C–H…X (X = F, Cl, Br, I) Versus π-Stacking in the Crystal Packing of Compounds Containing the {M(tpy)X3} Motif
Abstract
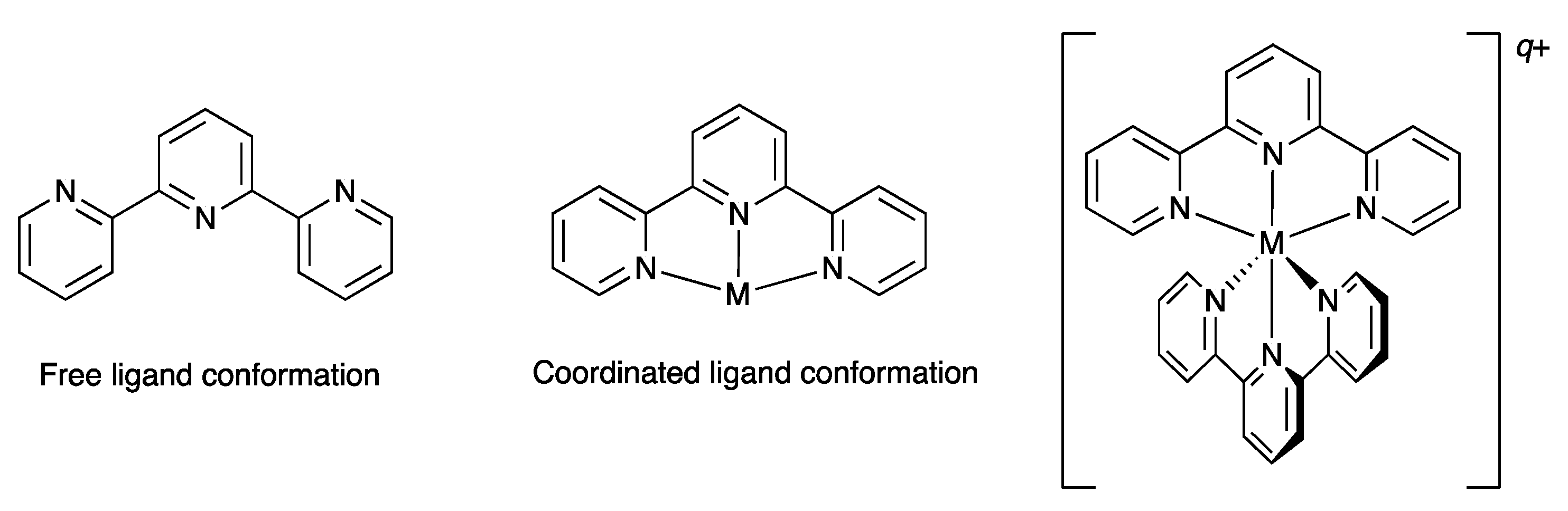
1. Introduction
2. Methods
3. Defining the Sets of Structures for Analysis
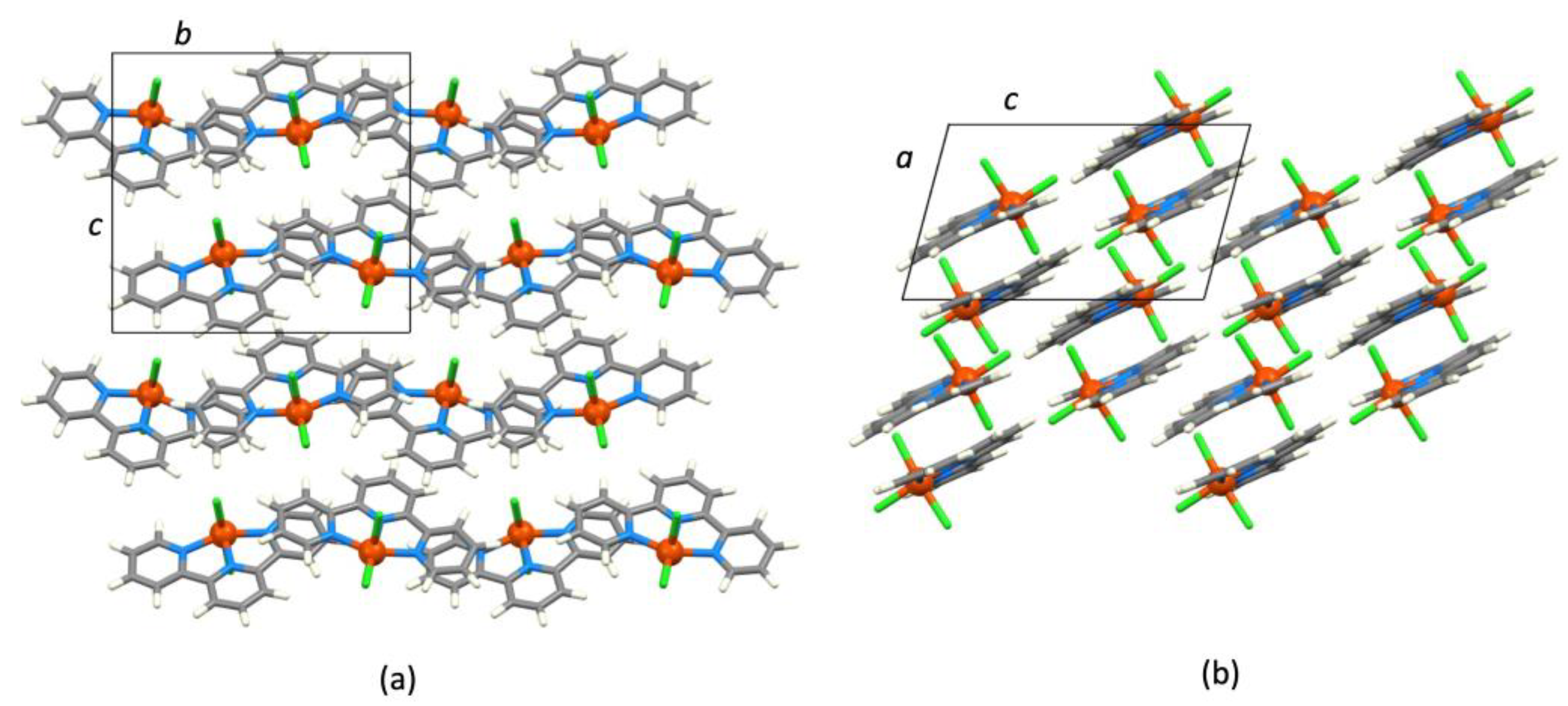
4. Mononuclear [M(tpy)X3], CN = 6
5. Mononuclear [M(tpy)X3(Y)], (Y = X, OH2, MeOH), CN = 7
6. Dinuclear [M2(tpy)2Xn(μ-X)2] (n = 2, 4), [M2(tpy)2X2(OH2)2(μ-X)2], [M2(tpy)2X4(OH2)2(μ-X)2] and [M2(tpy)2X2(DMF)2(μ-Cl)2] (DMF = dimethylformamide)
7. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Constable, E.C. The Coordination Chemistry of 2,2′:6′,2″-Terpyridine and Higher Oligopyridines. Adv. Inorg. Chem. 1986, 30, 69–121. [Google Scholar] [CrossRef]
- Schubert, U.S.; Hofmeier, H.; Newkome, G.R. Modern Terpyridine Chemistry; Wiley-VCH Verlag & Co.: Weinheim, Germany, 2006. [Google Scholar]
- Constable, E.C. 2,2′:6′,2″-Terpyridines: From chemical obscurity to common supramolecular motifs. Chem. Soc. Rev. 2007, 36, 246–253. [Google Scholar] [CrossRef] [PubMed]
- Wei, C.; He, Y.; Shi, X.; Song, Z. Terpyridine-metal complexes: Applications in catalysis and supramolecular chemistry. Coord. Chem. Rev. 2019, 385, 1–19. [Google Scholar] [CrossRef] [PubMed]
- McMurtie, J.; Dance, I. Crystal packing in metal complexes of 4’-phenylterpyridine and related ligands: Occurrence of the 2D and 1D terpy embrace arrays. CrystEngComm 2009, 11, 1141–1149. [Google Scholar] [CrossRef]
- McMurtie, J.; Dance, I. Alternative metal grid structures formed by [M(terpy)2]2+ and [M(terpyOH)2]2+ complexes with small and large tetrahedral dianions, and by [Ru(terpy)2]0. CrystEngComm 2010, 12, 2700–2710. [Google Scholar] [CrossRef]
- McMurtie, J.; Dance, I. Alternative two-dimensional embrace nets formed by metal complexes of 4’-phenylterpyridine crystallised with hydrophilic anions. CrystEngComm 2010, 12, 3207–3217. [Google Scholar] [CrossRef]
- Janiak, C. A critical account on π-π stacking in metal complexes with aromatic nitrogen-containing ligands. J. Chem. Soc. Dalton Trans. 2000, 21, 3885–3896. [Google Scholar] [CrossRef]
