Ceramic Bracket Surface Treated with Hydrofluoric Acid, Er, Cr: YSGG Laser, and Phthalocyanine Activated via Low-Level Laser Therapy on Surface Roughness and Shear Bond Strength Bonded to Enamel via Unmodified and Sepiolite-Modified Orthodontic Adhesive-A SEM, EDX, and DC Evaluation
Abstract
1. Introduction
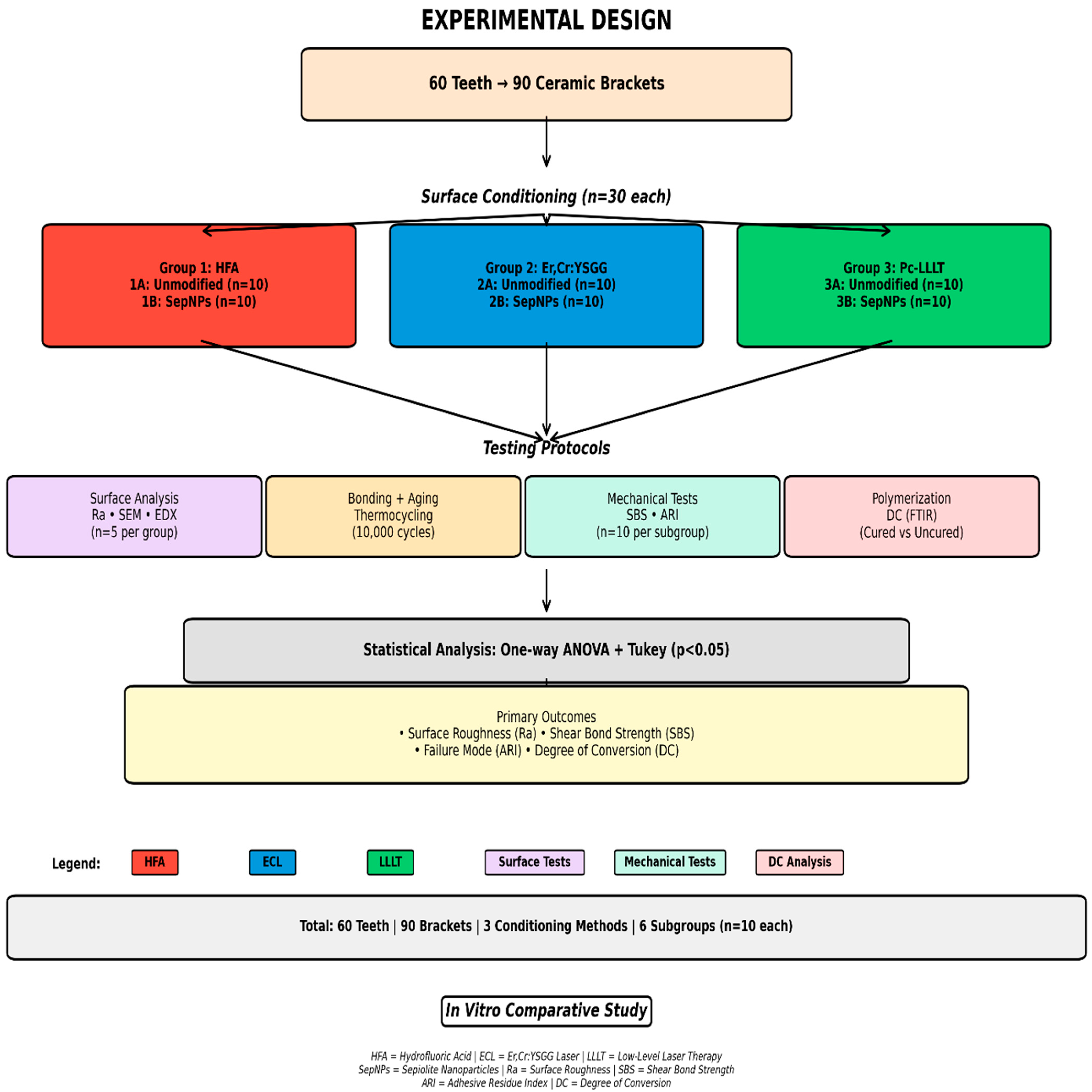
2. Materials and Methods
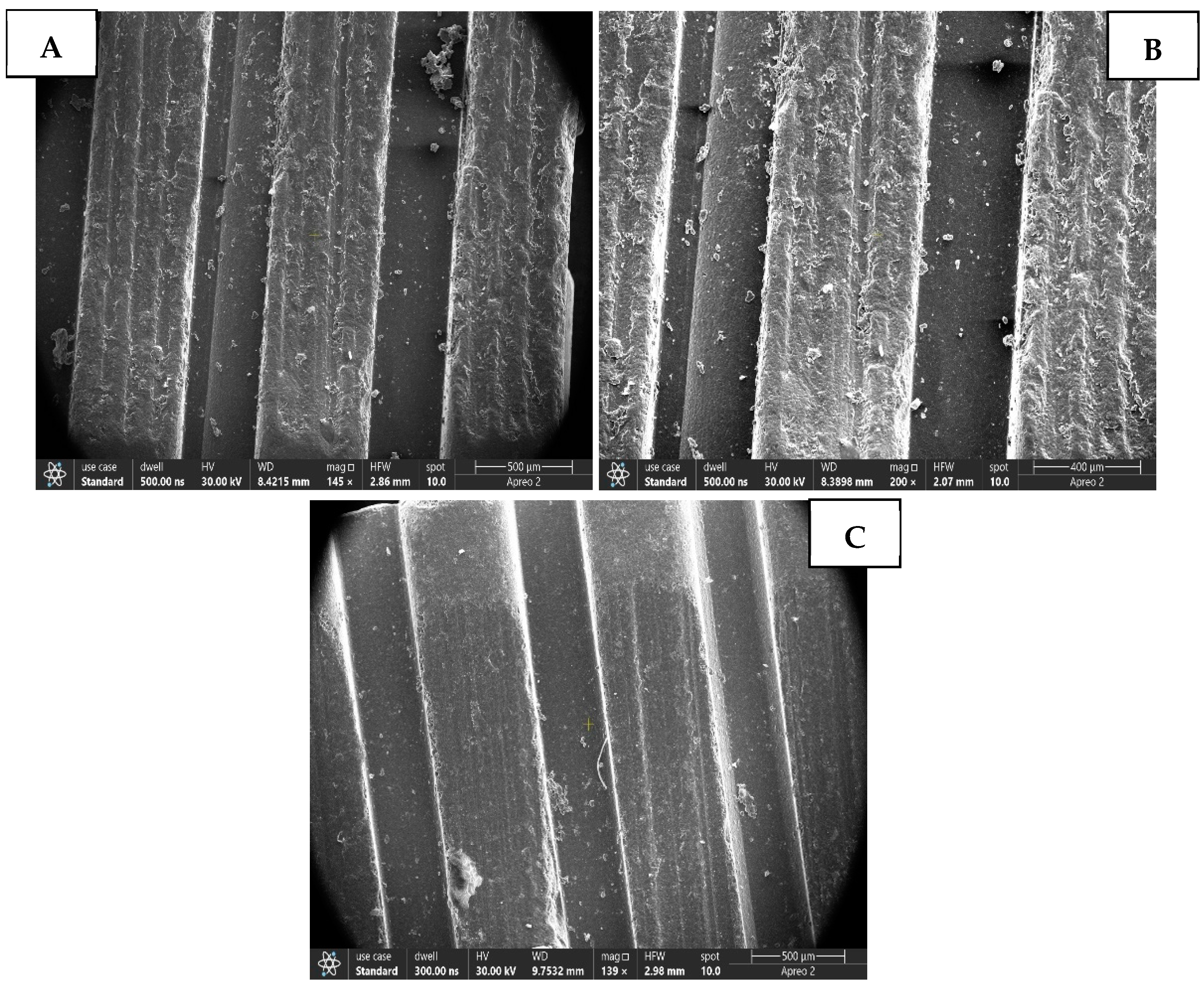
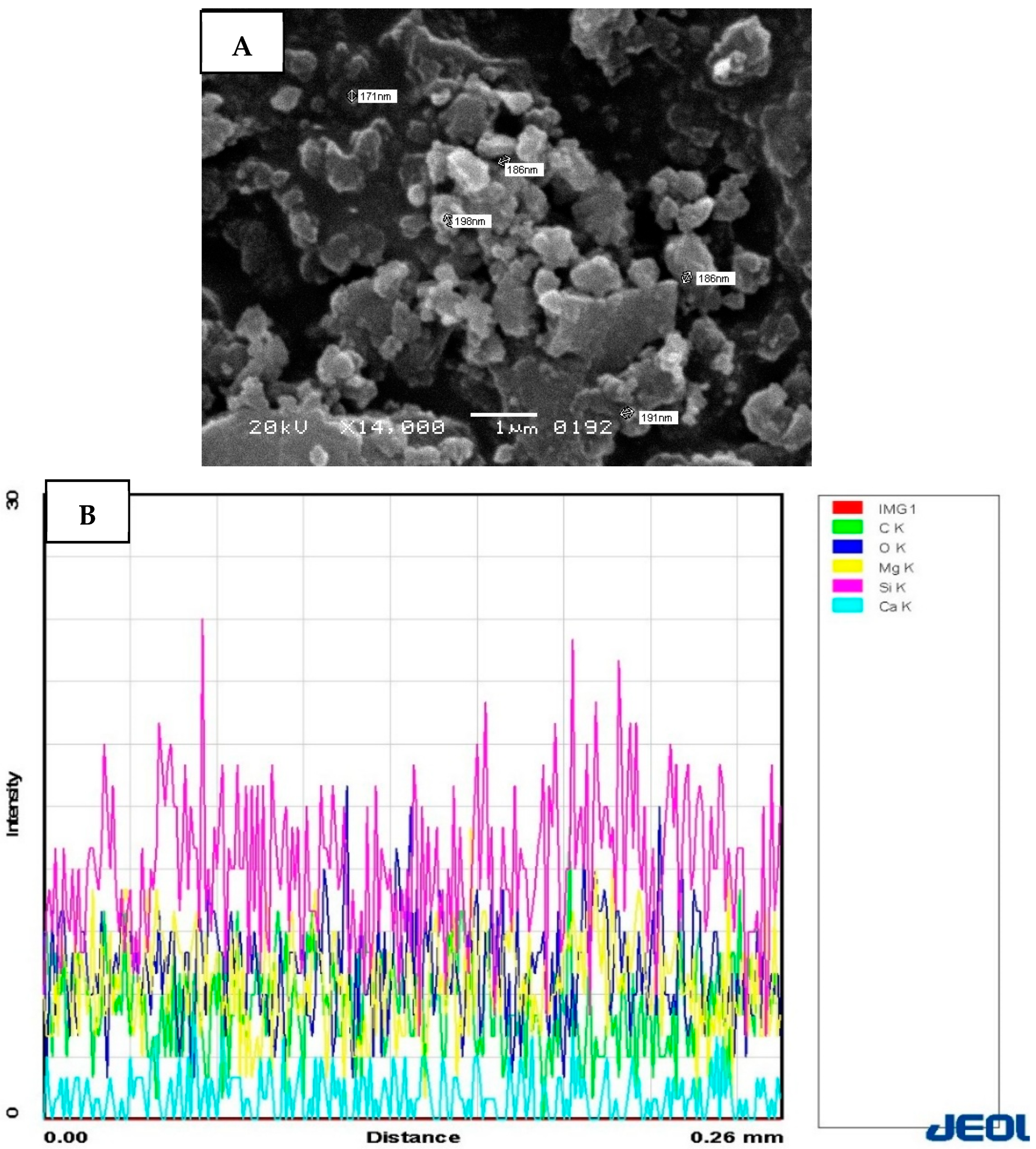
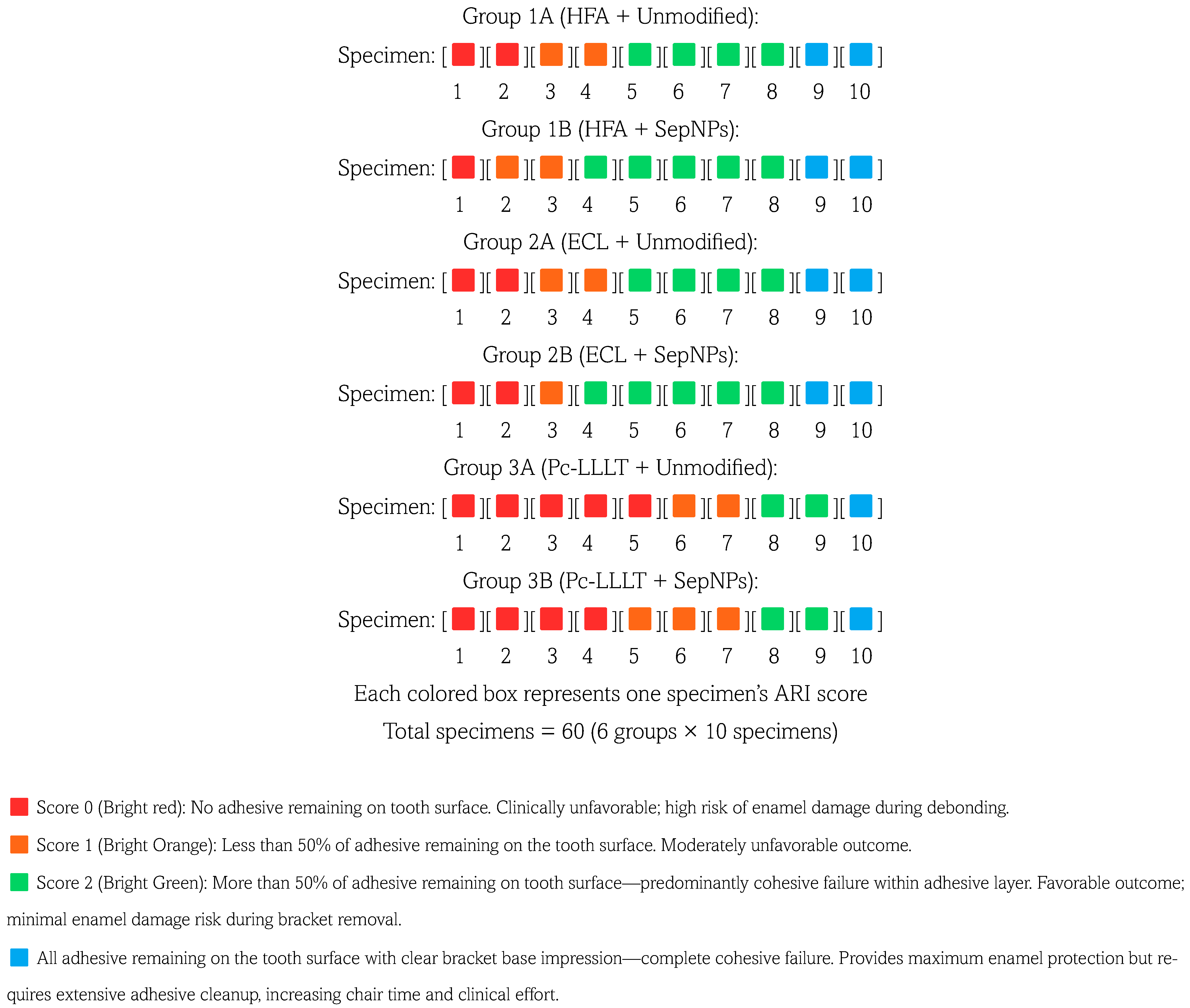
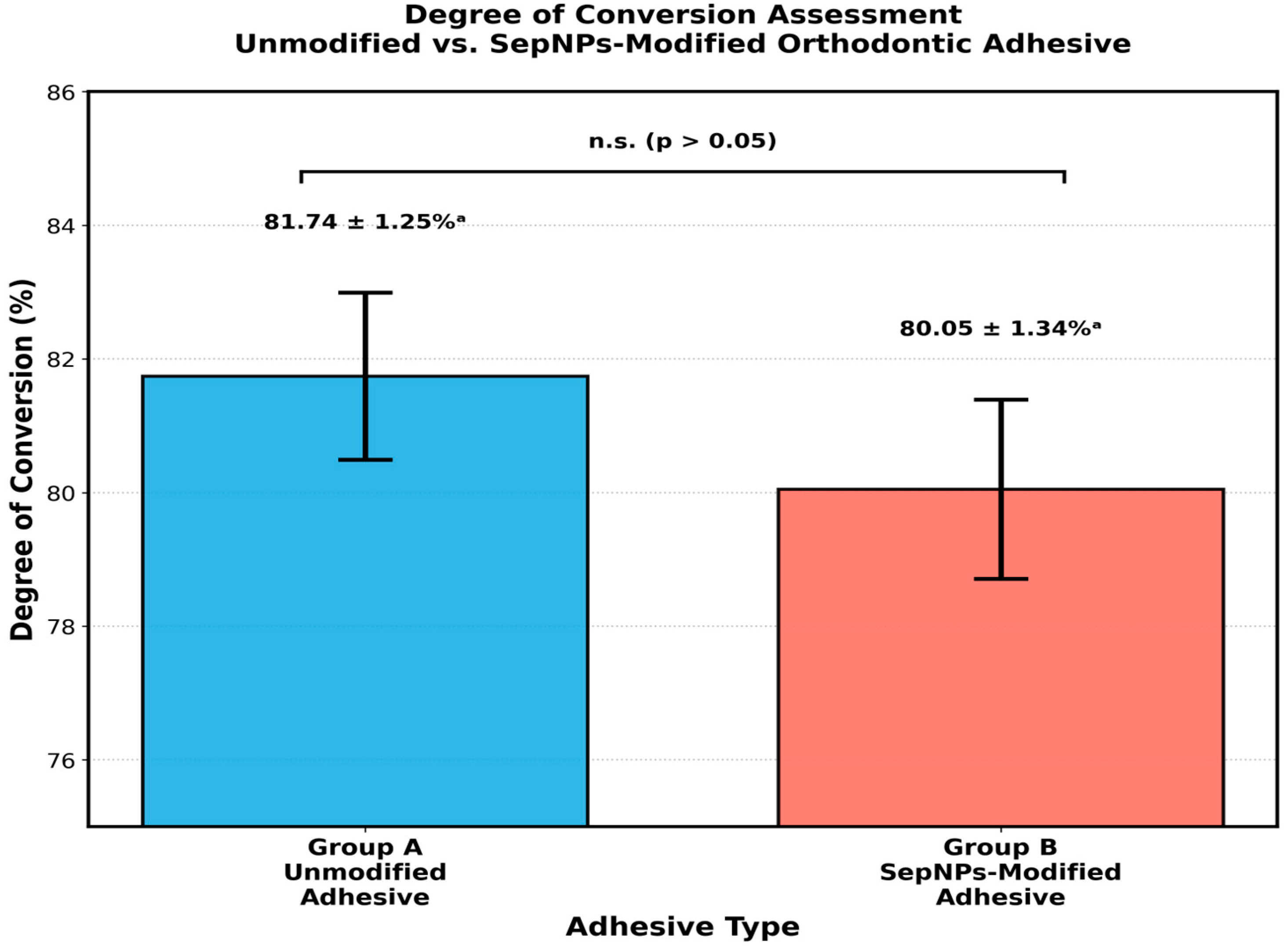
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Gudipaneni, R.K.; Aldahmeshi, R.F.; Patil, S.R.; Alam, M.K. The Prevalence of Malocclusion and the Need for Orthodontic Treatment among Adolescents in the Northern Border Region of Saudi Arabia: An Epidemiological Study. BMC Oral Health 2018, 18, 16. [Google Scholar] [CrossRef]
- Omran, R.; Dowie, A. Increased Demand for Orthodontic Treatments during the COVID-19 Pandemic: A Commentary. Br. Dent. J. 2023, 234, 84–87. [Google Scholar] [CrossRef]
- Koaban, A.M.; Alwadai, J.M.; Alghamdi, A.M.; Alsiwat, F.J.; Dashti, A.I.; Nasser, M.M.; Alhazmi, M.A.; Aljaroudi, E.M.; Alanazi, S.B.; Almanjhi, W.A. Recent Advances in Orthodontic Brackets: From Aesthetics to Smart Technologies. Cureus 2025, 17, e85385. [Google Scholar] [CrossRef] [PubMed]
- Reddy, Y.G.; Sharma, R.; Singh, A.; Agarwal, V.; Agrawal, V.; Saurab, C. The Shear Bond Strengths of Metal and Ceramic Brackets: An In-Vitro Comparative Study. J. Clin. Diagn. Res. 2013, 7, 1495–1497. [Google Scholar] [CrossRef] [PubMed]
- Nimplod, P.; Tansalarak, R.; Sornsuwan, T. Effect of the Different Debonding Strength of Metal and Ceramic Brackets on the Degree of Enamel Microcrack Healing. Dent. Press J. Orthod. 2021, 26, e2119177. [Google Scholar] [CrossRef]
- Türk, T.; Saraç, D.; Saraç, Y.Ş.; Elekdaǧ-Türk, S. Effects of Surface Conditioning on Bond Strength of Metal Brackets to All-Ceramic Surfaces. Eur. J. Orthod. 2006, 28, 450–456. [Google Scholar] [CrossRef] [PubMed]
- Alqahtani, S.A.; Alsaeed, A.Y.; Alqahtani, S.M.; Shafqat, S.; Niaz, M.O.; Ishtiaque, Y.; Abrar, E. Lithium Disilicate Ceramics Pretreated with Ti: Sapphire Fs, Er; Cr: YSGG, and Er: YAG Lasers: A Study on Mechanical Properties, Energy Dispersive Spectroscopy, Color Alterations, and Surface Topography. Microsc. Res. Tech. 2025, 88, 3029–3036. [Google Scholar] [CrossRef]
