.51 | 24.58 | 26.20 | 26.79 | 27.34 | 27.35 | ||||||
| Ni | 1011.07 | 1070.66 | 906.16 | 840.56 | 627.28 | 626.59 | 676.58 | 647.32 | ||||||
| Cu | 0.24 | 4.84 | 11.90 | 14.60 | 4.05 | 2.92 | 3.89 | 2.48 | ||||||
| Zn | 10.42 | 11.01 | 6.74 | 6.52 | 10.20 | 9.94 | 11.14 | 10.76 | ||||||
| Ga | 3.08 | 3.51 | 4.65 | 4.35 | 3.58 | 3.41 | 3.99 | 4.00 | ||||||
| Rb | 0.00 | 0.00 | 0.08 | 0.12 | 0.00 | 0.00 | 0.00 | 0.00 | ||||||
| Ba | 0.09 | 0.00 | 0.00 | 0.00 | 0.15 | 0.12 | 0.12 | 0.05 | ||||||
| Nb | 0.54 | 0.49 | 0.04 | 0.13 | 0.50 | 0.53 | 0.51 | 0.48 | ||||||
| La | 1.00 | 0.99 | 0.60 | 0.63 | 1.27 | 1.29 | 1.54 | 1.49 | ||||||
| Ce | 2.95 | 2.79 | 2.69 | 2.74 | 0.00 | 0.00 | 0.00 | 0.00 | ||||||
| Pb | 0.09 | 0.04 | 0.03 | 0.02 | 0.10 | 0.07 | 0.07 | 0.08 | ||||||
| Pr | 0.43 | 0.47 | 0.80 | 0.81 | 0.79 | 0.73 | 1.01 | 1.00 | ||||||
| Sr | 56.27 | 57.99 | 62.49 | 70.17 | 65.65 | 66.72 | 86.16 | 85.82 | ||||||
| Nd | 2.51 | 3.12 | 5.46 | 5.95 | 4.34 | 4.42 | 6.21 | 6.51 | ||||||
| Zr | 13.23 | 14.22 | 42.91 | 44.36 | 24.62 | 25.30 | 27.18 | 29.08 | ||||||
| Hf | 0.43 | 0.28 | 1.60 | 1.27 | 0.77 | 0.77 | 0.94 | 0.98 | ||||||
| Sm | 0.92 | 1.08 | 2.46 | 2.70 | 1.38 | 1.78 | 2.28 | 2.29 | ||||||
| Eu | 0.27 | 0.42 | 1.15 | 1.16 | 0.64 | 0.65 | 0.76 | 0.75 | ||||||
| Gd | 1.13 | 1.10 | 3.92 | 3.29 | 1.98 | 2.52 | 2.44 | 2.48 | ||||||
| Tb | 0.31 | 0.25 | 0.60 | 0.70 | 0.41 | 0.43 | 0.42 | 0.44 | ||||||
| Dy | 2.33 | 2.31 | 4.30 | 4.21 | 3.10 | 3.30 | 2.77 | 2.84 | ||||||
| Y | 12.91 | 13.46 | 25.65 | 25.85 | 17.24 | 18.31 | 16.62 | 17.02 | ||||||
| Ho | 0.54 | 0.61 | 0.95 | 1.17 | 0.67 | 0.67 | 0.58 | 0.64 | ||||||
| Er | 1.59 | 1.54 | 2.84 | 2.76 | 1.92 | 2.16 | 1.89 | 2.04 | ||||||
| Tm | 0.17 | 0.21 | 0.40 | 0.40 | 0.25 | 0.35 | 0.23 | 0.24 | ||||||
| Yb | 1.77 | 1.59 | 2.05 | 1.74 | 1.73 | 1.85 | 1.62 | 1.83 | ||||||
| Lu | 0.26 | 0.18 | 0.38 | 0.35 | 0.25 | 0.28 | 0.28 | 0.22 | ||||||
| Sample | DMP208-1-3 | DMP208-1-4 | DMP208-1-5 | DMP208-1-6 | DMP208-2-2 | DMP208-2-3 | DMP208-2-4 | |||||||
| Sc | 82.81 | 92.92 | 94.82 | 99.05 | 82.47 | 99.13 | 108.78 | |||||||
| Ti | 4310 | 3970 | 3790 | 2920 | 4070 | 2850 | 2950 | |||||||
| V | 309.15 | 322.15 | 316.92 | 326.69 | 319.77 | 321.34 | 331.65 | |||||||
| Cr | 11,873.09 | 10,912.96 | 11,253.50 | 8890.56 | 8759.32 | 9442.91 | 10,770.85 | |||||||
| Co | 25.74 | 27.06 | 27.04 | 27.61 | 28.28 | 28.44 | 27.87 | |||||||
| Ni | 690.06 | 701.67 | 672.17 | 705.92 | 884.13 | 705.70 | 731.64 | |||||||
| Cu | 2.74 | 4.94 | 2.11 | 4.22 | 9.31 | 4.78 | 3.01 | |||||||
| Zn | 9.93 | 10.35 | 10.51 | 11.08 | 10.72 | 10.42 | 10.94 | |||||||
| Ga | 4.21 | 4.22 | 4.14 | 3.75 | 4.41 | 4.41 | 3.99 | |||||||
| Rb | 0.00 | 0.00 | 0.00 | 0.00 | 0.07 | 0.00 | 0.00 | |||||||
| Ba | 0.07 | 0.00 | 0.01 | 0.00 | 0.26 | 0.00 | 0.00 | |||||||
| Nb | 0.60 | 0.55 | 0.59 | 0.48 | 0.60 | 0.55 | 0.60 | |||||||
| La | 1.58 | 1.60 | 1.49 | 1.61 | 1.35 | 1.62 | 1.66 | |||||||
| Ce | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | |||||||
| Pb | 0.08 | 0.08 | 0.06 | 0.16 | 0.09 | 0.05 | 0.06 | |||||||
| Pr | 1.14 | 1.01 | 1.05 | 1.02 | 1.00 | 0.98 | 1.08 | |||||||
| Sr | 85.74 | 85.33 | 86.20 | 88.23 | 86.89 | 87.17 | 90.32 | |||||||
| Nd | 6.55 | 6.50 | 7.04 | 6.15 | 5.90 | 6.64 | 6.49 | |||||||
| Zr | 29.83 | 30.18 | 31.49 | 28.81 | 25.76 | 30.27 | 34.15 | |||||||
