Abstract
(1) Background: White spot lesions (WSLs) on enamel result from demineralization and are an early sign of dental caries. Glass ionomer cement (GIC) has been widely used for its remineralization potential, and zinc-containing GIC (zGIC) has been introduced to enhance this effect. However, its efficacy compared to conventional GIC (cGIC) remains unclear. This study aimed to evaluate and compare the remineralization effects of cGIC and zGIC on WSLs. (2) Methods: Thirty-six bovine enamel specimens were prepared, demineralized for four days, and divided into three groups: control, cGIC, and zGIC. Half of each specimen’s treated window was covered with varnish, and a two-week pH cycling protocol was conducted. Mineral density (MD) changes were assessed using microcomputed tomography (Micro-CT) at five time points: pre-demineralization, after demineralization, immediately after treatment, and 1- and 2-weeks post-treatment. Scanning electron microscopy (SEM) was also performed. (3) Results: MD values in the zGIC and cGIC groups were significantly higher than in the control group throughout every post-treatment assessment (p < 0.05). zGIC demonstrated significantly higher MD than cGIC (p < 0.05), and SEM images revealed inferior mineral deposition. (4) Conclusions: These findings suggest that zGIC is more effective in remineralizing WSLs than cGIC over two weeks.
Keywords:
biomaterials; crystallization; remineralization; glass ionomer cement; zinc; fluoride; pH cycling 1. Introduction
Enamel has a dynamic equilibrium between demineralization and remineralization, making it a rather stable structure [1,2]. Its structure consists predominantly of hydroxyapatite crystals arranged in tightly packed prisms, giving it remarkable strength and durability. Despite the fact that mature enamel is rich in minerals and functions as a protective layer to safeguard teeth against daily mechanical wear and acid erosion, as well as bacterial attack, it does not possess the capacity for self-repair or structural remodeling after damage [3]. This inherent limitation underscores the importance of early intervention to prevent the progression of dental caries and to preserve tooth integrity.
A white spot lesion (WSL) on enamel is the first visible sign of the caries process, characterized by subsurface porosity due to an imbalance between demineralization and remineralization [4,5]. Therefore, WSLs offer a crucial window for timely treatment to reverse demineralization and preserve enamel integrity. Under appropriate treatments, the reversal of WSL is possible, and several methods have been recommended for early therapeutic treatment [6,7]. WSL management should include strategies to prevent further demineralization and promote the remineralization of existing lesions [8]. Materials used for dental restorations, such as glass ionomer cement (GIC) and resin-based composites, are used in cavities as part of conventional dental caries therapy, and the demineralized tooth structure needs to be physically removed [9,10]. However, this process is not ideal for early enamel carious lesions like WSLs, because in the early stage of caries, the dynamic equilibrium of demineralization and remineralization can be altered, facilitating the recovery of the lesion without the mechanical removal of tooth structure by using a remineralizing agent containing dental materials. Therefore, the focus of treatment should now be on the early detection of carious lesions and their treatment with simple, low-cost techniques like remineralization, rather than restoration [11].
Enamel remineralization is a natural process that replenishes minerals that become depleted due to acidic microenvironments. This dynamic repair mechanism involves the redeposition of calcium and phosphate ions into demineralized enamel, facilitated primarily by saliva and aided by external agents such as fluoride. During remineralization, these ions integrate into the enamel’s crystalline structure, often forming acid-resistant fluorapatite in the presence of fluoride. This process not only halts the progression of early caries lesions, but also enhances enamel’s resistance to future demineralization [12]. However, the efficiency of enamel remineralization depends on the availability of remineralizing agents and the oral pH balance, as prolonged acidic conditions can overwhelm the repair process. Understanding and enhancing enamel remineralization are therefore fundamental in developing preventive and therapeutic strategies for maintaining dental health.
GICs have successfully been used as dental restorative materials for the minimally invasive treatment of enamel and dentin. These materials are favored for their strong chemical bonding to the tooth structure and their capability to release fluoride ions [13,14]. Fluoride is the first line of defense against dental caries and encourages the remineralization of teeth [15]. However, there is now an increasing demand for substances that either work better than fluoride or have preventive and therapeutic properties comparable to fluoride, as fluoride alone has limitations in terms of completely preventing or reversing dental caries, prompting the need for more advanced, comprehensive solutions.