- Almutairi, B.; Al-Qahtani, A.S.; Shabbir, T.; Leemani, M.J.; Unar, J.; Manzar, N.; Abduljabbar, T. A Surface Topographical Analysis of Lithium Disilicate Ceramics Pretreated with Rose Bengal, Er:YAG Laser, and Ceramic Primer on Bond Integrity, Surface Roughness, and Bond Failure to Composite Resin. Sci. Adv. Mater. 2024, 16, 800–806. [Google Scholar] [CrossRef]
- Faltermeier, A.; Reicheneder, C.; Götzfried, P.; Proff, P. Bonding Orthodontic Ceramic Brackets to Ceramic Restorations: Evaluation of Different Surface Conditioning Methods. Mater. Sci. Appl. 2013, 4, 10–14. [Google Scholar] [CrossRef]
- Kim, K.J.; Park, Y.T.; Yoo, J.H.; Park, T.H. Hydrofluoric Acid Burns. Exog. Dermatol. 2004, 3, 12–18. [Google Scholar] [CrossRef]
- García-Sanz, V.; Paredes-Gallardo, V.; Bellot-Arcís, C.; Mendoza-Yero, O.; Doñate-Buendía, C.; Montero, J.; Albaladejo, A. Effects of Femtosecond Laser and Other Surface Treatments on the Bond Strength of Metallic and Ceramic Orthodontic Brackets to Zirconia. PLoS ONE 2017, 12, e0186796. [Google Scholar] [CrossRef] [PubMed]
- Erdur, E.A.; Basciftci, F.A. Effect of Ti:Sapphire Laser on Shear Bond Strength of Orthodontic Brackets to Ceramic Surfaces. Lasers Surg. Med. 2015, 47, 512–519. [Google Scholar] [CrossRef]
- Alkhudhairy, F.; Naseem, M.; Bin-Shuwaish, M.; Vohra, F. Efficacy of Er Cr: YSGG Laser Therapy at Different Frequency and Power Levels on Bond Integrity of Composite to Bleached Enamel. Photodiagnosis Photodyn. Ther. 2018, 22, 34–38. [Google Scholar] [CrossRef] [PubMed]
- Hoteit, M.; Nammour, S.; Zeinoun, T. Evaluation of Enamel Topography after Debonding Orthodontic Ceramic Brackets by Different Er,Cr:YSGG and Er:YAG Lasers Settings. Dent. J. 2020, 8, 6. [Google Scholar] [CrossRef] [PubMed]
- Maawadh, A.M.; Almohareb, T.; Al-Hamdan, R.S.; Al Deeb, M.; Naseem, M.; Alhenaki, A.M.; Vohra, F.; Abduljabbar, T. Repair Strength and Surface Topography of Lithium Disilicate and Hybrid Resin Ceramics with LLLT and Photodynamic Therapy in Comparison to Hydrofluoric Acid. J. Appl. Biomater. Funct. Mater. 2020, 18, 2280800020966938. [Google Scholar] [CrossRef]
- Vohra, F.; Labban, N.; Al-Hussaini, A.; Al-Jarboua, M.; Zawawi, R.; Alrahlah, A.; Naseem, M. Influence of Er;Cr:YSGG Laser on Shear Bond Strength and Color Stability of Lithium Disilicate Ceramics: An In Vitro Study. Photobiomodul. Photomed. Laser Surg. 2019, 37, 483–488. [Google Scholar] [CrossRef]
- Alrabeah, G.; Alhamid, R.F.; Alamer, B.A.; Alrajhi, F.N.; Binrayes, A.; Habib, S.R. Impact of Various Surface Treatments on the Shear Bond Strength Between Lithium Disilicate Ceramics and Resin Cement. Front. Mater. 2024, 11, 1496749. [Google Scholar] [CrossRef]