| Hf | 0.74 | 1.10 | 1.01 | 0.89 | 0.79 | 0.95 | 0.90 | |||||||
| Sm | 2.13 | 2.22 | 2.33 | 2.29 | 1.80 | 2.23 | 1.84 | |||||||
| Eu | 0.84 | 0.74 | 0.87 | 0.82 | 0.79 | 0.67 | 0.67 | |||||||
| Gd | 2.88 | 2.71 | 2.93 | 2.49 | 2.07 | 2.76 | 2.82 | |||||||
| Tb | 0.42 | 0.40 | 0.51 | 0.39 | 0.36 | 0.40 | 0.52 | |||||||
| Dy | 3.13 | 2.89 | 3.37 | 3.15 | 2.32 | 2.92 | 3.04 | |||||||
| Y | 17.97 | 17.32 | 18.25 | 17.00 | 13.84 | 16.54 | 17.91 | |||||||
| Ho | 0.69 | 0.61 | 0.67 | 0.61 | 0.50 | 0.60 | 0.67 | |||||||
| Er | 2.00 | 2.12 | 1.94 | 1.62 | 1.60 | 2.15 | 2.02 | |||||||
| Tm | 0.27 | 0.23 | 0.26 | 0.29 | 0.20 | 0.25 | 0.25 | |||||||
| Yb | 1.73 | 1.94 | 1.93 | 1.70 | 1.26 | 1.70 | 1.63 | |||||||
| Lu | 0.25 | 0.27 | 0.32 | 0.29 | 0.18 | 0.24 | 0.27 | |||||||
References
- Zhao, G.; Wilde, S.; Cawood, P.; Sun, M. Archean blocks and their boundaries in the North China Craton: Lithological, geochemical, structural and P-T path constraints and tectonic evolution. Precambr. Res. 2001, 107, 45–73. [Google Scholar] [CrossRef]
- Zhao, G.; Sun, M.; Wilde, S.; Sanzhong, L. Late Archean to Paleoproterozoic evolution of the North China Craton: Key issues revisited. J. Asian Earth Sci. 2005, 24, 519–522. [Google Scholar]
- Chu, X.L.; Xu, J.H. Oxygen isotopes of mantle xenolith from Hannoba et al. Prog. Nat. Sci. Commun. State Key Lab. China 1999, 9, 5. [Google Scholar]
- Griffin, W.L.; Andi, Z.; O’Reilly, S.Y.; Ryan, C.G. Phanerozoic evolution of the lithosphere beneath the Sino-Korean Craton. Mantle Dyn. Plate Interact. E. Asia 1998, 27, 107–126. [Google Scholar]
- Zheng, J.; O’Reilly, S.Y.; Griffin, W.L.; Lu, F.; Pearson, N.J. Relict refractory mantle beneath the eastern North China block: Significance for lithosphere evolution. Lithos 2001, 57, 43–66. [Google Scholar] [CrossRef]
- Liu, Y.; Gao, S.; Jin, S.; Hu, S.; Sun, M.; Zhao, Z.; Feng, J. Geochemistry of lower crustal xenoliths from Neogene Hannuoba basalt, North China craton: Implications for petrogenesis and lower crustal composition. Geochim. Et Cosmochim. Acta 2001, 65, 2589–2604. [Google Scholar]
- Chen, S.; O’Reilly, S.Y.; Zhou, X.; Griffin, W.L.; Zhang, G.; Sun, M.; Feng, J.; Zhang, M. Thermal and petrological structure of the lithosphere beneath Hannuoba, Sino-Korean Craton, China: Evidence from xenoliths. Lithos 2001, 56, 267–301. [Google Scholar]
- Yang, H.; Liang, R.; Xu, C.; Zhao, J. Prospecting for the north-easterly periphery of Damaping peridot ore in Wanquan County, Hebi Province. China Acad. J. Electron. Publ. House 2017, 31, 936–940. [Google Scholar]
- Xu, X.; O’Reilly, S.Y.; Griffin, W.L.; Zhou, X. Genesis of Young Lithospheric Mantle in Southeastern China: An LAM–ICPMS Trace Element Study. J. Petrol. 2000, 41, 111–148. [Google Scholar] [CrossRef]
- Rudnick, R.L.; Gao, S.; Ling, W.L.; Liu, Y.S.; Mcdonough, W.F. Petrology and geochemistry of spinel peridotite xenoliths from Hannuoba and Qixia, North China craton. Lithos 2004, 77, 609–637. [Google Scholar]
- Gao, S.; Rudnick, R.L.; Carlson, R.W.; Mcdonough, W.F.; Liu, Y.S. Re-Os evidence for replacement of ancient mantle lithosphere beneath the North China craton. Earth Planet. Sci. Lett. 2002, 198, 307–322. [Google Scholar] [CrossRef]
- Yu, C.M.; Zheng, J.P.; Griffin, W.L. LAM-ICPMS analysis on clinopyroxenes of peridotite xenoliths from Hannuoba and its significance on lithospheric mantle evolution. Earth Sci. 2006, 31, 93–100. [Google Scholar]