- Dance, I.; Scudder, M. Molecular embracing in crystals. CrystEngComm 2009, 11, 2233–2247. [Google Scholar] [CrossRef]
- Groom, C.R.; Bruno, I.J.; Lightfoot, M.P.; Ward, S.C. The Cambridge Structural Database. Acta Crystallogr. Sect. B 2016, 72, 171–179. [Google Scholar] [CrossRef]
- Constable, E.C.; Housecroft, C.E. Halide Ion Embraces in Tris(2,2’-bipyridine)metal Complexes. Crystals 2020, 10, 671. [Google Scholar] [CrossRef]
- Constable, E.C.; Housecroft, C.E. Embracing [XY3]m– and [XY4]m– anions in salts of [M(bpy)3]q+. Crystals 2023, 13, 97. [Google Scholar] [CrossRef]
- Constable, E.C.; Housecroft, C.E. Packing motifs in [M(bpy)2X2] coordination compounds (bpy = 2,2′-bipyridine; X = F, Cl, Br, I). Crystals 2023, 13, 505. [Google Scholar] [CrossRef]
- Constable, E.C.; Seddon, K.R. A Deuterium Exchange Reaction of the Tris-(2,2′-bipyridine)ruthenium(II) Cation: Evidence for the Acidity of the 3,3′-Protons. J. Chem. Soc. Chem. Commun. 1982, 1, 34–36. [Google Scholar] [CrossRef]
- Bruno, I.J.; Cole, J.C.; Edgington, P.R.; Kessler, M.; Macrae, C.F.; McCabe, P.; Pearson, J.; Taylor, R. New software for searching the Cambridge Structural Database and visualising crystal structures. Acta Crystallogr. Sect. B 2002, 58, 389–397. [Google Scholar] [CrossRef] [PubMed]
- Macrae, C.F.; Sovago, I.; Cottrell, S.J.; Galek, P.T.A.; McCabe, P.; Pidcock, E.; Platings, M.; Shields, G.P.; Stevens, J.S.; Towler, M.; et al. Mercury 4.0: From visualization to analysis, design and prediction. J. Appl. Cryst. 2020, 53, 226–235. [Google Scholar] [CrossRef]
- Lhoste, J.; Perez-Campos, A.; Henry, N.; Loiseau, T.; Rabu, P.; Abraham, F. Chain-like and dinuclear coordination polymers in lanthanide (Nd, Eu)oxochloride complexes with 2,2’:6’,2”-terpyridine: Synthesis, XRD structure and magnetic properties. Dalton Trans. 2011, 40, 9136–9144. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.-F.; Zhang, X.; Sun, S.-W.; Yao, C.-Z.; Liu, Z.-R.; Wang, Y.-C.; Liu, Y.-Z. Synthesis and structural characterization of a novel copper(II)/lead(II) heterometallic organic–inorganic hybrid. Z. Naturforsch. B 2015, 70, 617–623. [Google Scholar] [CrossRef]