Recent advancements in restorative dentistry and preventive strategies have focused on enhancing enamel remineralization through therapeutic materials. Zinc has emerged as a valuable element due to its ability to enhance enamel remineralization [16], prevent root dentin demineralization [17], slow dentin collagen breakdown [18], suppress bacterial proliferation [19], reduce acid production [20], and inhibit biofilm development in vitro [21,22]. Zinc-ion-containing dental materials are currently drawing significant interest from both researchers and clinicians. GIC infused with zinc ions is expected to exhibit a particularly potent effect due to the presence of zinc, which can enhance remineralization in addition to fluorine [16]. However, while numerous studies have examined the individual benefits of zinc and fluoride in dental materials, the available evidence on the remineralization potential of zinc-containing GIC on WSLs under conditions that mimic oral pH fluctuations remains limited. Existing research has primarily focused on static models, which may not accurately represent the dynamic oral environment. To the best of our knowledge, no studies have evaluated the remineralization capacity of zinc-containing GIC using a pH cycling system to simulate the acidic challenges associated with cariogenic activity.
Therefore, this study aims to fill this gap by investigating the remineralization effect of zinc-containing GIC on WSLs under a pH cycling model. The null hypothesis is that conventional GIC will demonstrate greater remineralization effects on WSLs compared to zinc-containing GIC under standard pH cycling conditions.
2. Materials and Methods
2.1. Specimen Preparation
The treatment protocols and three-dimensional micro-computed tomography (μCT) (InspeXio SMX-100 CT; Shimadzu, Kyoto, Japan) images of the enamel–dentin block are illustrated in Figure 1 and Figure 2. All procedures in this study were conducted by a single researcher, including the random assignment of specimens, application of glass ionomer treatment, and conduction of pH cycling. G*Power software (version 3.1.9.7) was used to determine the sample size while keeping a 95% power level. In this study, a total of 36 bovine incisor enamel specimens were used. Bovine samples, aged below 30 months, were acquired as discarded specimens following approved protocols by the Food Safety Commission of Japan and the Ministry of Health, Labor, and Welfare. Each of the 36 bovine incisors was used to produce 36 enamel specimens; then, these were randomly assigned into 3 groups. A low-speed diamond saw (Isomet; Buehler, Lake Bluff, IL, USA) was utilized to horizontally section the teeth at the cervical margin, separating the root from the crown while being cooled with running water. A model trimmer was used to create enamel specimens (7 × 6 × 2 mm3) from the crown portion of the cervical third region. A reference landmark with a diameter of roughly 1 mm was created at the corner of the lateral surface of each specimen using a diamond bur (440SS ISO#010; Shofu, Kyoto, Japan) to enable the proper superimposition of serial images. All the specimens were immersed in epoxy resin (EpoxiCure 2; Buehler, Lake Bluff, IL, USA), with the enamel surface being exposed. The specimens were then left undisturbed for 24 h to allow the epoxy resin to fully cure and dry. The surfaces were then polished under running water using 250- to 1500-grit silicon carbide sandpaper (Fuji Star; Sankyo Rikagaku, Saitama, Japan), and a 5 × 4 mm2 experimental window was then defined by applying nail varnish (RED 680; Revlon, New York, NY, USA). To thoroughly clean the surface, the specimens were subjected to a 3 min ultrasonication process in deionized water (Milli-Q water; Millipore, Billerica, MA, USA).
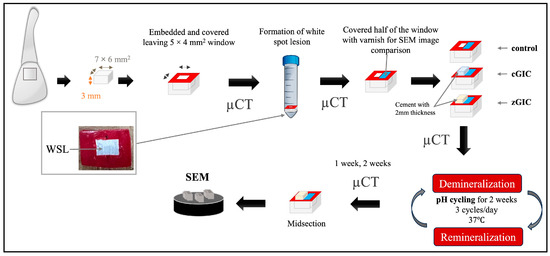
Figure 1.
Visual representation of specimen preparation and an image of WSL on enamel surface.
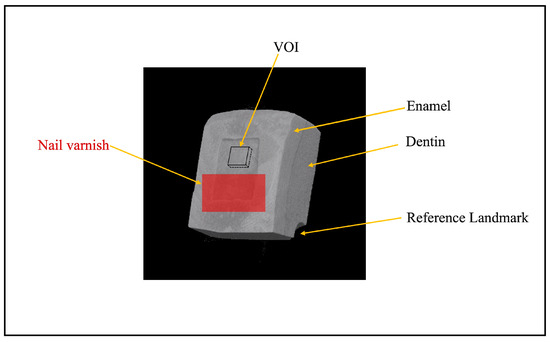
Figure 2.
A typical three-dimensional (3D) micro-focus X-ray computed tomography (μCT) image of enamel–dentin block after demineralization, with a reference landmark in the corner. VOI: volume of interest (200 × 200 × 15 μm3).
2.2. WSL Formation
In this study, a demineralization solution was used to develop artificial WSLs. Our preliminary investigation found that a lesion with no depth and softened enamel could be induced through a chemical method following four days of immersion in a demineralization solution (DS) (1.5 mM CaCl2, 0.9 mM KH2PO4, and 50 mM acetic acid, adjusted to pH 5.0 with 10 M KOH). Every specimen was submerged separately in 10 mL of the demineralizing solution at 37 °C, with 100 revolutions per minute. The demineralization solution was replenished with a fresh batch every 24 h to control pH fluctuations. After 4 days, all the specimens were retrieved, cleaned with deionized water, and analyzed for WSL formation using a mirrorless digital camera (X-S10, Fujifilm, Tokyo, Japan) and µCT.
