Investigation of In Vitro Corrosion and Wear Properties of Biomedical Coatings Applied to Ti6Al4V Alloy Manufactured by Selective Laser Melting
Abstract
1. Introduction
2. Materials and Methods
3. Results and Discussion
4. Conclusions
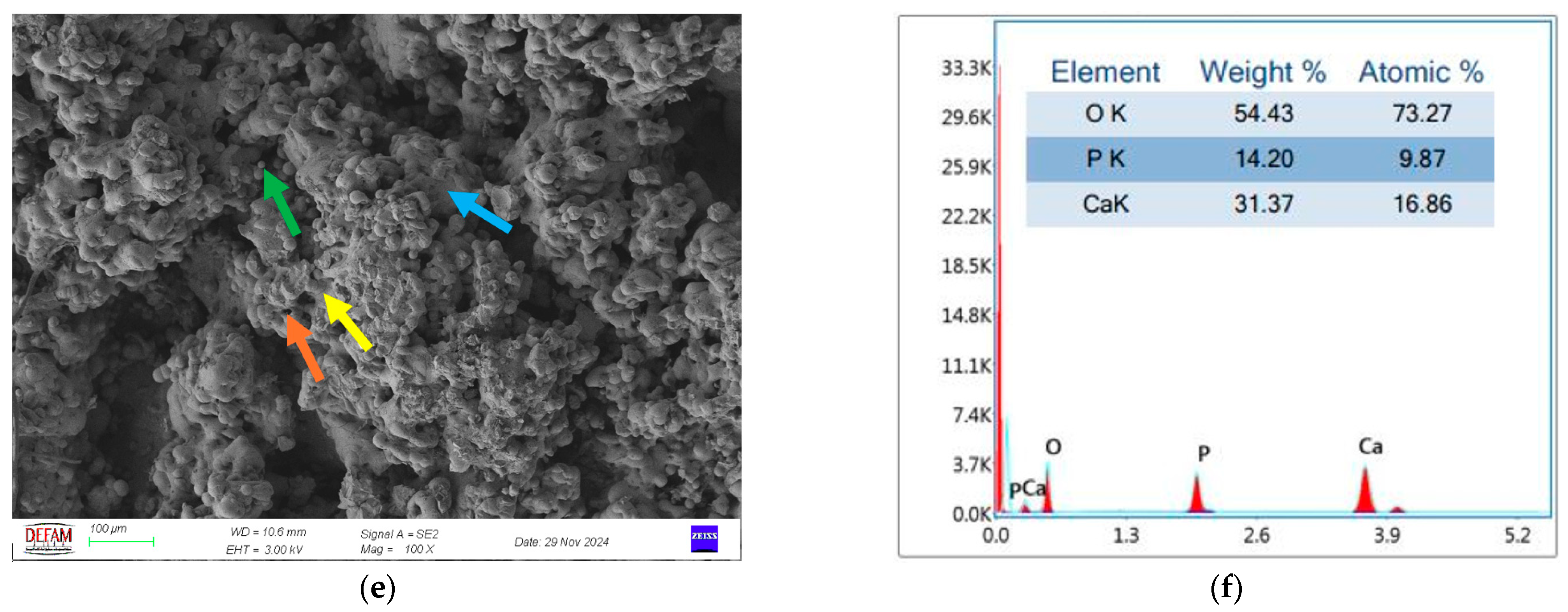
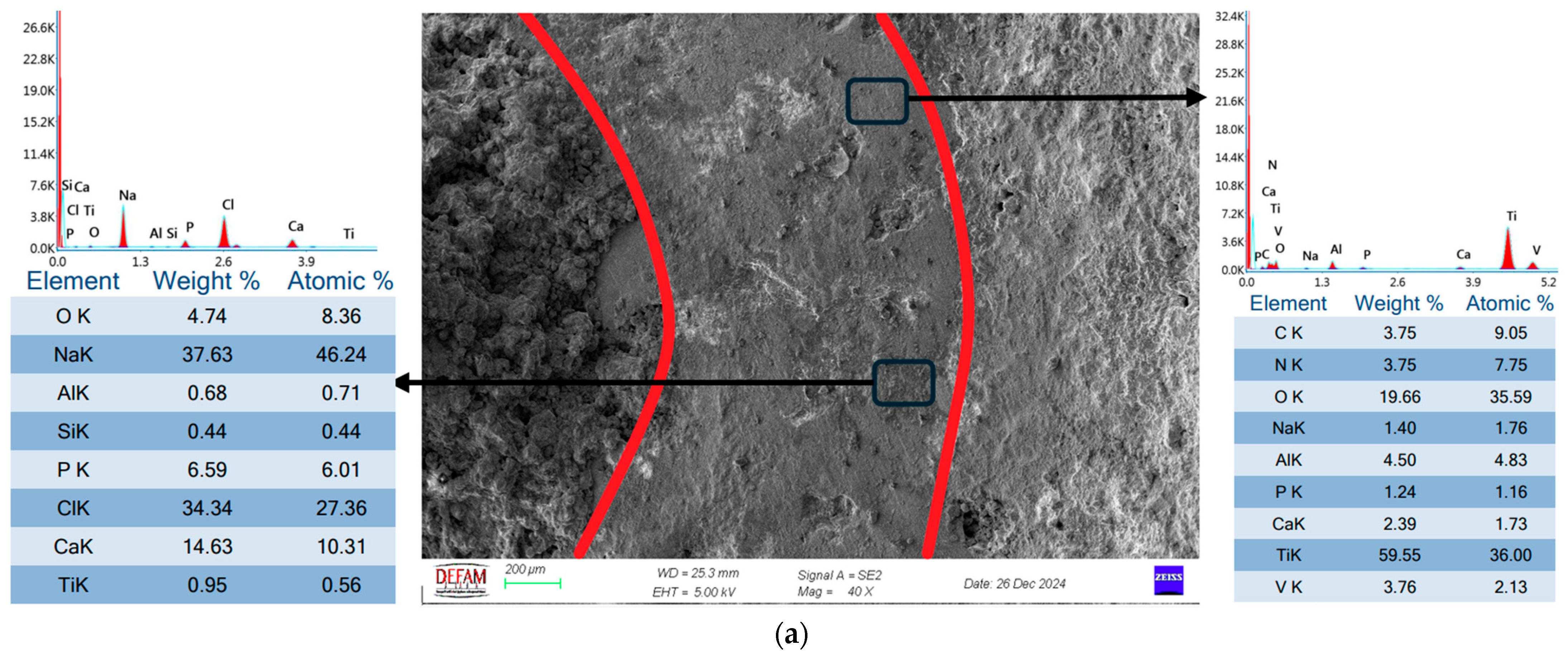
