Polymorphism of the Transition Metal Oxidotellurates NiTeO4 and CuTe2O5
Abstract
1. Introduction
2. Materials and Methods
2.1. Crystal Growth Experiments
2.2. X-Ray Diffraction Measurements and Crystal Structure Analysis
2.3. Raman Spectroscopy
3. Results and Discussion
3.1. Crystal Growth
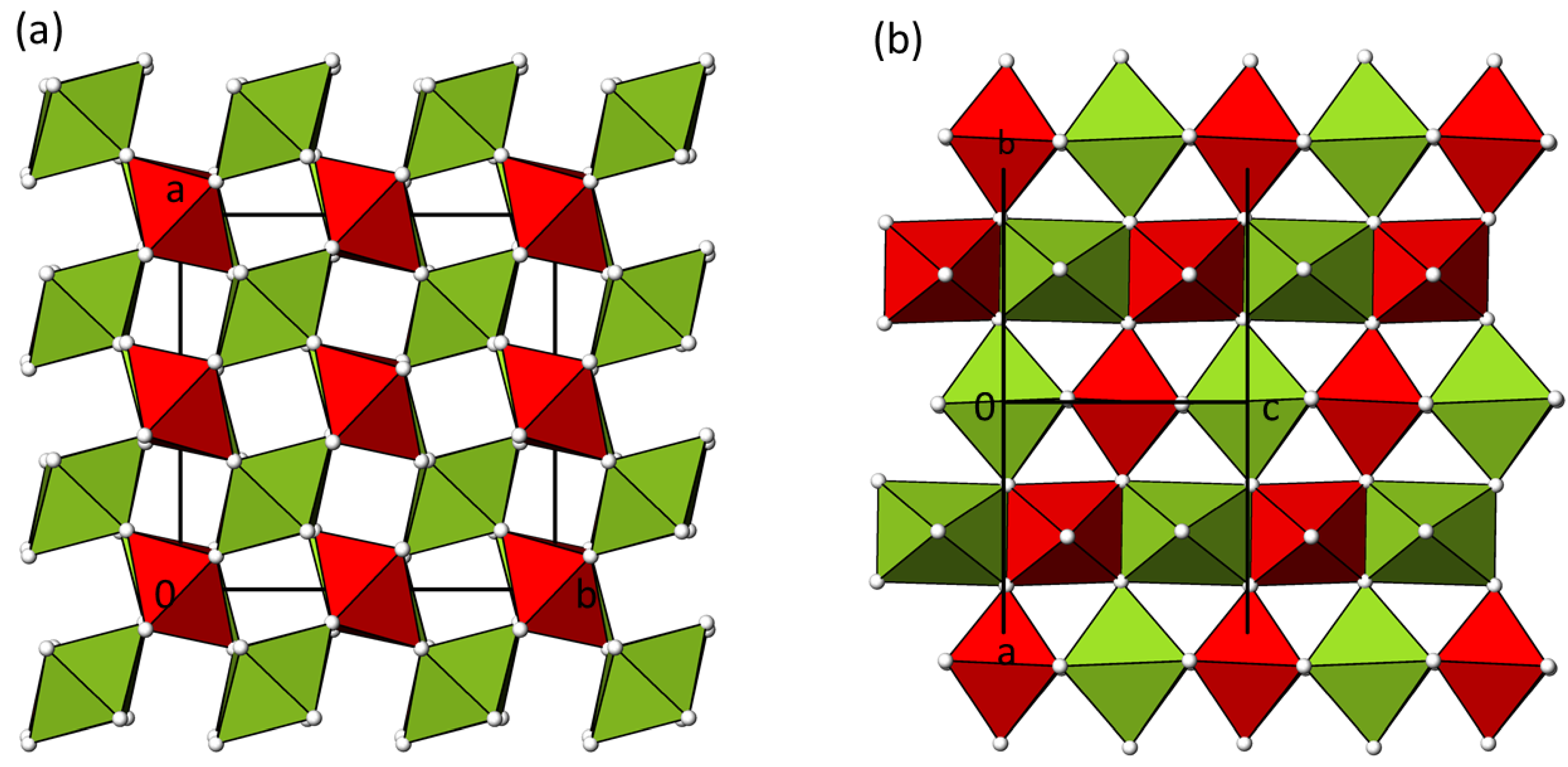
3.2. Structure Description of α-NiTeO4
3.3. Structure Description of β-NiTeO4
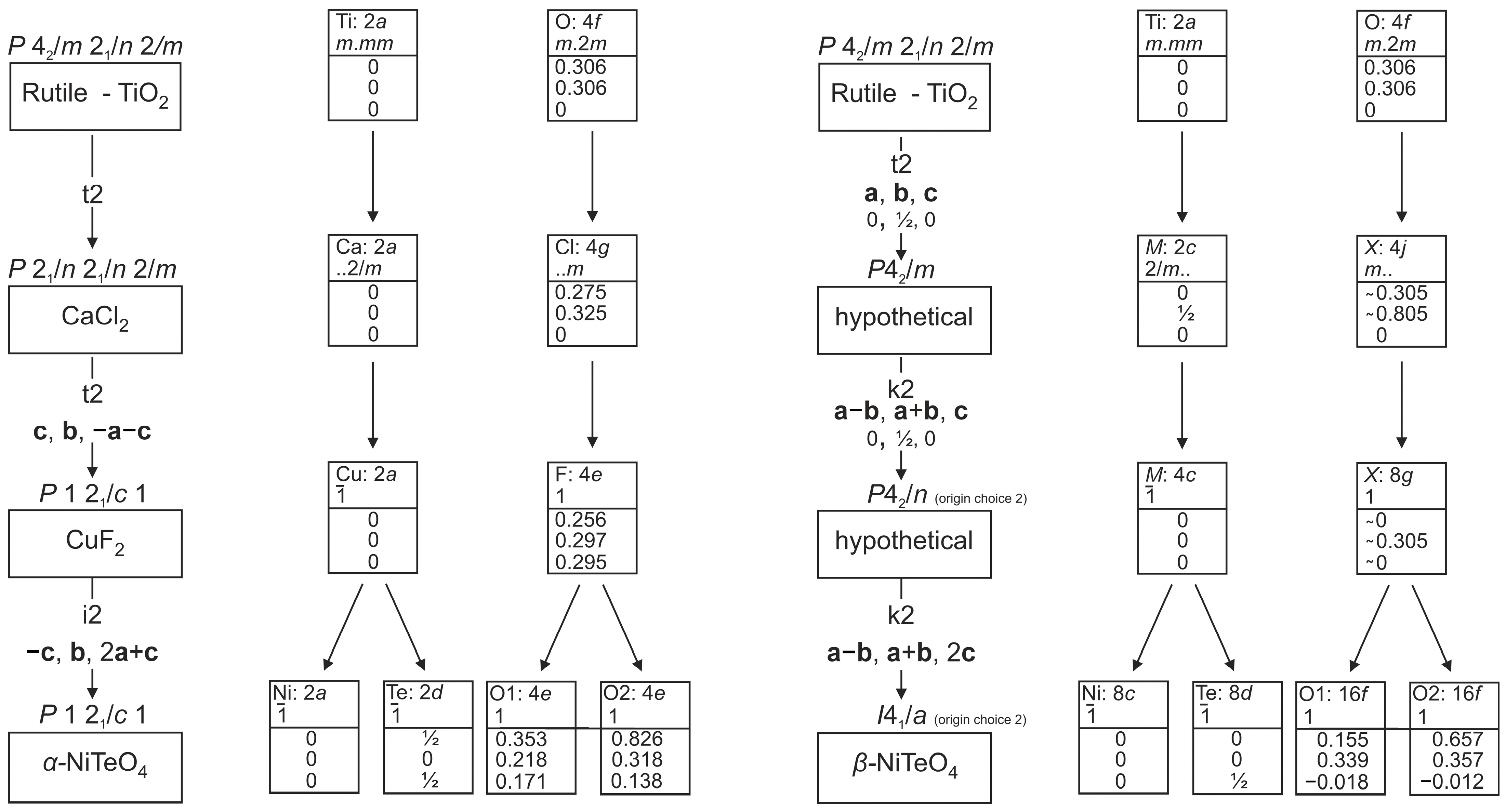
3.4. Structure Relation of α- and β-NiTeO4 to Rutile
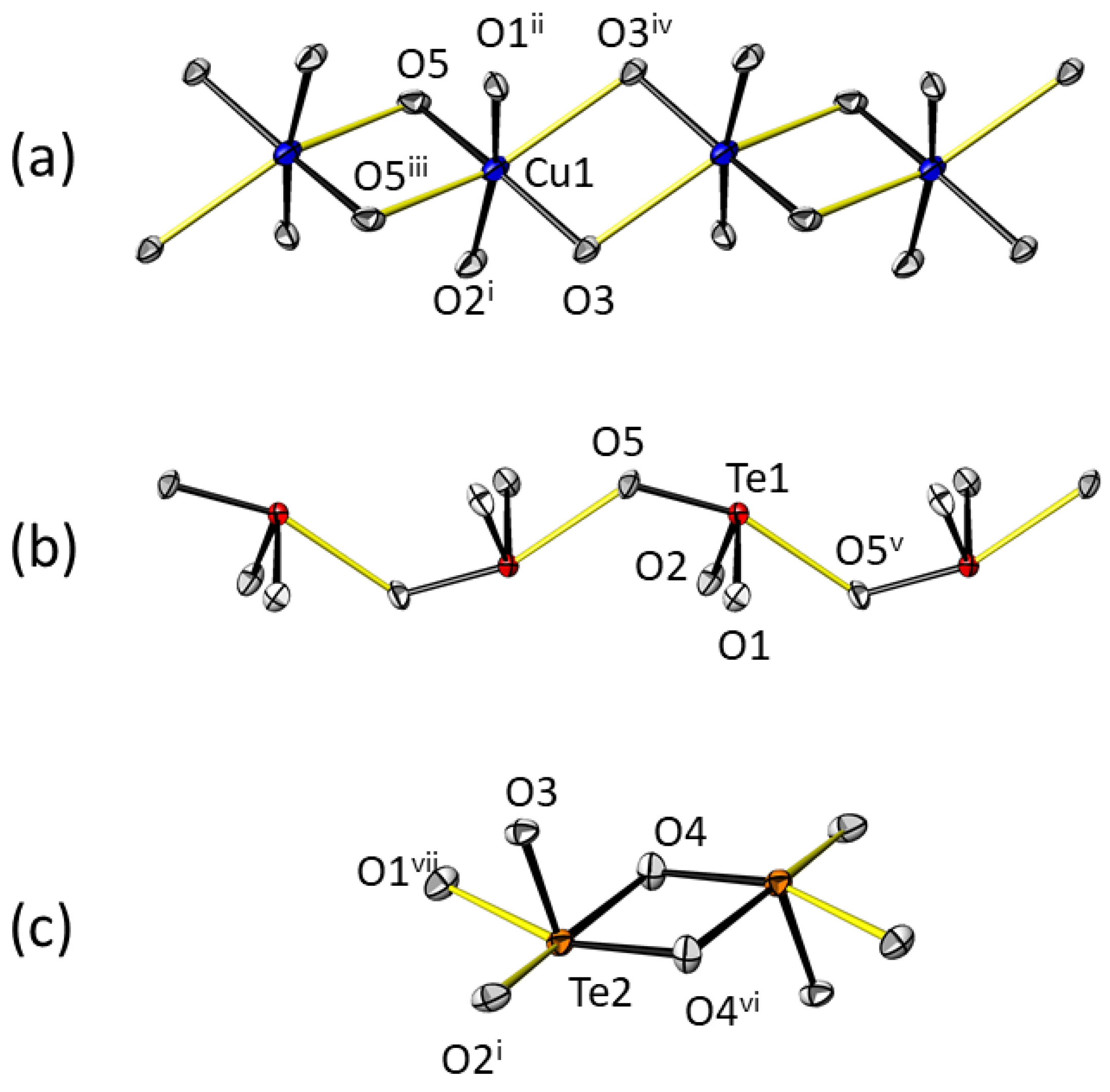
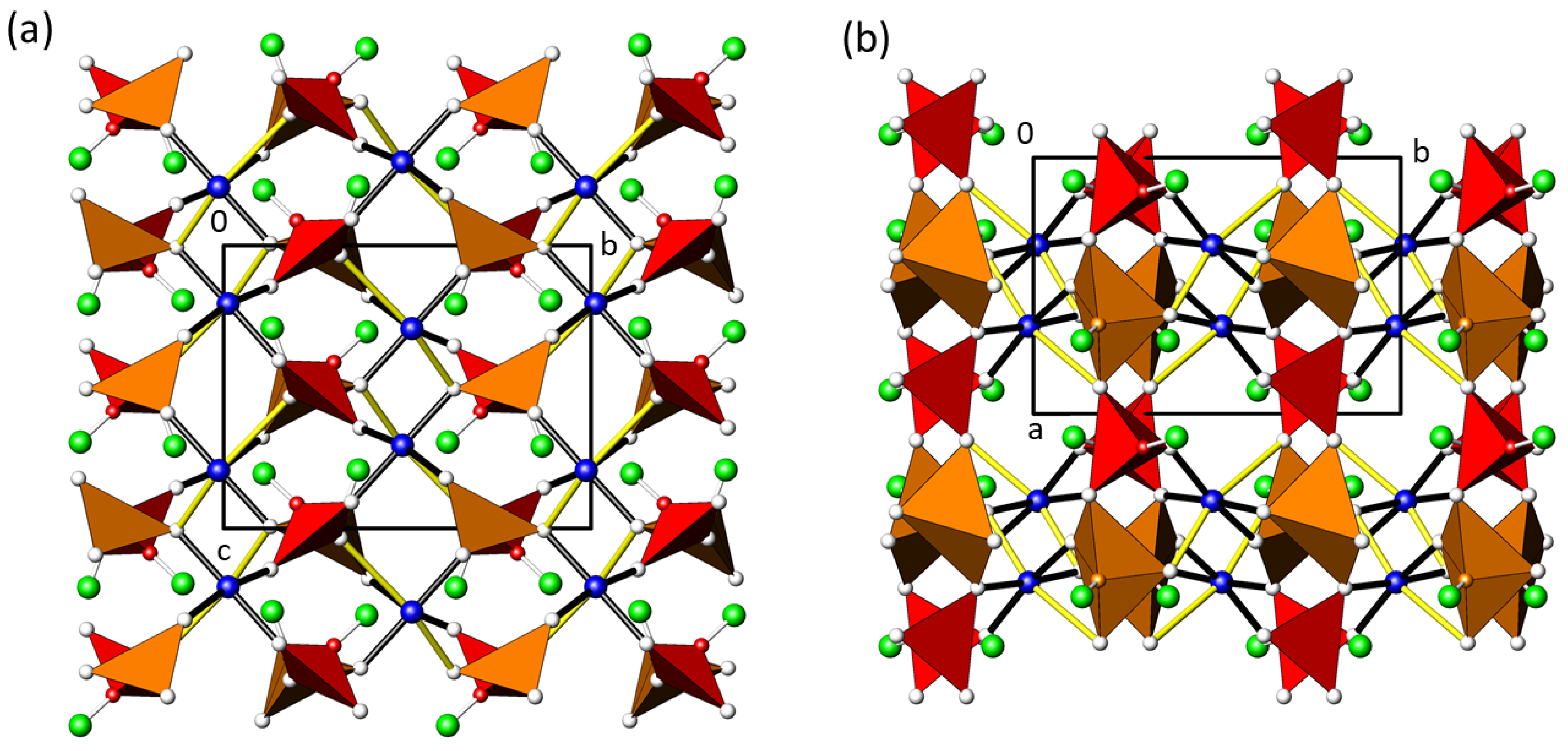
3.5. Structure Description of β-CuTe2O5
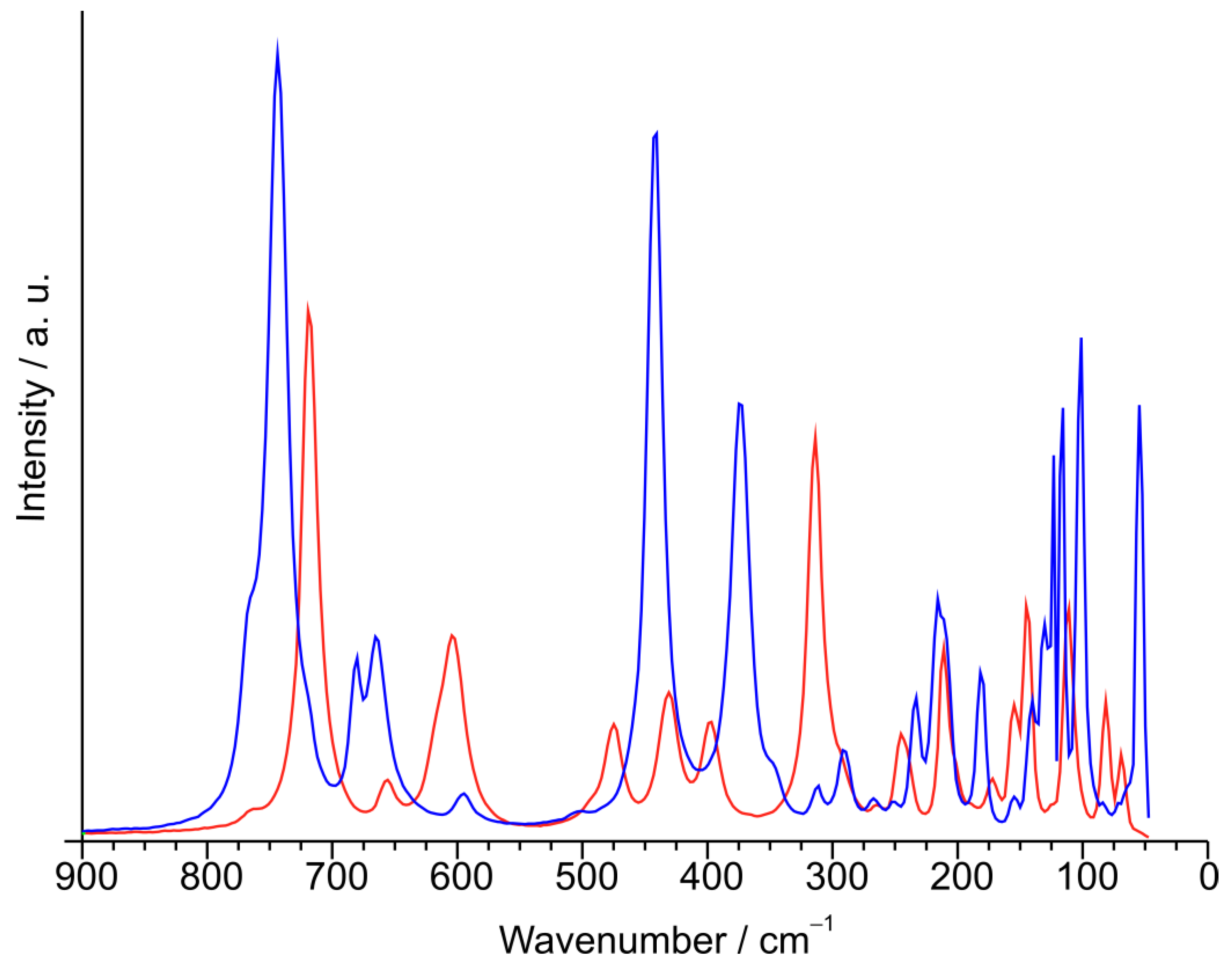
3.6. Raman Spectroscopy of α- and β-CuTe2O5
3.7. Polymorphism Observed in Oxidotellurates
| Phase | Space Group | a/Å | b/Å | c/Å | α/° | β/° | γ/° | V/ų | Z | Ref. |
|---|---|---|---|---|---|---|---|---|---|---|
| Ag2Te2O6 | P21/m | 5.4562(5) | 7.4009(7) | 6.9122(7) | 90 | 101.237(2) | 90 | 273.77 | 2 | [42] |
| P21/n | 5.9099(5) | 11.6831(8) | 8.0305(7) | 90 | 100.424(7) | 90 | 545.32 | 4 | [42] | |
| P21/c | 6.4255(10) | 6.9852(11) | 13.204(2) | 90 | 90.090(3) | 90 | 592.64 | 4 | [43] | |
| BaTeO3 | P21/m | 4.633(4) | 5.943(5) | 7.104(5) | 90 | 106.4(1) | 90 | 187.64 | 2 | [44] |
| Pnma | 14.784(2) | 6.129(1) | 12.350(2) | 90 | 90 | 90 | 1119.05 | 12 | [44] | |
| BaTe2O6 | Cmcm | 5.569(2) | 12.796(4) | 7.320(3) | 90 | 90 | 90 | 521.63 | 4 | [45] |
| HP | P21/m | 5.40473 | 7.18472 | 6.54906 | 90 | 115.2374 | 90 | 230.03 | 2 | [46] |
| CaTeO3 α | P43 | 12.1070(10) | 12.1070(10) | 11.0911(18) | 90 | 90 | 90 | 1625.73 | 20 | [47] |
| β | P1 | 25.6220(4) | 10.2426(2) | 11.3327(2) | 107.218(10) | 110.245(2) | 33.0190(10) | 1520.42 | 18 | [47] |
| β‘ 250 K | P | 25.6149(4) | 10.3921(2) | 11.2440(2) | 108.6080(10) | 112.394(2) | 32.3130(10) | 1479.25 | 18 | [47] |
| γ | P21 | 8.4010(17) | 5.6913(11) | 22.680(5) | 90 | 110.82(3) | 90 | 1013.58 | 12 | [47] |
| δ 673 K | P21ca | 13.3647(6) | 6.5330(3) | 8.1896(3) | 90 | 90 | 90 | 715.04 | 8 | [48] |
| CaTe2O5 | P21/c | 9.382(2) | 5.7095(14) | 11.132(3) | 90 | 115.109(4) | 90 | 539.95 | 4 | [49] |
| mica-like | [50] | |||||||||
| Ca4Te5O14 | Pbca | 10.9536(16) | 16.556(2) | 15.779(2) | 90 | 90 | 90 | 2861.49 | 8 | [51] |
| HP | P21/c | 11.0272(1) | 12.0588(1) | 10.1038(1) | 90 | 91.8490(10) | 90 | 1342.85 | 4 | [52] |
| CdTeO3 α | P21/c | 7.790(1) | 11.253(2) | 7.418(1) | 90 | 113.5(1) | 90 | 596.34 | 8 | [53] |
| β | Pnma | 7.45850(3) | 14.52185(6) | 11.04584(5) | 90 | 90 | 90 | 1196.37 | 16 | [54] |