- Yu, C.; Zheng, J.; Griffin, W.L. Petrography and Geochemistry of Peridotite Xenoliths from Hannuoba and Significance for Lithospheric Mantle Evolution. J. China Univ. Geosci. 2006, 17, 25–33. [Google Scholar] [CrossRef]
- Xia, Q.K. Low water content of the Cenozoic lithospheric mantle beneath the eastern part of the North China Craton. J. Geophys. Res. Solid. Earth 2010, 115, B7. [Google Scholar] [CrossRef]
- Beran, A.; Putnis, A. A model of the OH positions in olivine, derived from infrared-spectroscopic investigations. Phys. Chem. Miner. 1983, 9, 57–60. [Google Scholar] [CrossRef]
- Asimow, P.D.; Langmuir, C.H. The importance of water to oceanic mantle melting regimes. Nature 2003, 421, 815. [Google Scholar] [CrossRef]
- Dixon, J.E.; Dixon, T.H.; Bell, D.R.; Malservisi, R. Lateral variation in upper mantle viscosity: Role of water. Earth Planet. Sci. Lett. 2004, 222, 451–467. [Google Scholar] [CrossRef]
- Hirschmann, M.M.; Tenner, T.; Aubaud, C.; Withers, A.C. Dehydration melting of nominally anhydrous mantle: The primacy of partitioning. Phys. Earth Planet. Inter. 2009, 176, 54–68. [Google Scholar] [CrossRef]
- Yang, X.Z.; Xia, Q.K.; Deloule, E.; Dallai, L.; Fan, Q.C.; Feng, M. Water in minerals of the continental lithospheric mantle and overlying lower crust: A comparative study of peridotite and granulite xenoliths from the North China Craton. Chem. Geol. 2008, 256, 33–45. [Google Scholar] [CrossRef]
- Lihe, G.; Xingyuan, L.; Manze, X.; Jialin, F.; Shuqi, W.U. Water in Mantle-derived Xenoliths in the Hannuoba Basalt. Acta Geol. Sin.-Engl. Ed. 1998, 72, 224–229. [Google Scholar] [CrossRef]
- Qunke, X.; Daogong, C.; Lihe, G.; Xiachen, Z. Structural water in mantle-derived clinopyroxene megacrysts from hannuoba: FTIR investigations. ACTA Mineral. Sin. 1999, 19, 161–165. [Google Scholar]
- Xia, Q.K.; Hao, Y.T.; Liu, S.C.; Gu, X.Y.; Feng, M. Water contents of the Cenozoic lithospheric mantle beneath the western part of the North China Craton: Peridotite xenolith constraints. Gondwana Res. 2013, 23, 108–118. [Google Scholar] [CrossRef]
- Kusky, T.M. Geophysical and geological tests of tectonic models of the North China Craton. Gondwana Res. 2011, 20, 26–35. [Google Scholar]
- Zhao, G.; Cawood, P.; Wilde, S.; Sun, M.; Lu, L. Metamorphism of basement rocks in the Central Zone of the North China Craton: Implications for Paleoproterozoic tectonic evolution. Precambr. Res. 2000, 103, 55–88. [Google Scholar]
- Santosh, M. Assembling North China Craton within the Columbia supercontinent: The role of double-sided subduction. Precambr. Res. 2010, 178, 149–167. [Google Scholar]
- Liu, S.; Santosh, M.; Wang, W.; Bai, X.; Yang, P. Zircon U–Pb chronology of the Jianping Complex: Implications for the Precambrian crustal evolution history of the northern margin of North China Craton. Gondwana Res. 2011, 20, 48–63. [Google Scholar]
- Zhai, M.G.; Santosh, M. The early Precambrian odyssey of the North China Craton: A synoptic overview. Gondwana Res. 2011, 20, 6–25. [Google Scholar]
- Kroner, A.; Compston, W.; Guo-Wei, Z.; An-Lin, G.; Todt, W. Age and tectonic setting of Late Archean greenstone-gneiss terrain in Henan Province, China, as revealed by single-grain zircon dating. Geology 1988, 16, 211–215. [Google Scholar] [CrossRef]
- Wu, F.Y.; Yang, J.H.; Xu, Y.G.; Wilde, S.A.; Walker, R.J. Destruction of the North China Craton in the Mesozoic. Annu. Rev. 2019, 47, 173–195. [Google Scholar]