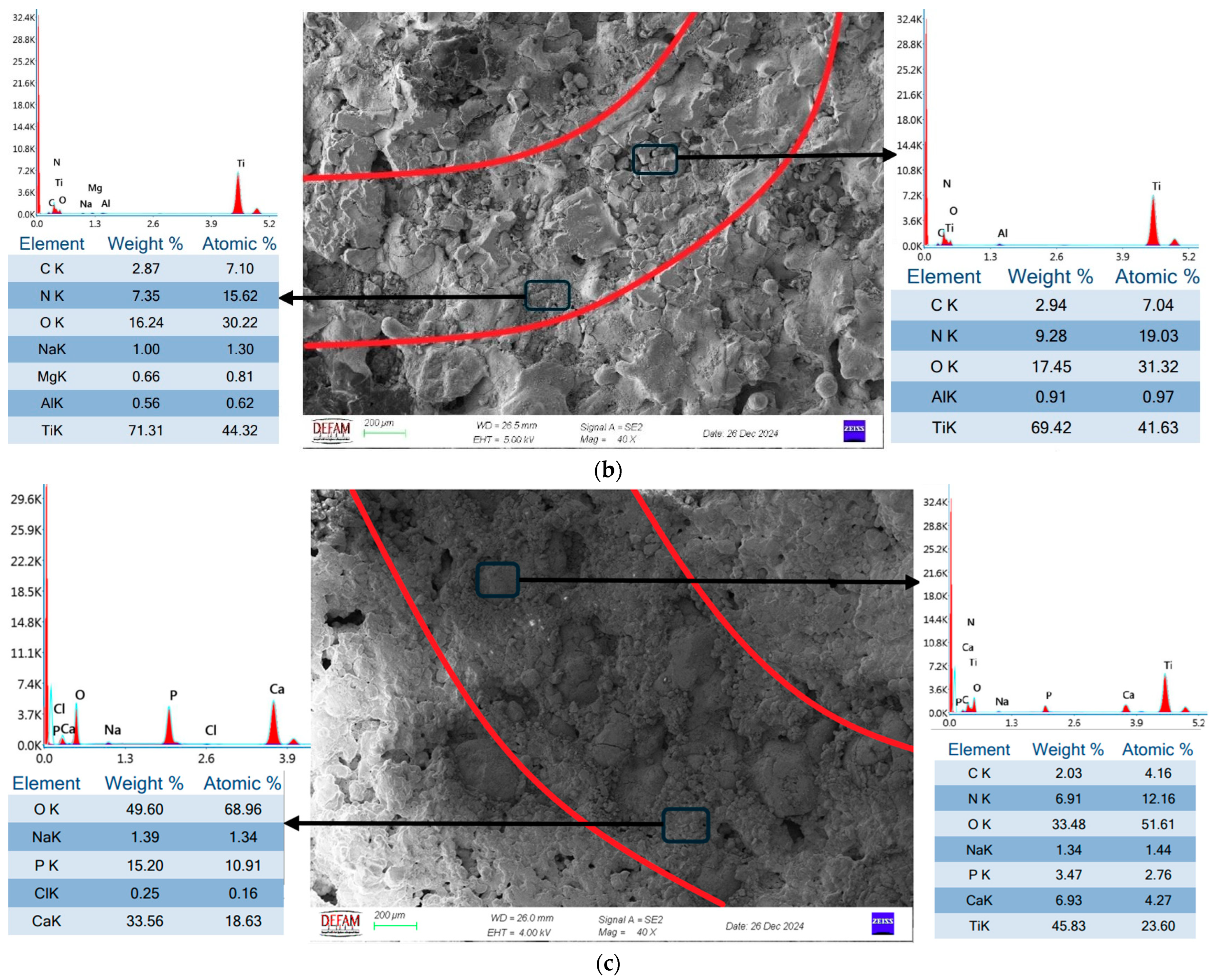
- SEM and EDS analyses demonstrated that bilayer Ti/HA coatings minimized microcrack formation, thereby enhancing mechanical durability and biocompatibility. The Ca/P ratio of 1.67, determined through EDS analysis, further validated the superior biocompatibility of these coatings.