| CdTe2O5 100 K | P21/c | 9.4535(5) | 5.5806(3) | 10.8607(5) | 90 | 114.4300(10) | 90 | 521.66 | 4 | [55] |
| mica-like | [50] | |||||||||
| Cd3TeO6 | P21/n | 5.4986(3) | 5.6383(3) | 8.0191(5) | 90 | 90.00(5) | 90 | 248.61 | 2 | [56] |
| R3 | 9.1620(2) | 9.1620(2) | 11.0736(3) | 90 | 90 | 120 | 805.00 | 6 | [57] | |
| Co3TeO6 | C2/c | 14.8167(18) | 8.8509(11) | 10.3631(14) | 90 | 94.900(10) | 90 | 1354.06 | [58] | |
| HP | R3 | 5.1937(6) | 5.1937(6) | 13.8237(17) | 90 | 90 | 120 | 322.93 | 3 | [59] |
| Co5TeO8 | Fd3m | 8.55719(19) | 8.55719(19) | 8.55719(19) | 90 | 90 | 90 | 626.61 | 12 | [60] |
| P4332 | 8.55185(4) | 8.55185(4) | 8.55185(4) | 90 | 90 | 90 | 625.42 | 4 | [60] | |
| Cs2TeO4 | Pcmn | 11.6982(15) | 6.6747(9) | 8.5018(9) | 90 | 90 | 90 | 663.83 | 4 | [61] |
| 773 K | P63/mmc | 6.8661(8) | 6.8661(8) | 8.897(1) | 90 | 90 | 120 | 363.24 | 2 | [61] |
| CuTeO3 | Pmcn | 7.604(6) | 5.837(4) | 12.705(6) | 90 | 90 | 90 | 563.91 | 8 | [62] |
| P21/n | 5.214(1) | 9.108(2) | 5.965(1) | 90 | 95.06(1) | 90 | 282.17 | 4 | [63] | |
| CuTe2O5 α | P21/c | 6.871(2) | 9.322(2) | 7.602(2) | 90 | 109.08(1) | 90 | 460.17 | 4 | [21] |
| β | P21/c | 6.6599(4) | 7.6233(4) | 8.8413(5) | 92.6910(10) | 448.38(4) | 4 | this work | ||
| Cu3TeO6 | Ia | 9.537(1) | 9.537(1) | 9.537(1) | 90 | 90 | 90 | 867.43 | 8 | [64] |
| Pcca | 9.745(3) | 9.749(2) | 9.771(2) | 90 | 90 | 90 | 928.28 | 8 | [65] | |
| HP | Ibca | 9.2444(3) | 9.3018(1) | 9.3757(4) | 90 | 90 | 90 | 806.21 | 8 | [66] |
| Hg2Te2O7 α | C2/c | 12.910(4) | 7.407(2) | 13.256(4) | 90 | 112.044(5) | 90 | 1174.93 | 8 | [67] |
| β | Aba2 | 7.4405(12) | 23.713(4) | 13.522(2) | 90 | 90 | 90 | 2385.78 | 16 | [67] |
| Li2Te2O5 | P21/n | 10.355(3) | 4.702(1) | 10.860(3) | 90 | 110.13(1) | 90 | 496.46 | 4 | [68] |
| Pnaa | 5.194(1) | 8.170(2) | 24.165(5) | 90 | 90 | 90 | 1025.44 | 8 | [68] | |
| Mg3TeO6 | R3 | 8.615(3) | 8.615(3) | 10.315(3) | 90 | 90 | 120 | 663.00 | 6 | [69] |
| HP | R3 | 5.1382(7) | 5.1382(7) | 13.8070(19) | 90 | 90 | 120 | 315.68 | 3 | [70] |
| MnTeO3 α | Pbca | 10.0662(3) | 8.0958(2) | 14.1654(4) | 90 | 90 | 90 | 1154.39 | 16 | [71] |
| β HP | Pnma | 6.1443(4) | 7.7866(7) | 5.4408(4) | 90 | 90 | 90 | 260.30 | 4 | [72] |
| γ | Pbca | 7.4535(12) | 6.4164(11) | 12.843(2) | 90 | 90 | 90 | 614.21 | 8 | [71] |
| MnTe2O5 α | P42/nbc | 8.82(5) | 8.82(5) | 13.04(5) | 90 | 90 | 90 | 1014.41 | 8 | [73] |
| β | Pbcn | 7.3114(4) | 10.9216(6) | 6.1711(3) | 90 | 90 | 90 | 492.78 | 4 | [74] |
| Mn2TeO6 HT | P42/mnm | 4.64205(11) | 4.64205(11) | 9.0750(2) | 90 | 90 | 90 | 195.54 | 2 | [75] |