- Zhu, R.; Yigang, X.U. The subduction of the west Pacific plate and the destruction of the North China Craton. Sci. China Earth Sciences 2019, 62, 11. [Google Scholar] [CrossRef]
- Zhou, X.; Armstrong, R.L. Cenozoic volcanic rocks of eastern China—secular and geographic trends in chemistry and strontium isotopic composition. Earth Planet. Sci. Lett. 1982, 58, 301–329. [Google Scholar]
- Song, Y.; Frey, F.A.; Zhi, X. Isotopic characteristics of Hannuoba basalts, eastern China: Implications for their petrogenesis and the composition of subcontinental mantle. Chem. Geol. 1990, 88, 35–52. [Google Scholar]
- Zhi, X.; Song, Y.; Frey, F.A.; Feng, J.; Zhai, M. Geochemistry of Hannuoba basalts, eastern China: Constraints on the origin of continental alkalic and tholeiitic basalt. Chem. Geol. 1990, 88, 1–33. [Google Scholar]
- Song, Y.; Frey, F.A. Geochemistry of peridotite xenoliths in basalt from Hannuoba, Eastern China: Implications for subcontinental mantle heterogeneity. Geochim. Et Cosmochim. Acta 1989, 53, 97–113. [Google Scholar]
- Xu, Y. Evidence for crustal components in the mantle and constraints on crustal recycling mechanisms: Pyroxenite xenoliths from Hannuoba, North China. Chem. Geol. 2002, 182, 301–322. [Google Scholar]
- Liu, Y.; Gao, S.; Lee, C.T.A.; Hu, S.; Liu, X.; Yuan, H. Melt–peridotite interactions: Links between garnet pyroxenite and high-Mg# signature of continental crust. Earth Planet. Sci. Lett. 2005, 234, 39–57. [Google Scholar]
- Liu, D.Y.; Nutman, A.P.; Compston, W.; Wu, J.S.; Shen, Q.H. Remnants of ≥3800 Ma crust in the Chinese part of the Sino-Korean craton. Geology 1992, 20, 339. [Google Scholar]
- Fan, W.M.; Zhang, H.F.; Baker, J.; Jarvis, K.E.; Mason, P.R.D.; Menzies, M.A. On and Off the North China Craton: Where is the Archaean Keel? J. Petrol. 2000, 41, 933–950. [Google Scholar]
- Tatsumoto, M.; Basu, A.R.; Wankang, H.; Junwen, W.; Guanghong, X. Sr, Nd, and Pb isotopes of ultramafic xenoliths in volcanic rocks of Eastern China: Enriched components EMI and EMII in subcontinental lithosphere. Earth Planet. Sci. Lett. 1992, 113, 107–128. [Google Scholar]
- Fan, Q.; Hooper, P.R. The Cenozoic Basaltic Rocks of Eastern China: Petrology and Chemical Composition. J. Petrol. 1991, 32, 765–810. [Google Scholar] [CrossRef]
- Gao, S.; Kern, H.; Liu, Y.S.; Jin, S.Y.; Popp, T.; Jin, Z.M.; Feng, J.L.; Sun, M.; Zhao, Z.B. Measured and calculated seismic velocities and densities for granulites from xenolith occurrences and adjacent exposed lower crustal sections: A comparative study from the North China craton. J. Geophys. Res. Solid Earth 2000, 105, 18965–18976. [Google Scholar] [CrossRef]
- Wilde, S.A.; Zhou, X.; Nemchin, A.A.; Sun, M. Mesozoic crust-mantle interaction beneath the North China craton: A consequence of the dispersal of Gondwanaland and accretion of Asia. Geology 2003, 31, 817. [Google Scholar] [CrossRef]
- Zhou, X.; Sun, M.; Zhang, G.; Chen, S. Continental crust and lithospheric mantle interaction beneath North China: Isotopic evidence from granulite xenoliths in Hannuoba, Sino-Korean craton. Lithos 2002, 62, 111–124. [Google Scholar] [CrossRef]
- Xu, B.; Yin, R.S.; Chiaradia, M.; Miao, Z.; Griffin, W.L.; Hou, Z.Q.; Yang, Z.M.; O’Reilly, S.Y. Mercury isotope evidence for the importance of recycled fluids in collisional ore systems. Sci. Adv. 2024, 10, eadp7383. [Google Scholar] [CrossRef]