- Birk, T.; Magnussen, M.J.; Piligkos, S.; Weihe, H.; Holten, A.; Bendix, J. Alkali metal cation complexation and solvent interactions by robust chromium(III) fluoride complexes. J. Fluor. Chem. 2010, 131, 898–906. [Google Scholar] [CrossRef]
- Engelhardt, L.M.; Harrowfield, J.M.; Miyamae, H.; Patrick, J.M.; Skelton, B.W.; Soudi, A.A.; White, A.H. Lewis-Base Adducts of Lead(II) Compounds. XVII Synthetic and Structural Studies of Some 1:1 Adducts of 2,2’:6’,2”-Terpyridine with Lead(II) Salts. Aust. J. Chem. 1996, 49, 1135–1146. [Google Scholar] [CrossRef]
- Tershansy, M.A.; Goforth, A.M.; Smith, M.D.; zur Loye, H.-C. The Synthesis and Crystal Structure of [BiI2(tpy)2][Bi2I7(tpy)]: A New Metal Halide Material. J. Chem. Cryst. 2008, 38, 453–459. [Google Scholar] [CrossRef]
- Lewis, K.M.; Kelley, J.; Petersen, L., Jr.; Smith, M.D.; Severance, R.C.; Vaughn, S.A.; zur Loye, H.-C. Synthesis and Crystal Structure of an Iodobismuthate Incorporating Both a Cationic and Anionic Bi(III) Complex Ion. J. Chem. Crystallogr. 2010, 40, 867–871. [Google Scholar] [CrossRef]
- Battaglia, L.P.; Corradi, A.B.; Pelosi, G.; Cantoni, A.; Alonzo, G.; Bertazzi, N. Crystal and Molecular Structure of AntimonyTrifluoride Terpyridine 1:1 Adduct: A Case of Pseudo-pentagonal-bipyramidal Geometry. J. Chem. Soc. Dalton Trans. 1991, 11, 3153–3155. [Google Scholar] [CrossRef]
- Beran, G.; Dymock, K.; Patel, H.A.; Carty, A.J.; Boorman, P.M. Solid-State Structures of Group IIIb Metal Chloride Adducts with 2,2’,2”-Terpyridyl. Inorg. Chem. 1972, 11, 896–898. [Google Scholar] [CrossRef]
- Beran, G.; Carty, A.J.; Patel, H.A.; Palenik, G.J. A trans-Effect in Gallium Complexes: The Crystal Structure of Trichloro-(2,2’,2”-terpyridyl)galliurn(III). J. Chem. Soc. D Chem. Comm. 1970, 222–223. [Google Scholar] [CrossRef]
- Palenik, G.J.; Dymock, K. American Crystallographic Association; Spring Meeting: Berkeley, CA, USA, 1974; p. 137. [Google Scholar]