| LT | P21/c | 9.102948(10) | 13.046326(9) | 6.465927(6) | 90 | 90.0331(4) | 90 | 767.88 | 8 | [75] |
| Mn3TeO6 | R | 8.8673(10) | 8.8673(10) | 10.6729(12) | 90 | 90 | 120 | 726.77 | 6 | [76] |
| HP | P21/n | 5.29370(1) | 5.45203(1) | 7.80894(1) | 90 | 89.62514(5) | 90 | 225.37 | 2 | [77] |
| MoTe2O7 α | P21/c | 4.286 | 8.618 | 15.945 | 90 | 95.68 | 90 | 586.06 | 4 | [78] |
| Pna21 | 17.5632(5) | 4.9107(2) | 12.9518(4) | 90 | 90 | 90 | 1117.06 | 8 | [79] | |
| Mo5TeO16 | P21/c | 10.038(2) | 14.431(2) | 8.1617(6) | 90 | 90.85(1) | 90 | 1182.16 | 4 | [80] |
| Pm2a | 20.010(1) | 4.0650(2) | 7.2254(4) | 90 | 90 | 90 | 587.72 | 2 | [81] | |
| Na2TeO4 α | P21/c | 10.632(5) | 5.161(2) | 13.837(11) | 90 | 103.27(4) | 90 | 738.99 | 8 | [82] |
| β | Pbcn | 5.798(6) | 12.24(1) | 5.214(5) | 90 | 90 | 90 | 370.02 | 4 | [83] |
| γ | Pnna | 13.5390(7) | 8.4169(4) | 6.7781(4) | 90 | 90 | 90 | 772.40 | 8 | [84] |
| NiTeO4 α | P21/c | 5.5285(6) | 4.6500(5) | 5.5449(6) | 90 | 113.120(2) | 90 | 131.10(2) | 2 | this work |
| β | I41/a | 9.2627(5) | 9.2627(5) | 6.0988(4) | 90 | 90 | 90 | 523.26(7) | 8 | this work |
| Ni3TeO6 | R3 | 5.103(2) | 5.103(2) | 13.755(10) | 90 | 90 | 120 | 310.20 | 3 | [18] |
| HP | Pnma | 5.9588(1) | 7.5028(1) | 5.2143(1) | 90 | 90 | 90 | 233.12 | 4 | [85] |
| PbTeO3 α | C2/c | 26.73 | 4.6 | 18.06 | 90 | 106 | 90 | 2134.60 | 24 | [86] |
| β | P41 | 5.304(3) | 5.304(3) | 11.900(6) | 90 | 90 | 90 | 334.78 | 4 | [87] |
| γ | P | 7.0185(3) | 10.6166(4) | 11.9616(5) | 78.548(3) | 82.992(2) | 84.048(2) | 864.12 | 10 | [88] |
| Sc2TeO6 | P321 | 8.7406(7) | 8.7406(7) | 4.7985(4) | 90 | 90 | 120 | 317.48 | 3 | [89] |
| HP | 7.2943(3) | 5.1252(2) | 10.9502(4) | 90 | 103.8800(10) | 90 | 397.41 | 4 | [90] | |
| SrTeO3 α | C2 | 28.151(6) | 5.8970(10) | 15.261(3) | 90 | 122.09(3) | 90 | 2146.35 | 24 | [91] |
| β 473 K | C2/c | 28.206(6) | 5.9210(10) | 28.528(6) | 90 | 114.16(3) | 90 | 4347.06 | 48 | [92] |
| γ 583 K | C2 | 28.262(6) | 5.9350(10) | 15.434(3) | 90 | 122.21(3) | 90 | 2190.40 | 24 | [93] |
| δ 780 K | C2/m | 28.438(6) | 5.9500(10) | 15.550(3) | 90 | 122.45(3) | 90 | 2220.32 | 24 | [94] |
| ε | P21/c | 6.7759(1) | 7.2188(1) | 8.6773(2) | 90 | 126.4980(7) | 90 | 341.20 | 4 | [95] |
| Te(OH)6 | P21/n | 6.495(1) | 9.320(1) | 11.393(1) | 90 | 133.88 | 90 | 497.10 | 4 | [96] |
| F4132 | 15.699(2) | 15.699(2) | 15.699(2) | 90 | 90 | 90 | 3869.15 | 32 | [97] | |
| Te3O3(PO4)2 | P21/c | 12.375(2) | 7.317(1) | 9.834(1) | 90 | 98.04(1) | 90 | 881.70 | 4 | [98] |
| β 203 K | P21/n | 11.115(5) | 4.7033(19) | 17.287(7) | 90 | 106.086(5) | 90 | 868.33 | 4 | [99] |