- Liu, Z.C.; Wu, F.Y.; Chu, Z.Y.; Xu, X.S. Isotopic compositions of the peridotitic xenoliths from the Nushan area, Anhui Province: Constraints on the age of subcontinental lithospheric mantle beneath the East China. Acta Petrol. Sin. 2010, 26, 1217–1240. [Google Scholar]
- Kovács, I.; Hermann, J.; O’Neill, H.S.C.; Gerald, J.F.; Sambridge, M.; Horváth, G. Quantitative absorbance spectroscopy with unpolarized light: Part II. Experimental evaluation and development of a protocol for quantitative analysis of mineral IR spectra. Am. Mineral. 2008, 93, 765–778. [Google Scholar]
- Poitrasson, F. Silicon Isotope Geochemistry. Rev. Mineral. Geochem. 2017, 82, 289–344. [Google Scholar] [CrossRef]
- Xu, B.; Hou, Z.-Q.; Griffin, W.L.; Yu, J.-X.; Long, T.; Zhao, Y.; Wang, T.; Fu, B.; Belousova, E.; O’Reilly, S.Y. Apatite halogens and Sr-O and zircon Hf-O isotopes: Recycled volatiles in Jurassic porphyry ore systems in southern Tibet. Chem. Geol. 2022, 605, 120924. [Google Scholar] [CrossRef]
- Gan, W.; Jin, Z.; Fang, Z.; Liu, W. Deformation of the Subcontinental Lithospheric Mantle in NE China: Constraints from Rheological and Fabric Study of Mantle Peridotite Xenoliths from Jiaohe, Jilin Province. J. Earth Sci. 2023, 34, 767–775. [Google Scholar] [CrossRef]
- Xu, Y.G.; Menzies, M.A.; Thirlwall, M.F.; Huang, X.L.; Liu, Y.; Chen, X.M. “Reactive” harzburgites from Huinan, NE China: Products of the lithosphere-asthenosphere interaction during lithospheric thinning?—ScienceDirect. Geochim. Et Cosmochim. Acta 2003, 67, 487–505. [Google Scholar] [CrossRef]
- Princivalle, F.; De Min, A.; Lenaz, D.; Scarbolo, M.; Zanetti, A. Ultramafic xenoliths from Damaping (Hannuoba region, NE-China): Petrogenetic implications from crystal chemistry of pyroxenes, olivine and Cr-spinel and trace element content of clinopyroxene. Lithos 2014, 188, 3–14. [Google Scholar]
- Sun, J.; Liu, C.Z.; Wu, F.Y.; Yang, Y.H.; Chu, Z.Y. Metasomatic origin of clinopyroxene in Archean mantle xenoliths from Hebi, North China Craton: Trace-element and Sr-isotope constraints. Chem. Geol. 2012, 328, 123–136. [Google Scholar]
- Zheng, J.; O'Reilly, S.Y.; Griffin, W.L.; Lu, F.; Zhang, M.; Pearson, N.J. Nature and evolution of Cenozoic lithospheric mantle beneath Shan dong peninsula, Sino-Korean craton, eastern China. Int. Geol. Rev. 1998, 40, 471–499. [Google Scholar]
- Hayashi, J.; Hideshi, H.; Chiba, C.T.; Bedini, R.M.; Bodinier, J.L. Distribution of incompatible trace elements between the constituents of spinel peridotite xenoliths: ICP-MS data from the East African rift. Geochim. Et Cosmochim. Acta 1999, 63, 3883–3900. [Google Scholar]
- McDonough, W.F.; Sun, S.S. The composition of the Earth—ScienceDirect. Chem. Geol. 1995, 120, 223–253. [Google Scholar]
- Mccoy-West, A.J.; Bennett, V.C.; O’Neill, H.S.C.; Jrg, H.; Puchtel, I.S. The Interplay between Melting, Refertilization and Carbonatite Metasomatism in Off-Cratonic Lithospheric Mantle under Zealandia: An Integrated Major, Trace and Platinum Group Element Study. J. Petrol. 2015, 56, 563–604. [Google Scholar] [CrossRef]
- Nimis, P.; Taylor, W.R. Single clinopyroxene thermobarometry for garnet peridotites. Part I. Calibration and testing of a Cr-in-Cpx barometer and an enstatite-in-Cpx thermometer. Contrib. Mineral. Petrol. 2000, 139, 541–554. [Google Scholar]