2.3. Reagent Application Protocols
The materials utilized in this study are summarized in Table 1. The specimens were randomly divided into three groups as follows:

Table 1.
Restorative materials employed in the study.
- Group 1 (control): No treatment;
- Group 2 (cGIC): FujiIX treatment;
- Group 3 (zGIC): Caredyne Restore treatment.
After WSL formation, all halves of the windows were covered with nail varnish for comparison with sound areas by μCT and scanning electron microscopy (SEM) (JSM-IT100; JEOL, Tokyo, Japan). The remaining halves of the windows were treated with GIC, as per the guidelines provided by the manufacturer. The standard ratio of powder to liquid for cGIC treatment was 3.6:1, implying that 3 specimens could be covered with about 0.2 g of material when one level scoop of powder and one drop of liquid were mixed [23]. For zGIC, the standard ratio of powder to liquid is 2.3:1; when mixing, one level scoop of powder with one drop of liquid was enough for 3 specimens. After treatment, the measured thicknesses of the cements were about 2 mm, and the specimens were shaped like a rectangular parallelepiped.
2.4. pH Cycling Condition
After the application, each group was immersed separately in artificial saliva (AS) (130 mM KCl, 1.5 mM CaCl2, 0.9 mM KH2PO4, and 20 mM HEPES, adjusted to pH 7.0 with 10 M KOH) [24] and DS, taking turns using an automatic pH cycling system [25]. The pH cycle was set as three cycles per day (Figure 3), simulating the normal meal cycle of a human. The pH cycle started at 7:00 A.M and ended at 11:30 P.M. The specimens were left in the AS from 11:30 P.M to 7:00 A.M the next morning. Therefore, the specimens were immersed for a total of 1.5 h in DS and 22.5 h in AS each day.
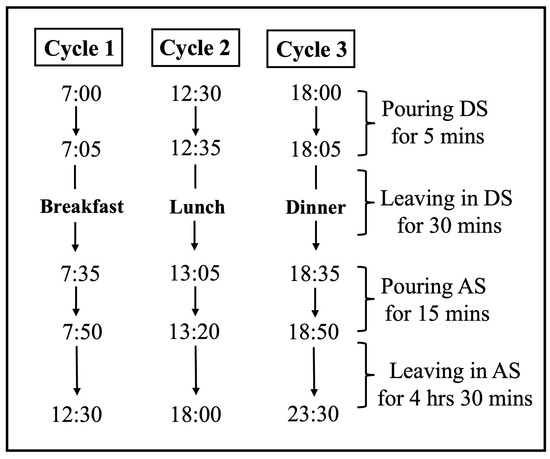
Figure 3.
The pH cycling schedule of each day.
2.5. μCT Scanning
μCT scans were performed on each specimen separately at five distinct time intervals: baseline, after white spot lesion formation, immediately after treatment, one week after treatment, and two weeks after treatment. A brass filter (Cu-Zn) with a thickness of 0.2 mm was positioned along the beam pathway to lessen the effect of beam hardening [26,27]. To keep the specimens from drying out while scanning, a wet cotton ball was placed on top of it. Each specimen was positioned perpendicular to the X-ray beam and placed on a computer-controlled turntable. All specimens were scanned under identical parameters, including a tube voltage of 90 kV, a current of 109 μA, isotropic voxel dimensions of 5.0 μm, and a normal isotropic resolution of 6 μm. The setup utilized an integration time of 240 s. The distances from the X-ray source to the specimen and the detector were maintained at 93 mm and 300 mm, respectively. Each scan involved a 360° rotation of the specimens with 0.3° steps. A mineral reference aluminum phantom (Phantoms; Ratoc System Engineering, Tokyo, Japan) was scanned using the same set-up and parameters. The mineral density (MD) of the enamel specimens was assessed using μCT. Three-dimensional analysis software (TRI/3D-BON; Ratoc System Engineering) was utilized to regenerate three-dimensional (3D) images from two-dimensional (2D) data. To evaluate changes in enamel lesions, the 3D images from all treatment stages of the same specimen were registered and aligned within a unified coordinate system. A volume of interest (VOI) measuring 200 × 200 × 15 μm3 was selected at the center of the treated window for assessment. Assuming that sound enamel has a maximum mineral density (MD) of 100 vol%, the MD profile was converted into an average MD profile for analysis.