| Tl2Te2O5 α | P21/n | 7.119(1) | 12.138(2) | 8.439(2) | 90 | 114.28(3) | 90 | 664.72 | 4 | [100] |
| β | [101] |
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Christy, A.G.; Mills, S.J.; Kampf, A.R. A review of the structural architecture of tellurium oxycompounds. Mineral. Mag. 2016, 80, 415–545. [Google Scholar] [CrossRef]
- Weil, M.; Pramanik, P.; Maltoni, P.M.; Clulow, R.; Rydh, A.; Wildner, M.; Blaha, P.; King, G.; Ivanov, S.A.; Mathieu, R.; et al. CoTeO4—A wide-bandgap material adopting the dirutile structure type. Mater. Adv. 2024, 5, 3001–3013. [Google Scholar] [CrossRef]
- Patel, A.K.; Panda, M.R.; Rani, E.; Singh, H.; Samatham, S.S.; Nagendra, A.; Jha, S.N.; Bhattacharyya, D.; Suresh, K.G.; Mitra, S. Unique Structure-Induced Magnetic and Electrochemical Activity in Nanostructured Transition Metal Tellurates Co1–XNixTeO4 (x = 0, 0.5, and 1). ACS Appl. Energy Mater. 2020, 3, 9436–9448. [Google Scholar] [CrossRef]
- Falck, L.; Lindquist, O.; Mark, W.; Philippot, E.; Moret, J. The crystal structure of CuTeO4. Acta Crystallogr. 1978, B34, 1450–1453. [Google Scholar] [CrossRef]
- Hasan, Z.; Zoghlin, E.; Winiarski, M.; Arpino, K.E.; Halloran, T.; Tran, T.T.; McQueen, T.M. Quasi-one-dimensional exchange interactions and short-range magnetic correlations in CuTeO4. Phys. Rev. B 2024, 109, 195168. [Google Scholar] [CrossRef]
- Chang, Y.H.R.; Yoon, T.L.; Lim, T.L.; Koh, P.W.; Goh, E.S. Effects of oxygen variation on the improved structural stability, electronic and optical properties of ZnTeO compounds. J. Phys. Condens. Matter 2020, 32, 225701. [Google Scholar] [CrossRef]
- Binnewies, M.; Glaum, R.; Schmidt, M.; Schmidt, P. Chemical Vapor Transport Reactions; De Gruyter: Berlin, Germany; Boston, MA, USA, 2012. [Google Scholar]
- Rabenau, A. The Role of Hydrothermal Synthesis in Preparative Chemistry. Angew. Chem. Int. Ed. Engl. 1985, 24, 1026–1040. [Google Scholar] [CrossRef]
- Schöneborn, M.; Hoffbauer, W.; Schmedt auf der Günne, J.; Glaum, R. Beiträge zur Kristallchemie und zum thermischen Verhalten von wasserfreien Phosphaten, XXXVII. Synthese, Kristallstruktur und kernresonanzspektroskopische Untersuchung von In2Ti6(PO4)6[Si2O(PO4)6]—Eine Hybride aus den NASICON und M4[Si2O(PO4)6] Strukturtypen. Z. Naturforsch. 2006, 61b, 741–748. [Google Scholar]
- Platte, C.; Trömel, M. Nickelditellurat(IV): Sauerstoffkoordinationszahl Fünf am vierwertigen Tellur. Acta Crystallogr. 1981, B37, 1276–1278. [Google Scholar] [CrossRef]
- APEX-4 and SAINT; Bruker AXS Inc.: Madison, WI, USA, 2021.