- KöHler, T.P.; Brey, G.P. Calcium exchange between olivine and clinopyroxene calibrated as a geothermobarometer for natural peridotites from 2 to 60 kb with applications. Geochim. Et Cosmochim. Acta 1990, 54, 2375–2388. [Google Scholar] [CrossRef]
- Bell, D.R.; Rossman, G.R. Water in Earth’s Mantle: The Role of Nominally Anhydrous Minerals. Science 1992, 255, 1391–1397. [Google Scholar]
- Grant, K.; Ingrin, J.; Lorand, J.P.; Dumas, P. Water partitioning between mantle minerals from peridotite xenoliths. Contrib. Mineral. Petrol. 2007, 154, 15–34. [Google Scholar] [CrossRef]
- Peslier, A.H.; Luhr, J.F.; Post, J. Low water contents in pyroxenes from spinel-peridotites of the oxidized, sub-arc mantle wedge. Earth Planet. Sci. Lett. 2002, 201, 69–86. [Google Scholar]
- Aubaud, C.; Hauri, E.H.; Hirschmann, M.M. Hydrogen partition coefficients between nominally anhydrous minerals and basaltic melts. Geophys. Res. Lett. 2004, 31, 20. [Google Scholar]
- Aubaud, C.; Withers, A.C.; Hirschmann, M.M.; Guan, Y.; Leshin, L.A.; Mackwell, S.J.; Bell, D.R. Intercalibration of FTIR and SIMS for hydrogen measurements in glasses and nominally anhydrous minerals. Am. Mineral. 2007, 92, 811–828. [Google Scholar]
- Hauri, E.H.; Gaetani, G.A.; Green, T.H. Partitioning of water during melting of the Earth’s upper mantle at H2O-undersaturated conditions. Earth Planet. Sci. Lett. 2006, 248, 715–734. [Google Scholar]
- Koga, K.; Hauri, E.H.; Hirschmann, M.M.; Bell, D. Hydrogen concentration analyses using SIMS and FTIR: Comparison and calibration for nominally anhydrous minerals. Geochem. Geophys. Geosyst. 2003, 4, 2. [Google Scholar] [CrossRef]
- Tenner, T.J.; Hirschmann, M.M.; Withers, A.C.; Hervig, R.L. Hydrogen partitioning between nominally anhydrous upper mantle minerals and melt between 3 and 5 GPa and applications to hydrous peridotite partial melting. Chem. Geol. 2009, 262, 42–56. [Google Scholar]
- Mattey, D.; Lowry, D.; Macpherson, C. Oxygen isotope composition of mantle peridotite. Earth Planet. Sci. Lett. 1994, 128, 231–241. [Google Scholar]
- Eiler, J.M. Oxygen Isotope Variations of Basaltic Lavas and Upper Mantle Rocks. Rev. Mineral. Geochem. 2001, 43, 319–364. [Google Scholar]
- Xu, Y.G.; Menzies, M.A.; Mattey, D.P.; Lowry, D.; Harte, B.; Hinton, R.W. The nature of the lithospheric mantle near the Tancheng-Lujiang fault, China: An integration of texture, chemistry and O-isotopes. Chem. Geol. 1996, 134, 67–81. [Google Scholar]
- Chazot, G.; Lowry, D.; Menzies, M.; Mattey, D. Oxygen isotopic composition of hydrous and anhydrous mantle peridotites. Geochim. Et Cosmochim. Acta 1997, 61, 161–169. [Google Scholar] [CrossRef]
- Norman, M.D. Melting and metasomatism in the continental lithosphere: Laser ablation ICPMS analysis of minerals in spinel lherzolites from eastern Australia. Contrib. Mineral. Petrol. 1998, 130, 240–255. [Google Scholar] [CrossRef]
- Johnson, K.T.M.; Dick, H.J.B.; Shimizu, N. Melting in the oceanic upper mantle: An ion microprobe study of diopsides in abyssal peridotites. J. Geophys. Res. 1990, 95, 2661. [Google Scholar] [CrossRef]
- Yaoling, N. Mantle Melting and Melt Extraction Processes beneath Ocean Ridges: Evidence from Abyssal Peridotites. J. Petrol. 1997, 38, 1047–1074. [Google Scholar]