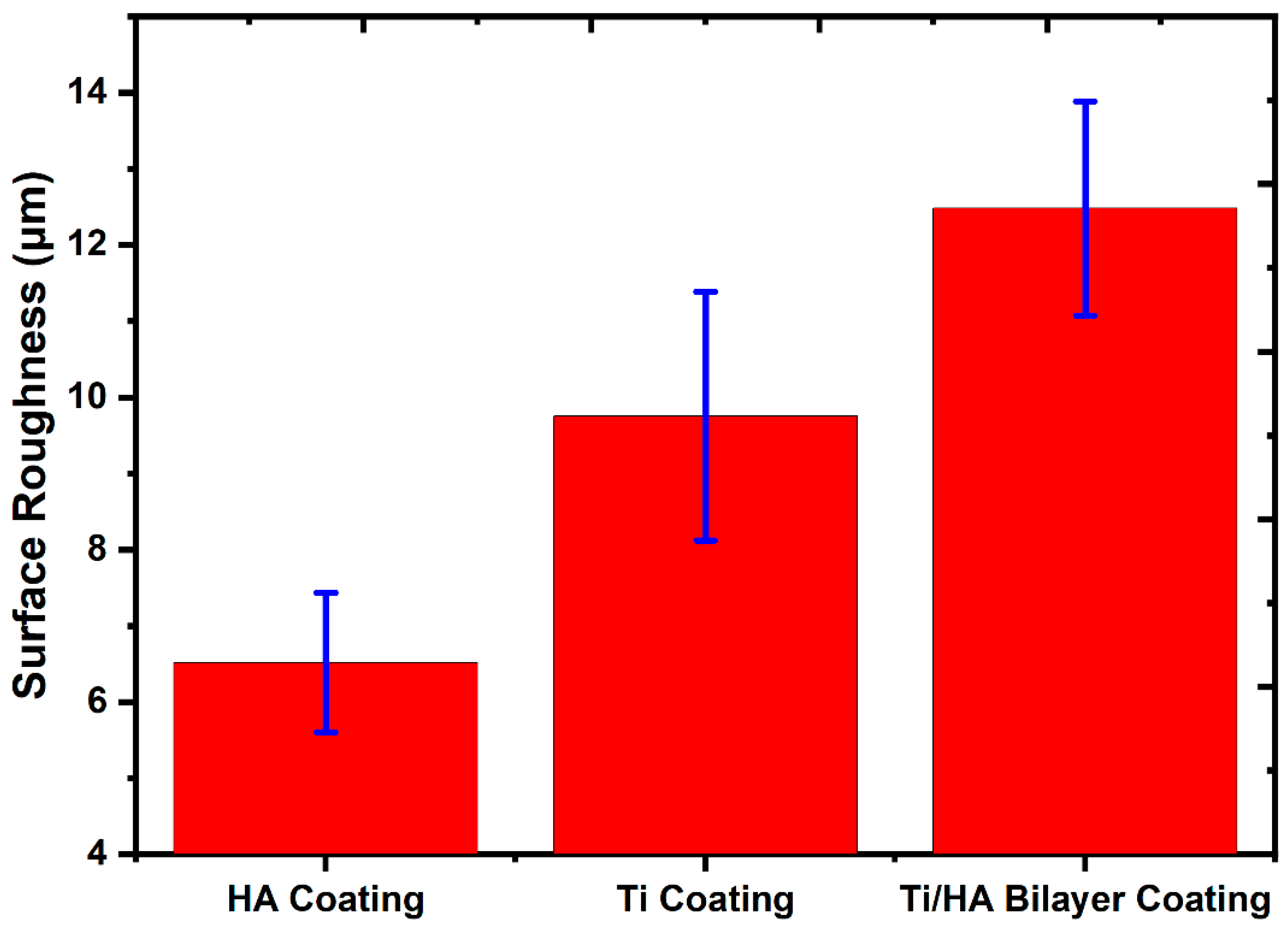
- Surface roughness analyses revealed that Ti coatings exhibited a roughness value of 9.75 ± 1.63 μm, HA coatings 6.51 ± 0.92 μm, and bilayer Ti/HA coatings 12.48 ± 1.4 μm. The highest surface roughness value observed in bilayer coatings is a critical feature that promotes cellular adhesion, making it advantageous for biomedical applications.