- Alsunbul, H.; Almutairi, B.; Aljanakh, M.; Abduljabbar, T. Hybrid Ceramic Repair Strength, Surface Roughness, and Bond Failure, Using Methylene Blue-Activated Low-Level Laser Therapy, Carbon Dioxide, and Ti: Al2O3 Laser. Photodiagnosis Photodyn. Ther. 2023, 43, 103693. [Google Scholar] [CrossRef]
- Alkhudhairy, F.; AlFawaz, Y.F. Pretreatment of Hybrid Ceramics Using Ho: YAG, Low-Level Laser Therapy Activated Malachite Green, and Non-Thermal Plasma on Surface Roughness, Bond Strength, and Color Change, SEM and EDX Analysis. Ceramics 2024, 7, 944–957. [Google Scholar] [CrossRef]
- Alhamdan, E.M. Repair Bond Strength and Surface Roughness of Zirconia Ceramics Treated via Carbon Dioxide Laser, Malachite Green, and Sandblasting. A Lab-Based Study. J. Ceram. Sci. Technol. 2025, 16, 85–92. [Google Scholar] [CrossRef]
- Alanazi, A.M.; Khan, N.A.; Khan, A.A.; Ansari, Z.; Shabbir, T.; Leemani, M.J. Lithium Disilicate Ceramics Surface Pre-Treatment Using Low-Level Laser Therapy-Activated Riboflavin, and Ti: Al2O3 Laser on the Colour Change, Surface Roughness, and Shear Bond Strength to Adhesive Cement: An In Vitro Sem Valuation. Ceram.-Silik. 2025, 69, 93–100. [Google Scholar] [CrossRef]
- Galstyan, A. Turning Photons into Drugs: Phthalocyanine-Based Photosensitizers as Efficient Photoantimicrobials. Chem. Eur. J. 2021, 27, 1903–1920. [Google Scholar] [CrossRef]
- Spesia, M.B.; Durantini, E.N. Evolution of Phthalocyanine Structures as Photodynamic Agents for Bacteria Inactivation. Chem. Rec. 2022, 22, e202100292. [Google Scholar] [CrossRef]
- Liu, M.; Zhang, S.; Wang, Z. Advanced Oxidation Processes of Organic Contaminants. Toxics 2024, 12, 579. [Google Scholar] [CrossRef]
- Shirase, S.; Shinohara, K.; Tsurugi, H.; Mashima, K. Oxidation of Alcohols to Carbonyl Compounds Catalyzed by Oxo-Bridged Dinuclear Cerium Complexes with Pentadentate Schiff-Base Ligands Under a Dioxygen Atmosphere. ACS Catal. 2018, 8, 6939–6947. [Google Scholar] [CrossRef]
- Al-Hamdan, R.S.; Almutairi, B.; Abduljabbar, T. Sandblasting, Bioactive Glass Particles, and Phthalocyanine Activated via Low-Level Laser Therapy on the Surface Roughness and Shear Bond Strength of Hybrid Ceramics Bonded to Different Cement Types. Photodiagnosis Photodyn. Ther. 2025, 55, 104682. [Google Scholar] [CrossRef]