- Nakayama, Y.; Baba, Y.; Yasuda, H.; Kawakita, K.; Ueyama, N. Stereospecific Polymerizations of Conjugated Dienes by Single Site Iron Complexes Having Chelating N,N,N-Donor Ligands. Macromolecules 2003, 36, 7953–7958. [Google Scholar] [CrossRef]
- Kazakov, I.V.; Bodensteiner, M.; Timoshkin, A.Y. Masking of Lewis acidity trends in the solid-state structures of trichlorido- and tribromido(2,2’:6’,2”-terpyridine-κ3N,N’,N”)gallium(III). Acta Crystallogr. Sect. C 2014, 70, 312–313. [Google Scholar] [CrossRef]
- Butcher, R.J.; George, C.; Muratore, N.; Purdy, A.P. Trichloro(2,6-di-2-pyridylpyridine-κ3N)indium(III). Acta Crystallogr. Sect. E 2003, 59, m1107–m1109. [Google Scholar] [CrossRef]
- Zhang, Y.; Yuan, S.; Yuan, Y.; Bao, Y.; Ran, Q.; Liu, E.; Fan, J.; Li, W. Alleviation of π-π* Transition Enabling Enhanced Luminescence in Emerging TpyInClx (x = 3, 5) Perovskite Single Crystals. Adv. Opt. Mater. 2022, 10, 2102041. [Google Scholar] [CrossRef]
- Curnock, E.; Levason, W.; Light, M.E.; Luthra, S.K.; McRobbie, G.; Monzittu, F.M.; Reid, G.; Williams, R.N. Group 3 metal trihalide complexes with neutral N-donor ligands—Exploring their affinity towards fluoride. Dalton Trans. 2018, 47, 6059–6068. [Google Scholar] [CrossRef]
- Mantel, C.; Chen, H.; Crabtree, R.H.; Brudvig, G.W.; Pécaut, J.; Collomb, M.-N.; Duboc, C. High-Spin Chloro Mononuclear MnIII Complexes: A Multifrequency High-Field EPR Study. ChemPhysChem 2005, 6, 541–546. [Google Scholar] [CrossRef]
- Cotton, S.A.; Franckevicius, V.; Fawcett, J. Syntheses and structures of iron(III) complexes of simple N-donor ligands. Polyhedron 2002, 21, 2055–2061. [Google Scholar] [CrossRef]
- Paraskevopoulos, J.N.; Smith, P.J.; Hoppe, H.C.; Chopra, D.; Govender, T.; Kruger, H.G.; Maguire, G.E.M. Terpyridyl Complexes as Antimalarial Agents. S. Afr. J. Chem. 2013, 66, 80–85. [Google Scholar]
- Laurent, F.; Plantalech, E.; Donnadieu, B.; Jiménez, A.; Hernández, F.; Martínez-Ripoll, M.; Biner, M.; Llobet, A. Synthesis, structure and redox properties of ruthenium complexes containing the tpm facial and the trpy meridional tridentate ligands. Crystal structures of [RuCl3(trpy)] and [Ru(tpm)(py)3](PF6)2. Polyhedron 1999, 18, 3321–3331. [Google Scholar] [CrossRef]