2.6. SEM
For SEM observations, eighteen specimens (n = 6/group) were chosen at random. Three specimens of each group were cut into halves with a low-speed diamond saw under running water. The cement and nail vanish of the other three specimens from each group were carefully removed with a scaler from the corner of the treated surface. After that, the specimens were ultrasonically cleaned in deionized water for one minute. The specimens were dehydrated in a desiccator for 24 h. Following gold-sputter coating (SC-701AT; SANYU ELECTRON, Yokohama, Japan), the lesions were examined under operating conditions of 1.0 kV. Both cross-sectional and surface views were analyzed to assess the lesion morphology using SEM.
2.7. Statistical Analysis
The mineral density obtained from the 3D analysis software is presented as an average with standard deviation. Statistical analyses were conducted with two-way ANOVAs with Bonferroni-adjusted post hoc tests and the Kruskal–Wallis test. A significance level of p < 0.05 was applied. All statistical analyses were performed with a computerized statistical program (SPSS for Windows Ver. 26.0; IBM, Chicago, IL, USA).
3. Results
3.1. μCT Analysis
Representative 2D images of all groups are summarized in Figure 4. In the control group, mineral recovery is visible in half of the window. The cGIC group demonstrated a mild chronological mineral recovery in the VOI, while the zGIC group exhibited significant mineral recovery over time in the VOI. These 2D image findings align with the results observed in the MD value graph.
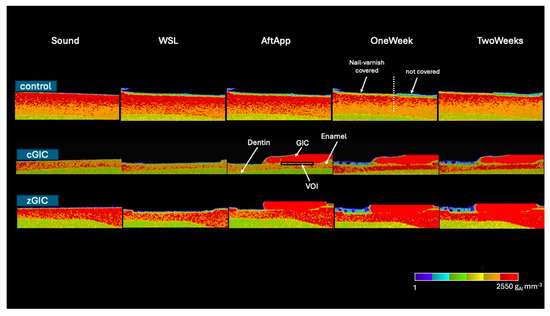
Figure 4.
Representative 2D images (color scale) for three groups at different time intervals. Black box represents VOI. White dotted line indicates the boundary between the nail varnish covered and uncovered areas.
MD value comparisons between the control, cGIC, and zGIC groups, considering up to 15 μm of depth from the lesion surface, are summarized in Table 2 in chronological order. The MD values in the zGIC group were significantly higher than those in both the control and cGIC groups after application, as well as at 1 week and 2 weeks of pH cycling, while the cGIC group also showed significantly higher MD values than the control group during the same periods. The zGIC group showed the highest degree of remineralization among all three groups.

Table 2.
The average MD values (gAl/mm3) of control, cGIC, and zGIC.
In chronological order, regarding the control group, there is a significant increase in MD from application to 1 week and 2 weeks of pH cycling. Treatment with cGIC significantly improved within 2 weeks of pH cycling. In the zGIC group, there was a significant increase in MD from application to 1 week and 2 weeks of pH cycling. However, there was no significant difference from 1 week to 2 weeks in any of the groups.
3.2. SEM Observations
The micrographs of the cross-sectional surfaces of the enamel samples are shown in Figure 5. The cross-section of untreated enamel showed an acid-eroded structure of enamel rods and inter-rods; within them, the crystals were aligned in a parallel configuration, and with noticeable inter-rod degradation and fragmented crystal structures (Figure 5a). After 2 weeks of pH cycling, in the control group, the cross-sectional analysis revealed a highly porous and disorganized microstructure, where crystals were packed somewhat closely to bundles (Figure 5b). After 2 weeks of pH cycling, in the cGIC group, the enamel damage was not fully repaired and mineral deposition remained incomplete, leaving visible gaps between crystals (Figure 5c). The zGIC group, after 2 weeks of pH cycling, exhibited a well-restored enamel architecture, where newly formed minerals densely occupied the exposed crystal spaces, effectively reinforcing the structure (Figure 5d).
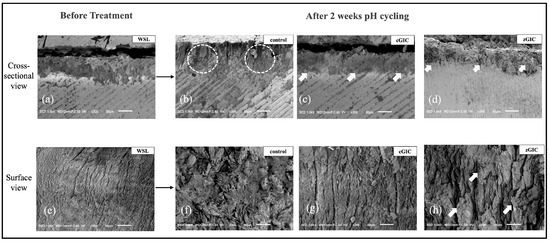
Figure 5.
SEM images of enamel: cross-sectional and surface views of WSLs after 2 weeks of pH cycling at 300 magnifications. (a) Cross-sectional SEM images after 4 days of demineralization. (b) Image of control group after 2 weeks of pH cycling. White circles indicate the remineralized enamel structure after 2 weeks of pH cycling. (c) Cross-sectional image of cGIC group 2 weeks after treatment. (d) Cross-sectional image of zGIC group 2 weeks after treatment. White arrows indicate the degree of new mineral gain from the GIC. (e) Surface-view images after 4 days of demineralization. (f) Images of control group after 2 weeks of pH cycling. (g) Images of cGIC group after 2 weeks of treatment. (h) Images of zGIC group after 2 weeks of treatment. White arrows indicate the enamel crystal protrusions from remineralization.