- Krause, L.; Herbst-Irmer, R.; Sheldrick, G.M.; Stalke, D. Comparison of silver and molybdenum microfocus X-ray sources for single-crystal structure determination. J. Appl. Cryst. 2015, 48, 3–10. [Google Scholar] [CrossRef] [PubMed]
- Sheldrick, G.M. SHELXT—Integrated space-group and crystal-structure determination. Acta Crystallogr. 2015, A71, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Crystallogr. 2015, C71, 3–8. [Google Scholar]
- ATOMS for Windows; Shape Software: Kingsport, TN, USA, 2006.
- Brown, I.D. The Chemical Bond in Inorganic Chemistry: The Bond Valence Model; Oxford University Press: Oxford, UK, 2002. [Google Scholar]
- Brese, N.E.; O’Keeffe, M. Bond-valence parameters for solids. Acta Crystallogr. 1991, B47, 192–197. [Google Scholar] [CrossRef]
- Mills, S.J.; Christy, A.G. Revised values of the bond-valence parameters for TeIV–O, TeVI–O and TeIV–Cl. Acta Crystallogr. 2013, B69, 145–149. [Google Scholar] [CrossRef] [PubMed]
- Newnham, R.E.; Meagher, E.P. Crystal structure of Ni3TeO6. Mater. Res. Bull. 1967, 2, 549–554. [Google Scholar] [CrossRef]
- Binnewies, M.; Schmidt, M.; Schmidt, P. Chemical Vapor Transport Reactions—Arguments for Choosing a Suitable Transport Agent. Z. Anorg. Allg. Chem. 2017, 643, 1295–1311. [Google Scholar] [CrossRef]
- Hanke, K.; Kupčik, V.; Lindqvist, O. The crystal structure of CuTe2O5. Acta Crystallogr. 1973, B29, 963–970. [Google Scholar] [CrossRef]
- Williams, S.A. Rajite, naturally occurring cupric pyrotellurite, a new mineral. Mineral. Mag. 1979, 43, 91–92. [Google Scholar] [CrossRef][Green Version]
- Isasi, J. New MM’O4 oxides derived from the rutile type: Synthesis, structure and study of magnetic and electronic properties. J. Alloys Comps. 2001, 322, 89–96. [Google Scholar] [CrossRef]
- Salinas-Sanchez, A.; Garcia-Muñoz, J.L.; Rodriguez-Carvajal, J.; Saez-Puche, R.; Martinez, J.L. Structural characterization of R2BaCuO5 (R = Y, Lu, Yb, Tm, Er, Ho, Dy, Gd, Eu and Sm) oxides by X-ray and neutron diffraction. J. Solid State Chem. 1992, 100, 201–211. [Google Scholar]
- Brown, I.D. Recent Developments in the Methods and Applications of the Bond Valence Model. Chem. Rev. 2009, 109, 6858–6919. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Wong, L.L.; Adams, S. SoftBV–a software tool for screening the materials genome of inorganic fast ion conductors. Acta Crystallogr. 2019, B75, 18–33. [Google Scholar] [CrossRef]
- Gagné, O.C.; Hawthorne, F.C. Bond-length distributions for ions bonded to oxygen: Results for the transition metals and quantification of the factors underlying bond-length variation in inorganic solids. IUCrJ 2020, 7, 581–629. [Google Scholar] [CrossRef] [PubMed]
- Gagné, O.C.; Hawthorne, F.C. Bond-length distributions for ions bonded to oxygen: Results for the non-metals and discussion of lone-pair stereoactivity and the polymerization of PO4. Acta Crystallogr. 2018, B74, 79–96. [Google Scholar]
- Baur, W.H. The rutile type and its derivatives. Crystallogr. Rev. 2007, 13, 65–113. [Google Scholar] [CrossRef]
- Müller, U. Symmetry Relationships Between Crystal Structures; Oxford University Press: Okford, UK, 2013. [Google Scholar]
- Bärnighausen, H. Group-Subgroup Relations between Space Groups: A useful Tool in Crystal Chemistry. MATCH Commun. Math. Chem. 1989, 9, 139–175. [Google Scholar]
- Halcrow, M.A. Jahn–Teller distortions in transition metal compounds, and their importance in functional molecular and inorganic materials. Chem Soc. Rev. 2013, 42, 1784–1795. [Google Scholar] [CrossRef] [PubMed]
- Hamani, D.; Masson, O.; Thomas, P. Localization and steric effect of the lone electron pair of the tellurium Te4+ cation and other cations of the p-block elements. A systematic study. J. Appl. Crystallogr. 2020, 53, 1243–1251. [Google Scholar] [CrossRef]
- Majzlan, J.; Notz, S.; Haase, P.; Kamitsos, E.I.; Tagiara, N.S.; Dachs, E. Thermodynamic properties of tellurite (β-TeO2), paratellurite (α-TeO2), TeO2 glass, and Te(IV) phases with stoichiometry M2Te3O8, MTe6O13, MTe2O5 (M2+ = Co, Cu, Mg, Mn, Ni, Zn). Geochemistry 2022, 82, 12591. [Google Scholar] [CrossRef]
- Baran, E.J. Das Schwingungsspektrum des Ditellurit-Ions. Z. Anorg Allg Chem. 1978, 442, 112–118. [Google Scholar] [CrossRef]
- Weil, M. Redetermination of MgTe2O5. Acta Crystallogr. 2005, E61, i237–i239. [Google Scholar] [CrossRef]
- Simon, A.; Paetzold, R. Untersuchungen an Selen–Sauerstoff-Verbindungen. III. Die Struktur des Pyroselenitions. Z. Anorg. Allg. Chem. 1960, 303, 39–45. [Google Scholar] [CrossRef]
- Frost, R.L.; Dickfos, M.J.; Keeffe, E.C. Raman spectroscopic study of the tellurite minerals: Rajite and denningite. Spectrochim. Acta 2008, 71A, 1512–1515. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Jezowska-Trzebiatowska, B. Theory and importance of oxygen bridge-bonding. Pure Appl. Chem. 1971, 27, 89–111. [Google Scholar] [CrossRef]
- Siebert, H. Anwendungen der Schingungsspektroskopie in der Anorganischen Chemie; Springer: Berlin/Heidelberg, Germany, 1966. [Google Scholar]
- Zagorac, D.; Müller, H.; Ruehl, S.; Zagorac, J.; Rehme, S. Recent developments in the Inorganic Crystal Structure Database: Theoretical crystal structure data and related features. J. Appl. Crystallogr. 2019, 52, 918–925. [Google Scholar] [CrossRef] [PubMed]
- Klein, W.; Curda, J.; Peters, E.M.; Jansen, M. Neue Silber(I)-oxotellurate(IV/VI). Z. Anorg. Allg. Chem. 2005, 631, 2893–2899. [Google Scholar] [CrossRef]
- Weil, M. New silver tellurates—The crystal structures of a third modification of Ag2Te2O6 and of Ag4TeO5. Z. Anorg. Allg. Chem. 2007, 633, 1217–1222. [Google Scholar] [CrossRef]