- Dobroschke, M.; Geldmacher, Y.; Ott, I.; Harlos, M.; Kater, L.; Wagner, L.; Gust, R.; Sheldrick, W.S.; Prokop, A. Cytotoxic Rhodium(III) and Iridium(III) Polypyridyl Complexes: Structure–Activity Relationships, Antileukemic Activity, and Apoptosis Induction. ChemMedChem 2009, 4, 177–187. [Google Scholar] [CrossRef] [PubMed]
- Demadis, K.D.; El-Samanody, E.-S.; Meyer, T.J.; White, P.S. Structural and redox chemistry of osmium(III) chloro complexes containing 2,2’:6’,2”-terpyridyl and tris-pyrazolyl borate ligands. Polyhedron 1999, 18, 1587–1594. [Google Scholar] [CrossRef]
- Chong, J.; Besnard, C.; Cruz, C.M.; Piguet, C.; Jiménez, J.-R. Heteroleptic mer-[Cr(N∩N∩N)(CN)3] complexes: Synthetic challenge, structural characterization and photophysical properties. Dalton Trans. 2022, 51, 4297–4309. [Google Scholar] [CrossRef]
- Bhalla, R.; Levason, W.; Luthra, S.K.; McRobbie, G.; Monzittu, F.M.; Palmer, J.; Reid, G.; Sanderson, G.; Zhang, W. Hydrothermal synthesis of Group 13 metal trifluoride complexes with neutral N-donor ligands. Dalton Trans. 2015, 44, 9569–9580. [Google Scholar] [CrossRef]
- Blower, P.J.; Levason, W.; Luthra, S.K.; McRobbie, G.; Monzittu, F.M.; Mules, T.O.; Reid, G.; Subhan, M.N. Exploring transition metal fluoride chelates—Synthesis, properties and prospects towards potential PET probes. Dalton Trans. 2019, 48, 6767–6776. [Google Scholar] [CrossRef]
- Mantel, C.; Hassan, A.K.; Pécaut, J.; Deronzier, A.; Collomb, M.-N.; Duboc-Toia, C. A High-Frequency and High-Field EPR Study of New Azide and Fluoride Mononuclear Mn(III) Complexes. J. Am. Chem. Soc. 2003, 125, 12337–12344. [Google Scholar] [CrossRef]
- Cloete, N.; Visser, H.G.; Roodt, A. mer-Trichloro(2,2’,2”-terpyridine)chromium(III) dimethyl sulfoxide solvate. Acta Crystallogr. Sect. E 2007, 63, m45–m47. [Google Scholar] [CrossRef]