- Alzahrani, A.H.; Ebrahim, M.I.; Felemban, M.F.; Alqarni, A.A.; Algahtani, F.S.; Shawli, H.T.; Al Humayyani, N.; Meshni, A.A.; Al Moaleem, M.M. Nanoparticle Augmentation of Adhesive Systems: Impact on Tensile Strength in Fiberglass Post Placement Within Root Dentin. Med. Sci. Monit. 2024, 30, e943502-1. [Google Scholar] [CrossRef]
- Fallahzadeh, F.; Safarzadeh-Khosroshahi, S.; Atai, M. Dentin Bonding Agent with Improved Bond Strength to Dentin Through Incorporation of Sepiolite Nanoparticles. J. Clin. Exp. Dent. 2017, 9, e738–e742. [Google Scholar] [CrossRef] [PubMed]
- Iqbal Khan, Z.; Habib, U.; Binti Mohamad, Z.; Razak Bin Rahmat, A.; Amira Sahirah Binti Abdullah, N. Mechanical and Thermal Properties of Sepiolite Strengthened Thermoplastic Polymer Nanocomposites: A Comprehensive Review. Alex. Eng. J. 2022, 61, 975–990. [Google Scholar] [CrossRef]
- Niazi, F.H.; Alotaibi, B.; Abdulla, A.M.; AlTowayan, S.A.R.; Ahmed, S.Z.; Alshehri, D.; Samran, A.; Alsuwayyigh, N.; Luddin, N. Modified Experimental Adhesive with Sepiolite Nanoparticles on Caries Dentin Treated with Femtosecond Laser and Photodynamic Activated Erythrosine. An In Vitro Study. Photodiagnosis Photodyn. Ther. 2024, 49, 104306. [Google Scholar] [CrossRef] [PubMed]
- Falkensammer, F.; Freudenthaler, J.; Pseiner, B.; Bantleon, H.P. Influence of Surface Conditioning on Ceramic Microstructure and Bracket Adhesion. Eur. J. Orthod. 2012, 34, 498–504. [Google Scholar] [CrossRef]
- Saraç, Y.Ş.; Külünk, T.; Elekdaǧ-Türk, S.; Saraç, D.; Türk, T. Effects of Surface-Conditioning Methods on Shear Bond Strength of Brackets Bonded to Different All-Ceramic Materials. Eur. J. Orthod. 2011, 33, 667–672. [Google Scholar] [CrossRef]
- Abdullah Kamran, M. Remineralization of Eroded Enamel for Improved Orthodontic Bracket Bonding: An In Vitro Study. Korean J. Orthod. 2025, 55, 244–253. [Google Scholar] [CrossRef]
- Samih, H.; Aboulazm, K.; Abd El-Motie, M. Debonding of Ceramic Brackets with ER,CR:YSGG Laser. Egypt. Orthod. J. 2014, 46, 27–39. [Google Scholar] [CrossRef]
- Mirhashemi, A.H.; Hossaini, S.M.H.; Etemadi, A.; Kharazifard, M.J.; Bahador, A.; Soudi, A. Effect of Er:YAG and Er,Cr:YSGG Lasers on Ceramic Bracket Debonding from Composite Blocks. Front. Dent. 2019, 16, 88–95. [Google Scholar] [CrossRef]
- Shabib, S. Use of Nd:YVO4 Laser, Photodynamic Therapy, Sulfuric Acid and Sand Blasting on Improving Bond Integrity of PEEK to Resin Cement with Adhesive. Photodiagnosis Photodyn. Ther. 2022, 39, 102865. [Google Scholar] [CrossRef]