- In wear tests, Ti coatings displayed the lowest wear rate at 6.32 × 10−3 (mm3/N⋅m), while HA coatings exhibited the highest wear rate at 73.83 × 10−3 (mm3/N⋅m). Bilayer Ti/HA coatings showed an intermediate wear performance with a wear rate of 34.22 × 10−3 (mm3/N⋅m). These findings highlight the contribution of the Ti layer to wear resistance and the role of the bilayer structure in improving durability.
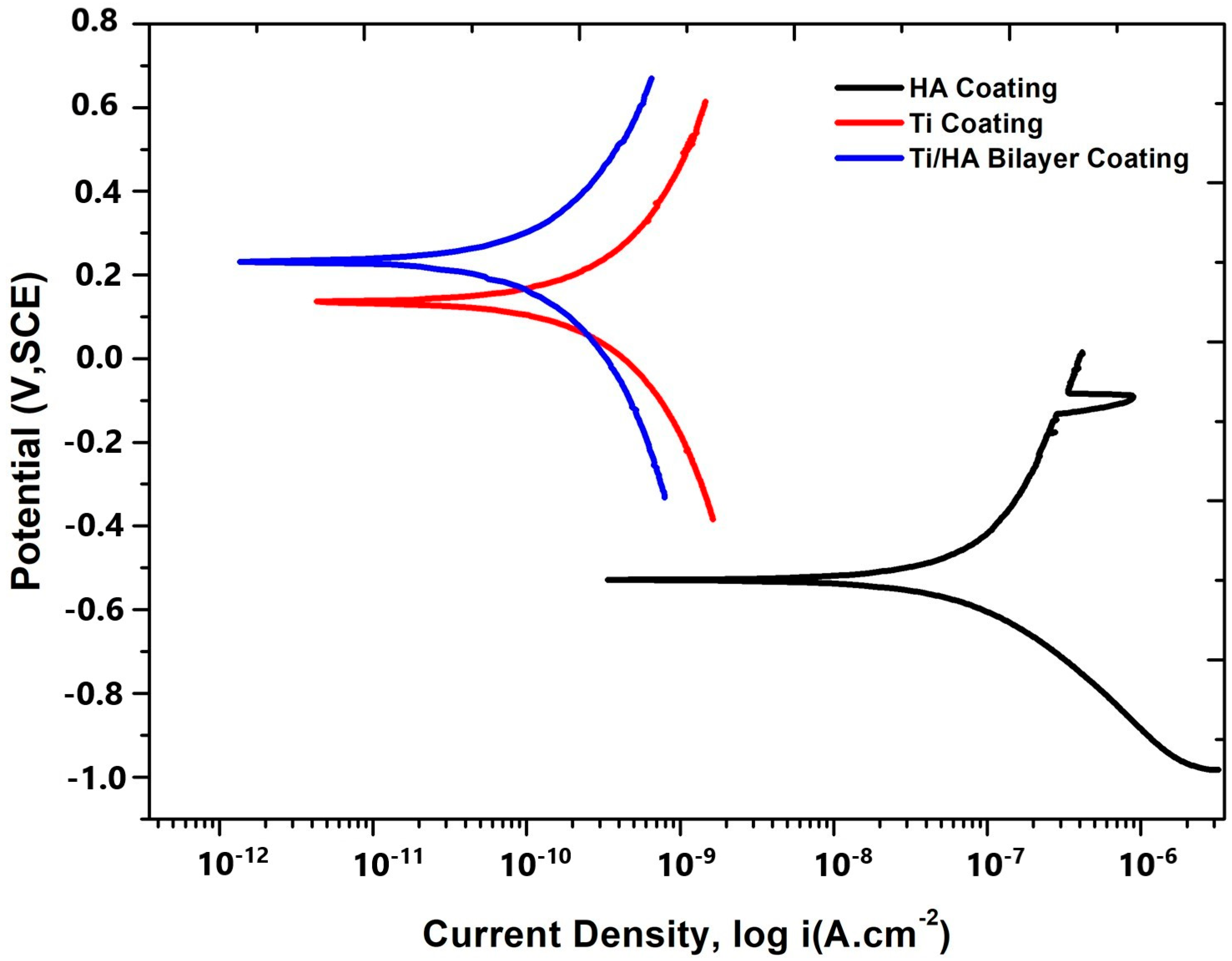
- In corrosion tests, the bilayer Ti/HA coating demonstrated the best performance with a corrosion current of 0.000153 μA and a corrosion rate of 0.00009 mm/year, Ti coatings exhibited a corrosion current of 0.000351 μA and a corrosion rate of 0.0002 mm/year, showing lower resistance compared to bilayer coatings but higher than HA coatings. HA coatings, with a corrosion current of 0.051 μA and a corrosion rate of 0.003 mm/year, showed the lowest corrosion resistance.