- Koçak, M.; Platte, C.; Trömel, M. Über verschiedene Formen von BaTeO3. Z. Anorg. Allg. Chem. 1979, 453, 93–97. [Google Scholar] [CrossRef]
- Koçak, M.; Platte, C.; Trömel, M. Bariumhexaoxoditellurat(IV,VI). Sauerstoffkoordinationszahl fünf am vierwertigen Tellur. Acta Crystallogr. 1979, B35, 1439–1441. [Google Scholar] [CrossRef]
- Mishra, K.K.; Achary, S.N.; Chandra, S.; Ravindran, T.R.; Pandey, K.K.; Tyagi, A.K.; Sharma, S.M. Study of Phase Transformation in BaTe2O6 by in Situ High-Pressure X-ray Diffraction, Raman Spectroscopy, and First-Principles Calculations. Inorg. Chem. 2016, 55, 227–238. [Google Scholar] [CrossRef] [PubMed]
- Stöger, B.; Weil, M.; Zobetz, E.; Giester, G. Polymorphism of CaTeO3 and solid solutions CaxSr1−xTeO3. Acta Crystallogr. 2009, B65, 167–181. [Google Scholar] [CrossRef] [PubMed]
- Poupon, M.; Barrier, N.; Petit, S.; Clevers, S.; Dupray, V. Hydrothermal Synthesis and Dehydration of CaTeO3(H2O): An Original Route to Generate New CaTeO3 Polymorphs. Inorg. Chem. 2015, 54, 5660–5670. [Google Scholar] [CrossRef]
- Weil, M.; Stöger, B. A non-twinned polymorph of CaTe2O5 from a hydrothermally grown crystal. Acta Crystallogr. 2008, C64, i79–i81. [Google Scholar]
- Redman, M.J.; Chen, J.H.; Binnie, W.P.; Mallio, W.J. Mica-Like Tellurites of Calcium, Strontium, and Cadmium. J. Am. Ceram. Soc. 1970, 53, 645–648. [Google Scholar] [CrossRef]
- Weil, M. New phases in the systems Ca–Te–O and Cd–Te–O: The calcium tellurite(IV) Ca4Te5O14 and the cadmium compounds Cd2Te3O9 and Cd2Te2O7 with mixed-valent oxotellurium(IV/VI) anions. Solid State Sci. 2004, 6, 29–37. [Google Scholar] [CrossRef]
- Weil, M.; Heymann, G.; Huppertz, H. The high-pressure polymorph of Ca4Te5O14 and the mixed-valent compound Ca13 TeVI2/3- TeIV3.75 O15 (BO3)4(OH)3. Eur. J. Inorg. Chem. 2016, 2016, 3574–3579. [Google Scholar] [CrossRef]
- Krämer, V.; Brandt, G. Structure of cadmium tellurate(IV), CdTeO3. Acta Crystallogr. 1985, C41, 1152–1154. [Google Scholar] [CrossRef]
- Poupon, M.; Barrier, N.; Petit, S.; Boudin, S. A new β-CdTeO3 polymorph with a structure related to α-CdTeO3. Dalton Trans. 2017, 46, 1927–1935. [Google Scholar] [CrossRef]
- Eder, F.; Weil, M. The crystal structure of a new CdTe2O5 polymorph, isotypic with ε-CaTe2O5. Acta Crystallogr. 2020, E76, 831–834. [Google Scholar]
- Burckhardt, H.G.; Platte, C.; Trömel, M. Cadmiumorthotellurat(VI) Cd3TeO6: Ein pseudoorthorhombischer Kryolith im Vergleich mit Ca3TeO6. Acta Crystallogr. 1982, B38, 2450–2452. [Google Scholar] [CrossRef]
- Weil, M.; Veyer, T. A new form of Cd3TeO6 revealing dimorphism. Acta Crystallogr. 2018, E74, 1561–1564. [Google Scholar]
- Becker, R.; Johnsson, M.; Berger, H. A new synthetic cobalt tellurate: Co3TeO6. Acta Crystallogr. 2006, C62, i67–i69. [Google Scholar]
- Selb, E.; Buttlar, T.; Janka, O.; Tribus, M.; Ebbinghaus, S.G.; Heymann, G. Multianvil high-pressure/high-temperature synthesis and characterization of magnetoelectric HP-Co3TeO6. J. Mater. Chem. C 2021, 9, 5486–5496. [Google Scholar] [CrossRef]
- Podchezertsev, S.; Barrier, N.; Pautrat, A.; Suard, E.; Retuerto, M.; Alonso, J.A.; Fernández-Díaz, M.T.; Rodríguez-Carvajal, J. Influence of polymorphism on the magnetic properties of Co5TeO8 spinel. Inorg. Chem. 2021, 60, 13990–14001. [Google Scholar] [CrossRef] [PubMed]
- Epifano, E.; Volfi, A.; Abbink, M.; Nieuwland, H.; van Eijck, L.; Wallez, G.; Banerjee, D.; Martin, P.M.; Smith, A.L. Investigation of the Cs(Mo,Te)O4 Solid Solution and Implications on the Joint Oxyde-Gaine System in Fast Neutron Reactors. Inorg. Chem. 2020, 59, 10172–10184. [Google Scholar] [CrossRef] [PubMed]
- Lindqvist, O. The Crystal Structure of CuTeO3. Acta Chem. Scand. 1972, 26, 1423–1430. [Google Scholar] [CrossRef]
- Pertlik, F. Dimorphism of hydrothermal synthesized copper tellurite CuTeO3: The structure of a monoclinic representative. J. Solid State Chem. 1987, 71, 291–295. [Google Scholar] [CrossRef]
- Hostachy, A.; Coing-Boyat, J. Structure cristalline de Cu3TeO6. C. R. Seances Acad. Sci. Ser. B 1968, 267, 1435–1438. [Google Scholar]
- Missen, O.P.; Mills, S.J.; Canossa, S.; Hadermann, J.; Nénert, G.; Weil, M.; Libowitzky, E.; Housley, R.M.; Artner, W.; Kampf, A.R.; et al. Polytypism in mcalpineite. A study of natural and synthetic Cu3TeO6. Acta Crystallogr. 2022, B78, 20–32. [Google Scholar] [CrossRef]
- Qu, J.; Yan, L.; Liu, H.; Tao, Q.; Zhu, P.; Li, Z.; Wang, X. Pressure-induced structural phase transition in corundum-related class Cu3TeO6. High Press. Res. 2021, 41, 318–327. [Google Scholar] [CrossRef]
- Weil, M. Dimorphism in mercury(II) tellurite(IV) tellurate(VI): Preparation and crystal structures of α- and β-Hg2Te2O7. Z. Kristallogr. 2003, 218, 691–698. [Google Scholar] [CrossRef]
- Cachau-Herreillat, D.; Norbert, A.; Maurin, M.; Philippot, E. Etude cristallochimique comparée et conductivité ionique des deux variétés Li2Te2O5 α et β. J. Solid State Chem. 1981, 37, 352–361. [Google Scholar] [CrossRef]
- Schulz, H.; Bayer, G. Structure determination of Mg3TeO6. Acta Crystallogr. 1971, B27, 815–821. [Google Scholar] [CrossRef]
- Selb, E.; Declara, L.; Bayarjargal, L.; Podewitz, M.; Tribus, M.; Heymann, G. Crystal Structure and Properties of a UV-Transparent High-Pressure Polymorph of Mg3TeO6 with Second Harmonic Generation Response. Eur. J. Inorg. Chem. 2019, 2019, 4668–4676. [Google Scholar] [CrossRef]
- Eder, F.; Weil, M. Phase formation studies and crystal structure refinements in the MnII/TeIV/O/(H) system. Z. Anorg. Allg. Chem. 2022, 648, e202200205. [Google Scholar] [CrossRef]