- Tianfu, L.; Hongwen, M.; Zhimin, B. Geochemical characteristics and genetic model of hannuoba basalts, hebei province. Acta Petrol. Et Mineral. 1999, 18, 217–228. [Google Scholar]
- Zhao, Y.; Zheng, J.-P.; Xiong, Q. Prolonged Slab-derived Silicate and Carbonate Metasomatism of a Cratonic Mantle Wedge (Maowu Ultramafic body, China). J. Petrol. 2021, 62, egab081. [Google Scholar] [CrossRef]
- Wulff-Pedersen, E.; Neumann, E.R.; Jensen, B.B. The upper mantle under La Palma, Canary Islands: Formation of SiKNa-rich melt and its importance as a metasomatic agent. Contrib. Mineral. Petrol. 1996, 125, 113–139. [Google Scholar] [CrossRef]
- Zhao, Y.; Zheng, J.; Xiong, Q.; Xu, B.; Zhang, Y.; Hou, Z. Survived carbonates in orogenic dunites from recycling of subduction-related sediments. Lithos 2023, 458, 14. [Google Scholar] [CrossRef]
- Bell, K.; Kjarsgaard, B.A.; Simonetti, A. Carbonatites—Into the Twenty-First Century. J. Petrol. 1998, 39, 1839–1845. [Google Scholar] [CrossRef]
- Peslier, A.H.; Luhr, J.F. Hydrogen loss from olivines in mantle xenoliths from Simcoe (USA) and Mexico: Mafic alkalic magma ascent rates and water budget of the sub-continental lithosphere. Earth Planet. Sci. Lett. 2006, 242, 302–319. [Google Scholar] [CrossRef]
- Li, Z.X.A.; Lee, C.T.A.; Peslier, A.H.; Lenardic, A.; Mackwell, S.J. Water contents in mantle xenoliths from the Colorado Plateau and vicinity: Implications for the mantle rheology and hydration-induced thinning of continental lithosphere. J. Geophys. Res. Solid. Earth 2008, 113, B9. [Google Scholar] [CrossRef]
- Gose, J.; Schm?Dicke, E.; Beran, A. Water in enstatite from Mid-Atlantic Ridge peridotite: Evidence for the water content of suboceanic mantle? Geology 2009, 37, 543–546. [Google Scholar]
- Qunke, X.; Yantao, H. The distribution of water in the continental lithospheric mantle and its implications for the stability of continents. Sci. Bull. 2013, 58, 3879–3889. [Google Scholar]
- Bell, D.R.; Rossman, G.R.; Moore, R.O. Abundance and Partitioning of OH in a High-pressure Magmatic System: Megacrysts from the Monastery Kimberlite, South Africa. J. Petrol. 2004, 45, 1539–1564. [Google Scholar]
- Schmädicke, E.; Gose, J.; Will, T.M. Heterogeneous mantle underneath the North Atlantic: Evidence from water in orthopyroxene, mineral composition and equilibrium conditions of spinel peridotite from different locations at the Mid-Atlantic Ridge. Lithos 2011, 125, 308–320. [Google Scholar]
- Yamamoto, J.; Ando, J.I.; Kagi, H.; Inoue, T.; Yamada, A.; Yamazaki, D.; Irifune, T. In situ strength measurements on natural upper-mantle minerals. Phys. Chem. Miner. 2008, 35, 249–257. [Google Scholar] [CrossRef]
- Dixon, J.E.; Leist, L.; Langmuir, C.; Schilling, J.G. Recycled dehydrated lithosphere observed in plume-influenced mid-ocean-ridge basalt. Nature 2002, 420, 385–389. [Google Scholar]
- Hellebrand, E.; Snow, J.E.; Dick, H.J.B.; Hofmann, A.W. Coupled major and trace elements as indicators of the extent of melting in mid-ocean-ridge peridotites. Nature 2001, 410, 677–681. [Google Scholar] [CrossRef]
- Michael, P. Regionally distinctive sources of depleted MORB: Evidence from trace elements and H2O. Earth Planet. Sci. Lett. 1995, 131, 301–320. [Google Scholar] [CrossRef]
- Roeder, P.L.; Emslie, R.F. Olivine-liquid equilibrium. Contrib. Mineral. Petrol. 1970, 29, 275–289. [Google Scholar]