- Pruchnik, F.P.; Jakimowicz, P.; Ciunik, Z.; Zakrzewska-Czerwińska, J.; Opolski, A.; Wietrzyk, J.; Wojdat, E. Rh dium(III) complexes with polypyridyls and pyrazole and their antitumor activity. Inorg. Chim. Acta 2002, 334, 59–66. [Google Scholar] [CrossRef]
- Taylor, S.D.; Shingade, V.M.; Muvirimi, R.; Hicks, S.D.; Krause, J.A.; Connick, W.B. Spectroscopic Characterization of Platinum(IV) Terpyridyl Complexes. Inorg. Chem. 2019, 58, 16364–16371. [Google Scholar] [CrossRef]
- Ayscue, R.L.; Vallet, V.; Bertke, J.A.; Réal, F.; Knope, K.E. Structure−Property Relationships in Photoluminescent Bismuth Halide Organic Hybrid Materials. Inorg. Chem. 2021, 60, 9727–9744. [Google Scholar] [CrossRef] [PubMed]
- Kepert, C.J.; Weimin, L.; Skelton, B.W.; White, A.H. Structural Systematics of Rare Earth Complexes. V The Hydrated 1:1 Adducts of 2,2’:6’,2”-Terpyridine with the Lanthanoid(III) Chlorides. Aust. J. Chem. 1994, 47, 365–384. [Google Scholar] [CrossRef]
- Waters, A.F.; White, A.H. Synthesis and Structural Systematics of Nitrogen Base Adducts of Group 2 Salts. X Some Mixed-Ligand Complexes of Group 2 Metal Halides with 2,2’:6’,2”-Terpyridine and Oxygen Donors. Aust. J. Chem. 1996, 49, 147–154. [Google Scholar] [CrossRef]
- Adcock, A.K.; Ayscue, R.L.; Breuer, L.M.; Verwiel, C.P.; Marwitz, A.C.; Bertke, J.A.; Vallet, V.; Réal, F.; Knope, K.E. Synthesis and photoluminescence of three bismuth(III)-organic compounds bearing heterocyclic N-donor ligands. Dalton Trans. 2020, 49, 11756–11771. [Google Scholar] [CrossRef]
- Aullón, G.; Bellamy, D.; Brammer, L.; Bruton, E.A.; Orpen, A.G. Metal-bound chlorine often accepts hydrogen bonds. Chem. Commun. 1998, 6, 653–654. [Google Scholar] [CrossRef]
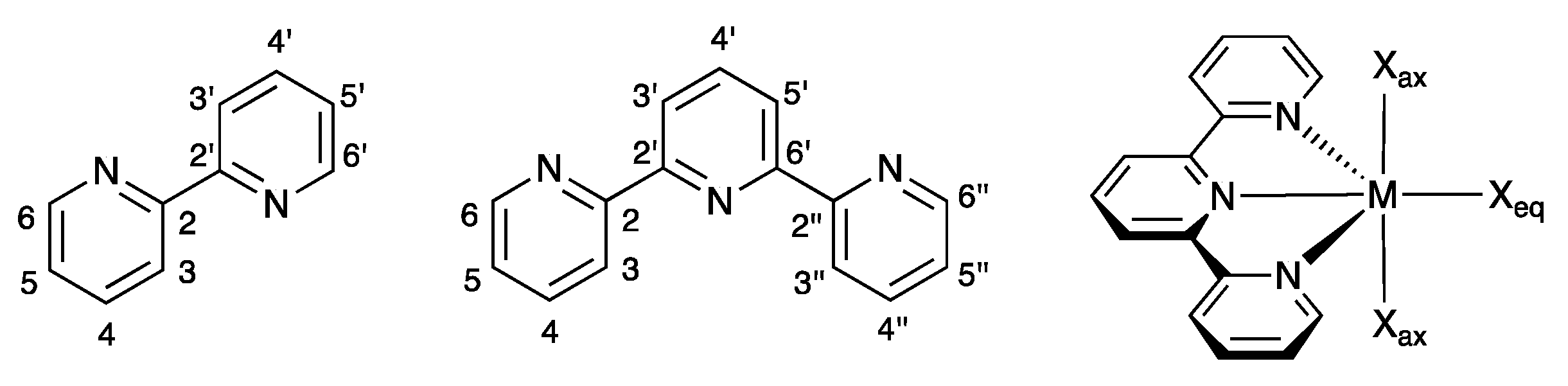
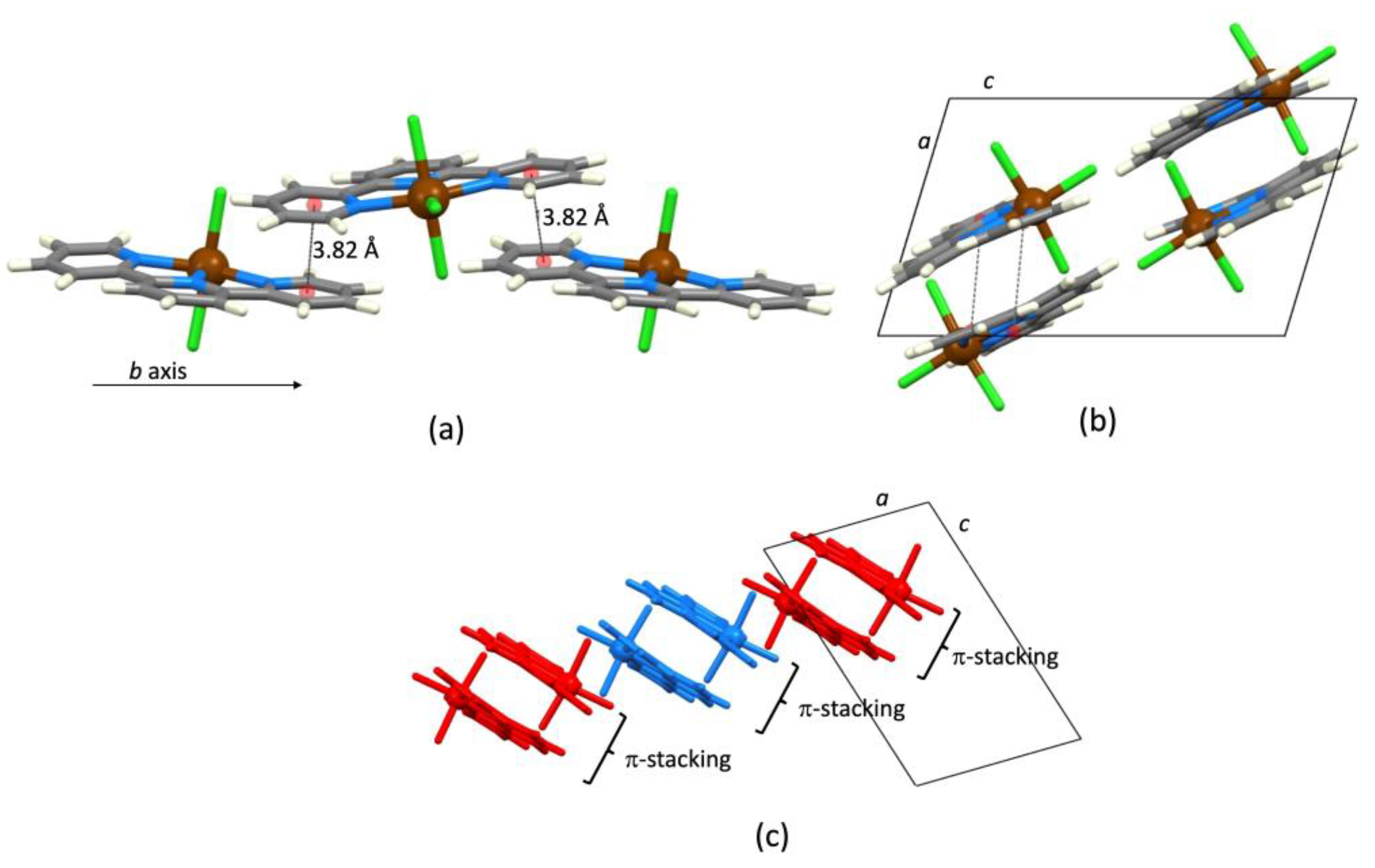
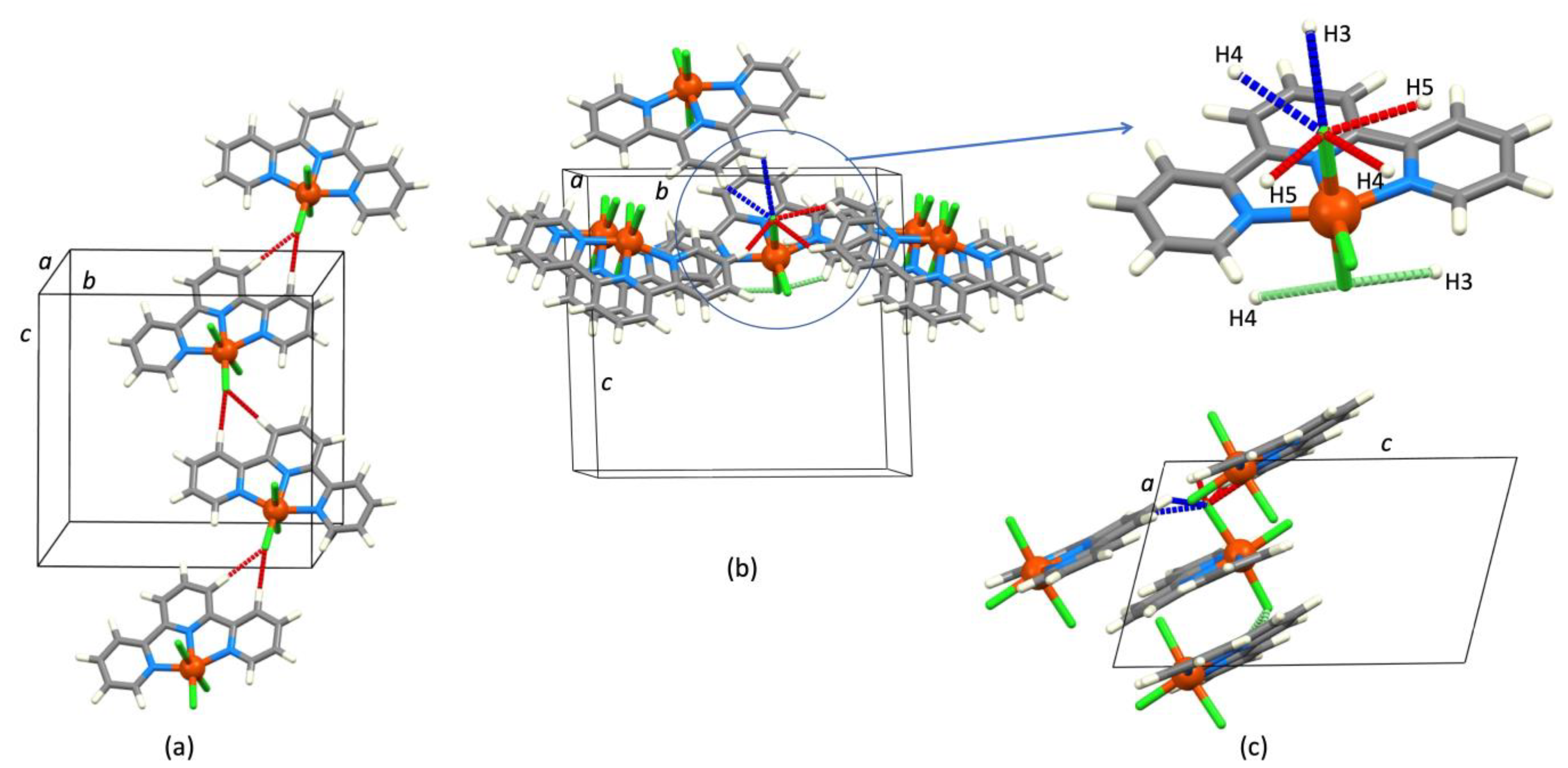
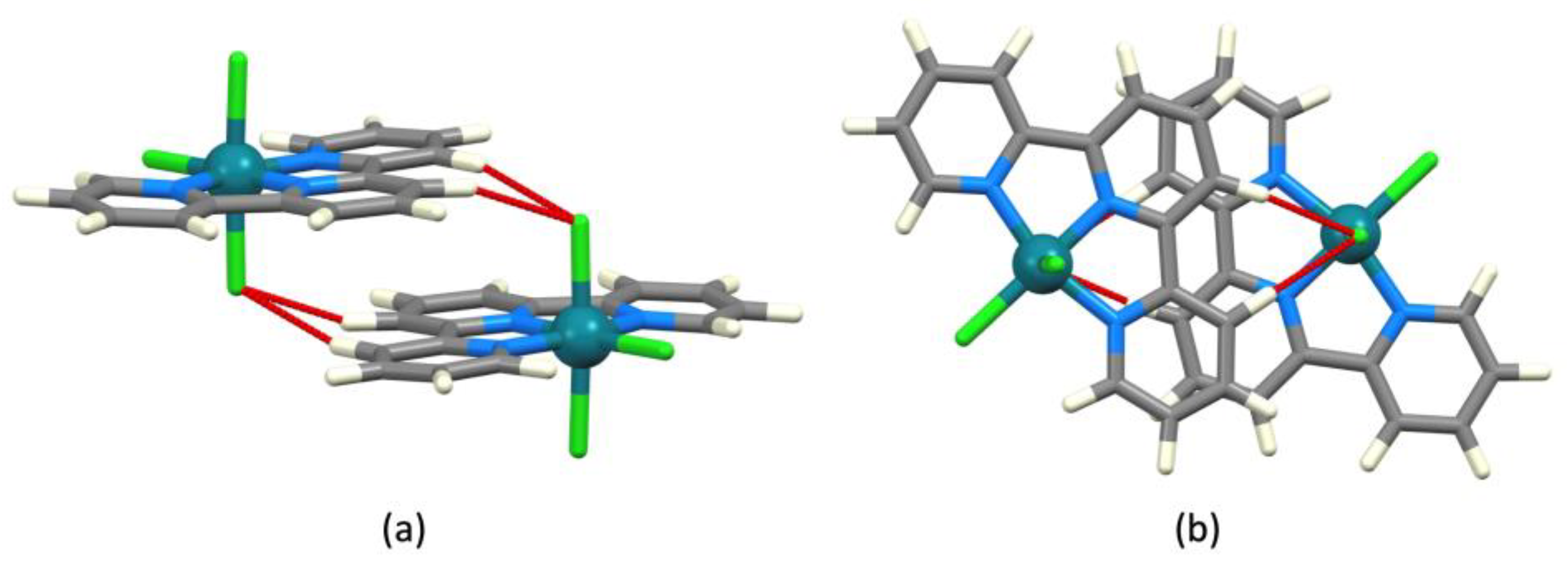
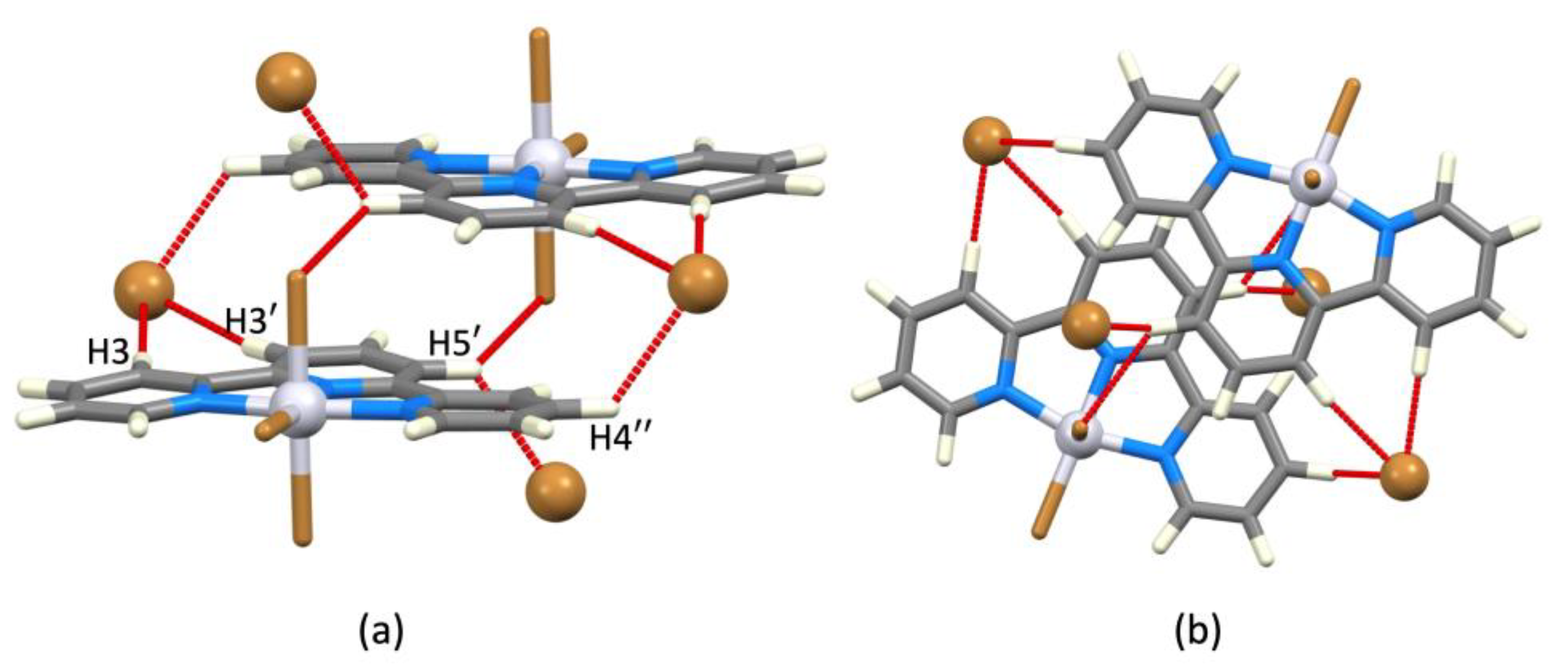
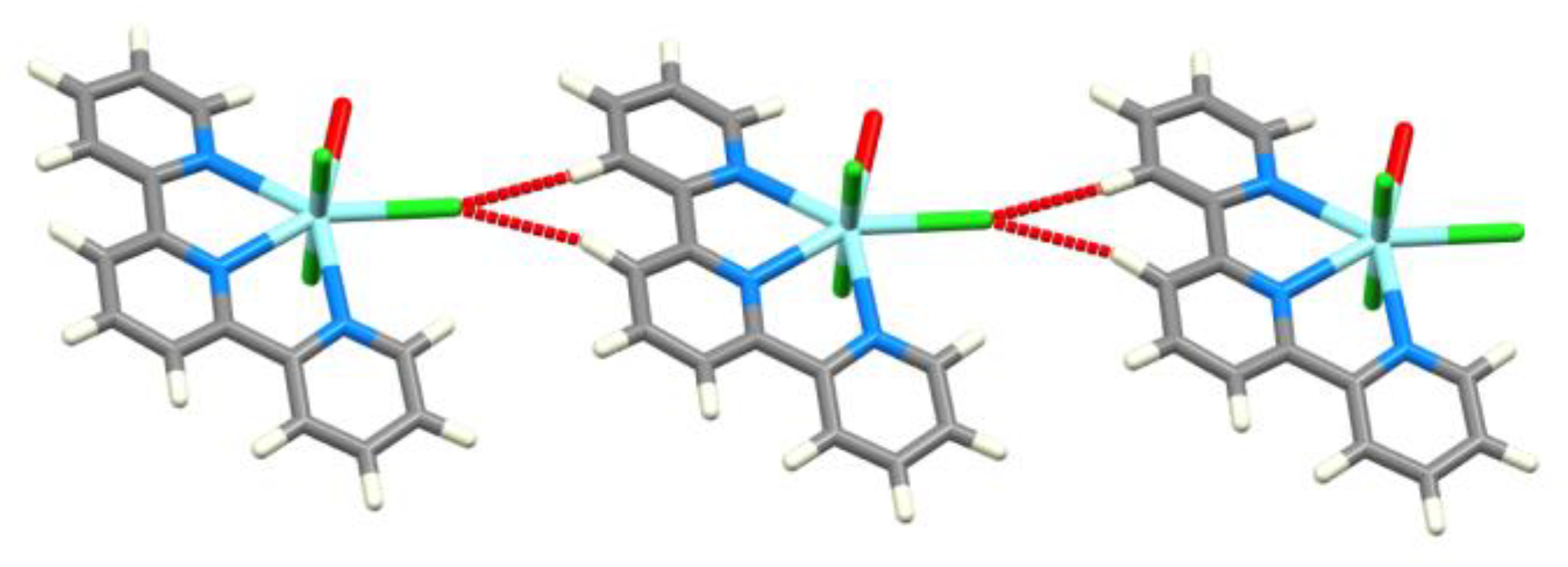
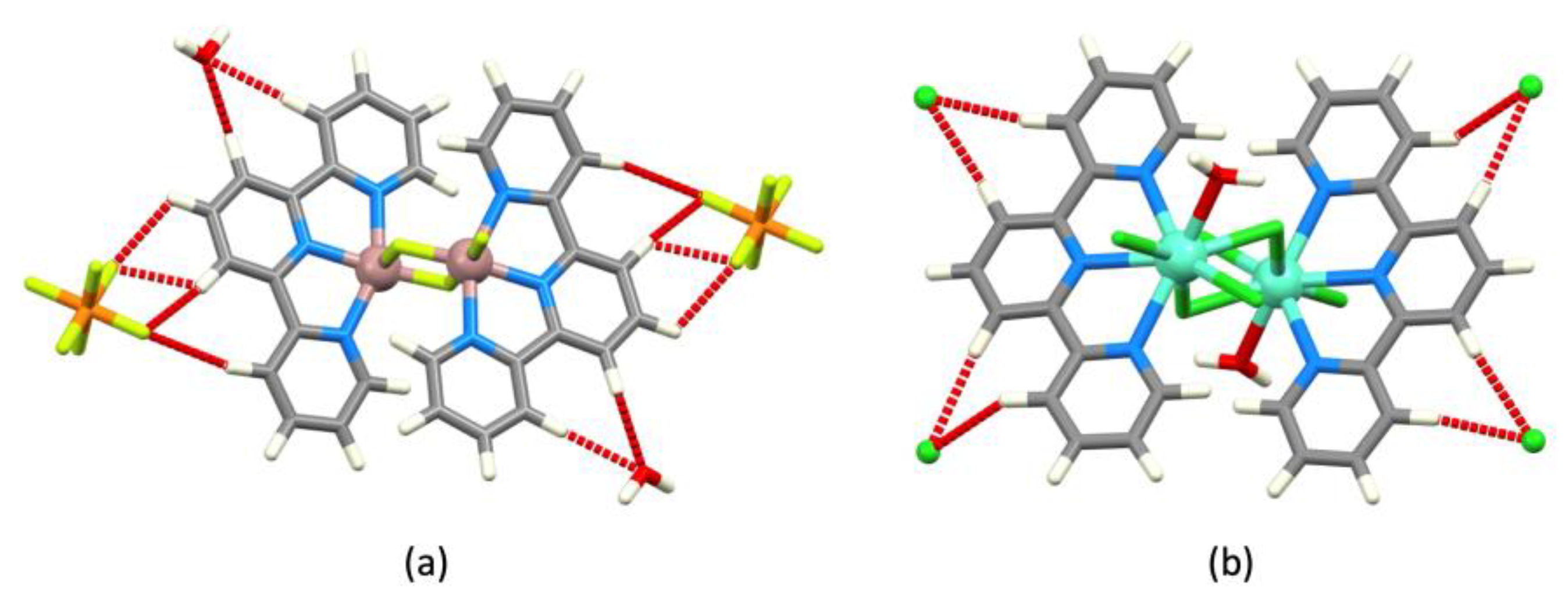
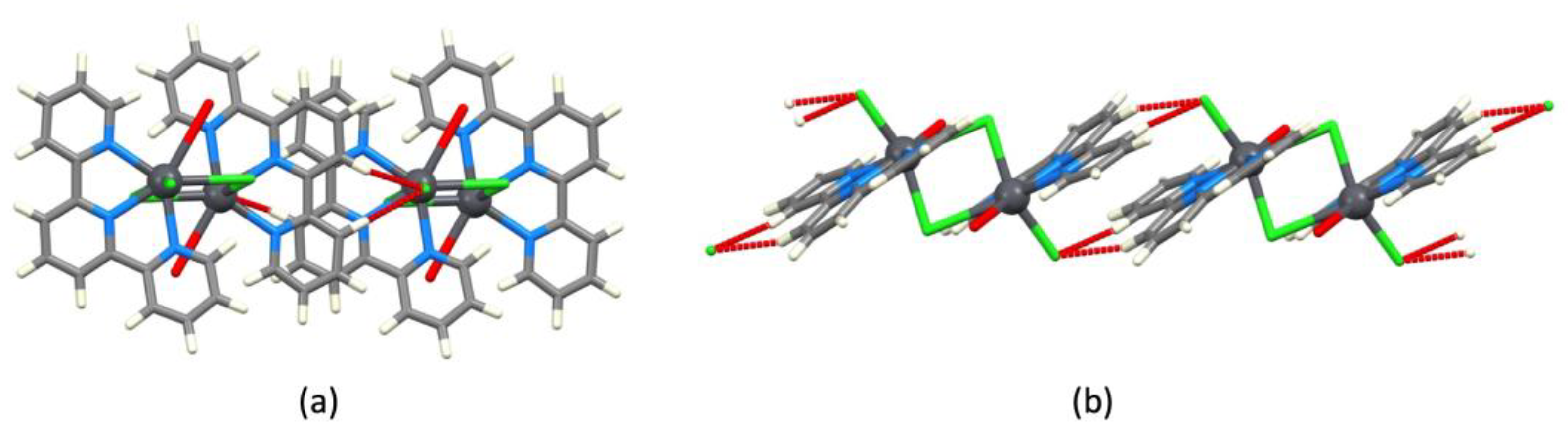
| Refcode Space Group | M | X | Centroid…Centroid Distance for tpy-tpy π-Stacking within Chain/Å a (Inter-Ring Plane Angle/°) a | Centroid…Centroid Distance for Inter-Chain Close tpy…tpy contacts/Å b (Interplane Angle/°) b | C–H3/3’…Xeq; C3/3’…Xeq/Å | C–H3/3’…Xeq/° | Ref. |