Images of enamel surfaces of all groups are also presented in Figure 5. The demineralized enamel surface displayed a characteristic of ‘waves’ (Figure 5e). The typical acid-etched features were no longer distinguishable after 2 weeks of pH cycling in the control group, and the presence of newly deposited mineral ions was evident (Figure 5f). In the cGIC group, the surface irregularities of the demineralized enamel became less pronounced, with a noticeable reduction in intercrystalline gaps (Figure 5g). The zGIC group, however, demonstrated a dense and compact mineral layer covering the enamel surface, effectively masking the previously eroded structures, which were no longer discernible (Figure 5h).
4. Discussion
WSLs with mildly demineralized surfaces can be restored using a variety of treatment techniques and remineralizing agents [28]. In this study, bovine enamel was subjected to controlled demineralization to create WSLs, enabling a comparative assessment of the mineral profile of enamel lesions following a two-week pH cycling regimen. The evaluation was conducted using μCT and SEM for detailed morphological observation. Bovine teeth are suited for creating WSLs because their larger and more uniform enamel surfaces allow for consistent lesion formation, and their similar enamel composition to human teeth provides reliable results when studying demineralization and remineralization processes.
As zinc-ion-containing dental materials have gained significant interest among researchers, our study analyzed remineralization effects on WSLs on bovine teeth to evaluate their effectiveness. In the present in vitro study, the results indicated that the zGIC group exhibited higher remineralization effects than the cGIC group. Therefore, the null hypothesis was rejected. This suggests that incorporating bioactive components, such as zinc ions, could further enhance the efficacy of materials like those used in the zGIC group, providing superior remineralization compared to traditional products like cGIC. The observed enhancement in the remineralization effect of cGIC within 2 weeks of pH cycling could be attributed to the material’s inherent properties, such as its ability to release fluoride ions, which are known to promote remineralization by enhancing the deposition of minerals in demineralized areas [29].
Remineralization is the process of restoring a structurally compromised tooth with mineral depletion via mineral re-deposition in enamel deficient in calcium and phosphate [30]. This process results in net mineral gain by depositing calcium and phosphate ions from plaque and saliva into the demineralized tooth areas that are missing their crystals [31]. The zGIC group in this study displayed not only the highest remineralization effect of all the groups, but also the best remineralization effect within 2 weeks of pH cycling. This indicates that certain ions released from the zGIC promoted the remineralization of demineralized enamel lesions.
Lynch et.al demonstrated that zinc has the potential to enhance fluoride-induced remineralization without acidic challenge in early caries lesions [16]. Remarkably, it was found that fluoride and zinc ions were released in a high amount: the amounts of fluorine and zinc at pH 4.5 were 2-fold and 33-fold greater than at pH 7.0, respectively, in the fluoro-zinc-silicate glass portion of the zGIC under an acidic environment [21,32]. In the present study, remineralization conditions involving a pH cycling condition were employed to specifically attribute any observed effects to the presence of zinc ions. pH cycling is a fundamental aspect of dental research that stimulates the natural process of demineralization and remineralization, mimicking the oral environment [33]. The use of pH cycling in our present study highlights the counteracting effect of BioUnionTM filler (GC, Tokyo, Japan) when placed in acidic environment. During the acidic phase of pH cycling, GIC undergoes surface dissolution, leading to the release of zinc ions and other ions. Zinc, fluoride, and calcium in the BiounionTM filler were able to enhance remineralization [16,34,35]. When the powder and liquid components are combined and applied to the enamel surface, they undergo an acid–base reaction, resulting in the formation of a mixture containing Zn3(PO4)2 and BioUnion™ filler.
BioUnion™ filler is an advanced dental material designed to promote the integration between the restorative material and natural tooth structure. It functions by mimicking the biochemical properties of enamel and dentin, creating a stronger bond, and enhancing the stability of the restoration. Its biocompatibility and ability to form a tight bond with enamel and dentin make it a promising innovation for minimally invasive restorative dentistry [36]. The synergistic effect of zinc and fluoride in the remineralization process highlights the potential of BioUnion™ filler as a therapeutic material in combating early caries lesions [37]. Zinc ions are known to inhibit demineralization by reducing enamel solubility and promoting the formation of a stable zinc-substituted hydroxyapatite layer, which acts as a protective barrier on the enamel surface [38]. Zinc ions can also replace calcium in the hydroxyapatite crystal lattice, increasing its resistance to acid dissolution and enhancing its stability [39,40,41]. Calcium and phosphate ions are extracted from the enamel and incorporated into the mixture, resulting in the formation of a protective layer on the enamel surface [42]. It is hypothesized that the outermost layer of enamel treated with zGIC consists of Zn3(PO4)2, CaF2, Ca3(PO4)2, and the BioUnion™ filler.