- Kohn, K.; Inoue, K.; Horie, O.; Akimoto, S.-I. Crystal chemistry of MSeO3 and MTeO3 (M = Mg, Mn, Co, Ni, Cu, and Zn). J. Solid State Chem. 1976, 18, 27–37. [Google Scholar] [CrossRef]
- Walitzi, E.M. Die Kristallstruktur von Denningit, (Mn,Ca,Zn)Te2O5. Ein Beispiel für die Koordination um vierwertiges Tellur. Tschermaks Mineral. Petrogr. Mitt. 1965, 10, 241–255. [Google Scholar] [CrossRef]
- Johnston, M.G.; Harrison, W.T.A. Manganese tellurite, β-MnTe2O5. Acta Crystallogr. 2002, E58, i59–i61. [Google Scholar] [CrossRef]
- Matsubara, N.; Damay, F.; Vertruyen, B.; Barrier, N.; Lebedev, O.I.; Boullay, P.; Elkaim, E.; Manuel, P.; Khalyavin, D.D.; Martin, C. Mn2TeO6: A distorted inverse trirutile structure. Inorg. Chem. 2017, 56, 9742–9753. [Google Scholar] [CrossRef]
- Weil, M. Mn3TeO6. Acta Crystallogr. 2006, E62, i244–i245. [Google Scholar] [CrossRef]
- Arévalo-López, A.M.; Solana-Madruga, E.; Aguilar-Maldonado, C.; Ritter, C.; Olivier Mentré, O.; Attfield, J.P. Magnetic frustration in the high-pressure Mn2MnTeO6 (Mn3TeO6-II) double perovskite. Chem. Commun. 2019, 55, 14470–14473. [Google Scholar] [CrossRef] [PubMed]
- Arnaud, Y.; Averbuch-Pouchot, M.T.; Durif, A.; Guidot, J. Structure cristalline de l’oxide mixte de molybdene-tellure: MoTe2O7. Acta Crystallogr. 1976, B32, 1417–1420. [Google Scholar] [CrossRef]
- Ling, J.; Zhang, H.; Yuan, K.; Burgess, D.; Hu, J.; Hu, M. Hydrothermal syntheses and crystal structures of molybdenum tellurites. J. Solid State Chem. 2020, 287, 121317. [Google Scholar] [CrossRef]
- Arnaud, Y.; Guidot, J. Structure cristalline de l’oxyde mixte de molybdene-tellure: Mo5TeO16. Acta Crystallogr. 1977, B33, 2151–2155. [Google Scholar] [CrossRef]
- Forestier, P.; Goreaud, M. Structure cristalline de l’oxyde a valence mixte TeMo5O16 orthorombique. C. R. Acad. Sci. Ser. II 1991, 312, 1141–1145. [Google Scholar]
- Daniel, F.; Maurin, M.; Moret, J.; Philippot, E. Etude structurale d’un nouveau tellurate alcalin: Na2TeO4. Evolution de la coordination du tellure(VI) et du cation quand on passe du cation lithium au sodium. J. Solid State Chem. 1977, 22, 385–391. [Google Scholar] [CrossRef]
- Kratochvil, B.; Jensovsky, L. The crystal structure of sodium metatellurate. Acta Crystallogr. 1977, B33, 2596–2598. [Google Scholar] [CrossRef]
- Weil, M.; Stöger, B.; Larvor, C.; Raih, I.; Gierl-Mayer, C. The hydrous sodium oxotellurates(VI) Na[TeO(OH)5], Na2[TeO2(OH)4], Na4[Te2O6(OH)4](H2O)6, and a third polymorph of anhydrous Na2[TeO4]. Z. Anorg. Allg. Chem. 2017, 643, 1888–1897. [Google Scholar] [CrossRef]
- Martínez-Lope, M.J.; Retuerto, M.; Alonso, J.A.; Sánchez-Benítez, J.; Fernández-Díaz, M.T. High-pressure synthesis and neutron diffraction investigation of the crystallographic and magnetic structure of TeNiO3 perovskite. Dalton Trans. 2011, 40, 4599–4604. [Google Scholar] [CrossRef]
- Mariolacos, K. Die Kristallstruktur von PbTeO3. Anz. Oesterr. Akad. Wiss. Math.-Naturwiss. Kl. 1969, 106, 129–131. [Google Scholar]
- Sciau, P.; Lapasset, J.; Moret, J. Structure de la phase quadratique de PbTeO3. Acta Crystallogr. 1986, C42, 1688–1690. [Google Scholar] [CrossRef]
- Weil, M.; Shirkhanlou, M.; Füglein, E.; Libowitzky, E. Determination of the correct composition of anhydrous lead(II) oxotellurate(IV) as PbTeO3, crystallizing as a new polymorph. Crystals 2018, 8, 51. [Google Scholar] [CrossRef]
- Höss, P.; Schleid, T. Sc2Te5O13 und Sc2TeO6: Die ersten Oxotellurate des Scandiums. Z. Anorg. Allg. Chem. 2007, 633, 1391–1396. [Google Scholar] [CrossRef]
- Ziegler, R.; Tribus, M.; Hejny, C.; Heymann, G. Single-crystal structure of HP-Sc2TeO6 prepared by high-pressure/high-temperature synthesis. Crystals 2021, 11, 1554. [Google Scholar] [CrossRef]
- Zavodnik, V.E.; Ivanov, S.A.; Stash, A.I. The α-phase of SrTeO3 at 295 K. Acta Crystallogr. 2007, E63, i75–i76. [Google Scholar] [CrossRef]
- Zavodnik, V.E.; Ivanov, S.A.; Stash, A.I. On the thermal evolution of the crystal structure of SrTeO3: The β-form at 473 K. Acta Crystallogr. 2007, E63, i111–i112. [Google Scholar] [CrossRef]
- Zavodnik, V.E.; Ivanov, S.A.; Stash, A.I. The γ-phase of SrTeO3 at 583 K. Acta Crystallogr. 2007, E63, i151. [Google Scholar]
- Zavodnik, V.E.; Ivanov, S.A.; Stash, A.I. The δ-phase of SrTeO3 at 780 K. Acta Crystallogr. 2008, E64, i52. [Google Scholar]
- Stöger, B.; Weil, M.; Baran, E.J.; Gonzalez Baro, A.C.; Malo, S.; Rueff, J.M.; Petit, S.; Lepetit, M.B.; Raveau, B.; Barrier, N. The dehydration of SrTeO3(H2O)—A topotactic reaction for preparation of the new metastable strontium oxotellurate(IV) phase ε-SrTeO3. Dalton Trans. 2011, 40, 5538–5548. [Google Scholar] [CrossRef]
- Lindqvist, O.; Lehmann, M.S. A neutron diffraction refinement of the crystal structure of telluric acid, Te(OH)6 (mon). Acta Chem. Scand. 1973, 27, 85–95. [Google Scholar] [CrossRef]
- Mullica, D.F.; Korp, J.D.; Mulligan, W.O.; Beall, G.W.; Bernal, I. Neutron Structural Refinement of Cubic Orthotelluric Acid. Acta Crystallogr. 1980, B36, 2565–2570. [Google Scholar] [CrossRef]
- Mayer, H.; Weil, M. Synthese und Kristallstruktur von Te3O3(PO4)2, einer Verbindung mit fünffach koordiniertem Tellur(IV). Z. Anorg. Allg. Chem. 2003, 629, 1068–1072. [Google Scholar] [CrossRef]
- Li, L.; Zhuang, R.-C.; Mi, J.-X.; Huang, Y.-X. A New Modification of Tellurite Phosphate: β-Te3O3(PO4)2. Chin. J. Struct. Chem. 2018, 37, 1417–1425. [Google Scholar]
- Jeansannetas, B.; Thomas, P.; Champarnaud-Mesjard, J.C.; Frit, B. Crystal structure of α-Tl2Te2O5. Mater. Res. Bull. 1998, 33, 1709–1716. [Google Scholar] [CrossRef]