- Al-Hamdan, R.S.; Almutairi, B.; Kattan, H.F.; Alsuwailem, N.A.; Farooq, I.; Vohra, F.; Abduljabbar, T. Influence of Hydroxyapatite Nanospheres in Dentin Adhesive on the Dentin Bond Integrity and Degree of Conversion: A Scanning Electron Microscopy (SEM), Raman, Fourier Transform-Infrared (FTIR), and Microtensile Study. Polymers 2020, 12, 2948. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; Xiao, Z.; Militz, H.; Hao, X. Silane Coupling Agents Used in Natural Fiber/Plastic Composites. In Handbook of Composites from Renewable Materials; Wiley: Hoboken, NJ, USA, 2017; Volume 1–8, pp. 407–430. ISBN 9781119441632. [Google Scholar]
- Sabzi, M.; Mirabedini, S.M.; Zohuriaan-Mehr, J.; Atai, M. Surface Modification of TiO2 Nano-Particles with Silane Coupling Agent and Investigation of Its Effect on the Properties of Polyurethane Composite Coating. Prog. Org. Coat. 2009, 65, 222–228. [Google Scholar] [CrossRef]
- Khan, A.S.; Alhamdan, Y.; Alibrahim, H.; Almulhim, K.S.; Nawaz, M.; Ahmed, S.Z.; Aljuaid, K.; Ateeq, I.S.; Akhtar, S.; Ansari, M.A.; et al. Analyses of Experimental Dental Adhesives Based on Zirconia/Silver Phosphate Nanoparticles. Polymers 2023, 15, 2614. [Google Scholar] [CrossRef]
- Kasraei, S.H.; Atai, M.; Khamverdi, Z.; Nejad, S.K. The effect of nanofiller addition to an experimental dentin adhesive on microtensile bond strength to human dentin. Front. Dent. 2009, 6, 36–41. [Google Scholar]
- Marovic, D.; Tarle, Z.; Hiller, K.A.; Müller, R.; Ristic, M.; Rosentritt, M.; Skrtic, D.; Schmalz, G. Effect of Silanized Nanosilica Addition on Remineralizing and Mechanical Properties of Experimental Composite Materials with Amorphous Calcium Phosphate. Clin. Oral Investig. 2014, 18, 783–792. [Google Scholar] [CrossRef]
- Chen, M.H. Critical Reviews in Oral Biology & Medicine: Update on Dental Nanocomposites. J. Dent. Res. 2010, 89, 549–560. [Google Scholar]
- Alqerban, A. Effectiveness of Riboflavin and Rose Bengal Photosensitizer Modified Adhesive Resin for Orthodontic Bonding. Pharmaceuticals 2021, 14, 48. [Google Scholar] [CrossRef]
- Messias, A.M.; Galvão, M.R.; Boaventura, J.M.C.; Jacomassi, D.P.; Bernardi, M.I.B.; Bagnato, V.S.; Rastelli, A.N.S.; Andrade, M.F. Degree of Conversion of Different Composite Resins Photo-Activated with Light-Emitting Diode and Argon Ion Laser. Laser Phys. 2015, 25, 025601. [Google Scholar] [CrossRef]