- In conclusion, bilayer Ti/HA coatings provide significant advantages in terms of biomedical performance by preventing microcrack formation and exhibiting superior wear and corrosion resistance. These findings contribute valuable insights to the limited literature on SLM-based coating applications and serve as a solid foundation for future research.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Zhou, L.; Yuan, T.; Li, R.; Tang, J.; Wang, M.; Mei, F. Microstructure and mechanical properties of selective laser melted biomaterial Ti-13Nb-13Zr compared to hot-forging. Mater. Sci. Eng. A 2018, 725, 329–340. [Google Scholar] [CrossRef]
- Munir, K.; Biesiekierski, A.; Wen, C.; Li, Y. Selective Laser Melting in Biomedical Manufacturing. In Metallic Biomaterials Processing and Medical Device Manufacturing; Elsevier: Amsterdam, The Netherlands, 2020; pp. 235–269. [Google Scholar] [CrossRef]
- Amirnejad, M.; Rajabi, M.; Jamaati, R. Importance of individual evaluation of crystallographic texture and microstructure effects on biocompatibility and corrosion performance of Ti6Al4V alloy. Met. Mater. Int. 2023, 29, 343–356. [Google Scholar] [CrossRef]
- Thomas, P.; Weik, T.; Roider, G.; Summer, B.; Thomsen, M. Influence of surface coating on metal ion release: Evaluation in patients with metal allergy. Orthopedics 2016, 39, S24–S30. [Google Scholar] [CrossRef]
- Levine, B.R.; Sporer, S.; Poggie, R.A.; Della Valle, C.J.; Jacobs, J.J. Experimental and clinical performance of porous tantalum in orthopedic surgery. Biomaterials 2006, 27, 4671–4681. [Google Scholar] [CrossRef]
- Kazemi, M.; Ahangarani, S.; Esmailian, M.; Shanaghi, A. Investigating the corrosion performance of Ti-6Al-4V biomaterial alloy with hydroxyapatite coating by artificial neural network. Mater. Sci. Eng. B 2022, 278, 115644. [Google Scholar] [CrossRef]
- Ritwik, A.; Saju, K.K.; Vengellur, A.; Saipriya, P.P. Development of thin-film hydroxyapatite coatings with an intermediate shellac layer produced by dip-coating process on Ti6Al4V implant materials. J. Coat. Technol. Res. 2022, 19, 597–605. [Google Scholar] [CrossRef]
- Singh, H.; Kumar, R.; Prakash, C.; Singh, S. HA-based coating by plasma spray techniques on titanium alloy for orthopedic applications. Mater. Today Proc. 2022, 50, 612–628. [Google Scholar] [CrossRef]
- Ghadami, F.; Hamedani, M.A.; Rouhi, G.; Saber-Samandari, S.; Dehghan, M.M.; Farzad-Mohajeri, S.; Mashhadi-Abbas, F. The correlation between osseointegration and bonding strength at the bone-implant interface: In-vivo & ex-vivo investigations on hydroxyapatite and hydroxyapatite/titanium coatings. J. Biomech. 2022, 144, 111310. [Google Scholar] [CrossRef]
- Kylmäoja, E.; Holopainen, J.; Abushahba, F.; Ritala, M.; Tuukkanen, J. Osteoblast attachment on titanium coated with hydroxyapatite by atomic layer deposition. Biomolecules 2022, 12, 654. [Google Scholar] [CrossRef]
- Van Hove, R.P.; Sierevelt, I.N.; van Royen, B.J.; Nolte, P.A. Titanium-nitride coating of orthopaedic implants: A review of the literature. BioMed Res. Int. 2015, 2015, 485975. [Google Scholar] [CrossRef]
- Piscanec, S.; Ciacchi, L.C.; Vesselli, E.; Comelli, G.; Sbaizero, O.; Meriani, S.; De Vita, A. Bioactivity of TiN-coated titanium implants. Acta Mater. 2004, 52, 1237–1245. [Google Scholar] [CrossRef]
- Liu, D.G.; Zheng, L.; Liang, Y.; Li, H.; Liu, J.Q.; Luo, L.M.; Wu, Y.C. Preparation, biocompatibility, and biotribological properties of TiN-incorporated graphite-like amorphous carbon bio-ceramic composite films. Ceram. Int. 2018, 44, 6810–6816. [Google Scholar] [CrossRef]
- Subramanian, B.; Muraleedharan, C.V.; Ananthakumar, R.; Jayachandran, M. A comparative study of titanium nitride (TiN), titanium oxy nitride (TiON) and titanium aluminum nitride (TiAlN), as surface coatings for bio implants. Surf. Coat. Technol. 2011, 205, 5014–5020. [Google Scholar] [CrossRef]