- Jeansannetas, B.; Marchet, P.; Thomas, P.; Champarnaud-Mesjard, J.C.; Frit, B. New investigations within the TeO2-rich part of the Tl2O–TeO2 system. J. Mater. Chem. 1998, 8, 1039–1042. [Google Scholar] [CrossRef]



| Formula | α-NiTeO4 | β-NiTeO4 | β-CuTe2O5 | |
|---|---|---|---|---|
| Temperature/°C | 23 | 23 | 23 | |
| Mr | 250.31 | 250.31 | 398.74 | |
| Crystal size/mm3 | 0.08 × 0.07 × 0.01 | 0.18 × 0.10 × 0.02 | 0.18 × 0.10 × 0.04 | |
| Crystal color, form | brownish-orange, plate | brownish-green, plate | green, plate | |
| Crystal system | monoclinic | tetragonal | monoclinic | |
| Space group, No. | P21/c, 14 | I41/a, 88 | P21/c, 14 | |
| a/Å | 5.5285(6) | 9.2627(5) | 6.6599(4) | |
| b/Å | 4.6500(5) | 9.2627(5) | 7.6233(4) | |
| c/Å | 5.5449(6) | 6.0988(4) | 8.8413(5) | |
| α/° | 90 | 90 | 90 | |
| β/° | 113.120(2) | 90 | 92.6910(10) | |
| γ/° | 90 | 90 | 90 | |
| V/Å3 | 131.10(2) | 523.26(7) | 448.38(4) | |
| Z | 2 | 8 | 4 | |
| Dcalcd, /g cm–3 | 6.341 | 6.355 | 5.907 | |
| μ(MoKα), mm–1 | 18.093 | 18.132 | 17.541 | |
| Theta range/° | 3.996–41.802 | 3.110–43.921 | 3.602–32.778 | |
| hkl range | −10 → 9 | −18 → 17 | −9 → 10 | |
| −8 → 8 | −18 → 18 | −11 → 11 | ||
| −10 → 10 | −11 → 11 | −12 → 12 | ||
| Reflections measured | 5409 | 17891 | 7179 | |
| Reflections unique | 943 | 1011 | 1580 | |
| Reflections [I > 2σ(I)] | 694 | 703 | 1573 | |
| Rint | 0.038 | 0.064 | 0.029 | |
| Extinction coefficient (Shelx) | 0.0319(13) | - | 0.0128(3) | |
| Parameters refined | 33 | 33 | 74 | |
| R(F2)/wR(F2) [I > 2σ(I)] | 0.0181/0.0343 | 0.0246/0.0604 | 0.0160/0.0381 | |
| R(F2)/wR(F2) [all reflections] | 0.0326/0.0378 | 0.0480/0.0680 | 0.0161/0.0382 | |
| GooF (F2) | 1.030 | 0.984 | 1.197 | |
| Δρfin (max/min)/e− Å–3 | 1.569/−1.718 | 1.041/−1.107 | 0.862/−0.874 | |
| CSD-number | 2418621 | 2418623 | 2418622 | |
| α-NiTeO4 (the italicized figures in brackets refer to a previous but incorrect model [3]) | |||
| Ni1–O2 i | 2.0371(16) [1.995] | Te1–O2 vi | 1.8615(16) [1.974] |
| Ni1–O2 ii | 2.0371(16) | Te1–O2 ii | 1.8615(16) |
| Ni1–O2 iii | 2.0689(15) [1.998] | Te1–O1 | 1.9617(16) [2.051] |
| Ni1–O2 iv | 2.0689(15) | Te1–O1 vii | 1.9618(15) |
| Ni1–O1 | 2.0691(16) [1.837] | Te1–O1vi | 1.9721(15) [2.079] |
| Ni1–O1 v | 2.0691(16) | Te1–O1 ii | 1.9721(15) |
| BVS: Ni1 2.01 [2.68], Te1 5.91 [4.71], O1 2.17 (CN 3; 1 Ni, 2 Te) [1.69], O2 1.79 (CN 3; 2 Ni, 1 Te) [1.99]. | |||
| Symmetry codes: (i) x−1, −y+1/2, z−1/2; (ii) −x+1, y−1/2, −z+1/2; (iii) −x+1, −y, −z; (iv) x−1, y, z; (v) −x, −y, −z; (vi) x, −y+1/2, z+1/2; (vii) −x+1, −y, −z+1. | |||
| β-NiTeO4 | |||
| Ni1–O1 i | 2.033(3) | Te1–O1 i | 1.861(3) |
| Ni1–O1 ii | 2.033(3) | Te1–O1 viii | 1.861(3) |
| Ni1–O2 iii | 2.068(3) | Te1–O2 iii | 1.955(3) |
| Ni1–O2 iv | 2.068(3) | Te1–O2 ix | 1.955(3) |
| Ni1–O1 v | 2.070(2) | Te1–O2 x | 1.970(2) |
| Ni1–O1 vi | 2.070(2) | Te1–O2 xi | 1.970(2) |
| BVS: Ni1 2.02, Te1 5.94, O1 (CN 3; 2 Ni, 1 Te), O2 2.18 (CN 3; 1 Ni, 2 Te). | |||
| Symmetry codes: (i) y−1/4, −x+1/4, −z+1/4; (ii) −y+1/4, x−1/4, z−1/4; (iii) −y+1/4, x−3/4, −z+1/4; (iv) y−1/4, −x+3/4, z−1/4; (v) −x, −y+1/2, z; (vi) x, y−1/2, −z; (vii) x, y, z−1; (viii) −y+1/4, x−1/4, z+3/4; (ix) y−1/4, −x+3/4, z+3/4; (x) −x+1/2, −y+1/2, −z+1/2; (xi) x−1/2, y−1/2, z+1/2. | |||
| Cu1–O3 | 1.9523(18) | O3–Te1–O5 | 98.32(6) |
| Cu1–O5 | 1.9626(18) | O3–Te1–O4 | 94.73(6) |
| Cu1–O2 i | 1.9694(18) | O5–Te1–O4 | 98.82(5) |
| Cu1–O1 ii | 1.9950(18) | O2–Te1–O5 | 92.16(8) |
| Cu1–O5 iii | 2.4727(19) | O2–Te1–O1 | 96.84(8) |
| Cu1–O3 iv | 2.5635(19) | O5–Te1–O1 | 93.96(8) |
| Te1–O2 | 1.8979(18) | O2–Te1–O5 v | 71.42(7) |
| Te1–O5 | 1.9010(17) | O5–Te1–O5 v | 160.50(5) |
| Te1–O1 | 1.9067(18) | O1–Te1–O5 v | 78.14(7) |
| Te1–O5 v | 2.4953(18) | O3–Te2–O4 | 93.31(8) |
| Te2–O3 | 1.8585(17) | O3–Te2–O4 vi | 93.25(8) |
| Te2–O4 | 1.9635(17) | O4–Te2–O4 vi | 77.75(8) |
| Te2–O4 vi | 2.0413(17) | O3–Te2–O1 vii | 78.88(7) |
| Te2–O1 vii | 2.3041(17) | O4–Te2–O1 vii | 85.69(7) |
| Te2–O2 i | 2.4026(18) | O4vi–Te2–O1 vii | 161.26(7) |
| O3–Te2–O2 i | 73.87(7) | ||
| O4–Te2–O2 i | 156.08(7) | ||
| O4vi–Te2–O2 i | 82.81(7) | ||
| O1vii–Te2–O2 i | 110.71(7) |
| α-CuTe2O5 | β-CuTe2O5 | ||
|---|---|---|---|
| Band position/cm−1 | Assignment | Band position/cm−1 | Assignment |
| 763 sh, 744 vs | νs(TeO2) | 767 sh, 719 vs | ν(Te–O) |
| 680 m, 665 m | νas(TeO2) | 656 w | νas(Te–O–Te) |
| 595 vw | νas(Te–O–Te) | 604 s | νs(Te–O–Te) |
| 507 vvw | νs(Te–O–Te) | 475 m, 431 m, 397 m | ν(Te2O2) + δ(TeOn) |
| 441 vs | δ(TeO2) | 314 vs | δ(TeOn) |
| 375 s, 348 sh, 311 vw, 292 w | “Rocking” | 265 sh, 245 m, 211 s, 175 w, 145 s, 111 s | δ(bridges) + τ + lattice vibrations |
| 233 m, 216 m, 182 m | τ + δ(TeOTe) + lattice vibrations |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Weil, M.; Baran, E.J. Polymorphism of the Transition Metal Oxidotellurates NiTeO4 and CuTe2O5. Crystals 2025, 15, 183. https://doi.org/10.3390/cryst15020183
Weil M, Baran EJ. Polymorphism of the Transition Metal Oxidotellurates NiTeO4 and CuTe2O5. Crystals. 2025; 15(2):183. https://doi.org/10.3390/cryst15020183
Chicago/Turabian StyleWeil, Matthias, and Enrique J. Baran. 2025. "Polymorphism of the Transition Metal Oxidotellurates NiTeO4 and CuTe2O5" Crystals 15, no. 2: 183. https://doi.org/10.3390/cryst15020183
APA StyleWeil, M., & Baran, E. J. (2025). Polymorphism of the Transition Metal Oxidotellurates NiTeO4 and CuTe2O5. Crystals, 15(2), 183. https://doi.org/10.3390/cryst15020183