|---|---|---|---|---|---|---|---|
| TPYGAC01 P21/n | Ga | Cl | 3.84 (5.7) | 4.55 (5.7) | 2.533, 2.677; 3.621(2), 3.756(2) | 178.4, 170.7 | [28] |
| TERPIN01 P21/n | In | Cl | 3.79 (5.8) | 4.85 (5.8) | 2.550, 2.575; 3.634 (3), 3.664(2) | 177.3, 173.5 | [29] |
| TERPIN02 P21/n | In | Cl | 3.83 (5.8) | 4.92 (5.8) | 2.592, 2.607; 3.676(4), 3.696(3) | 177.8, 173.6 | [30] |
| KEZZOH P21/n | Sc | Cl | 3.82 (6.3) | 4.88 (6.3) | 2.547, 2.573; 3.635(6), 3.658(7) | 176.5, 173.5 | [31] |
| PAXVAM P21/n | Mn | Cl | 3.79 (5.5) | 4.79 (5.5) | 2.583, 2.602; 3.671(3), 3.689(3) | 177.3, 176.7 | [32] |
| HUJKIG P21/n | Fe | Cl | 3.79 (5.6) | 4.61 (5.6) | 2.525, 2.608; 3.613(2), 3.689(2) | 178.0, 171.5 | [33] |
| HUJKIG02 P21/n | Fe | Cl | 3.80 (5.7) | 4.62 (5.7) | 2.535, 2.618; 3.623(2), 3.699(2) | 178.3, 171.6 | [34] |
| CUBQEV P21/n | Ru | Cl | 3.94 (6.8) | 4.59 (6.8) | 2.58, 2.90; 3.67(1), 3.96(1) | 173.0, 166.1 | [35] |
| BONRAY P21/n | Ir | Cl | 3.90 (6.5) | 4.49 (6.5) | 2.434, 2.692; 3.522(4), 3.771(4) | 177.0, 170.5 | [36] |
| WOLDOQ P21/n | Os | Cl | 3.88 (6.7) | 4.60 (6.7) | 2.468, 2.699; 3.538(4), 3.780(4) | 171.7, 167.2 | [37] |
| LOBXAD P21/n | Ga | Br | 3.93 (5.7) | 4.60 (5.7) | 2.653, 2.806; 3.740(3), 3.892(3) | 176.4, 174.6 | [28] |
| RARVIT P21/n | Cr | Br | 3.99 (6.7) | 4.65 (6.7) | 2.594, 2.828; 3.680(3), 3.913(3) | 174.6, 174.2 | [38] |
| BONREC P21/n | Ir | Br | 4.01 (7.5) | 4.59 (7.5) | 2.537, 2.867; 3.62(2), 3.95(2) | 175.8, 173.8 | [36] |
| Refcode Space Group | M | Centroid…Centroid/Å | C–H…X; C…X/Å | C–H…X/° | C–H…X; C…X/Å | C–H…X/° | Ref. |
|---|---|---|---|---|---|---|---|
| LEWKEE | Cr | 3.81 | 2.622; 3.644(3) | 156.0 | 2.629; 3.635(2) | 153.3 | [42] |
| IGAWIW | Rh | 3.81 | 2.595; 3.638(6) | 160.3 | 2.615; 3.663(7) | 161.2 | [43] |
| Refcode Space Group | M | C–H…Cl; C…Cl/Å | C–H…Cl/° | C–H…Cl; C…Cl/Å | C–H…Cl/° | Ref. |
|---|---|---|---|---|---|---|
| KEZZUN | Y | 2.634; 3.700(5) | 166.2 | 2.831; 3.862(6) | 157.9 | [31] |