While this study successfully observed a remineralized change in cGIC, there were some limitations. Firstly, the pH cycling period in our study was limited to only two weeks; it is anticipated that extending the duration of pH cycling would result in a greater degree of remineralization. Secondly, in this study, the composition of the remineralized area was hypothesized based on the findings. A Time-of-Flight Secondary Ion Mass Spectrometry (TOF-SIMS) analysis would be beneficial to support these results by providing detailed elemental analysis of the remineralized areas. Consequently, further clinical research incorporating extended pH cycling periods and TOF-SIMS analysis is recommended to provide a more comprehensive understanding of the methods employed.
5. Conclusions
The zGIC group showed higher mineral density than the other groups as time passed. Therefore, the results suggest that zinc ions released from GIC have the potential for early carious lesion treatment due to their remineralization-promoting properties.
Author Contributions
Conceptualization, K.M., G.I. and X.C.; methodology, K.M., G.I. and X.C.; software, K.M. and X.C.; validation, K.M., G.I. and X.C.; formal analysis, K.M.; investigation, K.M.; resources, K.M., G.I. and X.C.; data curation, K.M.; writing—original draft preparation, K.M.; writing—review and editing, K.M., G.I., X.C. and Y.S.; visualization, K.M.; supervision, G.I., X.C. and Y.S.; project administration, G.I. and X.C.; funding acquisition, G.I. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
The original contributions presented in this study are included in the article. Further inquiries can be directed to the corresponding author.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Huang, T.T.; Jones, A.S.; He, L.H.; Darendeliler, M.A.; Swain, M.V. Characterisation of enamel white spot lesions using X-ray micro-tomography. J. Dent. 2007, 35, 737–743. [Google Scholar] [CrossRef] [PubMed]
- Aoba, T. Solubility properties of human tooth mineral and pathogenesis of dental caries. Oral Dis. 2004, 10, 249–257. [Google Scholar] [CrossRef]
- Ruan, Q.; Moradian-Oldak, J. Amelogenin and enamel biomimetics. J. Mater. Chem. B 2015, 3, 3112–3129. [Google Scholar] [CrossRef]
- Featherstone, J.D. The science and practice of caries prevention. J. Am. Dent. Assoc. 2000, 131, 887–899. [Google Scholar] [CrossRef] [PubMed]
- Kidd, E.A.M.; Fejerskov, O. What Consitututes Dental Caries? What Constitutes Dental Caries? Histopathology of Carious Enamel and Dentin Related to the Action of Cariogenic Biofilms. J. Dent. Res. 2004, 83, 35–38. [Google Scholar] [CrossRef] [PubMed]
- Han, S.; Fan, Y.; Zhou, Z.; Tu, H.; Li, D.; Lv, X.; Ding, L.; Zhang, L. Promotion of enamel caries remineralization by an amelogenin-derived peptide in a rat model. Arch. Oral Biol. 2017, 73, 66–71. [Google Scholar] [CrossRef]
- Cassiano, L.; Pessan, J.; Comar, L.; Levy, F.; Cardoso, C.; Dionisio, A.; Manarelli, M.; Grizzo, L.; Magalhães, A.C.; Buzalaf, M. Frequency of intake and amount of fluoride in milk for remineralisation of artificial caries on enamel and dentine: Ex vivo/in situ study. Arch. Oral Biol. 2017, 73, 136–141. [Google Scholar] [CrossRef]
- Reynolds, E.C.; Cai, F.; Cochrane, N.J.; Shen, P.; Walker, G.D.; Morgan, M.V.; Reynolds, C. Fluoride and Casein Phosphopeptide-Amorphous Calcium Phosphate. J. Dent. Res. 2008, 87, 344–348. [Google Scholar] [CrossRef]
- Bayne, S.C.; Ferracane, J.L.; Marshall, G.W.; Marshall, S.J.; van Noort, R. The Evolution of Dental Materials over the Past Century: Silver and Gold to Tooth Color and Beyond. J. Dent. Res. 2019, 98, 257–265. [Google Scholar] [CrossRef]
- Cramer, N.; Stansbury, J.; Bowman, C. Recent Advances and Developments in Composite Dental Restorative Materials. J. Dent. Res. 2011, 90, 402–416. [Google Scholar] [CrossRef]