- Santecchia, E.; Hamouda, A.M.S.; Musharavati, F.; Zalnezhad, E.; Cabibbo, M.; Spigarelli, S. Wear resistance investigation of titanium nitride-based coatings. Ceram. Int. 2015, 41, 10349–10379. [Google Scholar] [CrossRef]
- Kumar, C.S.; Gupta, S.; Sharma, A.K. Characterization of TiN−HA nanocomposite developed through rapid microwave sintering route at high temperature. Int. J. Appl. Ceram. Technol. 2025, e15070. [Google Scholar] [CrossRef]
- Croitoru, C.; Roata, I.C.; Pascu, A.; Stanciu, E.M.; Hulka, I.; Stoian, G.; Lupu, N. Photocatalytic surfaces obtained through one-step thermal spraying of titanium. Appl. Surf. Sci. 2020, 504, 144173. [Google Scholar] [CrossRef]
- Amin, S.; Panchal, H. A review on thermal spray coating processes. Transfer 2016, 2, 556–563. [Google Scholar]
- Kahraman, N.; Gülenç, B. Abrasive wear behaviour of powder flame sprayed coatings on steel substrates. Mater. Des. 2002, 23, 721–725. [Google Scholar] [CrossRef]
- Quirama, A.; Echavarria, A.M.; Meza, J.M.; Osorio, J.; Bejarano, G. Improvement of the mechanical behavior of the calcium phosphate coatings deposited onto Ti6Al4V alloy using an intermediate TiN/TiO2 bilayer. Vacuum 2017, 146, 22–30. [Google Scholar] [CrossRef]
- Mohseni, E.; Zalnezhad, E.; Bushroa, A.R.; Hamouda, A.M.; Goh, B.T.; Yoon, G.H. Ti/TiN/HA coating on Ti–6Al–4V for biomedical applications. Ceram. Int. 2015, 41, 14447–14457. [Google Scholar] [CrossRef]
- Qi, J.; Chen, K.; Teng, S.; Li, X. Preparation and characterisation of gradient HA/TiN/Ti coatings for biomedical applications. Surf. Eng. 2024, 40, 826–837. [Google Scholar] [CrossRef]
- Lenis, J.A.; Bejarano, G.; Rico, P.; Ribelles, J.G.; Bolívar, F.J. Development of multilayer Hydroxyapatite-Ag/TiN-Ti coatings deposited by radio frequency magnetron sputtering with potential application in the biomedical field. Surf. Coat. Technol. 2019, 377, 124856. [Google Scholar]
- Sargin, F.; Erdogan, G.; Kanbur, K.; Turk, A. Investigation of in vitro behavior of plasma sprayed Ti, TiO2 and HA coatings on PEEK. Surf. Coat. Technol. 2021, 411, 126965. [Google Scholar] [CrossRef]
- Suntharavel Muthaiah, V.M.; Rajput, M.; Tripathi, A.; Suwas, S.; Chatterjee, K. Electrophoretic Deposition of Nanocrystalline Calcium Phosphate Coating for Augmenting Bioactivity of Additively Manufactured Ti-6Al-4V. ACS Mater. Au 2021, 2, 132–142. [Google Scholar] [CrossRef]
- Yan, C.; Hao, L.; Hussein, A.; Wei, Q.; Shi, Y. Microstructural and surface modifications and hydroxyapatite coating of Ti-6Al-4V triply periodic minimal surface lattices fabricated by selective laser melting. Mater. Sci. Eng. C 2017, 75, 1515–1524. [Google Scholar] [CrossRef]
- Luo, J.; Jia, X.; Gu, R.; Zhou, P.; Huang, Y.; Sun, J.; Yan, M. 316L stainless steel manufactured by selective laser melting and its biocompatibility with or without hydroxyapatite coating. Metals 2018, 8, 548. [Google Scholar] [CrossRef]
- Stango, S.A.X.; Karthick, D.; Swaroop, S.; Mudali, U.K.; Vijayalakshmi, U. Development of hydroxyapatite coatings on laser textured 316LSS and Ti-6Al-4V and its electrochemical behavior in SBF solution for orthopedic applications. Ceram. Int. 2018, 44, 3149–3160. [Google Scholar] [CrossRef]
- Fazel, M.; Salimijazi, H.R.; Shamanian, M.; Minneboo, M.; Modaresifar, K.; van Hengel, I.A.; Zadpoor, A.A. Osteogenic and antibacterial surfaces on additively manufactured porous Ti-6Al-4V implants: Combining silver nanoparticles with hydrothermally synthesized HA nanocrystals. Mater. Sci. Eng. C 2021, 120, 111745. [Google Scholar] [CrossRef]
- Yang, Y.; Zhao, M.; Wang, H.; Zhou, K.; He, Y.; Mao, Y.; Xie, D.; Lv, F.; Shen, L. Microstructure and fatigue performance of Ti6Al4V produced by laser powder bed fusion after post-heat treatment. Appl. Sci. 2023, 13, 1828. [Google Scholar] [CrossRef]
- EOS GmbH. EOS Titanium Ti64 Material Data Sheet. EOS. 2022. Available online: https://www.eos.info/03_system-related-assets/material-related-contents/metal-materials-and-examples/metal-material-datasheet/titan/ti64/eos_ti64_9011-0014_9011-0039_m290_mds_06-22_en.pdf (accessed on 13 March 2025).