- Bindeman, I.N.; Eiler, J.M.; Yogodzinski, G.M.; Tatsumi, Y.; Stern, C.R.; Grove, T.L.; Portnyagin, M.; Hoernle, K.; Danyushevsky, L.V. Oxygen isotope evidence for slab melting in modern and ancient subduction zones. Earth Planet. Sci. Lett. 2005, 235, 480–496. [Google Scholar]
- Peslier, A.H.; Woodland, A.B.; Bell, D.R.; Lazarov, M. Olivine water contents in the continental lithosphere and the longevity of cratons. Nature 2010, 467, 78–81. [Google Scholar] [PubMed]
- Xia, B.; Thybo, H.; Artemieva, I.M. Lithosphere mantle density of the North China Craton. J. Geophys. Res. Solid. Earth 2020, 125, e2020JB020296. [Google Scholar]
- Xia, Q.X.; Zhi, X.C.; Meng, Q.; Zheng, L.; Peng, Z.C. The trace element and Re-Os isotopic geochemistry of mantle-derived peridotite xenoliths from Hannuoba: Nature and age of SCLM beneath the area. Acta Petrol. Sin. 2004, 20, 1215–1224. [Google Scholar]
- Wu, F.Y.; Walker, R.J.; Yang, Y.H.; Yuan, H.L.; Yang, J.H. The chemical-temporal evolution of lithospheric mantle underlying the North China Craton. Geochim. Et Cosmochim. Acta 2006, 70, 5013–5034. [Google Scholar]
- Zhang, H.F.; Goldstein, S.L.; Zhou, X.H.; Sun, M.; Cai, Y. Comprehensive refertilization of lithospheric mantle beneath the North China Craton: Further Os-Sr-Nd isotopic constraints. J. Geol. Soc. 2009, 166, 249–259. [Google Scholar]








| Samples | Rock Type | Mineral Mode (%) | |||
|---|---|---|---|---|---|
| Cpx | Opx | Ol | Sp | ||
| DMP202-1 | Spinel lherzolite | 6.12 | 32.38 | 61.39 | 0.11 |
| DMP204-1 | Spinel lherzolite | 10.57 | 30.07 | 53.07 | 6.29 |
| DMP204-2 | Spinel lherzolite | 6.79 | 39.15 | 52 | 2.06 |
| DMP208-1 | Spinel lherzolite | 14.03 | 33.6 | 47.9 | 5.47 |
| DMP208-2 | Spinel lherzolite | 7.17 | 32.25 | 54.74 | 4.84 |
| Samples | TTaylor | TBKN | TCa-ol/cpx | Pkb |
|---|---|---|---|---|
| 202-1 | 1085 | 1380 | 1232 | 22 |
| 204-1 | 963 | 1307 | 1209 | 22 |
| 204-2 | 1068 | 1392 | 1199 | 29 |
| 208-1 | 1064 | 1390 | 1209 | 26 |
| 208-2 | 1071 | 1391 | 1213 | 19 |
| Sample | Water Content (ppm) | |||
|---|---|---|---|---|
| Cpx | Opx | Ol a | WR b | |
| DMP202-1 | 14 | 5 | 1 | 3 |
| DMP204-1 | 19 | 8 | 2 | 5 |
| DMP204-2 | 14 | 7 | 1 | 4 |
| DMP208-1 | 16 | 6 | 2 | 5 |
| DMP208-2 | 13 | 6 | 1 | 4 |
| Sample | δ18OCpx‰ |
|---|---|
| Mantle peridotite | 5.25–5.90 |
| Mid-ocean ridge basalts | 5.3–5.7 |
| DMP202 | 5.59 |
| DMP204 | 5.34 |
| DMP208 | 5.27 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, B.; Xu, B.; Zhao, Y.; Zhang, H. Lithosphere Modification Beneath the North China Craton: Geochemical Constraints of Water Contents from the Damaping Peridotite Xenoliths. Crystals 2025, 15, 349. https://doi.org/10.3390/cryst15040349
Yang B, Xu B, Zhao Y, Zhang H. Lithosphere Modification Beneath the North China Craton: Geochemical Constraints of Water Contents from the Damaping Peridotite Xenoliths. Crystals. 2025; 15(4):349. https://doi.org/10.3390/cryst15040349
Chicago/Turabian StyleYang, Baoyi, Bo Xu, Yi Zhao, and Hui Zhang. 2025. "Lithosphere Modification Beneath the North China Craton: Geochemical Constraints of Water Contents from the Damaping Peridotite Xenoliths" Crystals 15, no. 4: 349. https://doi.org/10.3390/cryst15040349
APA StyleYang, B., Xu, B., Zhao, Y., & Zhang, H. (2025). Lithosphere Modification Beneath the North China Craton: Geochemical Constraints of Water Contents from the Damaping Peridotite Xenoliths. Crystals, 15(4), 349. https://doi.org/10.3390/cryst15040349