- Alnazeh, A.A.; Kamran, M.A.; Almoammar, S.; Al Jearah, M.M.; Qasim, M.; Alshahrani, I. Visible light-activated curcumin-doped zinc oxide nanoparticles integrated into orthodontic adhesive on Micro-tensile bond strength, degree of conversion, and antibacterial effectiveness against Staphylococcus Aureus. An investigation using scanning electron microscopy and energy-dispersive X-ray spectroscopy. J. Photochem. Photobiol. B Biol. 2024, 253, 112888. [Google Scholar] [CrossRef]
- Kamran, M.A.; Asiri, A.M.; Alfaifi, A.M.A.; Almukawwi, A.H.A.; Mughaddi Alwadai, J.; Alqahtani, S.J. Fluoride-Activated Via Er:YAG, Diode, and Femtosecond Lasers for Reversing Bleached Enamel for Improved Orthodontic Bonding. Photobiomodulation Photomed. Laser Surg. 2025, 43–50. [Google Scholar] [CrossRef] [PubMed]
- Jacobson, A.; Shojaei, A.R.; Thompson, B.D.; Kulkarni, G.V.; Titley, K.C. Adhesive Remnant Index (ARI) Revisited. An In Vitro Assessment of Clinically Debonded Orthodontic Brackets. Am. J. Orthod. Dentofac. Orthop. 2006, 130, 120. [Google Scholar] [CrossRef]
- Reynolds, I.R. A Review of Direct Orthodontic Bonding. Br. J. Orthod. 1975, 2, 171–178. [Google Scholar] [CrossRef]
- Bishara, S.E.; Oonsombat, C.; Soliman, M.M.A.; Ajlouni, R.; Laffoon, J.F. The Effect of Tooth Bleaching on the Shear Bond Strength of Orthodontic Brackets. Am. J. Orthod. Dentofac. Orthop. 2005, 128, 755–760. [Google Scholar] [CrossRef]
- Scribante, A.; Contreras-Bulnes, R.; Montasser, M.A.; Vallittu, P.K. Orthodontics: Bracket Materials, Adhesives Systems, and Their Bond Strength. Biomed Res. Int. 2016, 2016, 1329814. [Google Scholar] [CrossRef]
- Ryf, S.; Flury, S.; Palaniappan, S.; Lussi, A.; Van Meerbeek, B.; Zimmerli, B. Enamel Loss and Adhesive Remnants Following Bracket Removal and Various Clean-up Procedures In Vitro. Eur. J. Orthod. 2012, 34, 25–32. [Google Scholar] [CrossRef]
- Kaygısız, E.; Eğilmez, F.; Ergün, G.; Yüksel, S.; Nagas, I.Ç. The Effects of Er,Cr:YSGG Laser on Shear Bond Strength of Orthodontic Lingual Brackets to CAD/CAM Ceramic Systems. Eur. Oral Res. 2023, 57, 122–127. [Google Scholar] [CrossRef]
- Alkhudhairy, F.; Naseem, M.; Ahmad, Z.H.; Alnooh, A.N.; Vohra, F. Efficacy of phototherapy with different conventional surface treatments on adhesive quality of lithium disilicate ceramics. Photodiagnosis Photodyn. Ther. 2019, 25, 292–295. [Google Scholar] [CrossRef]
- Alkan Demetoğlu, G.; Talay Çevlïk, E. Evaluation of the Effect of Etching with Ytterbium Fiber Laser on the Bond Strength, Color Stability, and Fracture Analysis of Lithium Disilicate Ceramics to Bovine Teeth: An In Vitro Study. BMC Oral Health 2025, 25, 607. [Google Scholar] [CrossRef] [PubMed]