| KIBBUV | Lu | 2.638; 3.706(3) | 166.7 | 2.773; 3.825(4) | 162.3 | [31] |
| Refcode Space Group | M | C–H3/H3’…Cl; C…Cl/Å | C–H3/H3’…Cl/° | C–H5’/H3”…Cl; C…Cl/Å | C–H5’/H3”…Cl/° | Ref. |
|---|---|---|---|---|---|---|
| KIBBOP | La | 2.734, 2.798; 3.530(3), 3.743(4) | 141.8, 173.0 | 2.792, 2.652; 3.713(3), 3.592(3) | 163.4, 170.1 | [31] |
| EXODIF | Nd | 2.93, 2.85; 3.554(2), 3.791(3) | 125, 171 | 2.75, 2.70; 3.741(2), 3.621(2) | 161, 168 | [17] |
| YECRUT | Sm | 2.70, 2.96; 3.551(4), 3.802(4) | 146, 170 | 2.83, 2.84; 3.737(4), 3.622(4) | 159, 165 | [46] |
| EXODOL | Eu | 2.661, 2.724; 3.568(3), 3.797(4) | 140.3, 168.6 | 2.678, 2.550; 3.726(3), 3.627(2) | 161.3, 170.0 | [17] |
| TAQSEJ C2/c | Ca | 2.524, 2.682; 3.61(1), 3.76(1) | 171.8, 169.9 | 2.675, 2.776; 3.75(3), 3.83(2) | 170.4, 163.0 | [47] |
| AVOVEP | Bi | 2.539, 2.781; 3.564(7), 3.862(7) | 156.4, 171.7 | 2.657, 2.652; 3.723(7), 3.722(8) | 166.2, 167.3 | [45] |
| QURDUF P21/n | Bi | 2.556, 2.643; 3.626(4), 3.706(4) | 167.5, 164.9 | [48] | ||
| RAQQEF | Pb | 2.658, 2.695; 3.668(7), 3.773(8) | 153.9, 170.7 | [20] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Housecroft, C.E.; Constable, E.C. C–H…X (X = F, Cl, Br, I) Versus π-Stacking in the Crystal Packing of Compounds Containing the {M(tpy)X3} Motif. Crystals 2023, 13, 885. https://doi.org/10.3390/cryst13060885
Housecroft CE, Constable EC. C–H…X (X = F, Cl, Br, I) Versus π-Stacking in the Crystal Packing of Compounds Containing the {M(tpy)X3} Motif. Crystals. 2023; 13(6):885. https://doi.org/10.3390/cryst13060885
Chicago/Turabian StyleHousecroft, Catherine E., and Edwin C. Constable. 2023. "C–H…X (X = F, Cl, Br, I) Versus π-Stacking in the Crystal Packing of Compounds Containing the {M(tpy)X3} Motif" Crystals 13, no. 6: 885. https://doi.org/10.3390/cryst13060885
APA StyleHousecroft, C. E., & Constable, E. C. (2023). C–H…X (X = F, Cl, Br, I) Versus π-Stacking in the Crystal Packing of Compounds Containing the {M(tpy)X3} Motif. Crystals, 13(6), 885. https://doi.org/10.3390/cryst13060885