- Kumrular, B.; Cicek, O.; Dağ, İ.E.; Avar, B.; Erener, H. Evaluation of the Corrosion Resistance of different types of Orthodontic fixed Retention Appliances: A preliminary Laboratory Study. J. Funct. Biomater. 2023, 14, 81. [Google Scholar] [CrossRef]
- Alontseva, D.L.; Abilev, M.B.; Zhilkashinova, A.M.; Voinarovych, S.G.; Kyslytsia, O.N.; Ghassemieh, E.; Russakova, A.; Łatka, L. Optimization of hydroxyapatite synthesis and microplasma spraying of porous coatings onto titanium implants. Adv. Mater. Sci. 2018, 18, 79–94. [Google Scholar] [CrossRef]
- Guillem-Marti, J.; Cinca, N.; Punset, M.; Cano, I.G.; Gil, F.J.; Guilemany, J.M.; Dosta, S. Porous titanium-hydroxyapatite composite coating obtained on titanium by cold gas spray with high bond strength for biomedical applications. Colloids Surf. B Biointerfaces 2019, 180, 245–253. [Google Scholar] [PubMed]
- Khor, K.A.; Li, H.; Cheang, P. Impact formation and microstructure characterization of thermal sprayed hydroxyapatite/titania composite coatings. Biomaterials 2003, 24, 2233–2243. [Google Scholar] [CrossRef]
- Sun, Y.; Zhang, X.; Luo, M.; Hu, W.; Zheng, L.; Huang, R.; Greven, J.; Hildebrand, F.; Yuan, F. Plasma Spray vs. Electrochemical Deposition: Toward a Better Osteogenic Effect of Hydroxyapatite Coatings on 3D-Printed Titanium Scaffolds. Front. Bioeng. Biotechnol. 2021, 9, 705774. [Google Scholar] [CrossRef]
- Farnoush, H.; Muhaffel, F.; Cimenoglu, H. Fabrication and characterization of nano-HA-45S5 bioglass composite coatings on calcium-phosphate containing micro-arc oxidized CP-Ti substrates. Appl. Surf. Sci. 2015, 324, 765–774. [Google Scholar] [CrossRef]
- Ansari, Z.; Kalantar, M.; Soriente, A.; Fasolino, I.; Kharaziha, M.; Ambrosio, L.; Raucci, M.G. In-situ synthesis and characterization of chitosan/hydroxyapatite nanocomposite coatings to improve the bioactive properties of Ti6Al4V substrates. Materials 2020, 13, 3772. [Google Scholar] [CrossRef] [PubMed]
- Qi, J.; Yang, Y.; Zhou, M.; Chen, Z.; Chen, K. Effect of transition layer on the performance of hydroxyapatite/titanium nitride coating developed on Ti-6Al-4V alloy by magnetron sputtering. Ceram. Int. 2019, 45, 4863–4869. [Google Scholar] [CrossRef]
- Kazemi, M.; Ahangarani, S.; Esmailian, M.; Shanaghi, A. Investigation on the corrosion behavior and biocompatibility of Ti-6Al-4V implant coated with HA/TiN dual layer for medical applications. Surf. Coat. Technol. 2020, 397, 126044. [Google Scholar] [CrossRef]
- Kobayashi, A. Formation of TiN coatings by gas tunnel type plasma reactive spraying. Surf. Coat. Technol. 2000, 132, 152–157. [Google Scholar] [CrossRef]
- Yanchun, D.; Dianran, Y.; Jining, H.; Jianxin, Z.; Lisong, X.; Xiangzhi, L. Studies on nanocrystalline TiN coatings prepared by reactive plasma spraying. J. Nanomater. 2008, 2008, 690951. [Google Scholar] [CrossRef]
- Dorozhkin, S.V. Bioceramics of calcium orthophosphates. Biomaterials 2010, 31, 1465–1485. [Google Scholar] [CrossRef] [PubMed]
- Gross, K.A.; Berndt, C.C. Biomedical application of apatites. Rev. Mineral. Geochem. 2002, 48, 631–672. [Google Scholar] [CrossRef]
- Sopyan, I.; Mel, M.; Ramesh, S.; Khalid, K.A. Porous hydroxyapatite for artificial bone applications. Sci. Technol. Adv. Mater. 2007, 8, 116. [Google Scholar] [CrossRef]
- Levingstone, T.J. Optimisation of Plasma Sprayed Hydroxyapatite Coatings. Ph.D. Thesis, Dublin City University, Dublin, Ireland, 2008. [Google Scholar]
- Albrektsson, T.; Johansson, C. Osteoinduction, osteoconduction and osseointegration. Eur. Spine J. 2001, 10, S96–S101. [Google Scholar] [CrossRef]