- Chen, X.; Inoue, G.; Ikeda, M.; Sadr, A.; Shimada, Y. Time-dependent structural changes and hypermineralisation of artificially demineralised dentine following treatment with silver diammine fluoride and glass ionomer cement. J. Dent. 2023, 131, 104452. [Google Scholar] [CrossRef] [PubMed]
- Farooq, I.; Bugshan, A. The role of salivary contents and modern technologies in the remineralization of dental enamel: A review. F1000Res 2020, 9, 171. [Google Scholar] [CrossRef] [PubMed]
- Hafshejani, T.M.; Zamanian, A.; Venugopal, J.R.; Rezvani, Z.; Sefat, F.; Saeb, M.R.; Vahabi, H.; Zarrintaj, P.; Mozafari, M. Antibacterial glass-ionomer cement restorative materials: A critical review on the current status of extended release formulations. J. Control. Release 2017, 262, 317–328. [Google Scholar] [CrossRef]
- Imataki, R.; Shinonaga, Y.; Nishimura, T.; Abe, Y.; Arita, K. Mechanical and Functional Properties of a Novel Apatite-Ionomer Cement for Prevention and Remineralization of Dental Caries. Materials 2019, 12, 3998. [Google Scholar] [CrossRef]
- Diefenderfer, C.E.K. Caries remineralization therapy: Implication for dental readiness. Mil. Med. 2008, 173, 48–50. [Google Scholar] [PubMed]
- Abdulkareem, F.A.; Alwaheb, A. The effect of Zinc Oxide nanoparticles with sodium fluoride in remineralization of enamel caries. Bionatura 2023, 8, 1–11. [Google Scholar] [CrossRef]
- Takatsuka, T.; Tanaka, K.; Iijima, Y. Inhibition of dentine demineralization by zinc oxide: In vitro and in situ studies. Dent. Mater. 2005, 21, 1170–1177. [Google Scholar] [CrossRef]
- Oh, S.; Jung, H.-S.; Kim, H.-J.; Jang, J.-H.; Kim, D.-S.; Choi, K.-K.; Kim, S.-Y. Effect of zinc on the collagen degradation in acid-etched dentin. J. Dent. Sci. 2018, 13, 97–102. [Google Scholar] [CrossRef]
- Toledano, M.; Yamauti, M.; Osorio, E.; Osorio, R. Zinc-Inhibited MMP-Mediated Collagen Degradation after Different Dentine Demineralization Procedures. Caries Res. 2012, 46, 201–207. [Google Scholar] [CrossRef]
- Hasegawa, T.; Takenaka, S.; Ohsumi, T.; Ida, T.; Ohshima, H.; Terao, Y.; Naksagoon, T.; Maeda, T.; Noiri, Y. Effect of a novel glass ionomer cement containing fluoro-zinc-silicate fillers on biofilm formation and dentin ion incorporation. Clin. Oral Investig. 2020, 24, 963–970. [Google Scholar] [CrossRef]
- Liu, Y.; Kohno, T.; Tsuboi, R.; Kitagawa, H.; Imazato, S. Acidity-induced release of zinc ion from BioUnionTM filler and its inhibitory effects against Streptococcus mutans. Dent. Mater. J. 2020, 39, 547–553. [Google Scholar] [CrossRef] [PubMed]
- Noach, N.; Lavy, E.; Reifen, R.; Friedman, M.; Kirmayer, D.; Zelinger, E.; Ritter, A.; Yaniv, D.; Reifen, E. Zinc chloride is effective as an antibiotic in biofilm prevention following septoplasty. Sci. Rep. 2023, 13, 1–12. [Google Scholar] [CrossRef]
- Chen, X.; Inoue, G.; Fan, L.; Sekizawa, O.; Nitta, K.; Ikeda, M.; Shimada, Y. Enhancement of mineral density and mechanical properties in root caries treated with silver diammine fluoride and glass ionomer cement, with emphasis on silver ion distribution. J. Dent. 2024, 146, 105041. [Google Scholar] [CrossRef]
- Cate, J.M.T. pH-cycling of enamel and dentin lesions in the presence of low concentrations of fluoride. Eur. J. Oral. Sci. 1995, 103, 362–367. [Google Scholar] [PubMed]
- Matsuda, Y.; Komatsu, H.; Murata, Y.; Tanaka, T.; Sano, H. A Newly Designed Automatic pH-cycling System to Simulate Daily pH Fluctuations. Dent. Mater. J. 2006, 25, 280–285. [Google Scholar]
- Hamba, H.; Nikaido, T.; Sadr, A.; Nakashima, S.; Tagami, J. Enamel Lesion Parameter Correlations between Polychromatic Micro-CT and TMR. J. Dent. Res. 2012, 91, 586–591. [Google Scholar] [CrossRef]