- Albaker, A.M.; Al Deeb, L.; Alhenaki, A.M.; Aldeeb, M.; Al Ahdal, K.; Abduljabbar, T.; Vohra, F. Bonding Integrity and Compressive Strength of Re-Bonded, Surface Conditioned and Er Cr YSGG Laser Treated Lithium Disilicate Ceramics. J. Appl. Biomater. Funct. Mater. 2020, 18, 2280800020910954. [Google Scholar] [CrossRef]
- Wainwright, M.; Phoenix, D.A.; Rice, L.; Burrow, S.M.; Waring, J. Increased Cytotoxicity and Phototoxicity in the Methylene Blue Series via Chromophore Methylation. J. Photochem. Photobiol. B Biol. 1997, 40, 233–239. [Google Scholar] [CrossRef] [PubMed]
- AlFawaz, Y.F.; Almutairi, B.; Kattan, H.F.; Zafar, M.S.; Farooq, I.; Naseem, M.; Vohra, F.; Abduljabbar, T. Dentin bond integrity of hydroxyapatite containing resin adhesive enhanced with graphene oxide nano-particles—An SEM, EDX, micro-Raman, and microtensile bond strength study. Polymers 2020, 12, 2978. [Google Scholar] [CrossRef] [PubMed]
- Baptista, M.S.; Cadet, J.; Di Mascio, P.; Ghogare, A.A.; Greer, A.; Hamblin, M.R.; Lorente, C.; Nunez, S.C.; Ribeiro, M.S.; Thomas, A.H.; et al. Type I and Type II Photosensitized Oxidation Reactions: Guidelines and Mechanistic Pathways. Photochem. Photobiol. 2017, 93, 912–919. [Google Scholar] [CrossRef]
- Kim, J.W.; Kim, L.U.; Kim, C.K. Size Control of Silica Nanoparticles and Their Surface Treatment for Fabrication of Dental Nanocomposites. Biomacromolecules 2007, 8, 215–222. [Google Scholar] [CrossRef]
- Kumar, U.; Kumar, D.; Gosai, K.N.; Dalal, D.; Pragnya, B.; Nagarajan, S. Effectiveness of nanoparticles in enhancing bond strength in adhesive dentistry. J. Pharm. Bioallied Sci. 2024, 16 (Suppl. S4), S3772–S3774. [Google Scholar] [CrossRef]
- Alhenaki, A.M.; Attar, E.A.; Alshahrani, A.; Farooq, I.; Vohra, F.; Abduljabbar, T. Dentin Bond Integrity of Filled and Unfilled Resin Adhesive Enhanced with Silica Nanoparticles—An Sem, Edx, Micro-Raman, Ftir and Micro-Tensile Bond Strength Study. Polymers 2021, 13, 1093. [Google Scholar] [CrossRef] [PubMed]
- Alqarawi, F.K.; Alkahtany, M.F.; Almadi, K.H.; Ben Gassem, A.A.; Alshahrani, F.A.; Alrefeai, M.H.; Farooq, I.; Vohra, F.; Abduljabbar, T. Influence of Different Conditioning Treatments on the Bond Integrity of Root Dentin to Rgo Infiltrated Dentin Adhesive. Sem, Edx, Ftir and Microraman Study. Polymers 2021, 13, 1555. [Google Scholar] [CrossRef] [PubMed]
| Investigated Groups | Mean ± SD Ra (µm) | p-Value! |