- Tirrell, M.; Kokkoli, E.; Biesalski, M. The role of surface science in bioengineered materials. Surf. Sci. 2002, 500, 61–83. [Google Scholar] [CrossRef]
- Roşu, R.A.; Şerban, V.A.; Bucur, A.I.; Dragoş, U. Deposition of titanium nitride and hydroxyapatite-based biocompatible composite by reactive plasma spraying. Appl. Surf. Sci. 2012, 258, 3871–3876. [Google Scholar] [CrossRef]
- Khethier Abbass, M.; Jasim, A.N.; Jasim, M.; Khashan, K.S.; Issa, M.J. Improving Bio Corrosion Resistance of the Single Layer of Nano Hydroxyapatite and Nano YSZ Coating on the Ti6Al4V Alloy Using Electrophoretic Deposition. Solid State Technol. 2020, 63, 18584–18597. [Google Scholar]
- Coşkun, M.İ.; Karahan, İ.H.; Yücel, Y.; Golden, T.D. Optimization of electrochemical step deposition for bioceramic hydroxyapatite coatings on CoCrMo implants. Surf. Coat. Technol. 2016, 301, 42–53. [Google Scholar] [CrossRef]
- Drevet, R.; Aaboubi, O.; Benhayoune, H. In vitro corrosion behavior of electrodeposited calcium phosphate coatings on Ti6Al4V substrates. J. Solid State Electrochem. 2012, 16, 3069–3077. [Google Scholar] [CrossRef]
- Butt, M.S.; Afsheen, R.; Saeed, H.; Javed, N.; Ghaffar, A. Promoting Surgical Pin Performance: Chitosan and Hydroxyapatite-Based Nano-composite Coatings for Antimicrobial and Corrosion Protection. Results Eng. 2024, 25, 103714. [Google Scholar] [CrossRef]
- Corona-Gomez, J.; Sandhi, K.K.; Yang, Q. Wear and corrosion behaviour of nanocrystalline TaN, ZrN, and TaZrN coatings deposited on biomedical grade CoCrMo alloy. J. Mech. Behav. Biomed. Mater. 2022, 130, 105228. [Google Scholar] [CrossRef] [PubMed]
- Hikov, T.; Nikolova, M.; Petrov, P. Effect of TiN/TiO2 multilayer coatings on the properties of stainless steel for biomedical applications. J. Phys. Conf. Ser. 2020, 1492, 012029. [Google Scholar] [CrossRef]




| Ti (Wt.%) | Al (Wt.%) | V (Wt.%) | O (ppm) | N (ppm) | C (ppm) | H (ppm) | Fe (ppm) | Density (g/cm3) |
|---|---|---|---|---|---|---|---|---|
| Balance | 5.5–6.75 | 3.5–4.5 | <2000 | <500 | <800 | <150 | <3000 | 4.41 |
| Spraying Parameter | Value (For HA Coating) | Value (for Ti Coating) |
|---|---|---|
| Propane flow rate (L·min−1) | 17 | 19 |
| Oxygen flow rate (L·min−1) | 20 | 23 |
| Air flow rate (L·min−1) | 10 | 10 |
| Spray distance (cm) | 40 | 40 |
| Spray angle | 90° | 90° |
| Coating Type | Ecorr (mV) | Beta a (mV/Decade) | Beta c | İcorr (μA) | Corrosion Rate (mm/Year) |
|---|---|---|---|---|---|
| HA Coating | −527.586 | 390.4 | 237.4 | 0.051 | 0.003 |
| Ti Coating | 137.658 | 726.7 | 705 | 0.000351 | 0.0002 |
| Ti/Ha Bilayer Coating | 237.259 | 648.9 | 713 | 0.000153 | 0.00009 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bahçepinar, A.İ.; Aydin, İ. Investigation of In Vitro Corrosion and Wear Properties of Biomedical Coatings Applied to Ti6Al4V Alloy Manufactured by Selective Laser Melting. Crystals 2025, 15, 316. https://doi.org/10.3390/cryst15040316
Bahçepinar Aİ, Aydin İ. Investigation of In Vitro Corrosion and Wear Properties of Biomedical Coatings Applied to Ti6Al4V Alloy Manufactured by Selective Laser Melting. Crystals. 2025; 15(4):316. https://doi.org/10.3390/cryst15040316
Chicago/Turabian StyleBahçepinar, Ali İhsan, and İbrahim Aydin. 2025. "Investigation of In Vitro Corrosion and Wear Properties of Biomedical Coatings Applied to Ti6Al4V Alloy Manufactured by Selective Laser Melting" Crystals 15, no. 4: 316. https://doi.org/10.3390/cryst15040316
APA StyleBahçepinar, A. İ., & Aydin, İ. (2025). Investigation of In Vitro Corrosion and Wear Properties of Biomedical Coatings Applied to Ti6Al4V Alloy Manufactured by Selective Laser Melting. Crystals, 15(4), 316. https://doi.org/10.3390/cryst15040316