- Hamba, H.; Nikaido, T.; Inoue, G.; Sadr, A.; Tagami, J. Effects of CPP-ACP with sodium fluoride on inhibition of bovine enamel demineralization: A quantitative assessment using micro-computed tomography. J. Dent. 2011, 39, 405–413. [Google Scholar] [CrossRef] [PubMed]
- De Rooij, J.; Nancollas, G. The Formation and Remineralization of Artificial White Spot Lesions: A Constant Composition Approach. J. Dent. Res. 1984, 63, 864–867. [Google Scholar]
- Amaral, M.T.; Guedes-Pinto, A.C.; Chevitarese, O. Effects of a glass-ionomer cement on the remineralization of occlusal caries-an in situ study Efeito de um cimento de ionômero de vidro sobre a remineralização de cárie na superfície oclusal-estudo in situ. Braz. Oral Res. 2006, 20, 91–96. [Google Scholar]
- Simeonov, M.; Gussiyska, A.; Mironova, J.; Nikolova, D.; Apostolov, A.; Sezanova, K.; Dyulgerova, E.; Vassileva, E. Novel hybrid chitosan/calcium phosphates microgels for remineralization of demineralized enamel—A model study. Eur. Polym. J. 2019, 119, 14–21. [Google Scholar] [CrossRef]
- Hamba, H.; Nakamura, K.; Nikaido, T.; Tagami, J.; Muramatsu, T. Remineralization of enamel subsurface lesions using toothpaste containing tricalcium phosphate and fluoride: An In Vitro µCT analysis. BMC Oral. Health 2020, 20, 292. [Google Scholar] [CrossRef]
- Naksagoon, T.; Ohsumi, T.; Takenaka, S.; Nagata, R.; Hasegawa, T.; Maeda, T.; Noiri, Y. Effect of water aging on the anti-biofilm properties of glass ionomer cement containing fluoro-zinc-silicate fillers. Biofouling 2020, 36, 1090–1099. [Google Scholar] [CrossRef] [PubMed]
- Cate, J.T.; Duijsters, P. Alternating Demineralization and Remineralization of Artificial Enamel Lesions. Caries Res. 1982, 16, 201–210. [Google Scholar] [CrossRef]
- Lippert, F. Dose-Response Effects of Zinc and Fluoride on Caries Lesion Remineralization. Caries Res. 2012, 46, 62–68. [Google Scholar] [CrossRef]
- Cate, J.M.T.; Cummins, D. Fluoride toothpaste containing 1.5% arginine and insoluble calcium as a new standard of care in caries prevention. J. Clin. Dent. 2013, 24, 79–87. [Google Scholar] [PubMed]
- Imazato, S.; Kohno, T.; Tsuboi, R.; Thongthai, P.; Xu, H.H.; Kitagawa, H. Cutting-edge filler technologies to release bio-active components for restorative and preventive dentistry. Dent. Mater. J. 2020, 39, 69–79. [Google Scholar] [CrossRef] [PubMed]
- Lynch, R.; Churchley, D.; Butler, A.; Kearns, S.; Thomas, G.; Badrock, T.; Cooper, L.; Higham, S. Effects of Zinc and Fluoride on the Remineralisation of Artificial Carious Lesions under Simulated Plaque Fluid Conditions. Caries Res. 2011, 45, 313–322. [Google Scholar] [CrossRef]
- Butera, A.; Maiorani, C.; Gallo, S.; Pascadopoli, M.; Quintini, M.; Lelli, M.; Tarterini, F.; Foltran, I.; Scribante, A. Biomimetic Action of Zinc Hydroxyapatite on Remineralization of Enamel and Dentin: A Review. Biomimetics 2023, 8, 71. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Mayer, I.; Featherstone, J. Dissolution studies of Zn-containing carbonated hydroxyapatites. J. Cryst. Growth 2000, 219, 98–101. [Google Scholar] [CrossRef]
- Li, M.; Xiao, X.; Liu, R.; Chen, C.; Huang, L. Structural characterization of zinc-substituted hydroxyapatite prepared by hydrothermal method. J. Mater. Sci. Mater. Med. 2008, 19, 797–803. [Google Scholar] [CrossRef]
- Dornelas, J.; Dornelas, G.; Rossi, A.; Piattelli, A.; Di Pietro, N.; Romasco, T.; Mourão, C.F.; Alves, G.G. The Incorporation of Zinc into Hydroxyapatite and Its Influence on the Cellular Response to Biomaterials: A Systematic Review. J. Funct. Biomater. 2024, 15, 178. [Google Scholar] [CrossRef] [PubMed]
- Matsuzaki, K.; Shimada, Y.; Shinno, Y.; Ono, S.; Yamaji, K.; Ohara, N.; Sadr, A.; Sumi, Y.; Tagami, J.; Yoshiyama, M. Assessment of Demineralization Inhibition Effects of Dentin Desensitizers Using Swept-Source Optical Coherence Tomography. Materials 2021, 14, 1876. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).