|---|---|---|
| Group 1: HFA | 1031.62 ± 0.72 a | <0.05 |
| Group 2: ECL | 1087.43 ± 0.43 a | |
| Group 3: Pc-LLLT | 706.53 ± 0.54 b |
| Investigated Groups | SBS Mean ± SD (MPa) | p-Value! |
|---|---|---|
| Group 1A: HFA + Unmodified adhesive | 7.86 ± 0.41 a | <0.05 |
| Group 1B: HFA + SepNPs modified adhesive | 8.63 ± 0.52 b | |
| Group 2A: ECL + Unmodified adhesive | 7.93 ± 0.33 a | |
| Group 2B: ECL + SepNPs modified adhesive | 8.79 ± 0.48 b | |
| Group 3A: Pc-LLLT + Unmodified adhesive | 5.23 ± 0.32 d | |
| Group 3B: Pc-LLLT+ SepNPs modified adhesive | 6.16 ± 0.48 c |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Almoammar, S.; Abdullah Kamran, M.; Alshehri, A.; Awadh, W.; Alshahrani, A.M.; Alshahrani, I. Ceramic Bracket Surface Treated with Hydrofluoric Acid, Er, Cr: YSGG Laser, and Phthalocyanine Activated via Low-Level Laser Therapy on Surface Roughness and Shear Bond Strength Bonded to Enamel via Unmodified and Sepiolite-Modified Orthodontic Adhesive-A SEM, EDX, and DC Evaluation. Crystals 2025, 15, 1010. https://doi.org/10.3390/cryst15121010
Almoammar S, Abdullah Kamran M, Alshehri A, Awadh W, Alshahrani AM, Alshahrani I. Ceramic Bracket Surface Treated with Hydrofluoric Acid, Er, Cr: YSGG Laser, and Phthalocyanine Activated via Low-Level Laser Therapy on Surface Roughness and Shear Bond Strength Bonded to Enamel via Unmodified and Sepiolite-Modified Orthodontic Adhesive-A SEM, EDX, and DC Evaluation. Crystals. 2025; 15(12):1010. https://doi.org/10.3390/cryst15121010
Chicago/Turabian StyleAlmoammar, Salem, Muhammad Abdullah Kamran, Abdulrahman Alshehri, Wael Awadh, Amirah Mesfer Alshahrani, and Ibrahim Alshahrani. 2025. "Ceramic Bracket Surface Treated with Hydrofluoric Acid, Er, Cr: YSGG Laser, and Phthalocyanine Activated via Low-Level Laser Therapy on Surface Roughness and Shear Bond Strength Bonded to Enamel via Unmodified and Sepiolite-Modified Orthodontic Adhesive-A SEM, EDX, and DC Evaluation" Crystals 15, no. 12: 1010. https://doi.org/10.3390/cryst15121010
APA StyleAlmoammar, S., Abdullah Kamran, M., Alshehri, A., Awadh, W., Alshahrani, A. M., & Alshahrani, I. (2025). Ceramic Bracket Surface Treated with Hydrofluoric Acid, Er, Cr: YSGG Laser, and Phthalocyanine Activated via Low-Level Laser Therapy on Surface Roughness and Shear Bond Strength Bonded to Enamel via Unmodified and Sepiolite-Modified Orthodontic Adhesive-A SEM, EDX, and DC Evaluation. Crystals, 15(12), 1010. https://doi.org/10.3390/cryst15121010







